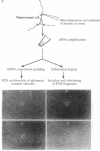
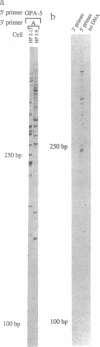
Abstract
Neurons are highly polarized cells with a mosaic of cytoplasmic and membrane proteins differentially distributed in axons, dendrites, and somata. In Drosophila and Xenopus, mRNA localization coupled with local translation is a powerful mechanism by which regionalized domains of surface or cytoplasmic proteins are generated. In neurons, there is substantial ultrastructural evidence positing the presence of protein synthetic machinery in neuronal processes, especially at or near postsynaptic sites. There are, however, remarkably few reports of mRNAs localized to these regions. We now present direct evidence that an unexpectedly large number of mRNAs, including members of the glutamate receptor family, second messenger system, and components of the translational control apparatus, are present in individual processes of hippocampal cells in culture.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainger K., Avossa D., Morgan F., Hill S. J., Barry C., Barbarese E., Carson J. H. Transport and localization of exogenous myelin basic protein mRNA microinjected into oligodendrocytes. J Cell Biol. 1993 Oct;123(2):431–441. doi: 10.1083/jcb.123.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D. G., Witter M. P. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31(3):571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- BODIAN D. A SUGGESTIVE RELATIONSHIP OF NERVE CELL RNA WITH SPECIFIC SYNAPTIC SITES. Proc Natl Acad Sci U S A. 1965 Feb;53:418–425. doi: 10.1073/pnas.53.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D. Branching patterns of individual sympathetic neurons in culture. J Cell Biol. 1973 Mar;56(3):702–712. doi: 10.1083/jcb.56.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchhalter J. R., Dichter M. A. Electrophysiological comparison of pyramidal and stellate nonpyramidal neurons in dissociated cell culture of rat hippocampus. Brain Res Bull. 1991 Mar;26(3):333–338. doi: 10.1016/0361-9230(91)90003-3. [DOI] [PubMed] [Google Scholar]
- Burgin K. E., Waxham M. N., Rickling S., Westgate S. A., Mobley W. C., Kelly P. T. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci. 1990 Jun;10(6):1788–1798. doi: 10.1523/JNEUROSCI.10-06-01788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicurel M. E., Terrian D. M., Potter H. mRNA at the synapse: analysis of a synaptosomal preparation enriched in hippocampal dendritic spines. J Neurosci. 1993 Sep;13(9):4054–4063. doi: 10.1523/JNEUROSCI.13-09-04054.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A. M., Blackstone C. D., Huganir R. L., Banker G. The distribution of glutamate receptors in cultured rat hippocampal neurons: postsynaptic clustering of AMPA-selective subunits. Neuron. 1993 Jun;10(6):1055–1068. doi: 10.1016/0896-6273(93)90054-u. [DOI] [PubMed] [Google Scholar]
- Davis L., Banker G. A., Steward O. Selective dendritic transport of RNA in hippocampal neurons in culture. Nature. 1987 Dec 3;330(6147):477–479. doi: 10.1038/330477a0. [DOI] [PubMed] [Google Scholar]
- Davis L., Burger B., Banker G. A., Steward O. Dendritic transport: quantitative analysis of the time course of somatodendritic transport of recently synthesized RNA. J Neurosci. 1990 Sep;10(9):3056–3068. doi: 10.1523/JNEUROSCI.10-09-03056.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L., Dou P., DeWit M., Kater S. B. Protein synthesis within neuronal growth cones. J Neurosci. 1992 Dec;12(12):4867–4877. doi: 10.1523/JNEUROSCI.12-12-04867.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberwine J. H., Spencer C., Newell D., Hoffman A. R. mRNA structure, in situ, as assessed by microscopic techniques. Microsc Res Tech. 1993 May 1;25(1):19–28. doi: 10.1002/jemt.1070250105. [DOI] [PubMed] [Google Scholar]
- Eberwine J., Yeh H., Miyashiro K., Cao Y., Nair S., Finnell R., Zettel M., Coleman P. Analysis of gene expression in single live neurons. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3010–3014. doi: 10.1073/pnas.89.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner C. C., Tucker R. P., Matus A. Selective localization of messenger RNA for cytoskeletal protein MAP2 in dendrites. Nature. 1988 Dec 15;336(6200):674–677. doi: 10.1038/336674a0. [DOI] [PubMed] [Google Scholar]
- Kislauskis E. H., Li Z., Singer R. H., Taneja K. L. Isoform-specific 3'-untranslated sequences sort alpha-cardiac and beta-cytoplasmic actin messenger RNAs to different cytoplasmic compartments. J Cell Biol. 1993 Oct;123(1):165–172. doi: 10.1083/jcb.123.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman R., Banker G., Steward O. Differential subcellular localization of particular mRNAs in hippocampal neurons in culture. Neuron. 1990 Dec;5(6):821–830. doi: 10.1016/0896-6273(90)90341-c. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Goto S., Anzai K. Brain-specific small RNA transcript of the identifier sequences is present as a 10 S ribonucleoprotein particle. J Biol Chem. 1991 Mar 15;266(8):4726–4730. [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Macdonald P. M., Struhl G. cis-acting sequences responsible for anterior localization of bicoid mRNA in Drosophila embryos. Nature. 1988 Dec 8;336(6199):595–598. doi: 10.1038/336595a0. [DOI] [PubMed] [Google Scholar]
- Mackler S. A., Eberwine J. H. Diversity of glutamate receptor subunit mRNA expression within live hippocampal CA1 neurons. Mol Pharmacol. 1993 Aug;44(2):308–315. [PubMed] [Google Scholar]
- Malinow R., Miller J. P. Postsynaptic hyperpolarization during conditioning reversibly blocks induction of long-term potentiation. Nature. 1986 Apr 10;320(6062):529–530. doi: 10.1038/320529a0. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Kater S. B. Development and selective neurodegeneration in cell cultures from different hippocampal regions. Brain Res. 1989 Jun 19;490(1):110–125. doi: 10.1016/0006-8993(89)90436-8. [DOI] [PubMed] [Google Scholar]
- Mohr E., Fehr S., Richter D. Axonal transport of neuropeptide encoding mRNAs within the hypothalamo-hypophyseal tract of rats. EMBO J. 1991 Sep;10(9):2419–2424. doi: 10.1002/j.1460-2075.1991.tb07781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Mowry K. L., Melton D. A. Vegetal messenger RNA localization directed by a 340-nt RNA sequence element in Xenopus oocytes. Science. 1992 Feb 21;255(5047):991–994. doi: 10.1126/science.1546297. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Powell S. K. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- Schuman E. M., Madison D. V. Locally distributed synaptic potentiation in the hippocampus. Science. 1994 Jan 28;263(5146):532–536. doi: 10.1126/science.8290963. [DOI] [PubMed] [Google Scholar]
- Spear D. H., Kutsunai S. Y., Correll C. C., Edwards P. A. Molecular cloning and promoter analysis of the rat liver farnesyl diphosphate synthase gene. J Biol Chem. 1992 Jul 15;267(20):14462–14469. [PubMed] [Google Scholar]
- Steward O., Davis L., Dotti C., Phillips L. L., Rao A., Banker G. Protein synthesis and processing in cytoplasmic microdomains beneath postsynaptic sites on CNS neurons. A mechanism for establishing and maintaining a mosaic postsynaptic receptive surface. Mol Neurobiol. 1988 Winter;2(4):227–261. doi: 10.1007/BF02935634. [DOI] [PubMed] [Google Scholar]
- Surmeier D. J., Eberwine J., Wilson C. J., Cao Y., Stefani A., Kitai S. T. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10178–10182. doi: 10.1073/pnas.89.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedge H., Fremeau R. T., Jr, Weinstock P. H., Arancio O., Brosius J. Dendritic location of neural BC1 RNA. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2093–2097. doi: 10.1073/pnas.88.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre E. R., Steward O. Demonstration of local protein synthesis within dendrites using a new cell culture system that permits the isolation of living axons and dendrites from their cell bodies. J Neurosci. 1992 Mar;12(3):762–772. doi: 10.1523/JNEUROSCI.12-03-00762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox K. S., Dichter M. A. Paired pulse depression in cultured hippocampal neurons is due to a presynaptic mechanism independent of GABAB autoreceptor activation. J Neurosci. 1994 Mar;14(3 Pt 2):1775–1788. doi: 10.1523/JNEUROSCI.14-03-01775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm J. E., Vale R. D. RNA on the move: the mRNA localization pathway. J Cell Biol. 1993 Oct;123(2):269–274. doi: 10.1083/jcb.123.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]