Abstract
Aims/Introduction
The present study was designed to evaluate the effect of chromium malate on glycometabolism, glycometabolism-related enzyme levels and lipid metabolism in type 2 diabetic rats, and dose–response and curative effects.
Materials and Methods
The model of type 2 diabetes rats was developed, and daily treatment with chromium malate was given for 4 weeks. A rat enzyme-linked immunosorbent assay kit was used to assay glycometabolism, glycometabolism-related enzyme levels and lipid metabolism changes.
Results
The results showed that the antihyperglycemic activity increased with administration of chromium malate in a dose–dependent manner. The serum insulin level, insulin resistance index and C-peptide level of the chromium malate groups at a dose of 17.5, 20.0 and 20.8 μg chromium/kg bodyweight were significantly lower than that of the model, chromium trichloride and chromium picolinate groups. The hepatic glycogen, glucose-6-phosphate dehydrogenase and glucokinase levels of the chromium malate groups at a dose of 17.5, 20.0 and 20.8 μg chromium/kg bodyweight were significantly higher than that of the model, chromium trichloride and chromium picolinate groups. Chromium malate at a dose of 20.0 and 20.8 μg chromium/kg bodyweight significantly changed the total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides levels compared with the chromium trichloride and chromium picolinate groups.
Conclusions
The results showed that chromium malate exhibits greater benefits in treating type 2 diabetes, and the curative effect of chromium malate is superior to chromium trichloride and chromium picolinate.
Keywords: Chromium malate, Glycometabolism, Lipid metabolism
Introduction
Diabetes mellitus has been identified as one of the three diseases (cardiovascular diseases, cancer and diabetes mellitus) difficult to cure by the World Health Organization. It was mainly divided into type 1 diabetes and type 2 diabetes1. Type 2 diabetes, which appears in middle-aged people and the elderly, was the focus of the present research. It accounts for more than 90% of diabetes cases2. Furthermore, the age of type 2 diabetes patients showed a downward trend. The occurrence of type 2 diabetes has been associated with genetic and acquired factors3. Type 2 diabetes is accompanied by glycometabolism, glycometabolism-related enzymes, lipid metabolism disorders and so on. The complications of type 2 diabetes are coronary heart disease, low learning deficits, low cognitive ability and cardiovascular disease4–7. However, type 2 diabetes and its complications cannot be cured completely, and prescribed medication and supplements should be given priority8. Chromium (Cr3+), magnesium, multivitamins, calcium and aspirin are used as over-the-counter supplements9–11.
Studies have shown that Cr3+ and its complex can decrease fasting blood glucose level12–15. Cr can enhance insulin sensitivity and regulate lipid metabolism in diabetes9,16–19. Studies have reported that supplemental Cr3+ propionate complex can significantly decrease serum insulin levels and increase the insulin sensitivity index. Similar results were reported that Cr3+ propionate complex can significantly reduce serum triacylglycerols, total cholesterols and low-density lipoprotein (LDL) cholesterol levels. Chromium supplementation can significantly reduce total cholesterol (TC), triglyceride (TG) and LDL levels in type 2 diabetes patients19. Currently, inorganic chromium and organic chromium are two categories of chromium supplements. Inorganic chromium complex, including chromium trichloride and chromium nitrate, have low absorption and high toxicity compared with organic chromium complex20,21. Chromium picolinate, chromium nicotinate and chromium yeast are organic chromium complexes, and have been widely used as supplements22,23. However, studies have shown that chromium picolinate can result in genotoxicity and cytotoxicity, thus its safety is a concern. The poor solubility and unstable structure of chromium nicotinate and chromium yeast have limited their application, respectively. Therefore, a novel and non-toxic organic chromium complex with antihyperglycemic activity has become an important issue.
Chromium malate is a new type of organic chromium complex that has been synthesized by our research group24. Chromium malate was synthesized by chelating Cr3+ with L-malic acid, which was selected as the natural ligand. Its chemical formula and molecular weight are Cr2C12H22O20 (or Cr2[C4H4O5]3.5H2O) and 590.18 g/mol, respectively. Chromium malate can control blood glucose levels in alloxan-induced diabetic mice and does not cause oxidative DNA damage, and was tested as non-toxic in acute and subacute toxicity studies25. The dose–response relationship of chromium malate can describe the curative effects in type 2 diabetes and provide a reference for clinical medication. However, few studies on the dose–response relationship in the antihyperglycemic activity of chromium malate have been reported. The relationship between chromium malate intake and the curative effects in type 2 diabetes was examined in the present study. The effect of chromium malate on improving glycometabolism, glycometabolism-related enzymes and lipid metabolism in type 2 diabetic rats was also studied.
Methods
Ethics Statement
All the experimental procedures were carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans, the EC Directive 86/609/EEC for animal experiments, and were approved by the Jiangsu University Committee on Animal Care and Use. Sprague–Dawley rats were procured from Jiangsu University (license number SYXK [SU] 2013–0036). The Sprague–Dawley rat is not a protected or endangered species. Our experiments complied with the laws and ethical recommendations currently in effect in China where the experiments were carried out.
Materials and Chemicals
The chromium malate was synthesized in our previous study25. The raw materials were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Streptozotocin was obtained from Sigma Chemical Co. (St. Louis, MO, USA) Distilled water was used throughout all the experiments. The “glycogen kit” was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Rat enzyme-linked immunosorbent assay (ELISA) kits were purchased from Hefei Bomei Biotechnology Co., Ltd (Hefei, China).
Animals and Diet
A total of 200 Sprague–Dawley male rats with an average weight of 180 ± 10 g were purchased from Jiangsu University for all the experiments. Before the experiment, the animals were allowed 3 days for environmental and trainer handling acclimatization. The temperatures and relative humidity of the animal house were 24 ± 1°C and 55–60%, respectively. Rats consumed distilled water during the entire experimental period.
Models of Type 2 Diabetes
The models of type 2 diabetes were developed using the method of Li et al. and Xu et al. with slight modification26,27. A high-sugar and high-fat diet was supplied to rats in the first 2 months. Then the rats were injected with streptozotocin at a dose of 30 mg/kg bodyweight through intraperitoneal injection. Fasting blood glucose (FBG) level was measured by a one touch glucometer 3 days later. The oral glucose tolerance test and insulin resistance test were carried out. The FBG level of 33.3 mmol/L > FBG ≥ 11.1 mmol/L, accompanied by oral glucose tolerance and reduced insulin resistance (IR) in rats was taken as a successful induction of type 2 diabetes.
Dose–Response Relationship
Antihyperglycemic Activity
The type 2 diabetic rats were randomly divided into 12 groups of 10 animals. The experiment design, and the dose of chromium malate, chromium trichloride and chromium picolinate are shown in Table1. Chromium malate was given by oral gavage to rats. Chromium trichloride and chromium picolinate were used as a positive control. The content of Cr in a standard pellet diet is 4.63 μg/kg. The Cr content, which was consumed by rats, is less than 1 μg, therefore, there has no obvious effect on this test. Normal rats were used as experimental animals in a blank control group and chromium malate control group. The type 2 diabetic rats were treated with chromium malate daily by gavage for 4 weeks. FBG and body mass were tested once a week. Blood samples were collected and centrifuged at the end of the experiment. The anticoagulated blood and the blood serum were used for hematological and biochemical analyses.
Table 1.
Experiment design and the dose of chromium malate, chromium trichloride and chromium picolinate
| Group | Rats | Dose (Cr, μg/kg bw) |
|---|---|---|
| Normal control group | Normal rats | – |
| Model group | Type 2 diabetic rats | – |
| Chromium trichloride-treated group | Type 2 diabetic rats | 20.8 |
| Chromium picolinate-treated group | Type 2 diabetic rats | 20.8 |
| Chromium malate-treated group 1 | Type 2 diabetic rats | 2.5 |
| Chromium malate-treated group 2 | Type 2 diabetic rats | 5.0 |
| Chromium malate-treated group 3 | Type 2 diabetic rats | 7.5 |
| Chromium malate-treated group 4 | Type 2 diabetic rats | 10.0 |
| Chromium malate-treated group 5 | Type 2 diabetic rats | 12.5 |
| Chromium malate-treated group 6 | Type 2 diabetic rats | 15.0 |
| Chromium malate-treated group 7 | Type 2 diabetic rats | 17.5 |
| Chromium malate-treated group 8 | Type 2 diabetic rats | 20.0 |
| Chromium malate-treated group 9 | Type 2 diabetic rats | 20.8 |
| Chromium malate control group | Normal rats | 20.8 |
BW, bodyweight
Cr, chromium.
Glycometabolism and Glycometabolism-Related Enzymes
The serum and liver of rats were collected when the rats were killed. Serum insulin and C-peptide (C-P) were assayed by rat ELISA kits. The IR index was calculated by the following formula:28.
A glycogen kit was used to assay liver glycogen levels. The livers of rats were homogenized and centrifuged at 5,000 g for 10 min at 4°C, and then supernatant was collected. Rat ELISA kits were used to estimate glucose-6-phosphate dehydrogenase (G6PD) and glucokinase (GCK) activity in the liver supernatant.
Lipid Metabolism
The serum of rats, which was collected in the previous step, was used to measure lipid metabolism analysis. TC, LDL, high-density lipoprotein cholesterol (HDL) and TG levels in blood serum were assayed by a rat reagent kit (Dong'ou Jinma Technology, Co., Ltd., Zhejiang, China).
Cr Content
The Vista-MPX Simultaneous Inductively coupled plasma (ICP) method (Varian, Inc., Palo Alto, CA, USA) was used to measure Cr content in the serum and organs (heart, liver, spleen, lung, kidney, brain) of rats. Cr was determined after wet digestion according to the method described by Wu et al.24 and Sereshti et al.29 The serum and organs of rats were joined in nitric acid and perchloric acid (1:4, v/v) to digestion.
Organ Index
The organs (heart, liver, spleen, lung, kidney, brain) of rats, which were collected in the previous step, were weighed. Relative heart/liver/spleen/lung/kidney/brain weights were expressed as the ratio of the heart/liver/spleen/lung/kidney/brain to bodyweight (mg/g).
Statistical Analysis
Statistical analyses were carried out by the program spss 16.0 (Chicago, IL, USA). One-way analysis of variance (anova) was used for data analysis. The Tukey test for multiple comparisons among the groups was carried out to determine significant differences. P < 0.05 was considered as statistically significant.
Results
Dose–Response Relationship of Chromium Malate
Antihyperglycemic Activity
The dose–response relationship of chromium malate in antihyperglycemic activity during the fourth week is shown in Figure1a. It can be observed that the FBG level of the chromium malate-treated groups showed a trend of decline in type 2 diabetic rats after the administration of chromium malate. The antihyperglycemic activity increased with an increase in dose of chromium malate. The FBG level of type 2 diabetic rats remained at 11–12 mmol/L after administration with chromium malate at a dose of 20.0 and 20.8 μg Cr/kg bodyweight. The antihyperglycemic activity of chromium malate shows a dose dependency. The FBG level of chromium malate-treated groups at a dose of 7.5, 10.0, 12.5, 15.0, 17.5, 20.0 and 20.8 μg Cr/kg bodyweight was significantly lower than that of the model group (Figure1b). It can be observed that chromium trichloride and chromium picolinate can reduce FBG level. The FBG level of the chromium picolinate-treated group and chromium trichloride-treated group was significantly decreased when compared with the model group. From these results, it can be deduced that the FBG level of the chromium malate-treated groups at a dose of 15.0, 17.5, 20.0 and 20.8 μg Cr/kg bodyweight was significantly lower than that of the chromium trichloride-treated group at a dose of 20.8 μg Cr/kg bodyweight (20.82 ± 2.04 mmol/L) and the chromium picolinate-treated group at a dose of 20.0 μg Cr/kg bodyweight (18.57 ± 1.88 mmol/L). The antihyperglycemic activity of chromium malate was better than that of chromium trichloride and chromium picolinate.
Figure 1.
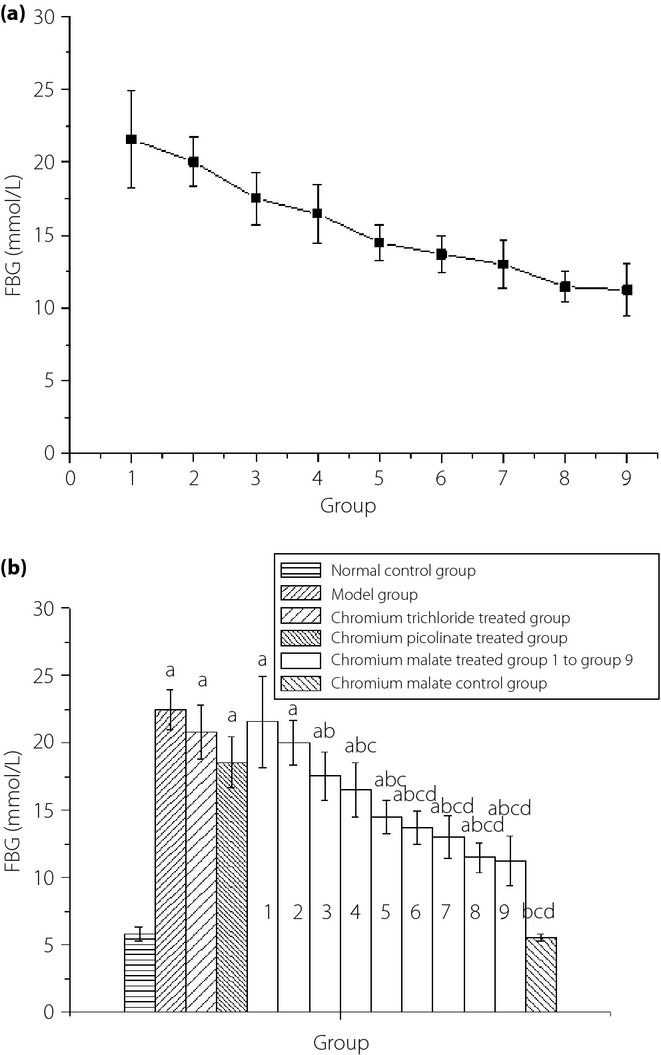
(a) Dose–response relationship of chromium malate in antihyperglycemic activity in the fourth week. Groups 1–9 are the chromium malate-treated groups. (b) The fasting blood glucose (FBG) level changes were treated with chromium malate, chromium trichloride, and chromium picolinate in normal and type 2 diabetic rats. Chromium trichloride and chromium picolinate was used as a positive control. Each value is presented as mean ± standard deviation (n = 10). aSignificantly different from normal the control group (P < 0.05). bSignificantly different from the model group (P < 0.05). cSignificantly different from the chromium trichloride-treated group (P < 0.05). dSignificantly different from the chromium picolinate-treated group (P < 0.05).
Glycometabolism
The changes in serum insulin, IR, C-P and hepatic glycogen levels of normal rats and type 2 diabetic rats after administration of chromium malate, chromium trichloride and chromium picolinate are shown in Figure2. Chromium malate, chromium trichloride and chromium picolinate can reduce serum insulin, IR and C-P levels in type 2 diabetic rats (Figure2a–c). The serum insulin, IR and C-P levels of chromium malate-treated groups at a dose of 17.5, 20.0 and 20.8 μg Cr/kg bodyweight was significantly different when compared with the model group, chromium trichloride-treated group and chromium picolinate-treated group. However, the chromium trichloride- and chromium picolinate-treated groups showed no significant change when compared with the model group. The serum insulin, IR and C-P level reduced with an increase in dose of chromium malate. The hepatic glycogen level was increased after administration of chromium malate and chromium picolinate in type 2 diabetic rats (Figure2d). Chromium trichloride cannot increase the hepatic glycogen level in type 2 diabetic rats. The hepatic glycogen level increased with an increase in dose of chromium malate. The hepatic glycogen level of the chromium malate-treated groups at a dose of 12.5, 15.0, 17.5, 20.0 and 20.8 μg Cr/kg bodyweight was significantly higher than that of the model group, chromium trichloride-treated group and chromium picolinate-treated group. The hepatic glycogen level of the chromium picolinate-treated group showed no significant increase when compared with the model group. The curative effects of chromium malate on the changes of serum insulin, IR, C-P and hepatic glycogen levels are better than those of chromium trichloride and chromium picolinate.
Figure 2.
The changes of (a) serum insulin, (b) IR, (c) C-peptide (C-P), and (d) hepatic glycogen levels in normal rats and type 2 diabetic rats after administration chromium malate, chromium trichloride and chromium picolinate. Chromium trichloride and chromium picolinate were used as positive controls. Each value is presented as mean ± standard deviation (n = 10). aSignificantly different from normal control group (P < 0.05). bSignificantly different from model group (P < 0.05). cSignificantly different from chromium trichloride-treated group (P < 0.05). dSignificantly different from chromium picolinate-treated group (P < 0.05).
Glycometabolism-Related Enzymes
The changes in G6PD and GCK levels of normal rats and type 2 diabetic rats after administration of chromium malate, chromium trichloride and chromium picolinate are shown in Figure3. Chromium trichloride and chromium picolinate can increase the content of G6PD and GCK in type 2 diabetic rats. However, the G6PD and GCK levels of chromium trichloride-treated group and chromium picolinate-treated group showed no significantly increase when compared with model group. The results indicated that chromium malate can increase the G6PD and GCK level in type 2 diabetic rats. The G6PD and GCK levels increased with an increase in dose of chromium malate. The G6PD level of the chromium malate-treated groups at a dose of 17.5, 20.0 and 20.8 μg Cr/kg bodyweight was significantly increased when compared with the model group, chromium trichloride-treated group and chromium picolinate-treated group. The GCK level of the chromium malate-treated groups at a dose of 12.5, 15.0, 17.5, 20.0 and 20.8 μg Cr/kg bodyweight was significantly increased when compared with the model group, chromium trichloride-treated group and chromium picolinate-treated group. The curative effects of chromium malate on increased G6PD and GCK levels are better than those of chromium trichloride and chromium picolinate.
Figure 3.

The changes of (a) glucose-6-phosphate dehydrogenase (G6PD) and (b) glucokinase (GCK) levels in normal rats and type 2 diabetic rats after administration of chromium malate, chromium trichloride and chromium picolinate. Chromium trichloride and chromium picolinate were used as positive controls. Each value is presented as mean ± SD (n = 10). aSignificantly different from normal control group (P < 0.05). bSignificantly different from model group (P < 0.05). cSignificantly different from chromium trichloride-treated group (P < 0.05). dSignificantly different from chromium picolinate-treated group (P < 0.05).
Lipid Metabolism
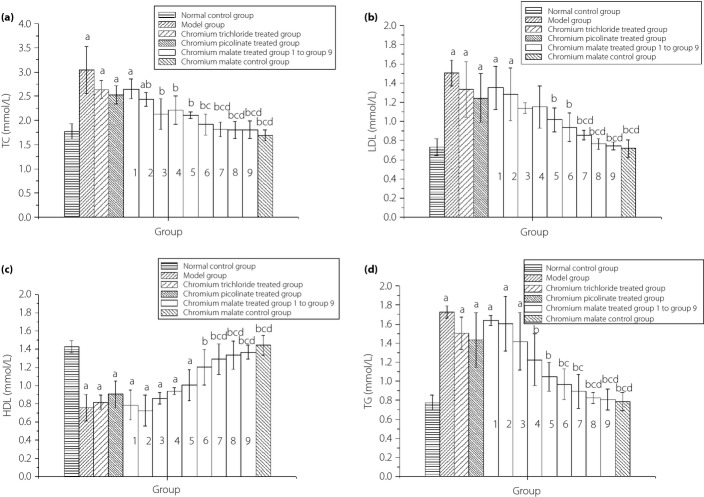
The changes in TC, LDL, HDL and TG levels of normal rats and type 2 diabetic rats after administration of chromium malate, chromium trichloride and chromium picolinate are shown in Figure4. Chromium trichloride and chromium picolinate can reduce serum TC, LDL and TG levels, and increase serum HDL levels in type 2 diabetic rats. However, chromium trichloride and chromium picolinate cannot significantly reduce serum TC, LDL and TG levels, or increase serum HDL levels when compared with the model group. The results showed that chromium malate can reduce the serum TC, LDL and TG levels, and increase serum HDL levels. The TC, LDL and TG levels reduced with an increase in dose of chromium malate. Serum HDL levels increased with an increase in dose of chromium malate. The TC, HDL and TG levels of the chromium malate-treated groups at a dose of 17.5, 20.0 and 20.8 μg Cr/kg bodyweight significantly changed when compared with the model group, chromium trichloride-treated group and chromium picolinate-treated group. The LDL level of chromium malate-treated groups at a dose of 20.0 and 20.8 μg Cr/kg bodyweight was significantly changed when compared with the model group, chromium trichloride-treated group and chromium picolinate-treated group. The curative effects of chromium malate on reducing TC, LDL and TG levels, and increasing HDL levels are better than those of chromium trichloride and chromium picolinate.
Figure 4.
The changes of serum total cholesterol (TC), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), and triglycerides (TG) in normal rats and type 2 diabetic rats after administration chromium malate, chromium trichloride and chromium picolinate. Chromium trichloride and chromium picolinate were used as positive controls. Each value was presented as mean ± SD (n = 10). aSignificantly different from normal control group (P < 0.05). bSignificantly different from model group (P < 0.05). cSignificantly different from chromium trichloride-treated group (P < 0.05). dSignificantly different from chromium picolinate-treated group (P < 0.05).
Cr Content
The changes in Cr content in the serum and organs (heart, liver, spleen, lung, kidney, brain) of normal rats and type 2 diabetic rats after administration chromium malate, chromium trichloride and chromium picolinate are shown in Table2. The serum Cr content of the normal control group was significantly higher than that of the model group. The results showed that type 2 diabetes can reduce serum Cr content, and chromium malate, chromium trichloride and chromium picolinate can increase the serum Cr content in type 2 diabetic rats. The serum Cr content of the chromium malate-treated group at a dose of 15.0, 17.5, 20.0 and 20.8 μg Cr/kg bodyweight, and the chromium picolinate-treated group was significantly increased when compared with the chromium trichloride-treated group. The results showed that the absorption of chromium malate and chromium picolinate was higher than that of chromium trichloride, and the absorption rate of chromium malate was the same as chromium picolinate. The Cr content in organs (heart, liver, spleen, lung, kidney, brain) of the model group showed no significant decrease when compared with the normal control group, chromium malate-treated group, chromium trichloride-treated group and chromium picolinate-treated group. The Cr content of the chromium malate-treated group at a dose of 20.8 μg Cr/kg bodyweight is spleen > lung > kidney > brain > heart > liver.
Table 2.
Chromium content of normal rats and type 2 diabetic rats after administration of chromium malate, chromium trichloride and chromium picolinate, and changes in serum and organs
| Group | Parameter | ||||||
|---|---|---|---|---|---|---|---|
| μg/mL | μg/g | ||||||
| Serum | Heart | Liver | Spleen | Lung | Kidney | Brain | |
| Normal control group | 15.28 ± 1.06 | 6.29 ± 2.01 | 5.97 ± 2.12 | 10.45 ± 2.49 | 14.54 ± 2.01 | 9.32 ± 2.27 | 9.98 ± 2.27 |
| Model group | 10.01 ± 1.05† | 5.91 ± 1.38 | 5.88 ± 1.24 | 9.74 ± 2.75 | 13.98 ± 2.92 | 7.58 ± 2.50 | 8.59 ± 2.13 |
| Chromium trichloride-treated group | 12.03 ± 1.13† | 5.93 ± 2.17 | 6.01 ± 2.19 | 11.00 ± 3.62 | 13.06 ± 4.30 | 8.64 ± 3.06 | 9.46 ± 3.27 |
| Chromium picolinate-treated group | 17.14 ± 1.51‡§ | 7.12 ± 4.28 | 5.84 ± 1.87 | 11.97 ± 4.19 | 14.56 ± 4.31 | 10.37 ± 3.24 | 10.58 ± 3.25 |
| Chromium malate-treated group 1 | 10.47 ± 1.27†¶ | 5.30 ± 2.23 | 5.84 ± 2.58 | 9.86 ± 2.59 | 13.91 ± 2.67 | 7.93 ± 2.48 | 8.51 ± 2.02 |
| Chromium malate-treated group 2 | 10.94 ± 1.47†¶ | 5.72 ± 2.14 | 5.97 ± 1.98 | 9.92 ± 3.01 | 13.98 ± 2.64 | 8.55 ± 2.30 | 8.69 ± 2.85 |
| Chromium malate-treated group 3 | 11.01 ± 1.05†¶ | 6.02 ± 2.01 | 5.91 ± 2.24 | 10.06 ± 3.21 | 13.98 ± 3.82 | 8.99 ± 3.01 | 8.82 ± 3.02 |
| Chromium malate-treated group 4 | 11.25 ± 1.34†¶ | 6.18 ± 2.26 | 5.95 ± 2.33 | 10.58 ± 3.51 | 14.12 ± 2.52 | 9.49 ± 2.15 | 9.03 ± 2.34 |
| Chromium malate-treated group 5 | 13.77 ± 1. 29¶ | 6.50 ± 2.12 | 5.97 ± 2.56 | 11.36 ± 3.90 | 14.15 ± 3.92 | 10.01 ± 2.59 | 9.20 ± 3.09 |
| Chromium malate-treated group 6 | 16.42 ± 1.65‡§ | 6.63 ± 2.48 | 6.00 ± 3.53 | 13.36 ± 5.04 | 14.22 ± 4.43 | 10.48 ± 3.43 | 10.04 ± 3.21 |
| Chromium malate-treated group 7 | 17.04 ± 1.53‡§ | 7.16 ± 3.47 | 6.00 ± 2.62 | 14.55 ± 3.37 | 14.39 ± 3.21 | 10.76 ± 3.41 | 10.21 ± 3.35 |
| Chromium malate-treated group 8 | 17.68 ± 1.35‡§ | 7.91 ± 3.14 | 6.03 ± 2.21 | 15.58 ± 4.53 | 14.59 ± 4.03 | 11.00 ± 3.62 | 10.34 ± 3.41 |
| Chromium malate-treated group 9 | 17.98 ± 1.17‡§ | 8.09 ± 3.54 | 6.06 ± 1.29 | 16.09 ± 5.21 | 14.60 ± 5.01 | 11.02 ± 4.23 | 10.82 ± 4.05 |
| Chromium malate control group | 15.45 ± 1.24‡§ | 6.39 ± 2.10 | 5.97 ± 2.67 | 12.31 ± 4.53 | 14.41 ± 4.86 | 10.69 ± 4.41 | 11.26 ± 4.16 |
Data is presented as mean ± standard deviation (n = 10). Chromium trichloride and chromium picolinate were used as positive controls.
Significantly different from normal control group (P < 0.05).
Significantly different from model group (P < 0.05).
Significantly different from chromium trichloride-treated group (P < 0.05).
Significantly different from chromium picolinate-treated group (P < 0.05).
Dose–Response Relationship of Chromium Malate in Body Mass
The dose–response relationship of chromium malate in the body mass of normal rats and type 2 diabetic rats in the fourth week is shown in Figure5. It can be observed that the body mass of type 2 diabetic rats increased with an increase in chromium malate. The body mass of the chromium malate-treated groups at a dose of 15.0, 17.5, 20.0 and 20.8 μg Cr/kg bodyweight showed a significant increase when compared with the model group. However, there was no significant increase when compared with the chromium trichloride-treated group and chromium picolinate-treated group. Chromium trichloride and chromium picolinate can increase the body mass of type 2 diabetic rats. However, there was no significant change when compared with the model group.
Figure 5.
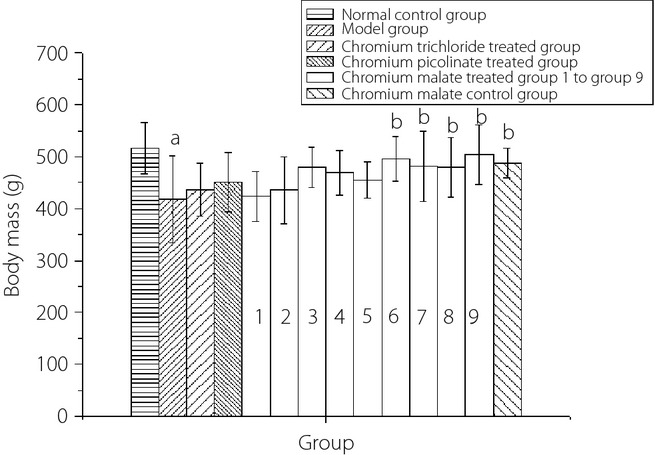
The dose–response relationship of chromium malate in the body mass of normal and type 2 diabetic rats in the fourth week. Chromium trichloride and chromium picolinate were used as positive controls. Each value was presented as mean ± SD (n = 10). aSignificantly different from normal control group (P < 0.05). bSignificantly different from model group (P < 0.05). cSignificantly different from chromium trichloride-treated group (P < 0.05). dSignificantly different from chromium picolinate-treated group (P < 0.05).
Biochemical and Hematological Analyses
The biochemical and hematological analyses of chromium malate by oral gavage to normal rats and type 2 diabetic rats are shown in Tables3 and 4, respectively. The results showed that type 2 diabetes can lead to alkaline phosphatase and blood urea nitrogen rising in type 2 diabetic rats. Chromium malate, chromium trichloride and chromium picolinate can reduce this biochemical index. However, there was no significant change when compared with the model group. The results of hematological analyses are similar to the results of biochemical analyses. These results showed that chromium malate did not significantly affect the biochemical and hematological index.
Table 3.
Biochemical analyses in normal rats and type 2 diabetic rats after administration of chromium malate, chromium trichloride and chromium picolinate
| Group | Parameter | |||||
|---|---|---|---|---|---|---|
| ALT (IU/L) | AST (IU/L) | ALP (IU/L) | TP (g/L) | BUN (mmol/L) | Crea (μmol/L) | |
| Normal control group | 45.28 ± 6.36 | 95.29 ± 11.72 | 155.57 ± 28.95 | 67.92 ± 5.46 | 4.45 ± 0.77 | 33.90 ± 7.12 |
| Model group | 41.11 ± 5.11 | 121.53 ± 34.38 | 242.83 ± 30.42† | 70.24 ± 1.17 | 5.82 ± 0.58† | 45.85 ± 9.05 |
| Chromium trichloride-treated group | 47.13 ± 8.89 | 123.63 ± 17.48 | 243.33 ± 29.91† | 71.00 ± 2.16 | 5.90 ± 0.13 | 41.65 ± 6.20 |
| Chromium picolinate-treated group | 40.41 ± 5.21 | 120.30 ± 14.28 | 224.20 ± 37.88† | 69.77 ± 1.96 | 5.67 ± 0.36 | 37.88 ± 4.21 |
| Chromium malate-treated group 1 | 44.71 ± 7.17 | 124.77 ± 19.32 | 231.00 ± 31.85† | 69.66 ± 2.35 | 6.11 ± 0.74† | 43.80 ± 4.88 |
| Chromium malate-treated group 2 | 45.83 ± 7.17 | 120.78 ± 29.41 | 234.67 ± 20.98† | 71.00 ± 2.65 | 5.99 ± 0.76† | 43.33 ± 4.20 |
| Chromium malate-treated group 3 | 49.25 ± 5.08 | 102.72 ± 11.36 | 230.00 ± 28.76† | 68.76 ± 2.13 | 5.88 ± 0.82† | 40.37 ± 3.67 |
| Chromium malate-treated group 4 | 46.50 ± 9.99 | 105.78 ± 26.27 | 214.75 ± 21.36 | 69.78 ± 1.78 | 5.81 ± 0.25 | 40.05 ± 5.63 |
| Chromium malate-treated group 5 | 47.70 ± 9.82 | 110.00 ± 12.27 | 211.75 ± 13.65 | 68.83 ± 2.04 | 5.57 ± 0.39 | 35.18 ± 2.43 |
| Chromium malate-treated group 6 | 46.42 ± 6.55 | 106.03 ± 18.45 | 211.67 ± 19.35 | 68.33 ± 3.06 | 5.62 ± 0.44 | 35.86 ± 3.13 |
| Chromium malate-treated group 7 | 45.84 ± 7.39 | 107.56 ± 10.47 | 206.33 ± 26.26 | 68.50 ± 1.73 | 5.52 ± 1.12 | 36.78 ± 6.94 |
| Chromium malate-treated group 8 | 49.30 ± 7.55 | 100.83 ± 14.53 | 201.75 ± 18.01 | 68.67 ± 2.03 | 5.65 ± 0.22 | 35.58 ± 3.70 |
| Chromium malate-treated group 9 | 48.58 ± 7.02 | 103.38 ± 15.45 | 196.00 ± 19.29 | 68.25 ± 4.44 | 5.20 ± 0.42 | 34.10 ± 3.29 |
| Chromium malate control group | 48.15 ± 7.45 | 93.33 ± 10.25 | 152.92 ± 17.60‡§¶ | 67.74 ± 3.94 | 4.01 ± 0.68‡ | 29.78 ± 1.74 |
Data is presented as mean ± standard deviation (n = 10). Chromium trichloride and chromium picolinate were used as positive controls.
ALT, alanine transaminase
ALP, alkaline phosphatase
AST, aspartate transaminase
BUN, blood urea nitrogen
Crea, creatinine
TP, total protein.
Significantly different from normal control group (P < 0.05).
Significantly different from model group (P < 0.05).
Significantly different from chromium trichloride-treated group (P < 0.05).
Significantly different from chromium picolinate-treated group (P < 0.05).
Table 4.
Hematological analyses in normal rats and type 2 diabetic rats after administration of chromium malate, chromium trichloride and chromium picolinate
| Group | Parameter | ||||||
|---|---|---|---|---|---|---|---|
| WBC (109/L) | LYMPH (109/L)† | MNCC (109/L) | NCC (109/L) | RBC (109/L) | HCT (%) | MCV (fL) | |
| Normal control group | 8.04 ± 1.99 | 8.15 ± 2.11 | 0.85 ± 0.49 | 1.40 ± 0.18 | 8.71 ± 0.10 | 46.76 ± 4.38 | 55.00 ± 1.71 |
| Model group | 13.45 ± 1.93 | 5.87 ± 0.94 | 0.62 ± 0.21 | 2.42 ± 0.26 | 9.61 ± 0.31 | 52.66 ± 1.43 | 57.33 ± 1.75 |
| Chromium trichloride-treated group | 12.41 ± 1.61 | 5.27 ± 1.53a | 0.61 ± 0.20 | 2.07 ± 0.40 | 9.63 ± 0.16 | 52.73 ± 3.69 | 57.25 ± 2.06 |
| Chromium picolinate-treated group | 11.96 ± 1.43 | 6.83 ± 0.95 | 0.66 ± 0.38 | 2.26 ± 0.25 | 9.31 ± 0.92 | 50.50 ± 3.56 | 57.50 ± 1.91 |
| Chromium malate-treated group 1 | 12.72 ± 1.87 | 5.84 ± 0.56 | 0.58 ± 0.18 | 2.20 ± 0.16 | 9.27 ± 0.16 | 52.58 ± 4.97 | 57.50 ± 1.73 |
| Chromium malate-treated group 2 | 13.03 ± 1.07 | 6.20 ± 0.72 | 0.58 ± 0.11 | 2.31 ± 0.31 | 9.32 ± 0.18 | 51.87 ± 1.90 | 57.00 ± 3.00 |
| Chromium malate-treated group 3 | 11.29 ± 4.54 | 6.84 ± 1.92 | 0.65 ± 0.23 | 1.87 ± 0.15 | 8.47 ± 0.39 | 49.35 ± 1.96 | 56.17 ± 1.47 |
| Chromium malate treated group 4 | 10.83 ± 2.30 | 6.72 ± 1.88 | 0.73 ± 0.11 | 1.92 ± 0.34 | 8.35 ± 0.45 | 49.58 ± 2.44 | 56.33 ± 1.97 |
| Chromium malate-treated group 5 | 10.84 ± 2.22 | 6.33 ± 0.58 | 0.78 ± 0.23 | 1.89 ± 0.16 | 8.99 ± 0.02 | 49.70 ± 1.31 | 56.00 ± 2.65 |
| Chromium malate-treated group 6 | 10.62 ± 1.64 | 7.00 ± 1.00 | 0.74 ± 0.22 | 1.89 ± 0.10 | 8.79 ± 0.23 | 49.97 ± 1.14 | 55.50 ± 1.52 |
| Chromium malate-treated group 7 | 10.06 ± 1.69 | 7.95 ± 1.59 | 0.78 ± 0.44 | 1.76 ± 0.55 | 8.74 ± 0.09 | 49.58 ± 3.14 | 55.83 ± 1.72 |
| Chromium malate-treated group 8 | 10.08 ± 1.71 | 7.68 ± 1.48 | 0.75 ± 0.16 | 1.61 ± 0.18 | 8.77 ± 0.65 | 47.80 ± 2.34 | 55.20 ± 1.84 |
| Chromium malate-treated group 9 | 9.40 ± 1.59 | 7.47 ± 1.02 | 0.75 ± 0.12 | 1.59 ± 0.37 | 8.72 ± 0.26 | 47.70 ± 2.55 | 55.50 ± 2.74 |
| Chromium malate control group | 8.07 ± 1.65 | 9.61 ± 2.03 | 0.88 ± 0.26 | 1.19 ± 0.12 | 8.72 ± 0.23 | 46.98 ± 1.97 | 55.20 ± 1.84 |
| Group | Parameter | |||||||
|---|---|---|---|---|---|---|---|---|
| Hb (g/L) | MHbC (Pg) | MCHC (g/L) | PLT (109/L) | PDW (fL) | MPV (fL) | PCT (%) | RDW (fL) | |
| Normal control group | 159.69 ± 16.79 | 17.63 ± 1.15 | 315.60 ± 24.72 | 852.67 ± 37.61 | 13.65 ± 2.45 | 8.43 ± 1.39 | 0.69 ± 0.14 | 14.89 ± 1.05 |
| Model group | 167.50 ± 12.26 | 19.65 ± 1.42 | 336.33 ± 28.15 | 968.33 ± 86.07 | 15.91 ± 1.46 | 10.80 ± 0.74 | 1.33 ± 0.26 | 16.50 ± 1.36 |
| Chromium trichloride-treated group | 165.67 ± 14.04 | 19.05 ± 2.76 | 335.67 ± 24.73 | 964.00 ± 83.72 | 16.30 ± 2.85 | 10.33 ± 0.84 | 1.28 ± 0.27 | 16.73 ± 1.64 |
| Chromium picolinate-treated group | 162.75 ± 12.42 | 19.43 ± 1.42 | 324.00 ± 21.87 | 947.72 ± 95.79 | 15.79 ± 2.34 | 10.05 ± 1.25 | 1.13 ± 0.10 | 16.41 ± 1.04 |
| Chromium malate-treated group 1 | 167.00 ± 15.66 | 19.44 ± 1.39 | 342.00 ± 25.57 | 965.50 ± 29.18 | 15.88 ± 1.59 | 10.64 ± 1.47 | 1.31 ± 0.38 | 16.35 ± 1.67 |
| Chromium malate-treated group 2 | 168.50 ± 12.12 | 19.55 ± 1.21 | 339.00 ± 25.29 | 964.25 ± 71.57 | 15.70 ± 1.85 | 10.60 ± 1.28 | 1.31 ± 0.19 | 16.40 ± 1.70 |
| Chromium malate-treated group 3 | 166.25 ± 14.35 | 19.52 ± 1.34 | 327.33 ± 24.16 | 947.00 ± 51.07 | 15.63 ± 1.33 | 10.37 ± 1.29 | 1.22 ± 0.18 | 16.13 ± 1.72 |
| Chromium malate-treated group 4 | 165.20 ± 13.03 | 19.33 ± 1.57 | 324.67 ± 21.53 | 942.00 ± 41.55 | 15.78 ± 1.10 | 10.28 ± 0.86 | 1.09 ± 0.11 | 16.07 ± 1.70 |
| Chromium malate-treated group 5 | 165.20 ± 13.70 | 18.43 ± 1.37 | 322.14 ± 28.03 | 938.50 ± 28.99 | 14.97 ± 2.68 | 9.67 ± 1.21 | 0.97 ± 0.12 | 15.72 ± 1.42 |
| Chromium malate-treated group 6 | 162.17 ± 16.01 | 18.23 ± 1.67 | 322.50 ± 23.79 | 928.50 ± 30.42 | 14.81 ± 1.01 | 9.43 ± 1.21 | 0.89 ± 0.15 | 15.95 ± 1.07 |
| Chromium malate-treated group 7 | 162.30 ± 16.11 | 18.26 ± 1.07 | 321.70 ± 13.17 | 892.67 ± 56.45 | 14.67 ± 1.47 | 9.09 ± 1.95 | 0.87 ± 0.39 | 15.76 ± 1.85 |
| Chromium malate-treated group 8 | 161.60 ± 13.97 | 18.30 ± 1.17 | 320.75 ± 23.86 | 889.67 ± 61.33 | 14.50 ± 1.71 | 8.87 ± 1.62 | 0.76 ± 0.47 | 15.64 ± 1.39 |
| Chromium malate-treated group 9 | 161.38 ± 13.29 | 18.57 ± 1.06 | 320.00 ± 24.24 | 874.75 ± 33.86 | 14.47 ± 2.67 | 8.73 ± 1.95 | 0.76 ± 0.19 | 15.44 ± 1.29 |
| Chromium malate control group | 158.40 ± 17.12 | 17.47 ± 1.59 | 317.25 ± 24.79 | 795.00 ± 24.68 | 13.73 ± 1.95 | 8.25 ± 1.69 | 0.61 ± 0.15 | 14.49 ± 1.51 |
Data is presented as mean ± standard deviation (n = 10). Chromium trichloride and chromium picolinate were used as positive controls.
Hb, hemoglobin
HCT, hemataocrit
LYMPH, lymphocyte count
MCHC, mean corpuscular hemoglobin concentration
MCV, mean corpuscular volume
MHbC, mean hemoglobin content
MNCC, mononuclear cell count
MPV, platelet mean volume
NCC, neutral cell count
PCT, platelet deposited
PDW, platelet distribution width
PLT, platelet count
RBC, red blood cell
RDW, red cell distribution width
WBC, white blood cell.
Significantly different from normal control group (P < 0.05).
Change of Absolute and Relative Organ Weights in Rats
The changes in absolute and relative organ weights (heart, liver, spleen, lung, kidney, brain) of normal rats and type 2 diabetic rats after administration of chromium malate, chromium trichloride and chromium picolinate are shown in Table5. Chromium malate, chromium trichloride and chromium picolinate can reduce the absolute and relative organ weights. However, there was no significant change when compared with the model group. These results showed that chromium malate did not have a significant effect on absolute and relative organ weights in rats.
Table 5.
The changes of absolute and relative organ weights in normal rats and type 2 diabetic rats after administration chromium malate, chromium trichloride and chromium picolinate
| Absolute organ weight | ||||||
|---|---|---|---|---|---|---|
| Group | Parameter | |||||
| Heart | Liver | Spleen | Lung | Kidney | Brain | |
| Normal control group | 1.46 ± 0.19 | 15.07 ± 2.11 | 0.77 ± 0.14 | 1.94 ± 0.31 | 2.48 ± 0.41 | 1.59 ± 0.28 |
| Model group | 1.41 ± 0.21 | 16.38 ± 2.80 | 0.76 ± 0.25 | 2.03 ± 0.35 | 3.77 ± 0.32† | 1.74 ± 0.16 |
| Chromium trichloride-treated group | 1.37 ± 0.12 | 14.43 ± 1.78 | 0.54 ± 0.11 | 1.98 ± 0.11 | 3.70 ± 0.36† | 1.82 ± 0.14 |
| Chromium picolinate-treated group | 1.36 ± 0.15 | 16.31 ± 3.06 | 0.82 ± 0.25 | 1.91 ± 0.32 | 3.53 ± 0.36† | 1.76 ± 0.18 |
| Chromium malate-treated group 1 | 1.37 ± 0.14 | 17.40 ± 2.42 | 0.90 ± 0.27 | 2.05 ± 0.17 | 3.58 ± 0.30† | 1.66 ± 0.16 |
| Chromium malate-treated group 2 | 1.47 ± 0.19 | 16.36 ± 2.21 | 0.65 ± 0.11 | 2.19 ± 0.44 | 3.58 ± 0.43† | 1.82 ± 0.17 |
| Chromium malate-treated group 3 | 1.33 ± 0.16 | 16.32 ± 0.79 | 0.83 ± 0.05 | 2.00 ± 0.36 | 3.51 ± 0.22† | 1.50 ± 0.15 |
| Chromium malate-treated group 4 | 1.44 ± 0.14 | 17.98 ± 1.75 | 0.86 ± 0.13 | 2.03 ± 0.11 | 3.33 ± 0.66† | 1.84 ± 0.17 |
| Chromium malate-treated group 5 | 1.44 ± 0.25 | 14.33 ± 1.14 | 0.57 ± 0.07 | 2.07 ± 0.28 | 3.50 ± 0.13† | 1.77 ± 0.21 |
| Chromium malate-treated group 6 | 1.41 ± 0.23 | 15.68 ± 2.19 | 0.59 ± 0.14 | 2.01 ± 0.19 | 3.40 ± 0.08 | 1.82 ± 0.18 |
| Chromium malate-treated group 7 | 1.35 ± 0.21 | 16.53 ± 2.48 | 0.77 ± 0.27 | 2.09 ± 0.39 | 3.25 ± 0.37 | 1.73 ± 0.19 |
| Chromium malate-treated group 8 | 1.37 ± 0.10 | 15.21 ± 1.98 | 0.67 ± 0.24 | 2.01 ± 0.13 | 3.07 ± 0.76 | 1.79 ± 0.19 |
| Chromium malate-treated group 9 | 1.27 ± 0.11 | 16.11 ± 2.87 | 0.87 ± 0.16 | 1.81 ± 0.28 | 3.02 ± 0.57 | 1.76 ± 0.15 |
| Chromium malate control group | 1.54 ± 0.33 | 15.05 ± 1.52 | 0.88 ± 0.15 | 2.07 ± 0.26 | 2.41 ± 0.21‡§¶ | 1.82 ± 0.28 |
| Relative organ weight | ||||||
|---|---|---|---|---|---|---|
| Group | Parameter | |||||
| Relative heart weight | Relative liver weight | Relative spleen weight | Relative lung weight | Relative kidney weight | Relative brain weight | |
| Normal control group | 0.0028 ± 0.00039 | 0.029 ± 0.0043 | 0.0015 ± 0.00029 | 0.0038 ± 0.00063 | 0.0048 ± 0.00084 | 0.0031 ± 0.00057 |
| Model group | 0.0034 ± 0.00025 | 0.039 ± 0.0053 | 0.0018 ± 0.00030 | 0.0049 ± 0.00042 | 0.0090 ± 0.00038† | 0.0042 ± 0.00019 |
| Chromium trichloride-treated group | 0.0034 ± 0.00024 | 0.036 ± 0.0035 | 0.0013 ± 0.00022 | 0.0049 ± 0.00022 | 0.0092 ± 0.00071 | 0.0045 ± 0.00028 |
| Chromium picolinate-treated group | 0.0031 ± 0.00026 | 0.037 ± 0.0053 | 0.0019 ± 0.00043 | 0.0044 ± 0.00056 | 0.0081 ± 0.00063 | 0.0040 ± 0.00031 |
| Chromium malate-treated group 1 | 0.0032 ± 0.00029 | 0.041 ± 0.0051 | 0.0021 ± 0.00057 | 0.0048 ± 0.00036 | 0.0085 ± 0.00063† | 0.0039 ± 0.00034 |
| Chromium malate-treated group 2 | 0.0034 ± 0.00041 | 0.038 ± 0.0034 | 0.0015 ± 0.00017 | 0.0050 ± 0.00068 | 0.0082 ± 0.00066† | 0.0042 ± 0.00026 |
| Chromium malate-treated group 3 | 0.0028 ± 0.00033 | 0.034 ± 0.0020 | 0.0017 ± 0.00013 | 0.0042 ± 0.00092 | 0.0073 ± 0.00056† | 0.0031 ± 0.00038 |
| Chromium malate-treated group 4 | 0.0031 ± 0.00032 | 0.038 ± 0.0041 | 0.0018 ± 0.00030 | 0.0043 ± 0.00026 | 0.0071 ± 0.00015† | 0.0039 ± 0.00040 |
| Chromium malate-treated group 5 | 0.0032 ± 0.00071 | 0.031 ± 0.0033 | 0.0012 ± 0.00020 | 0.0045 ± 0.00080 | 0.0077 ± 0.00037† | 0.0039 ± 0.00060 |
| Chromium malate-treated group 6 | 0.0028 ± 0.00053 | 0.032 ± 0.0051 | 0.0012 ± 0.00032 | 0.0040 ± 0.00044 | 0.0069 ± 0.00019 | 0.0037 ± 0.00042 |
| Chromium malate-treated group 7 | 0.0028 ± 0.00027 | 0.035 ± 0.0032 | 0.0016 ± 0.00035 | 0.0044 ± 0.00050 | 0.0069 ± 0.00048 | 0.0037 ± 0.00024 |
| Chromium malate-treated group 8 | 0.0029 ± 0.00017 | 0.032 ± 0.0035 | 0.0014 ± 0.00042 | 0.0042 ± 0.00023 | 0.0064 ± 0.00013 | 0.0037 ± 0.00033 |
| Chromium malate-treated group 9 | 0.0025 ± 0.00019 | 0.032 ± 0.0050 | 0.0017 ± 0.00028 | 0.0036 ± 0.00049 | 0.0060 ± 0.00099 | 0.0035 ± 0.00026 |
| Chromium malate-control group | 0.0032 ± 0.00012 | 0.031 ± 0.0053 | 0.0018 ± 0.00052 | 0.0042 ± 0.00091 | 0.0049 ± 0.00073‡§¶ | 0.0037 ± 0.00098 |
Data is presented as mean ± standard deviation (n = 10). Chromium trichloride and chromium picolinate was used as a positive control.
Significantly different from normal control group (P < 0.05).
Significantly different from model group (P < 0.05).
Significantly different from chromium trichloride-treated group (P < 0.05).
Significantly different from chromium picolinate-treated group (P < 0.05).
Discussion
Diabetes mellitus is a chronic disease affecting millions of people globally and resulting in significant death rates each year. Type 2 diabetes is one type of diabetes mellitus and is found all over the world including China. It is also characterized by glycometabolism disorders, glycometabolism-related enzymes disorders and lipid metabolism disorders. Its can increase the risk of developing learning and memory deficits, diabetic nephropathy, atherosclerotic arterial disease, and so on6,30. Type 2 diabetic rats showed significant hyperglycemia in the present study. The FBG level of type 2 diabetic rats was 22.46 ± 1.47 mmol/L and significantly higher than that of normal rats (5.82 ± 0.53 mmol/L).
Cr is an important micronutrient that can reduce the FBG level in diabetes mellitus. Similar results were reported by Pattar et al.31, who found that chromium picolinate can enhance glucose metabolism; however, the safety and dose problems of chromium picolinate have been a concern. Chromium malate reduced FBG levels in type 2 diabetic rats after administration of chromium malate in the present study. Hepatic glycogen is composed of glucose and stored in the liver. Its accumulation is promoted by hyperglycemia and insulin, and in diabetes patients it is often accompanied by lower hepatic glycogen level32. The results of the present study showed that the hepatic glycogen levels of type 2 diabetic rats were significantly lower than that of normal rats. Insulin is a type of protein hormone and is secreted from pancreatic β-cells to control the blood glucose level33. It is a marker in the diagnosis and monitoring of type 2 diabetes, and is often used to control postprandial glucose levels in diabetics. Type 2 diabetes is also associated with insulin resistance and higher IR34. C-P is a small polypeptide produced and secreted from pancreatic β-cells during insulin synthesis. The concentration of C-P, which was secreted by islet β-cells, is equal to the insulin in the blood35. It is an important index for detection of insulin36. The results of the present study showed that the insulin, IR and C-P levels of type 2 diabetic rats were significantly higher than that of normal rats, and the results were similar to those in the literature. G6PD and GCK are key enzymes in glucose metabolism, and exist in the liver. The present results showed that the G6PD and GCK levels of type 2 diabetic rats were significantly lower than that of normal rats. Serum lipids, which include TC, LDL, HDL and TG, in type 2 diabetic rats changed significantly compared with the normal rats. Serum TC, LDL and TG levels in type 2 diabetic rats were significantly higher than that of normal rats. Serum HDL was significantly lower than that of normal rats. Król et al.18 found that chromium (III) propionate complex can significantly reduce serum TG, TC and LDL levels. Sharma et al.19 reported that chromium supplementation can significantly reduce TC, TG and LDL levels in type 2 diabetes. Sahin et al.37 reported that chromium picolinate (80 μg/kg bodyweight per day) can significantly reduce TC and TG when compared with type 2 diabetes mellitus. In conclusion, chromium malate shows beneficial effects against type 2 diabetes-induced glycometabolism, glycometabolism-related enzymes and lipid metabolism disorders. The antihyperglycemic activity of chromium malate is superior to chromium trichloride and chromium picolinate.
The loss of body mass often accompanies type 2 diabetes. Rodbard et al.38 reported that body mass control is a recommended treatment goal in type 2 diabetes patients: any weight gain of diabetes is associated with anti-diabetic therapy. Chromium malate can maintain the body mass of type 2 diabetic rats, whereas chromium trichloride and chromium picolinate cannot. Studies have shown that diabetes can cause biochemical and hematological changes39,40, and the results were similar to the present results. However, chromium malate has no significant effect on the biochemical and hematological index.
In conclusion, chromium malate shows greater benefits in treating type 2 diabetes, and can improve glycometabolism, glycometabolism-related enzymes and lipid metabolism in type 2 diabetic rats. The dose–response relationship of chromium malate showed that antihyperglycemic activity was increased with administration of chromium malate in a dose–dependent manner.
Acknowledgments
This work was supported financially by the Specialized Research Fund for the Natural Science Foundation of China (31271850) and Graduate innovative projects in Jiangsu University (CXLX13_685). The authors declare no conflict of interest.
References
- 1.Sudagani J, Hitman GA. Diabetes Mellitus: Etiology and Epidemiology. Encyclopedia of Human Nutrition. 3rd edn. New York: Academic Press; 2013. [Google Scholar]
- 2.Caballero AE. Long-term benefits of insulin therapy and glycemic control in overweight and obese adults with type 2 diabetes. J Diabetes Complications. 2009;23:143–152. doi: 10.1016/j.jdiacomp.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Cortez-Espinosa N, García-Hernández MH, Reynaga-Hernández E, et al. Abnormal expression and function of Dectin-1 receptor in type 2 diabetes mellitus patients with poor glycemic control (HbA1c>8%) Metabolism. 2012;61:1538–1546. doi: 10.1016/j.metabol.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Kelly PJ, Clarke PM, Hayes AJ, et al. Research: complications predicting mortality in people with Type 2 diabetes mellitus after major complications: a study using Swedish National Diabetes Register data. Diabet Med. 2014;18:954–962. doi: 10.1111/dme.12468. [DOI] [PubMed] [Google Scholar]
- 5.Suge R, Shimazu T, Hasegawa H, et al. Cerebral antioxidant enzyme increase associated with learning deficit in type 2 diabetes rats. Brain Res. 2012;1481:97–106. doi: 10.1016/j.brainres.2012.08.056. [DOI] [PubMed] [Google Scholar]
- 6.Michelle CE, Monette MA, Anne B, et al. A meta-analysis of cognitive functioning in nondemented adults with Type 2 diabetes mellitus. Can J Diabetes. 2014;38:401–408. doi: 10.1016/j.jcjd.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Halimi S. Do not forget that type 2 diabetes does not only expose to cardiovascular complications. Diabetes Metab. 2014;40:167–168. doi: 10.1016/j.diabet.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Ryan EA, Pick ME, Marceau C. Use of alternative medicines in diabetes mellitus. Diabet Med. 2001;18:242–245. doi: 10.1046/j.1464-5491.2001.00450.x. [DOI] [PubMed] [Google Scholar]
- 9.Król E, Krejpcio Z. Chromium(III) propionate complex supplementation improves carbohydrate metabolism in insulin-resistance rat model. Food Chem Toxicol. 2010;48:2791–2796. doi: 10.1016/j.fct.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Guerrero-Romero F, Tamez-Perez HE, González-González G, et al. Oral magnesium supplementation improves insulin sensitivity in non-diabetic subjects with insulin resistance. A double-blind placebo-controlled randomized trial. Diabetes Metab. 2004;30:253–258. doi: 10.1016/s1262-3636(07)70116-7. [DOI] [PubMed] [Google Scholar]
- 11.Yu YM, Chang WC, Chang CT, et al. Effects of young barley leaf extract and antioxidative vitamins on LDL oxidation and free radical scavenging activities in type 2 diabetes. Diabetes Metab. 2002;28:107–114. [PubMed] [Google Scholar]
- 12.Lin C, Chen PW, Chen WY, et al. Glucagon and insulin have opposite effects on tissue chromium distribution in an obese mouse model. J Diabetes Invest. 2013;6:528–532. doi: 10.1111/jdi.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Refaie FM, Esmat AY, Mohamed AF, et al. Effect of chromium supplementation on the diabetes induced-oxidative stress in liver and brain of adult rats. Biometals. 2009;22:1075–1087. doi: 10.1007/s10534-009-9258-8. [DOI] [PubMed] [Google Scholar]
- 14.Díaz-Medina EM, Martín-Herrera D, Rodríguez-Rodríguez EM, et al. Chromium(III) in cactus pad and its possible role in the antihyperglycemic activity. J Funct Foods. 2012;4:311–314. [Google Scholar]
- 15.Ghosh D, Bhattacharya B, Mukherjee B, et al. Role of chromium supplementation in Indians with type 2 diabetes mellitus. J Nutr Biochem. 2002;13:690–697. doi: 10.1016/s0955-2863(02)00220-6. [DOI] [PubMed] [Google Scholar]
- 16.Chen PW, Lin C, Chen CD, et al. Chromium levels in insulin-sensitive tissues and the thigh bone are modulated by prednisolone and high-fat diets in mice. Biometals. 2013;26:347–354. doi: 10.1007/s10534-013-9621-7. [DOI] [PubMed] [Google Scholar]
- 17.Anderson RA. Chromium in the prevention and control of diabetes. Diabetes Metab. 2000;26:22–27. [PubMed] [Google Scholar]
- 18.Król E, Krejpcio Z. Evaluation of anti-diabetic potential of chromium(III) propionate complex in high-fat diet fed and STZ injected rats. Food Chem Toxicol. 2011;49:3217–3223. doi: 10.1016/j.fct.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Sharma S, Agrawal RP, Choudhary M, et al. Beneficial effect of chromium supplementation on glucose, HbA1C and lipid variables in individuals with newly onset type-2 diabetes. J Trace Elem Med Biol. 2011;25:149–153. doi: 10.1016/j.jtemb.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Vincent JB. Recent advances in the nutritional biochemistry of trivalent chromium. Proc Nutr Soc. 2004;63:41–47. doi: 10.1079/PNS2003315. [DOI] [PubMed] [Google Scholar]
- 21.Gammelgaardi B, Jensen K, Steffansen B. In vitro metabolism and permeation studies in rat jejunum: organic chromium compared to inorganic chromium. J Trace Elem Med Biol. 1999;13:82–88. doi: 10.1016/S0946-672X(99)80028-5. [DOI] [PubMed] [Google Scholar]
- 22.Whittaker P, San RHC, Clarke JJ, et al. Mutagenicity of chromium picolinate and its components in Salmonella typhimurium and L5178Y mouse lymphoma cells. Food Chem Toxicol. 2005;43:1619–1625. doi: 10.1016/j.fct.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Proctor DM, Suh M, Campleman SL, et al. Assessment of the mode of action for hexavalent chromium-induced lung cancer following inhalation exposures. Toxicology. 2014;325:160–179. doi: 10.1016/j.tox.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Wu XY, Li F, Xu WD, et al. Anti-hyperglycemic activity of chromium(III) malate complex in alloxan-induced diabetic rats. Biol Trace Elem Res. 2011;143:1031–1043. doi: 10.1007/s12011-010-8916-6. [DOI] [PubMed] [Google Scholar]
- 25.Li F, Wu XY, Zou YM, et al. Comparing anti-hyperglycemic activity and acute oral toxicity of three different trivalent chromium complexes in mice. Food Chem Toxicol. 2012;50:1623–1631. doi: 10.1016/j.fct.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Skovsø S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Invest. 2014;4:349–358. doi: 10.1111/jdi.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokokawa H, Kinoshita I, Hashiguchi T, et al. Enhanced exercise-induced muscle damage and muscle protein degradation in streptozotocin-induced type 2 diabetic rats. J Diabetes Invest. 2011;6:423–428. doi: 10.1111/j.2040-1124.2011.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto Y, Hirose H, Saito I, et al. Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population. Clin Sci. 2002;103:137–142. doi: 10.1042/cs1030137. [DOI] [PubMed] [Google Scholar]
- 29.Sereshti H, Khojeh V, Samadi S. Optimization of dispersive liquid–liquid microextraction coupled with inductively coupled plasma-optical emission spectrometry with the aid of experimental design for simultaneous determination of heavy metals in natural waters. Talanta. 2011;83:885–890. doi: 10.1016/j.talanta.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 30.Meral I, Donmez N, Baydas B, et al. Effect of Nigella sativa L. on heart rate and some haematological values of alloxan-induced diabetic rabbits. Scand. J Lab Anim Sci. 2004;1:49–53. [Google Scholar]
- 31.Pattar GR, Tackett L, Liu P, et al. Chromium picolinate positively influences the glucose transporter system via affecting cholesterol homeostasis in adipocytes cultured under hyperglycemic diabetic conditions. Mutat Res. 2006;610:93–100. doi: 10.1016/j.mrgentox.2006.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomihira M, Kawasaki E, Nakajima H, et al. Intermittent and recurrent hepatomegaly due to glycogen storage in a patient with type 1 diabetes: genetic analysis of the liver glycogen phosphorylase gene (PYGL) Diabetes Res Clin Pract. 2004;65:175–182. doi: 10.1016/j.diabres.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen MG, Cobelli C. Insulin Modelling. Modelling Methodology for Physiology and Medicine. 2nd edn. New York: Academic Press; 2014. [Google Scholar]
- 34.Ahna J, Lee H, Im SW, et al. Allyl isothiocyanate ameliorates insulin resistance through the regulation of mitochondrial function. J Nutr Biochem. 2014;25:1026–1034. doi: 10.1016/j.jnutbio.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Grueter CC, Deschner T, Behringer V, et al. Socioecological correlates of energy balance using urinary C-peptide measurements in wild female mountain gorillas. Physiol Behav. 2014;127:13–19. doi: 10.1016/j.physbeh.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Liu TC, Chen MJ, Ren ZQ, et al. Development of an improved time-resolved fluoroimmunoassay for simultaneous quantification of C-peptide and insulin in human serum. Clin Biochem. 2014;47:439–444. doi: 10.1016/j.clinbiochem.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 37.Sahin K, Onderci M, Tuzcu M, et al. Effect of chromium on carbohydrate and lipid metabolism in a rat model of type 2 diabetes mellitus: the fat-fed, streptozotocin-treated rat. Metabolism. 2007;56:1233–1240. doi: 10.1016/j.metabol.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 38.Rodbard HW, Blonde L, Braithwaite SS, et al. AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13:1–68. doi: 10.4158/EP.13.S1.1. [DOI] [PubMed] [Google Scholar]
- 39.Erukainure OL, Ebuehi OAT, Adeboyejo FO, et al. Hematological and biochemical changes in diabetic rats fed with fiber-enriched cake. J Acute Med. 2013;3:39–44. [Google Scholar]
- 40.Oyedemi SO, Adewusi EA, Aiyegoro OA, et al. Antidiabetic and haematological effect of aqueous extract of stem bark of Afzelia africana (Smith) on streptozotocin-induced diabetic Wistar rats. Asian Pac J Trop Med. 2011;1:353–358. doi: 10.1016/S2221-1691(11)60079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]