Abstract
The present brief review discusses recent progress with corneal confocal microscopy for the evaluation of diabetic sensorimotor polyneuropathy. Corneal confocal microscopy is a new, non-invasive and reproducible diagnostic modality, and it can also be easily applied for patient follow up. It enables new perspectives of studying the natural history of diabetic sensorimotor polyneuropathy, severity of nerve fiber pathology and documenting early nerve fiber regeneration after therapeutic intervention. It shows moderate to high sensitivity and specificity for the timely diagnosis of diabetic sensorimotor polyneuropathy. Currently, corneal confocal microscopy is mainly used in specialized centers, but deserves more widespread application for the assessment of diabetic sensorimotor polyneuropathy. Finally, further progress is required in terms of technical improvements for automated nerve fiber quantification and for analysis of larger images.
Keywords: Corneal confocal microscopy, Diabetic polyneuropathy, Small fibers
Introduction
Chronic diabetic sensorimotor polyneuropathy (DSPN) is the most common diabetic complication involving the nervous system1,2. Its diagnosis mainly rests on careful clinical examination including sensory and motor modalities1, while new practical tests have been developed during the past 15 years to improve its diagnosis in clinical reality3,4. In vivo corneal confocal microscopy (CCM) of the human eye is one of these new diagnostic modalities5. It is best used in the expert setting, and enables early demonstration of nerve fiber loss5. The aim of the present brief review was to discuss recent progress with CCM in the evaluation of DSPN.
Search Strategy
We carried out an electronic search through the PubMed, Embase and Google scholar databases up to 10 January 2015 using the following key words in various combinations: ‘complications,’ ‘cornea,’ ‘corneal confocal microscopy,’ ‘diabetes,’ ‘diabetic,’ ‘diagnosis,’ ‘neuropathy,’ ‘polyneuropathy’ and ‘small-fiber.’ Articles written in English were studied in full, whereas those written in other languages were only studied in abstract form.
Corneal Nerve Fibers and CCM
The cornea of the human eye harbors a multitude of nerve fibers originating from the ophthalmic division of the trigeminal nerve, and is organized in three main groups: the sub-basal plexus, the sub-epithelial plexus and the stromal nerves6. Of these, it is the sub-basal nerve plexus lying underneath the basal epithelium that has received the most attention by CCM for the evaluation of diabetic neuropathy7. In addition, corneal Langerhans cells, which represent antigen-presenting cells, have attracted some interest during the study of diabetic neuropathy by CCM8.
In regard to the technique involved, CCM makes use of a light beam, which passes through an aperture and is appropriately focused by an objective lens into the examined cornea layer9,10. At the same time, all light coming from other points is eliminated by a beam splitter and a photodetection device9,10. Three methodological variants have been developed: the tandem scanning CCM (TSCM), the slit-scanning CCM (SSCM) and the more recent laser scanning CCM (LSCM)7–12. SSCM and LSCM have been used for the detection of corneal nerve pathology in diabetic patients with and without DSPN7. The former enables rapid visualization of all points along the axis of a given slit7,9,10,12. The latter has been used more recently, and employs a laser beam as a light source, offering higher resolution and clearer visualization of corneal epithelium and stroma7,9,10,12,13. Even more recent technological progress in LSCM includes automated software, higher magnification lenses, real-time images, and 3-D reconstruction14–19.
Role of CCM in the Assessment of Diabetic Neuropathy
The following parameters have been mainly used in the assessment of corneal nerve pathology: (i) corneal nerve fiber density (CNFD), defined as the total number of major nerves per mm2; (ii) corneal nerve fiber length (CNFL), defined as the total length of all nerve fibers and branches (mm/mm2); (iii) corneal nerve branch density (CNBD), defined as the number of branches emanating from major nerves per mm2; and (iv) corneal nerve fiber tortuosity (CNFTo), mathematically calculated as total nerve fiber curvature reflecting the variability of nerve fiber directions7. Importantly, these parameters, especially CNFL7, have now proved to be highly reproducible20–22. More recently, Petropoulos et al.23 have shown that manual CNFD and automated CNFL yielded the highest diagnostic performance for DSPN. An interesting novel parameter is tortuosity-standardized CNFL24. With this approach, standardized CNFL shows higher correlations with established measures and risk factors for DSPN as compared with classical CNFL24.
In CCM studies, diagnosis and staging of DSPN has rested on clinical examination, mainly neuropathic deficits, occasionally also nerve conduction studies (NCS)5,8,25–31. Other measures of DSPN have included intra-epidermal nerve fiber density (IENFD) and the Neuropad indicator test of sudomotor function5,27,32,33. Of particular note, Halpern et al.34 have recently shown in patients with type 1 diabetes that CNFL was diagnostically valid against various DSPN definitions, exhibiting the highest diagnostic performance against NCS criteria. Furthermore, CCM has been compared against measures of cardiac autonomic neuropathy (CAN)27,31,35. Other works have studied the association of CCM with diabetic retinopathy36–39 and reduced corneal sensation5,8,28,29,31,38.
We will now briefly discuss the role of CCM in several aspects related to diabetic neuropathies. Recent studies are summarized in Table1. Earlier works have been reviewed in more detail previously7.
Table 1.
Recent studies on corneal confocal microscopy for the evaluation of diabetic polyneuropathy
| Authors [Ref.] | n (DM/controls) | Main findings |
|---|---|---|
| Ziegler et al.5 | 86 recently diagnosed T2DM/48 | 1) Reductions in T2DM vs controls: CNFL-MNF (P = 0.001), CNFD-MNF (P < 0.001), CNBD-MNF (P < 0.001), CNCP (P = 0.006), IENFD (P < 0.001), NCS (P < 0.001), QST (P < 0.001) and VR (P = 0.006) |
| 2) CNFD-MNF among T2DM: reduced below the 2.5th percentile in 21% | ||
| 3) IENFD among T2DM: reduced below the 2.5th percentile in 14% | ||
| 4) Vast majority of patients with abnormal CNFD: concomitantly normal IENFD | ||
| 5) Vast majority of patients with abnormal IENFD: concomitantly normal CNFD | ||
| Petropoulos et al.23 | 186/55 | 1) Increasing DSPN severity: significant reduction in manual and automated CNFD (P < 0.0001), CNBD (P < 0.001), and CNFL (P < 0.0001) |
| 2) Manual and automated analysis: correlated for CNFD (r = 0.9, P < 0.0001), CNFL (r = 0.89, P < 0.0001) and CNBD (r = 0.75, P < 0.0001) | ||
| 3) Highest diagnostic performance: manual CNFD and automated CNFL | ||
| Edwards et al.24 | 231/61 | 1) Tortuosity standardized CNFL vs classical CNFL in DM: better in showing differences between DSPN and no DSPN |
| 2) Tortuosity standardized CNFL in DM: 70.5 ± 27.3 (DSPN) vs 84.9 ± 28.7 mm/mm2 (no DSPN), P < 0.001, ROC area under the curve = 0.67 | ||
| 3) Classical CNFL in DM: 15.9 ± 6.9 (DSPN) vs 18.4 ± 6.2 mm/mm2 (no DSPN), P = 0.004, ROC area under the curve = 0.64 | ||
| 4) Tortuosity standardized CNFL vs classical CNFL: 94.3 ± 27.1 (DM without DSPN) vs 84.9 ± 28.7 mm/mm2 (controls) (P = 0.028) | ||
| 5) Classical CNFL: 20.1 ± 6.3 (DM without DSPN) vs 18.4 ± 6.2 mm/mm2 (controls) (P = 0.084) | ||
| 6) Tortuosity standardized CNFL vs classical CNFL in DM: stronger correlations with DSPN attributes | ||
| 7) Tortuosity standardized CNFL vs classical CNFL in DM: stronger correlations with risk factors for DSPN | ||
| Halpern et al.34 | 89 T1DM/0 | 1) Comparable areas under the ROC curve for CNFL against various definitions of DSPN (except for clinical definition) |
| 2) DSPN definitions including NCS: optimal CNFL threshold 14 mm/mm2 | ||
| 3) Clinical DSPN definition: optimal CNFL threshold 15.4 mm/mm2 | ||
| Maddaloni et al.35 | 36 T1DM/20 | 1) T1DM vs controls: 45.4 ± 20.2 vs 92.0 ± 22.7 fibers/mm2 (P < 0.001), more tortuous corneal nerve fibers (P = 0.022), 15.1 ± 3.5 vs 20.6 ± 5.0 beadings (P < 0.001) |
| 2) In T1DM, CAN vs no CAN: CNFD 32.8 ± 16.4 vs 51.7 ± 18.9 fibers/mm2 (P = 0.008); CNFL 5.5 ± 2.4 vs 9.2 ± 3.8 mm/mm2 (P = 0.005) | ||
| 3) In T1DM, NS differences between CAN vs no CAN: branching grade (1.4 ± 0.8 vs 1.9 ± 0.7, P = 0.06), nerve tortuosity (36.4 vs 64%; P = 0.159), nerve beadings (14.8 ± 4.2 vs 15.3 ± 3.2, P = 0.719) | ||
| Zhivov et al.39 | 18 T2DM/20 | 1) Corneal sensation: 59 ± 18 mm in healthy volunteers and 43 ± 11 mm in T2DM (P < 0.001) |
| 2) Reductions in T2DM vs controls: component pixels (P < 0.001), skeleton pixels (P < 0.001), component ratio (P < 0.001), single nerve fibers (P < 0.001), single nerve fibers per component (P < 0.001), total fiber length (P < 0.001), CNFD (P < 0.001), connectivity points (P < 0.001), number of branches (P < 0.001), homogeneity of component pixels (P = 0.001) and average single fiber length (P = 0.08) | ||
| 3) T2DM: NS differences in the aforementioned CCM nerve parameters between patients with DR and those without DR | ||
| Stem et al.43 | 25 T1DM without DSPN and 18 T2DM with DSPN/9 | 1) Severe DSPN: lower CNFL vs controls (12.5 ± 6.1 mm/mm2 vs 20.7 ± 2.2 mm/mm2, P = 0.009) |
| 2) T1DM without DSPN: lower CNFL vs controls (15.1 ± 4.7 mm/mm2 vs 20.7 ± 2.2 mm/mm2, P = 0.033) | ||
| Petropoulos et al.46 | 111/47 | 1) CNFD, CNBD and CNFL: symmetrical pathology (except in patients with severe DSPN) |
| 2) CNFD: significant (P < 0.001) reduction between controls and DM with increasing DSPN severity | ||
| 3) CNBD: significant (P < 0.001) reduction between controls and DM with increasing DSPN severity | ||
| 4) CNFL: significant (P < 0.001) reduction between controls and DM with increasing DSPN severity | ||
| Ishibashi et al.47 | 78 T2DM/28 | 1) DSPN vs no DSPN: reductions in CNFD (P < 0.001), CNFL (P < 0.001) and beading frequency (P < 0.0001) with increased CNFTo (P < 0.0001) |
| 2) Sudomotor function: negative correlations with CNFD (P < 0.002) and CNBD (P < 0.01) | ||
| 3) Sweat gland duct size: correlated with triglycerides (P < 0.02), uric acid (P < 0.01), CNBD (P < 0.03), sudomotor function (P < 0.03) and DSPN severity (P < 0.03) | ||
| Dehghani et al.48 | 147 Τ1DM/60 | 1) DSPN vs controls: significant (P = 0.01) linear decline of CNFD, in association with age (P = 0.04) and T1DM duration (P = 0.03) |
| 2) DSPN: modest correlation between CNBD and peroneal conduction velocity (r = 0.38, P = 0.05) | ||
| 3) DSPN: modest correlation between CNFL and CDT (r = 0.40, P = 0.03) | ||
| Sivaskandarajah et al.49 | 96 T1DM/64 | 1) In T1DM, DSPN vs no DSPN: lower CDT values (P < 0.0001), smaller LDIFLARE areas (P = 0.0002), and lower HRV values (P < 0.0001) |
| 2) In T1DM, reduction of CNFL by every 1 mm/mm2: association with a 0.61°C lower CDT, a 0.07 cm2 lower LDIFLARE area, and a 1.78% lower HRV | ||
| 3) CNFL in T1DM: significant positive correlations with CDT (P = 0.0002), LDIFLARE (P = 0.002) and HRV (P < 0.0001) | ||
| 4) CNFD in T1DM: significant positive correlations with CDT (P = 0.002), LDIFLARE (P = 0.0002) and HRV (P = 0.003) | ||
| 5) CNBD in T1DM: significant positive correlations with CDT (P = 0.0002), LDIFLARE (P = 0.01) and HRV (P = 0.001) | ||
| 6) CNFTo in T1DM: no association with small-fiber function | ||
| Dehghani et al.54 | 0/64 | 1) Age: significant (P = 0.02) linear CNFL reduction by 0.05 mm/mm2 per added year |
| 2) CNFL: NS change in over 36 months (P = 0.41) | ||
| Pritchard et al.51 | 242 T1DM (76 with DSPN, 166 without DSPN)/154 | 1) CNFL: lower in T1DM with DSPN (14.0 ± 6.4 mm/mm2) vs T1DM without DSPN (19.1 ± 5.8 mm/mm2) and controls (23.2 ± 6.3 mm/mm2) (P < 0.001) |
| 2) CNFL: lower in T1DM without DSPN (19.1 ± 5.8 mm/mm2) vs controls (23.2 ± 6.3 mm/mm2) (P < 0.001) | ||
| 3) CNBD: lower in T1DM with DSPN (40.1 ± 32.1 branches/mm2) vs T1DM without DSPN (61.7 ± 37.2 branches/mm2) and controls (83.5 ± 45.8 branches/mm2) (P < 0.001) | ||
| 4) CNBD: lower in T1DM without DSPN (61.7 ± 37.2 branches/mm2) vs controls (83.5 ± 45.8 branches/mm2) (P = 0.001) | ||
| Asghar et al.52 | 37 IGT/20 | 1) IGT vs controls: significantly increased NSP (P < 0.001), McGill pain index (P < 0.001), NDS (P = 0.001), VPT (P = 0.002), WDT (P = 0.006) and CDT (P = 0.03) |
| 2) IGT vs controls: reductions in IENFD (P = 0.03), CNFD (P < 0.001), CNBD (P = 0.002) and CNFL (P = 0.05) | ||
| Pritchard et al.55 | 90 T1DM without DSPN | 1) Development of DSPN after 4 years: associations with lower CNFL (P = 0.041) |
| 2) Development of DSPN after 4 years: associations with longer T1DM duration (P = 0.002), higher triglycerides (P = 0.023), retinopathy (P = 0.008), nephropathy (P = 0.001), higher NDS (P = 0.037), lower CDT (P = 0.001), higher WDT (P = 0.008), higher VPT (P = 0.003), impaired monofilament response (P = 0.003), NCS impairments (P < 0.05) | ||
| 3) CNFL cut-off of 14.1 mm/mm2: 63% sensitivity and 74% specificity for the prediction of DSPN after 4 years | ||
| Azmi et al.56 | 49 T1DM (18 CSII, 31 MDI)/40 | T1DM CSII vs T1DM MDI: increase in CNFD (P = 0.05), CNBD (P = 0.006) and CNFL (P = 0.003), NS difference in VPT, CDT, WDT, NCS or IENFD |
| Brines et al.57 | 48 T2DM/55 | ARA 290 vs placebo: improvement of neuropathic symptoms (P = 0.037), increase in CNFD (P = 0.02) and reduction in HbA1c (P = 0.002) |
| Tavakoli et al.58 | 34/18 | 1) CNFD (best cut-off <23.26 nerves per mm2): 86% sensitivity and 78% specificity for the diagnosis of DAN (AUC = 0.915, P = 0.0001) |
| 2) CNBD (best cut-off <19.53 branches per mm2): 100% sensitivity and 56% specificity for the diagnosis of DAN (AUC = 0.889, P = 0.0001) | ||
| 3) CNFL (best cut-off <4.78 mm/mm2): 86% sensitivity and 78% specificity for the diagnosis of DAN (AUC = 0.907, P = 0.0001) | ||
| 4) CNFD, CNBD, CNFL: significant (P < 0.001) correlations with CASS and COMPASS |
ARA 290, a peptide derived from erythropoietin
AUC, area under the curve
CAN, cardiac autonomic neuropathy
CASS, composite autonomic scoring scale
CCM, corneal confocal microscopy
CDT, cooling detection threshold
CNBD, corneal nerve fiber branch density
CNFD, corneal nerve fiber density
CNFL, corneal nerve fiber length
CNFTo, corneal nerve fiber tortuosity
CNCP, corneal nerve connecting points
COMPASS, composite autonomic symptom scale
CSII, continuous subcutaneous insulin infusion
DAN, diabetic autonomic neuropathy
DR, diabetic retinopathy
DSPN, diabetic polyneuropathy
HbA1c, glycated hemoglobin
HRV, heart rate variability
IENFD, intra-epidermal nerve fiber density
IGT, impaired glucose tolerance
LDIFLARE, laser Doppler imaging flare
MDI, multiple daily insulin injections
MNF, major nerve fibers
NCS, nerve conduction study
NDS, neuropathy disability score
NS, not significant
NSP, neuropathy symptom profile
QST, quantitative sensory testing
T1DM, type 1 diabetes mellitus
T2DM, type 2 diabetes mellitus
VPT, vibration perception threshold
VR, Valsalva ratio
WDT, warm detection threshold.
Role in Detecting DSPN
Given the fact that corneal nerve fiber pathology is more severe in the presence of DSPN5,7,8,25–28,33, it has been suggested to use CCM for the diagnosis of this complication7,8. Of particular note, CCM is sensitive enough to detect corneal nerve fiber perturbations as early as in recently diagnosed diabetes5 (Figure1) and before clinical neuropathic deficits develop33, encouraging the hope that this modality might prove useful for the earliest diagnosis of DSPN.
Figure 1.
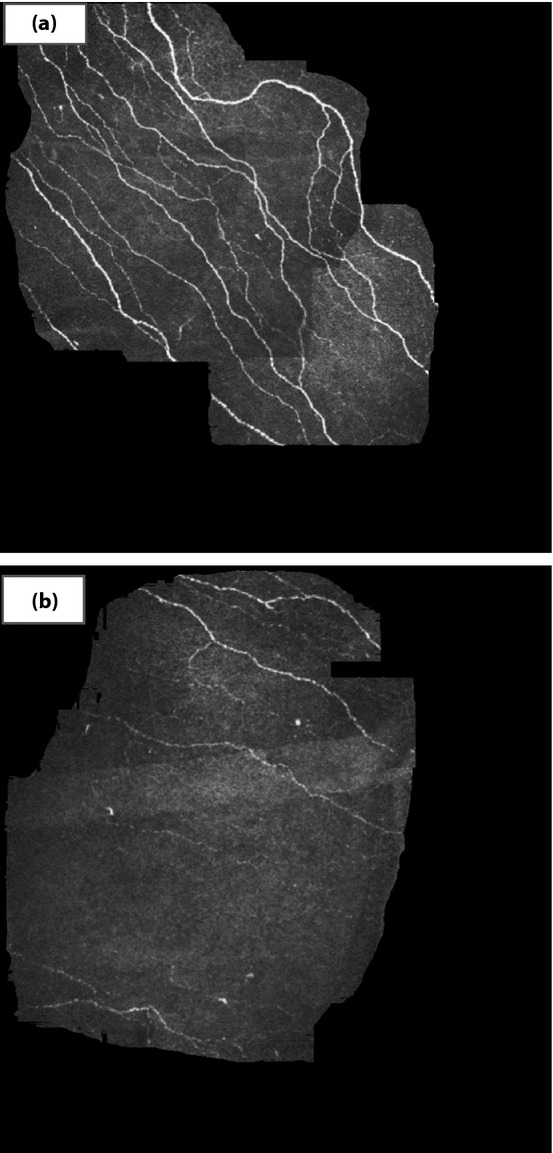
Corneal confocal microscopy showing the sub-basal nerve plexus. (a) Normal structure corneal nerve fibers in a healthy subject. (b) Loss of corneal nerve fibers in a recently diagnosed subject with type 2 diabetes.
In this context, some works have examined the sensitivity and specificity of CCM for the detection of DSPN28,32,40. Sensitivity and specificity are summarized in Table2. Compared with IENFD, CNFD was more specific but less sensitive32. Of note, Ahmed et al.40 identified the two best diagnostic cut-off points: (i) CNFL ≤14.0 mm/mm2 with 85% sensitivity, 84% specificity, positive likelihood ratio of 5.3 and negative likelihood ratio of 0.18; and (ii) CNFL ≥15.8 mm/mm2 with 91% sensitivity, 93% specificity, positive likelihood ratio of 8.5 and negative likelihood ratio of 0.1640.
Table 2.
Sensitivity and specificity of corneal confocal microscopy and skin biopsy
| Tavakoli et al.28 | ||||
|---|---|---|---|---|
| CNFD | CNBD | |||
| Diagnosis | Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) |
| DSPN | 82 | 52 | 91 | 45 |
| At-risk foot | 71 | 64 | 71 | 71 |
| Quattrini et al.32 | ||||
|---|---|---|---|---|
| CNFD | IENFD | |||
| Diagnosis | Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) |
| DSPN | 56 | 75 | 78 | 56 |
| At-risk foot | 63 | 72 | 88 | 54 |
| Small-fiber neuropathy | 50 | 84 | 69 | 63 |
| Severe small-fiber neuropathy | 86 | 69 | 86 | 46 |
CCM, corneal confocal microscopy
CNBD, corneal nerve fiber branch density
CNFD, corneal nerve fiber density
CNFL, corneal nerve fiber length
DSPN, diabetic polyneuropathy
IENFD, intra-epidermal nerve fiber density.
Role in Staging for DSPN Severity
CCM parameters typically deteriorate progressively with increasing severity of neuropathy5,8,25–29,33,41–43. CNFD, CNBD and CNFL show a significant negative correlation with neuropathic deficits8,27,29,41,44–46 and Neuropad response33,47, as well as a positive correlation with upper5 and lower extremity nerve conduction velocities5,29,48–50. Edwards et al.45 found that CNFL and CNBD most strongly correlated with NCS attributes and modestly with the other tests of neuropathy. There is also evidence for a negative correlation between corneal nerve fiber pathology and small-fiber dysfunction: impairment of pain/thermal perception27,48,49 and diminution of axon reflex-mediated neurogenic vasodilatation in response to cutaneous heating (laser Doppler imaging flare [LDIFLARE])49. In type 1 diabetes, every 1-mm/mm2 reduction of CNFL was linked with a 0.61°C reduction of cold perception threshold and a 0.07-cm2 reduction of the LDIFLARE area49. Furthermore, a positive correlation between CNFD and IENFD has been found in some27,31,50, but not in all5 studies.
Manifestation Patterns
Corneal nerve fiber pathology has been found to be symmetrical46, similar to DSPN in general1,2, except in patients with very severe DSPN46. In both diabetes types, corneal nerve fibers might be affected before DSPN becomes clinically manifest5,33,51. In type 1 diabetes, CNFL even among patients without DSPN might be lower than among controls43,51. Stem et al.43 have carried out an interesting comparison between the two types of diabetes. In type 1 diabetes without DSPN, CNFL was significantly reduced compared with controls, whereas among patients with type 2 diabetes, only those with severe DSPN showed a significant reduction of CNFL compared with controls43. Thus, it has been argued that CNFL might be reduced in type 1 diabetes earlier than in type 2 diabetes43. A further interesting observation in recently diagnosed diabetic patients is that they frequently show abnormal CNFD with concomitant normal IENFD and vice versa, suggesting that small nerve fibers are not simultaneously affected in all organs5.
Role in Impaired Glucose Tolerance
An initial small series of subjects with small-fiber neuropathy showed perturbations in CNFL, CNFD, CNBD and CNFTo, but there were no differences in corneal parameters between subjects with and those without impaired glucose tolerance (IGT)29. However, these observations were based on just eight IGT subjects29. Indeed, a larger series including 37 subjects with IGT and 20 age-matched control subjects found that the former showed significant reductions in CNFD, CNBD, CNFL and IENFD along with other manifestations of predominantly small-fiber neuropathy52. These findings add to the growing knowledge on the early beginning of neuropathy, as early as in prediabetes53.
Natural History
In healthy individuals, age induced a slight but significant (P = 0.02) linear diminution of CNFL: this was calculated as a linear decrease by 0.05 mm/mm2 for every added year of age54. Despite this, however, CNFL remained fairly constant over 36 months54. In type 1 diabetes, after a 4-year follow up there was a significant reduction of CNFD in association with age (P = 0.04) and type 1 diabetes duration (P = 0.03)48. Impressively, small changes in corneal nerve fibers are indicative of clinical DSPN after 4 years in type 1 diabetes55: lower CNFL has been found to be associated with DSPN after 4 years (P = 0.041). A CNFL cut-off of 14.1 mm/mm2 could predict DSPN after 4 years with 63% sensitivity and 74% specificity55.
Effects of Therapeutic Interventions
There is evidence that CCM can objectify early nerve fiber improvements. In an observational study enrolling 25 diabetic patients with mild/moderate DSPN, improved cholesterol levels after 24 months were linked to significant improvements in CNFD, CNBD and CNFTo, and glycated hemoglobin reduction was significantly correlated with the increase in CNFD30. In another setting, simultaneous pancreas and kidney transplantation in patients with type 1 diabetes induced significant improvements in CNFD and CNFL at 6 months, as well as in CNFD, CNFL, CNBD at 12 months31,50. Among all diagnostic modalities investigated, only CCM showed improvement after 12 months31. Very recently, the effect of continuous subcutaneous insulin infusion on DSPN was compared with that of multiple insulin injections in type 1 diabetes56. After 24 months, significant increases in CNFD (P = 0.05), CNBD (P = 0.006) and CNFL (P = 0.003) were noted only in the group under continuous subcutaneous insulin infusion, despite suboptimal and comparable glycemic control in both treatment arms56. Arguably, the stability of glycemic control accomplished with continuous subcutaneous insulin infusion exerted a beneficial effect on small nerve fibers, and CCM was accurate enough to show this effect56. Brines et al.57 have shown that administration of ARA 290, a peptide derived from erythropoietin, for 28 days could significantly improve neuropathic symptoms (P = 0.037) and CNFD (P = 0.02) in type 2 diabetes.
Associations with Retinopathy and Corneal Sensation
Corneal nerve fiber pathology has been found to be associated with both the presence and severity of diabetic retinopathy (background vs proliferative retinopathy)36–38. Conversely, Zhivov et al.39 have reported no difference in corneal nerve morphology between patients with vs without diabetic retinopathy. Patients with DSPN frequently have reduced corneal sensation as well, and a positive correlation between CNFD and corneal sensation has been reported41. Tavakoli et al.8 have reawakened the interest in the immune component of DSPN by showing a significant increase of corneal Langerhans cells in diabetic patients as well as an inverse correlation between these cells and the clinical severity of DSPN. The authors’ interpretation was that the increase of Langerhans cells in early DSPN pointed to the role of immune mechanisms in the first steps of its pathogenesis, although other factors became later more decisive in advanced DSPN8.
Role in Detecting Autonomic Neuropathy
CCM parameters show a positive correlation with heart rate variability on deep breathing27,49. In type 1 diabetes, every 1-mm/mm2 reduction of CNFL has been reported to be linked with a reduction of heart rate variability by 1.78%49. In a study comparing 36 type 1 diabetic patients with 20 age- and sex-matched controls, the former showed fewer (P < 0.001) and more tortuous corneal nerve fibers (P = 0.022), and fewer beadings (P < 0.001) than the latter35. Among patients with type 1 diabetes, CNFD was significantly lower (P = 0.008) in the presence of CAN, and this difference remained significant after adjustment for age, sex, type 1 diabetes duration, insulin dosage and severity of DSPN35. Similarly, CNFL was significantly lower in the presence of CAN (P = 0.005), and this difference remained significant after adjustment for type 1 diabetes duration, insulin dosage and severity of DSPN, but it lost significance after adjustment for age and sex35. Tavakoli et al.58 have recently reported that CNFD, CNBD and CNFL yielded very high (86–100%) sensitivity and moderate to high specificity (56–78%) for the diagnosis of autonomic neuropathy (Table1). Furthermore, these parameters showed significant (P < 0.001) correlations with the gravity of autonomic symptoms58.
Future Perspectives
There has been considerable progress with CCM for the evaluation of DSPN, and more knowledge is still accumulating. Future improvements should be mainly pursued in the following areas.
Widespread Use of CCM
For the time being, CCM is mainly used in specialized centers7. Given the evidence for its moderate to high sensitivity and specificity for the diagnosis of DSPN, it is reasonable that this modality could be of increased clinical utility, once technology and know-how become more widely available. Further arguments in favor of its wider availability include its non-invasive nature and the ability to repeat the examination for patient follow up30,31,50,56,57.
Normative Database
Especially if CCM becomes more widely used, we need a database of normative values, similar to the one reported for IENFD59. Such normal values are now for the first time becoming available60, but their application in clinical practice is awaited.
Revisiting the Efficacy of Pathogenetic Treatments Through CCM
The effect of neuroprotective, disease-modifying agents on peripheral nerve structure can now be revisited utilizing CCM. This suggestion is based on the ability of CCM to visualize nerve fiber regeneration30,31,50,56,57.
More Experience in Children and Adolescents
After the interesting pilot study by Sellers et al.61, additional experience in diabetic children and adolescents is highly welcome.
Technical Improvements in Automated Nerve Fiber Quantification and Image Analysis
Improved automated fiber measurement10,23 and wider-area image analysis5,17,19 are expected to increase accuracy and to enable the acquisition of more representative images.
Conclusions
There are now more than 10 years of experience with CCM for the evaluation of DSPN7,62. The advantages of CCM include its non-invasive nature, its high reproducibility20–22 and its easy application for patient follow up30,31,50,56,57. CCM opens new perspectives of studying the natural history of DSPN, staging nerve fiber pathology5,8,25–29,33,41–43,45,46,49 and documenting incipient nerve fiber regeneration after therapeutic intervention30,31,50,56,57. Importantly, it is useful for the early detection of nerve pathology5,33 and high-risk foot32 with moderate to high sensitivity and specificity28,32,34,40.
Based on this ample evidence, more widespread application of CCM for the evaluation of DSPN can be advocated. Such a broad utilization should serve both diagnostic and prognostic purposes in terms of DSPN evaluation at baseline and/or after therapeutic interventions5,30,31,33,50,56,57. Importantly, normal values for CCM parameters are now becoming available60 and need to be applied in practice. Finally, technical improvements in automated nerve fiber quantification and wider-area image analysis5,10,17,19,23 are more than welcome to increase diagnostic performance.
Acknowledgments
N Papanas wrote the first draft of the manuscript; Dan Ziegler edited and provided valuable insight and critical comments. N Papanas and D Ziegler have been scientific advisory board members for TrigoCare International, distributor of Neuropad.
References
- 1.Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler D, Papanas N, Vinik AI, et al. Epidemiology of polyneuropathy in diabetes and prediabetes. Handb Clin Neurol. 2014;126:3–22. doi: 10.1016/B978-0-444-53480-4.00001-1. [DOI] [PubMed] [Google Scholar]
- 3.Papanas N, Ziegler D. New diagnostic tests for diabetic distal symmetric polyneuropathy. J Diabetes Complications. 2011;25:44–51. doi: 10.1016/j.jdiacomp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Papanas N, Ziegler D. New vistas in the diagnosis of diabetic polyneuropathy. Endocrine. 2014;47:690–698. doi: 10.1007/s12020-014-0285-z. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler D, Papanas N, Zhivov A, et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014;63:2454–2463. doi: 10.2337/db13-1819. [DOI] [PubMed] [Google Scholar]
- 6.Al-Aqaba MA, Fares U, Suleman H, et al. Architecture and distribution of human corneal nerves. Br J Ophthalmol. 2010;94:784–789. doi: 10.1136/bjo.2009.173799. [DOI] [PubMed] [Google Scholar]
- 7.Papanas N, Ziegler D. Corneal confocal microscopy: a new technique for early detection of diabetic neuropathy. Curr Diab Rep. 2013;13:488–499. doi: 10.1007/s11892-013-0390-z. [DOI] [PubMed] [Google Scholar]
- 8.Tavakoli M, Boulton AJ, Efron N, et al. Increased Langerhans cell density and corneal nerve damage in diabetic patients: role of immune mechanisms in human diabetic neuropathy. Cont Lens Anterior Eye. 2011;34:7–11. doi: 10.1016/j.clae.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jalbert I, Stapleton F, Papas E, et al. In vivo confocal microscopy of the human cornea. Br J Ophthalmol. 2003;87:225–236. doi: 10.1136/bjo.87.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guthoff RF, Zhivov A, Stachs O. In vivo confocal microscopy, an inner vision of the cornea - a major review. Clin Experiment Ophthalmol. 2009;37:100–117. doi: 10.1111/j.1442-9071.2009.02016.x. [DOI] [PubMed] [Google Scholar]
- 11.Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 2010;25:171–177. doi: 10.3109/08820538.2010.518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel DV, McGhee CN. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol. 2009;93:853–860. doi: 10.1136/bjo.2008.150615. [DOI] [PubMed] [Google Scholar]
- 13.Zhivov A, Stachs O, Kraak R, et al. In vivo confocal microscopy of the ocular surface. Ocul Surf. 2006;4:81–93. doi: 10.1016/s1542-0124(12)70030-7. [DOI] [PubMed] [Google Scholar]
- 14.Holmes TJ, Pellegrini M, Miller C, et al. Automated software analysis of corneal micrographs for peripheral neuropathy. Invest Ophthalmol Vis Sci. 2010;51:4480–4491. doi: 10.1167/iovs.09-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hume DA, Lovblom LE, Ahmed A, et al. Higher magnification lenses versus conventional lenses for evaluation of diabetic neuropathy by corneal in vivo confocal microscopy. Diabetes Res Clin Pract. 2012;97:e37–e40. doi: 10.1016/j.diabres.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Zhivov A, Guthoff R, Stachs O. On-line mapping of corneal structures with in vivo laser scanning microscopy. Klin Monbl Augenheilkd. 2009;226:980–983. doi: 10.1055/s-0028-1109802. [DOI] [PubMed] [Google Scholar]
- 17.Zhivov A, Blum M, Guthoff R, et al. Real-time mapping of the subepithelial nerve plexus by in vivo confocal laser scanning microscopy. Br J Ophthalmol. 2010;94:1133–1135. doi: 10.1136/bjo.2009.175489. [DOI] [PubMed] [Google Scholar]
- 18.Allgeier S, Zhivov A, Eberle F, et al. Image reconstruction of the subbasal nerve plexus with in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2011;52:5022–5028. doi: 10.1167/iovs.10-6065. [DOI] [PubMed] [Google Scholar]
- 19.Edwards K, Pritchard N, Gosschalk K, et al. Wide-field assessment of the human corneal subbasal nerve plexus in diabetic neuropathy using a novel mapping technique. Cornea. 2012;31:1078–1082. doi: 10.1097/ico.0b013e318245c012. [DOI] [PubMed] [Google Scholar]
- 20.Efron N, Edwards K, Roper N, et al. Repeatability of measuring corneal subbasal nerve fiber length in individuals with type 2 diabetes. Eye Contact Lens. 2010;36:245–248. doi: 10.1097/ICL.0b013e3181eea915. [DOI] [PubMed] [Google Scholar]
- 21.Petropoulos IN, Manzoor T, Morgan P, et al. Repeatability of in vivo corneal confocal microscopy to quantify corneal nerve morphology. Cornea. 2013;32:e83–e89. doi: 10.1097/ICO.0b013e3182749419. [DOI] [PubMed] [Google Scholar]
- 22.Hertz P, Bril V, Orszag A, et al. Reproducibility of in vivo corneal confocal microscopy as a novel screening test for early diabetic sensorimotor polyneuropathy. Diabet Med. 2011;28:1253–1260. doi: 10.1111/j.1464-5491.2011.03299.x. [DOI] [PubMed] [Google Scholar]
- 23.Petropoulos IN, Alam U, Fadavi H, et al. Rapid automated diagnosis of diabetic peripheral neuropathy with in vivo corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2014;55:2071–2078. doi: 10.1167/iovs.13-13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards K, Pritchard N, Vagenas D, et al. Standardizing corneal nerve fibre length for nerve tortuosity increases its association with measures of diabetic neuropathy. Diabet Med. 2014;31:1205–1209. doi: 10.1111/dme.12466. [DOI] [PubMed] [Google Scholar]
- 25.Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46:683–688. doi: 10.1007/s00125-003-1086-8. [DOI] [PubMed] [Google Scholar]
- 26.Kallinikos P, Berhanu M, O'Donnell C, et al. Corneal nerve tortuosity in diabetic patients with neuropathy. Invest Ophthalmol Vis Sci. 2004;45:418–422. doi: 10.1167/iovs.03-0637. [DOI] [PubMed] [Google Scholar]
- 27.Quattrini C, Tavakoli M, Jeziorska M, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56:2148–2154. doi: 10.2337/db07-0285. [DOI] [PubMed] [Google Scholar]
- 28.Tavakoli M, Quattrini C, Abbott C, et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010;33:1792–1797. doi: 10.2337/dc10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tavakoli M, Marshall A, Pitceathly R, et al. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol. 2010;223:245–250. doi: 10.1016/j.expneurol.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavakoli M, Kallinikos P, Iqbal A, et al. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet Med. 2011;28:1261–1267. doi: 10.1111/j.1464-5491.2011.03372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tavakoli M, Mitu-Pretorian M, Petropoulos IN, et al. Corneal Confocal Microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes. 2013;62:254–260. doi: 10.2337/db12-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quattrini C, Tavakoli M, Kallinikos P, et al. Comparing skin biopsy with corneal confocal microscopy: diagnostic yield of nerve fibre density. Diabetologia. 2010;53(Suppl 1):A1114. [Google Scholar]
- 33.Tavakoli M, Quattrini C, Begum P, et al. Neuropad and corneal confocal microscopy: new indications for human diabetic neuropathy. Diabetologia. 2010;53(Suppl 1):A1112. [Google Scholar]
- 34.Halpern EM, Lovblom LE, Orlov S, et al. The impact of common variation in the definition of diabetic sensorimotor polyneuropathy on the validity of corneal in vivo confocal microscopy in patients with type 1 diabetes: a brief report. J Diabetes Complications. 2013;27:240–242. doi: 10.1016/j.jdiacomp.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Maddaloni E, Sabatino F, Del Toro R, et al. In vivo corneal confocal microscopy as a novel non-invasive tool to investigate cardiac autonomic neuropathy in Type 1 diabetes. Diabet Med. 2015;32:262–266. doi: 10.1111/dme.12583. [DOI] [PubMed] [Google Scholar]
- 36.Mocan MC, Durukan I, Irkec M, et al. Morphologic alterations of both the stromal and subbasal nerves in the corneas of patients with diabetes. Cornea. 2006;25:769–773. doi: 10.1097/01.ico.0000224640.58848.54. [DOI] [PubMed] [Google Scholar]
- 37.De Cillà S, Ranno S, Carini E, et al. Corneal subbasal nerves changes in patients with diabetic retinopathy: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2009;50:5155–5158. doi: 10.1167/iovs.09-3384. [DOI] [PubMed] [Google Scholar]
- 38.Messmer EM, Schmid-Tannwald C, Zapp D, et al. In vivo confocal microscopy of corneal small fiber damage in diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 2010;248:1307–1312. doi: 10.1007/s00417-010-1396-8. [DOI] [PubMed] [Google Scholar]
- 39.Zhivov A, Winter K, Hovakimyan M, et al. Imaging and quantification of subbasal nerve plexus in healthy volunteers and diabetic patients with or without retinopathy. PLoS One. 2013;8:e52157. doi: 10.1371/journal.pone.0052157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed A, Bril V, Orszag A, et al. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes Care. 2012;35:821–828. doi: 10.2337/dc11-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenberg ME, Tervo TM, Immonen IJ, et al. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;41:2915–2921. [PubMed] [Google Scholar]
- 42.Midena E, Brugin E, Ghirlando A, et al. Corneal diabetic neuropathy: a confocal microscopy study. J Refract Surg. 2006;22(9 Suppl):S1047–S1052. doi: 10.3928/1081-597X-20061102-08. [DOI] [PubMed] [Google Scholar]
- 43.Stem MS, Hussain M, Lentz SI, et al. Differential reduction in corneal nerve fiber length in patients with type 1 or type 2 diabetes mellitus. J Diabetes Complications. 2014;28:658–661. doi: 10.1016/j.jdiacomp.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nitoda E, Kallinikos P, Pallikaris A, et al. Correlation of diabetic retinopathy and corneal neuropathy using confocal microscopy. Curr Eye Res. 2012;37:898–906. doi: 10.3109/02713683.2012.683507. [DOI] [PubMed] [Google Scholar]
- 45.Edwards K, Pritchard N, Vagenas D, et al. Utility of corneal confocal microscopy for assessing mild diabetic neuropathy: baseline findings of the LANDMark study. Clin Exp Optom. 2012;95:348–354. doi: 10.1111/j.1444-0938.2012.00740.x. [DOI] [PubMed] [Google Scholar]
- 46.Petropoulos IN, Alam U, Fadavi H, et al. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care. 2013;36:3646–3651. doi: 10.2337/dc13-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishibashi F, Kojima R, Kawasaki A, et al. Correlation between sudomotor function, sweat gland duct size and corneal nerve fiber pathology in patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5:588–596. doi: 10.1111/jdi.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dehghani C, Pritchard N, Edwards K, et al. Natural history of corneal nerve morphology in mild neuropathy associated with type 1 diabetes: development of a potential measure of diabetic peripheral neuropathy. Invest Ophthalmol Vis Sci. 2014;55:7982–7990. doi: 10.1167/iovs.14-15605. [DOI] [PubMed] [Google Scholar]
- 49.Sivaskandarajah GA, Halpern EM, Lovblom LE, et al. Structure-function relationship between corneal nerves and conventional small-fiber tests in type 1 diabetes. Diabetes Care. 2013;36:2748–2755. doi: 10.2337/dc12-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mehra S, Tavakoli M, Kallinikos PA, et al. Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care. 2007;30:2608–2612. doi: 10.2337/dc07-0870. [DOI] [PubMed] [Google Scholar]
- 51.Pritchard N, Edwards K, Dehghani C, et al. Longitudinal assessment of neuropathy in type 1 diabetes using novel ophthalmic markers (LANDMark): study design and baseline characteristics. Diabetes Res Clin Pract. 2014;104:248–256. doi: 10.1016/j.diabres.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 52.Asghar O, Petropoulos IN, Alam U, et al. Corneal confocal microscopy detects neuropathy in subjects with impaired glucose tolerance. Diabetes Care. 2014;37:2643–2646. doi: 10.2337/dc14-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papanas N, Vinik AI, Ziegler D. Neuropathy in prediabetes: does the clock start ticking early? Nat Rev Endocrinol. 2011;7:682–690. doi: 10.1038/nrendo.2011.113. [DOI] [PubMed] [Google Scholar]
- 54.Dehghani C, Pritchard N, Edwards K, et al. Morphometric stability of the corneal subbasal nerve plexus in healthy individuals: a 3-year longitudinal study using corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2014;55:3195–3199. doi: 10.1167/iovs.14-13959. [DOI] [PubMed] [Google Scholar]
- 55.Pritchard N, Edwards K, Russell AW, et al. Corneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetes. Diabetes Care. 2015;pii:dc142114. doi: 10.2337/dc14-2114. doi: 10.2337/dc14-2114. [DOI] [PubMed] [Google Scholar]
- 56.Azmi S, Ferdousi M, Petropoulos IN, et al. Corneal confocal microscopy shows an improvement in small-fiber neuropathy in subjects with type 1 diabetes on continuous subcutaneous insulin infusion compared with multiple daily injection. Diabetes Care. 2015;38:e3–e4. doi: 10.2337/dc14-1698. [DOI] [PubMed] [Google Scholar]
- 57.Brines M, Dunne AN, Van Velzen M, et al. ARA 290, a non-erythropoietic peptide engineered from erythropoietin, improves metabolic control and neuropathic symptoms in patients with type 2 diabetes. Mol Med. 2014 doi: 10.2119/molmed.2014.00215. doi: 10.2119/molmed.2014.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tavakoli M, Begum P, McLaughlin J, et al. Corneal confocal microscopy for the diagnosis of diabetic autonomic neuropathy. Muscle Nerve. 2014 doi: 10.1002/mus.24553. doi: 10.1002/mus.24553. [DOI] [PubMed] [Google Scholar]
- 59.Lauria G, Bakkers M, Schmitz C, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst. 2010;15:202–207. doi: 10.1111/j.1529-8027.2010.00271.x. [DOI] [PubMed] [Google Scholar]
- 60.Tavakoli M, Ferdousi M, Petropoulos I, et al. Normative values for corneal nerve morphology assessed using corneal confocal microscopy: a worldwide normative dataset. Diabetes Care. 2015;pii:dc142311. doi: 10.2337/dc14-2311. doi: 10.2337/dc14-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sellers EA, Clark I, Tavakoli M, et al. The acceptability and feasibility of corneal confocal microscopy to detect early diabetic neuropathy in children: a pilot study. Diabet Med. 2013;30:630–631. doi: 10.1111/dme.12125. [DOI] [PubMed] [Google Scholar]
- 62.HJiang MS, Yuan Y, Gu ZX, et al. Corneal confocal microscopy for assessment of diabetic peripheral neuropathy: a meta-analysis. Br J Ophthalmol. 2015;pii doi: 10.1136/bjophthalmol-2014-306038. bjophthalmol-2014-306038. doi: 10.1136/bjophthalmol-2014-306038. [DOI] [PubMed] [Google Scholar]


