Abstract
Aims/Introduction
Elevation of the branched-chain amino acids (BCAAs), valine, leucine and isoleucine; and the aromatic amino acids, tyrosine and phenylalanine, has been observed in obesity-related insulin resistance. However, there have been few studies on Asians, who are generally less obese and less insulin-resistant than Caucasian or African-Americans. In the present study, we investigated the relationship between homeostasis model assessment of insulin resistance (HOMA-IR) and plasma amino acid concentration in non-diabetic Japanese participants.
Materials and Methods
A total of 94 healthy men and women were enrolled, and plasma amino acid concentration was measured by liquid chromatography/mass spectrometry after overnight fasting. The associations between HOMA-IR and 20 amino acid concentrations, and anthropometric and clinical parameters of lifestyle-related diseases were evaluated.
Results
The mean age and body mass index were 40.1 ± 9.6 years and 22.7 ± 3.9, respectively. Significantly positive correlations were observed between HOMA-IR and valine, isoleucine, leucine, tyrosine, phenylalanine and total BCAA concentration. Compared with the HOMA-IR ≤ 1.6 group, the HOMA-IR > 1.6 group showed significantly exacerbated anthropometric and clinical parameters, and significantly elevated levels of valine, isoleucine, leucine, tyrosine, phenylalanine and BCAA.
Conclusions
The present study shows that the insulin resistance-related change in amino acid profile is also observed in non-diabetic Japanese subjects. These amino acids include BCAAs (valine, isoleucine and leucine) and aromatic amino acids (tyrosine and phenylalanine), in agreement with previous studies carried out using different ethnic groups with different degrees of obesity and insulin resistance.
Keywords: Amino acids, Homeostasis model assessment of insulin resistance, Insulin resistance
Introduction
Hyperaminoacidemia as a manifestation of the insulin resistance characteristic of obesity was first reported in 19691. Of 20 plasma amino acids measured, the concentrations of valine, leucine, isoleucine, tyrosine and phenylalanine were elevated in obese subjects even after overnight fasting and correlated directly with serum insulin, which diminished after glucose infusion1. Since the late 2000s, metabolomic technologies have been applied to diabetes research2, and alterations in metabolites, such as amino acids, fatty acids and organic acids, have been shown to be associated with future development of diabetes3–5.
Branched-chain amino acids (BCAAs), namely valine, leucine and isoleucine, are among the nine essential amino acids (EAAs), accounting for 35% of the EAAs in muscle proteins and 40% of the preformed amino acids required by mammals6. Several studies have shown that insulin resistance plays a key role in the underlying mechanism linking obesity with increased circulating concentrations of BCAAs7–13. Insulin is known as a regulator of branched-chain α-keto acid dehydrogenase (BCKDH) complex14, a rate-limiting enzyme of BCAA catabolism, which catalyzes the irreversible oxidative decarboxylation of branched-chain a-keto acids generated by reversible transamination of BCAAs by branched-chain aminotransferase (BCAT)15. Reduced enzyme activity of BCKDH complex in obesity and/or diabetes has been shown16–19, and suppression of BCAA catabolism by insulin resistance is considered as a plausible etiology of elevated BCAA concentration in obesity. However, although the degree of obesity and insulin resistance might differ among different ethnic groups, there have been few studies on insulin resistance and amino acid profiles in Asian populations. Furthermore, it is unclear whether obesity-related changes in specific amino acids are also observed in healthy Japanese people with lower body mass index (BMI) than Western people.
In the present study using 94 non-diabetic Japanese men and women, we investigated the relationship between homeostasis model assessment of insulin resistance (HOMA-IR) and plasma amino acid concentration, as well as the anthropometric and clinical parameters of lifestyle-related diseases.
Materials and Methods
Participants
Healthy volunteers aged between 20 and 65 years were recruited, and 94 individuals (48 men and 46 women) were enrolled in the present cross-sectional study. The examinations were carried out in February 2013. Written informed consent was obtained from all the participants to use their health records for analysis. The present study was approved by the Ethical Committee of Tokai University, and was carried out in accordance with the Declaration of Helsinki.
Anthropometric Measurements
All measurements were carried out after overnight fasting. Height and weight were measured in the standing position, and BMI was calculated as weight/height2 (kg/m2). Waist circumference was assessed at the end of expiration, measuring the minimum circumference at the level of the umbilicus. Blood pressure (BP) was measured in the sitting position. Visceral and subcutaneous fat area was measured at the level of the umbilicus in the spine position using computed tomography. Bioelectrical impedance analysis (Inbody 720; Biospace Co., Ltd., Tokyo, Japan) was used for evaluating body fat mass, fat-free mass and percentage body fat.
Biochemical Measurements
Fasting serum immunoreactive insulin was measured by chemiluminescent enzyme immunoassay (CLEIA). Glycated hemoglobin (HbA1c) was determined by the latex agglutination immunoassay. HOMA-IR was calculated as: fasting plasma glucose (in mg/dL) × insulin (in mU/mL)/40520. HOMA-IR ≤ 1.6 was considered as non-insulin-resistant according to the definition of the Japan Diabetes Society21. Low-density lipoprotein cholesterol (LDL-C) was measured directly, and high-density lipoprotein cholesterol (HDL-C) and triglycerides were determined enzymatically. Leptin was measured by double antibody radioimmunoassay. High molecular adiponectin was measured using the CLEIA method. High-sensitivity C-reactive protein (hsCRP) was measured by turbidimetric immunoassay.
Plasma amino acid concentrations were measured by liquid chromatography/mass spectrometry (LC/MC). Of 39 amino acids measured (taurine, aspartic acid, hydroxyproline, threonine, serine, asparagine, glutamic acid, glutamine, sarcosine, α-aminoadipic acid, proline, glycine, alanine, citrulline, α-aminobutyric acid, valine, cysteine, cystathionine, methionine, isoleucine, leucine, tyrosine, phenylalanine, γ-amino β-hydroxybutyric acid, β-alanine, β-amino-iso-butyric acid, γ-aminobutyric acid, monoethanolamine, homocysteine, histidine, 3-methylhistidine, 1-methylhistidine, carnosine, anserine, tryptophan, hydroxylysine, ornithine, lysine, arginine), 20 amino acids were at detectable levels and eligible for analysis. Levels of total amino acid (total AA), non-essential amino acid (NEAA), EAA and BCAA were also obtained and used for analysis.
Statistical Analyses
Data are expressed as mean ± SD. SPSS Statistics (version 22.0; SPSS Inc., Chicago, IL, USA) was used for the statistical analyses. Pearson's correlation coefficient was calculated as a measure of association. Statistical significance of comparisons between the HOMA-IR ≤ 1.6 group and HOMA-IR > 1.6 group was determined by the chi squared-test or Student's t-test. All P-values were two-tailed, and P < 0.05 was considered significant.
Results
The clinical characteristics of the participants are shown in Table1. The mean age was 40.1 ± 9.6 years (42.0 ± 10.2 for men and 38.1 ± 8.6 for women). The age of the participants ranged from 22 to 62 years (24–62 for men and 22–55 for women). The participants were not obese on average (BMI 22.7 ± 3.9). Mean fasting glucose and HbA1c were within the range of normal glucose tolerance. There were no participants who exceeded both fasting glucose ≥ 126 mg/dL and HbA1c ≥ 6.5% or were under diabetes treatment. The mean HOMA-IR was 1.12 ± 0.2 (1.33 ± 0.85 for men and 0.90 ± 0.46 for women). A total of 38 men (79.1%) and 42 women (91.3%) were HOMA-IR ≤ 1.6. Average concentrations of 20 amino acids, total amino AA, NEAA, EAA and BCAA concentrations were within the normal ranges (Table2).
Table 1.
Background characteristics of the study participants
| All | Men | Women | |
|---|---|---|---|
| No. participants | 94 | 48 | 46 |
| Age (years) | 40.1 ± 9.6 | 42.0 ± 10.2 | 38.1 ± 8.6 |
| Bodyweight (kg) | 63.2 ± 14.5 | 72.5 ± 12.4 | 53.4 ± 9.3 |
| BMI (kg/m2) | 22.7 ± 3.9 | 24.1 ± 3.8 | 21.2 ± 3.5 |
| Waist circumference (cm) | 80.4 ± 10.6 | 85.4 ± 9.9 | 75.2 ± 8.7 |
| Visceral fat (cm2) | 47.4 ± 40.9 | 70.5 ± 41.4 | 23.3 ± 22.3 |
| Subcutaneous fat (cm2) | 136.3 ± 80.8 | 139.4 ± 90.2 | 133.0 ± 70.4 |
| Body fat mass (kg) | 15.6 ± 7.4 | 16.4 ± 8.3 | 14.8 ± 6.4 |
| Fat free mass (kg) | 44.9 ± 9.7 | 53.0 ± 5.5 | 36.3 ± 4.2 |
| %Body fat (%) | 24.3 ± 7.5 | 21.8 ± 7.0 | 26.9 ± 7.1 |
| Systolic BP (mmHg) | 119.6 ± 15.2 | 124.8 ± 12.2 | 114.1 ± 16.1 |
| Diastolic BP (mmHg) | 77.6 ± 13.2 | 82.5 ± 11.7 | 72.4 ± 12.8 |
| Fasting glucose (mg/dL) | 89.8 ± 8.7 | 93.2 ± 9.2 | 86.3 ± 6.7 |
| HbA1c (%) | 5.3 ± 0.3 | 5.3 ± 0.3 | 5.3 ± 0.3 |
| Fasting insulin (μU/mL) | 4.97 ± 2.92 | 5.69 ± 3.41 | 4.21 ± 2.06 |
| HOMA-IR | 1.12 ± 0.72 | 1.33 ± 0.85 | 0.90 ± 0.46 |
| LDL-C (mg/dL) | 113.5 ± 32.2 | 120.2 ± 31.4 | 106.5 ± 31.9 |
| HDL-C (mg/dL) | 65.1 ± 16.8 | 57.3 ± 13.2 | 73.2 ± 16.4 |
| Triglyceride (mg/dL) | 107.7 ± 84.0 | 134.7 ± 99.6 | 79.5 ± 51.4 |
| AST (U/L) | 20.6 ± 5.6 | 22.9 ± 5.7 | 18.3 ± 4.5 |
| ALT (U/L) | 19.9 ± 12.4 | 25.6 ± 14.0 | 14.0 ± 6.5 |
| γGT (U/L) | 33.5 ± 30.7 | 45.9 ± 37.4 | 20.5 ± 12.0 |
| Uric acid (mg/dL) | 5.3 ± 1.3 | 6.1 ± 1.1 | 4.5 ± 0.9 |
| Leptin (ng/mL) | 7.92 ± 6.24 | 5.46 ± 3.78 | 10.49 ± 7.23 |
| Adiponectin (μg/mL) | 3.65 ± 2.46 | 2.28 ± 1.30 | 5.08 ± 2.58 |
| hsCRP (ng/mL) | 1055.3 ± 4929.6 | 1564.3 ± 6792.4 | 524.2 ± 1212.2 |
Data are mean ± SD.
γGT, γ-glutamyl transpeptidase
ALT, alanine aminotransferase
AST, aspartate aminotransferase
BMI, body mass index
BP, blood pressure
HbA1c, glycated hemoglobin
HDL-C, high-density lipoprotein cholesterol
HOMA-IR, homeostasis model assessment of insulin resistance
hsCRP, high-sensitivity C-reactive protein
LDL-C, low-density lipoprotein cholesterol.
Table 2.
Average amino acid concentrations
| (nmol/mL) | All | Men | Women |
|---|---|---|---|
| Glycine | 209 ± 50.3 | 213.1 ± 43.9 | 206.0 ± 56.5 |
| Alanine | 329.0 ± 62.3 | 355.7 ± 59.0 | 301.2 ± 53.4 |
| Serine | 112.7 ± 20.3 | 109.6 ± 19.9 | 115.9 ± 20.3 |
| Threonine | 123.5 ± 24.7 | 123.2 ± 19.4 | 123.7 ± 29.5 |
| Valine | 206.7 ± 37.8 | 229.0 ± 29.1 | 183.3 ± 31.3 |
| Isoleucine | 59.6 ± 12.8 | 68.0 ± 10.6 | 50.9 ± 8.2 |
| Leucine | 118.3 ± 23.6 | 135.6 ± 15.5 | 100.3 ± 15.7 |
| Lysine | 172.0 ± 31.6 | 189.8 ± 25.1 | 153.4 ± 26.8 |
| Arginine | 51.0 ± 16.8 | 51.9 ± 15.8 | 50.1 ± 17.9 |
| Histidine | 78.9 ± 8.1 | 83.2 ± 7.3 | 74.4 ± 6.3 |
| Tyrosine | 57.7 ± 10.4 | 62.0 ± 9.2 | 53.2 ± 9.8 |
| Phenylalanine | 55.1 ± 8.4 | 59.8 ± 7.6 | 50.2 ± 6.1 |
| Tryptophan | 55.7 ± 8.8 | 59.6 ± 8.7 | 51.7 ± 7.1 |
| Methionine | 23.4 ± 4.0 | 25.1 ± 3.6 | 21.6 ± 3.7 |
| Cysteine | 12.7 ± 4.1 | 13.8 ± 4.4 | 11.5 ± 3.4 |
| Proline | 137.2 ± 39.7 | 157.6 ± 40.0 | 115.8 ± 26.0 |
| Glutamine | 507.2 ± 70.9 | 539.0 ± 58.5 | 474.0 ± 67.9 |
| Glutamic acid | 40.7 ± 18.4 | 50.2 ± 18.0 | 30.9 ± 13.0 |
| Asparagine | 47.0 ± 6.9 | 48.5 ± 7.4 | 45.5 ± 6.1 |
| Aspartic acid | 2.9 ± 0.8 | 3.0 ± 1.0 | 2.7 ± 0.5 |
| Total AA | 2570.7 ± 298.1 | 2759.4 ± 191.4 | 2373.7 ± 260.2 |
| NEAA | 1677.5 ± 209.6 | 1786.2 ± 148.3 | 1564.1 ± 205.0 |
| EAA | 893.1 ± 121.0 | 973.2 ± 83.2 | 809.6 ± 95.2 |
| BCAA | 384.6 ± 71.0 | 432.6 ± 50.8 | 334.5 ± 52.0 |
Data are mean ± SD.
BCAA, branched-chain amino acid
EAA, essential amino acid
NEAA, non-essential amino acid; Total AA, total amino acid.
The correlations between insulin resistance and clinical parameters are shown in Table3. The influence of age on HOMA-IR was insignificant. HOMA-IR showed a significantly positive correlation with all the values of anthropometric measurements (bodyweight, BMI, waist circumference, fat-free mass and percentage body fat, visceral and subcutaneous fat area) in both men and women. HOMA-IR was also significantly positively associated with BP in both sexes. Insulin showed a higher correlation with HOMA-IR than fasting glucose. Among lipid parameters, HDL-C and triglycerides, but not LDL-C, were significantly positively correlated with HOMA-IR in men and women. Alanine aminotransferase, which is often used as a surrogate marker for fatty liver, was significantly positively correlated with HOMA-IR in both sexes. There was a significantly negative correlation between adiponectin and HOMA-IR in the whole population and in men. High-sensitivity C-reactive protein was significantly positively correlated with HOMA-IR.
Table 3.
Correlations between homeostasis model assessment of insulin resistance and clinical parameters
| All | Men | Women | ||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Age | 0.045 | 0.667 | 0.013 | 0.930 | −0.084 | 0.580 |
| Bodyweight | 0.471 | <0.001 | 0.326 | 0.024 | 0.547 | <0.001 |
| BMI | 0.511 | <0.001 | 0.393 | 0.006 | 0.612 | <0.001 |
| Waist circumference | 0.542 | <0.001 | 0.479 | 0.001 | 0.510 | <0.001 |
| Visceral fat | 0.626 | <0.001 | 0.627 | <0.001 | 0.430 | 0.003 |
| Subcutaneous fat | 0.374 | <0.001 | 0.307 | 0.034 | 0.576 | <0.001 |
| Body fat mass | 0.509 | <0.001 | 0.478 | 0.001 | 0.585 | <0.001 |
| Fat free mass | 0.299 | 0.003 | 0.006 | 0.968 | 0.301 | 0.042 |
| %Body fat | 0.374 | <0.001 | 0.565 | <0.001 | 0.534 | <0.001 |
| Systolic BP | 0.410 | <0.001 | 0.412 | 0.004 | 0.318 | 0.031 |
| Diastolic BP | 0.391 | <0.001 | 0.357 | 0.013 | 0.289 | 0.051 |
| Fasting glucose | 0.435 | <0.001 | 0.388 | 0.006 | 0.303 | 0.041 |
| HbA1c | 0.194 | 0.060 | 0.137 | 0.354 | 0.263 | 0.077 |
| Fasting insulin | 0.985 | <0.001 | 0.985 | <0.001 | 0.989 | <0.001 |
| LDL-C | 0.132 | 0.206 | 0.096 | 0.514 | 0.038 | 0.802 |
| HDL-C | −0.485 | <0.001 | −0.344 | 0.017 | −0.597 | <0.001 |
| Triglyceride | 0.595 | <0.001 | 0.618 | <0.001 | 0.303 | 0.041 |
| AST | 0.348 | 0.001 | 0.231 | 0.113 | 0.340 | 0.021 |
| ALT | 0.482 | <0.001 | 0.365 | 0.011 | 0.586 | <0.001 |
| γGT | 0.408 | <0.001 | 0.381 | 0.008 | 0.048 | 0.750 |
| Uric acid | 0.319 | 0.002 | 0.348 | 0.015 | −0.199 | 0.184 |
| Leptin | 0.392 | <0.001 | 0.621 | <0.001 | 0.792 | <0.001 |
| Adiponectin | −0.336 | 0.001 | −0.305 | 0.035 | −0.213 | 0.155 |
| hsCRP | 0.296 | 0.004 | 0.286 | 0.049 | 0.375 | 0.010 |
γGT, γ-glutamyl transpeptidase
ALT, alanine aminotransferase
AST, aspartate aminotransferase
BMI, body mass index
BP, blood pressure
HbA1c, glycated hemoglobin
HDL-C, high-density lipoprotein cholesterol
hsCRP, high-sensitivity C-reactive protein
LDL-C, low-density lipoprotein cholesterol.
Table4 shows the correlations between HOMA-IR and amino acid levels. There were several amino acids that were significantly positively correlated with HOMA-IR. Most notably, BCAAs (valine, isoleucine and leucine) were positively associated with insulin resistance, although valine in men did not reach statistical significance. Furthermore, tyrosine and phenylalanine, which are aromatic amino acids, were significantly positively correlated with HOMA-IR. Total BCAA was also significantly positively associated with HOMA-IR in both men and women. Correlation diagrams for valine, isoleucine, leucine, tyrosine, phenylalanine and BCAA in the whole population are shown in Figure1. We found that isoleucine, leucine, tyrosine, phenylalanine and BCAA showed significantly positive correlations with HOMA-IR (P < 0.05) even after adjusted for BMI. Valine tended to increase as HOMA-IR increased, although its level did not reach statistical significance (P = 0.086) after adjustment for BMI.
Table 4.
Correlations between homeostasis model assessment of insulin resistance and plasma amino acid levels
| All | Men | Women | ||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Glycine | −0.120 | 0.248 | −0.326 | 0.024 | 0.084 | 0.578 |
| Alanine | 0.439 | <0.001 | 0.478 | 0.001 | 0.140 | 0.354 |
| Serine | −0.231 | 0.025 | −0.374 | 0.009 | 0.114 | 0.451 |
| Threonine | −0.113 | 0.276 | −0.222 | 0.129 | −0.016 | 0.918 |
| Valine | 0.384 | <0.001 | 0.245 | 0.094 | 0.354 | 0.016 |
| Isoleucine | 0.495 | <0.001 | 0.385 | 0.007 | 0.520 | <0.001 |
| Leucine | 0.430 | <0.001 | 0.323 | 0.025 | 0.383 | 0.009 |
| Lysine | 0.153 | 0.141 | −0.017 | 0.908 | −0.040 | 0.792 |
| Arginine | 0.192 | 0.063 | 0.265 | 0.068 | 0.079 | 0.600 |
| Histidine | 0.294 | 0.004 | 0.152 | 0.301 | 0.209 | 0.164 |
| Tyrosine | 0.462 | <0.001 | 0.462 | 0.001 | 0.313 | 0.034 |
| Phenylalanine | 0.398 | <0.001 | 0.292 | 0.044 | 0.304 | 0.040 |
| Tryptophan | 0.153 | 0.141 | −0.021 | 0.886 | 0.132 | 0.383 |
| Methionine | 0.157 | 0.131 | 0.026 | 0.862 | 0.058 | 0.704 |
| Cysteine | 0.204 | 0.049 | 0.151 | 0.305 | 0.088 | 0.563 |
| Proline | 0.396 | <0.001 | 0.312 | 0.031 | 0.249 | 0.095 |
| Glutamine | 0.075 | 0.474 | −0.154 | 0.297 | 0.047 | 0.758 |
| Glutamic acid | 0.461 | <0.001 | 0.448 | 0.001 | 0.195 | 0.195 |
| Asparagine | −0.184 | 0.077 | −0.229 | 0.117 | −0.370 | 0.011 |
| Aspartic acid | 0.400 | <0.001 | 0.383 | 0.011 | 0.303 | 0.087 |
| Total AA | 0.324 | 0.001 | 0.202 | 0.168 | 0.194 | 0.197 |
| NEAA | 0.266 | 0.010 | 0.162 | 0.271 | 0.128 | 0.396 |
| EAA | 0.336 | 0.001 | 0.176 | 0.230 | 0.254 | 0.089 |
| BCAA | 0.436 | <0.001 | 0.319 | 0.027 | 0.410 | 0.005 |
BCAA, branched-chain amino acid
EAA, essential amino acid
NEAA, non-essential amino acid
Total AA, total amino acid.
Figure 1.
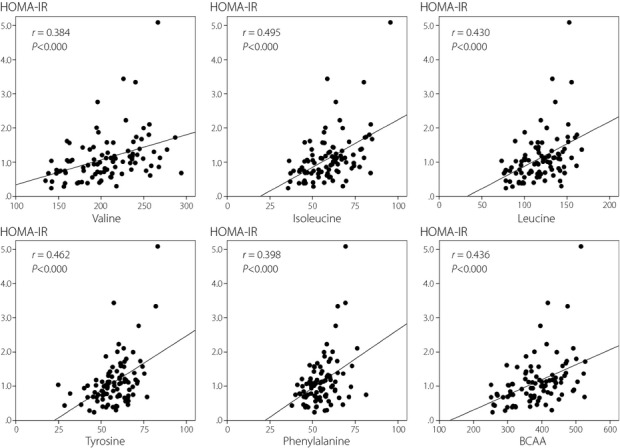
Correlation between homeostasis model assessment of insulin resistance (HOMA-IR) and branched-chain amino acids (BCAAs) and aromatic amino acids. Significant correlations were observed (P < 0.01) between HOMA-IR and valine, isoleucine, leucine, tyrosine, phenylalanine and BCAA. Correlation diagrams for the whole population are shown.
Next, we compared clinical characteristics between the HOMA-IR ≤ 1.6 group and HOMA-IR > 1.6 group (Tables5 and 6). Compared with the HOMA-IR ≤ 1.6 group, the HOMA-IR > 1.6 group was significantly higher in weight, BP, fasting glucose and triglycerides. HDL-C and adiponectin were significantly lower, and hsCRP was significantly higher in the HOMA-IR > 1.6 group than in the HOMA-IR ≤ 1.6 group. Among the amino acids measured, valine, isoleucine, leucine, tyrosine, phenylalanine and BCAA were significantly elevated in the HOMA-IR > 1.6 group than in the HOMA-IR ≤ 1.6 group in both sexes.
Table 5.
Comparison of clinical parameters between homeostasis model assessment of insulin resistance ≤1.6 and homeostasis model assessment of insulin resistance >1.6 groups
| HOMA-IR ≤ 1.6 | HOMA-IR > 1.6 | P | |
|---|---|---|---|
| No. men/women | 38 (47%)/42 (53%) | 10 (71%)/4 (29%) | 0.086 |
| Age (years) | 40.5 ± 9.7 | 37.9 ± 9.1 | 0.360 |
| Body weight (kg) | 60.5 ± 11.9 | 78.6 ± 18.7 | <0.001 |
| BMI (kg/m2) | 21.8 ± 2.9 | 27.5 ± 5.1 | <0.001 |
| Waist circumference (cm) | 78.1 ± 8.2 | 93.2 ± 13.6 | <0.001 |
| Visceral fat (cm2) | 39.2 ± 33.1 | 94.4 ± 50.1 | <0.001 |
| Subcutaneous fat (cm2) | 121.1 ± 57.7 | 222.9 ± 130.3 | <0.001 |
| Body fat mass (kg) | 14.0 ± 5.2 | 25.1 ± 10.8 | <0.001 |
| Fat free mass (kg) | 43.9 ± 9.3 | 50.5 ± 10.2 | 0.017 |
| %Body fat (%) | 23.1 ± 6.8 | 31.2 ± 7.7 | <0.001 |
| Systolic BP (mmHg) | 117.4 ± 14.4 | 131.9 ± 13.7 | 0.001 |
| Diastolic BP (mmHg) | 75.8 ± 11.8 | 87.9 ± 16.2 | 0.001 |
| Fasting glucose (mg/dL) | 88.7 ± 8.1 | 96.2 ± 9.6 | 0.003 |
| HbA1c (%) | 5.29 ± 0.26 | 5.40 ± 0.33 | 0.164 |
| Fasting insulin (μU/mL) | 4.08 ± 1.47 | 10.00 ± 3.94 | <0.001 |
| HOMA-IR | 0.90 ± 0.34 | 2.38 ± 0.98 | <0.001 |
| LDL-C (mg/dL) | 111.8 ± 31.9 | 123.1 ± 33.5 | 0.226 |
| HDL-C (mg/dL) | 67.4 ± 16.7 | 51.9 ± 9.5 | 0.001 |
| Triglyceride (mg/dL) | 95.8 ± 65.9 | 175.4 ± 135.3 | 0.001 |
| AST (U/L) | 19.9 ± 5.0 | 25.0 ± 7.1 | 0.001 |
| ALT (U/L) | 17.9 ± 9.4 | 31.5 ± 19.8 | <0.001 |
| γGT (U/L) | 29.2 ± 26.6 | 57.9 ± 40.8 | 0.001 |
| Uric acid (mg/dL) | 5.2 ± 1.2 | 5.9 ± 1.5 | 0.074 |
| Leptin (ng/mL) | 6.64 ± 4.13 | 15.25 ± 10.36 | <0.001 |
| Adiponectin (μg/mL) | 3.90 ± 2.49 | 2.23 ± 1.78 | 0.019 |
| hsCRP (ng/mL) | 461.6 ± 945.2 | 4448.3 ± 12403.6 | 0.005 |
Data are mean ± SD, unless otherwise stated.
γGT, γ-glutamyl transpeptidase
ALT, alanine aminotransferase
AST, aspartate aminotransferase
BMI, body mass index
BP, blood pressure
HbA1c, glycated hemoglobin
HDL-C, high-density lipoprotein cholesterol
HOMA-IR, homeostasis model assessment of insulin resistance
hsCRP, high-sensitivity C-reactive protein
LDL-C, low-density lipoprotein cholesterol.
Table 6.
Comparison of amino acid profile by insulin resistance
| (nmol/mL) | HOMA-IR ≤ 1.6 | HOMA-IR > 1.6 | P |
|---|---|---|---|
| Glycine | 211.1 ± 51.7 | 201.1 ± 42.4 | 0.495 |
| Alanine | 321.8 ± 58.3 | 370.2 ± 70.8 | 0.007 |
| Serine | 114.2 ± 20.2 | 104.3 ± 19.1 | 0.092 |
| Threonine | 123.9 ± 26.2 | 120.8 ± 14.2 | 0.662 |
| Valine | 202.2 ± 36.8 | 232.0 ± 34.3 | 0.006 |
| Isoleucine | 57.5 ± 11.3 | 72.0 ± 14.0 | <0.001 |
| Leucine | 115.2 ± 22.6 | 135.7 ± 22.0 | 0.002 |
| Lysine | 171.4 ± 31.9 | 175.1 ± 30.9 | 0.695 |
| Arginine | 49.4 ± 16.6 | 60.2 ± 15.8 | 0.027 |
| Histidine | 78.3 ± 8.1 | 82.7 ± 6.9 | 0.060 |
| Tyrosine | 56.2 ± 10.0 | 66.0 ± 8.9 | 0.001 |
| Phenylalanine | 53.9 ± 8.1 | 61.6 ± 7.2 | 0.001 |
| Tryptophan | 55.3 ± 8.4 | 58.2 ± 10.8 | 0.256 |
| Methionine | 23.2 ± 4.1 | 24.2 ± 3.8 | 0.418 |
| Cysteine | 12.5 ± 4.1 | 13.8 ± 3.6 | 0.272 |
| Proline | 131.9 ± 34.4 | 167.2 ± 54.3 | 0.002 |
| Glutamine | 505.6 ± 72.7 | 515.9 ± 61.2 | 0.619 |
| Glutamic acid | 38.5 ± 16.2 | 53.6 ± 25.1 | 0.004 |
| Asparagine | 47.6 ± 6.6 | 43.8 ± 7.7 | 0.057 |
| Aspartic acid | 2.8 ± 0.6 | 3.4 ± 1.4 | 0.014 |
| Total AA | 2544.0 ± 299.2 | 2723.1 ± 249.6 | 0.037 |
| NEAA | 1663.0 ± 211.7 | 1760.9 ± 182.1 | 0.107 |
| EAA | 881.0 ± 120.1 | 962.1 ± 105.4 | 0.020 |
| BCAA | 374.9 ± 67.7 | 439.7 ± 66.6 | 0.001 |
Data are mean ± SD.
BCAA, branched-chain amino acid
EAA, essential amino acid
HOMA-IR, homeostasis model assessment of insulin resistance
NEAA, non-essential amino acid
Total AA, total amino acid.
Discussion
In the present study, the association between HOMA-IR and plasma amino acid concentration was examined in 94 non-diabetic Japanese men and women. We identified that the insulin resistance-related change in amino acid profile was also observed in our participants, and BCAAs (valine, isoleucine and leucine) and aromatic amino acids (tyrosine and phenylalanine) were found to be significantly related to insulin resistance.
Pioneering application of metabolomics technologies by Newgard et al.7 has shown that a cluster of amino acids including BCAAs and aromatic amino acids was strongly associated with obesity-related insulin resistance, followed by several reports supporting the association between insulin resistance and increased circulating concentrations of BCAAs8–12. The degree of obesity and insulin resistance greatly differs among different ethnic groups; Asians are generally less obese and less insulin-resistant than Caucasian or African-Americans. In a study of Americans by Newgard et al.7, the average BMI and HOMA-IR were 36.6 and 5.73 in the obese group, and 23.3 and 2.51 in the lean group. The HOMA-IR value of even the lean American subjects with normal BMI exceeds the Japanese criterion for insulin resistance (HOMA-IR ≥ 2.5)21, which is comparable with the 97.5 percentile value of the Japanese reference individuals22. In the present study, the BMI and HOMA-IR values were 22.7 and 1.12 in the whole population, 21.8 and 0.90 in the HOMA-IR ≤ 1.6 group, and 27.5 and 2.38 in the HOMA-IR > 1.6 group. There is only one Asian study on the insulin resistance-related increase in amino acids using Chinese and Asian-Indian men living in Singapore. The mean BMI and HOMA-IR values for Chinese men were 23.9 and 0.75 for the low HOMA group (below the lower tertile), and 24.5 and 3.02 for the high HOMA group (above the upper tertile)9. We have investigated the insulin resistance-related change in amino acid profile in both men and women, whereas Tai et al. only studied in men. Although Tai et al. were the first who reported this phenotype in Asians, they emphasized the need to analyze Chinese and Asian-Indians separately to find the differences among the Asian ethnic groups. Ours is the first report using Japanese men and women, and has a significant role in the development for the studies of diabetes in Asians. Although heterogeneity in ethnicity might contribute to discrepancies in the degree of obesity and insulin resistance, the influence of insulin resistance on amino acids profile in our Japanese study was consistent with that of previous other studies7–12, regardless of ethnic background. Furthermore, the extent of BCAA elevation was 10–20% between the low and high HOMA groups in the present study, comparable with other studies7,9.
Currently, suppression of BCAA catabolism by insulin resistance is considered as a plausible etiology of elevated BCAA concentration in obesity. As BCAAs and phenylalanine are EAAs, and cannot be synthesized in the human body, it is highly doubtful whether dietary protein has any impact on their plasma concentration. Although we did not evaluate dietary nutrient intake in the present study, the limited numbers of previous reports showed that increased protein intake was not observed as evidence for higher BCAA levels in obese insulin-resistant subjects3,9,12. There is another intervention study supporting that BCAAs are uniquely correlated with insulin resistance, where BCAA levels were associated with improvement in insulin resistance with weight loss11. We and other investigators examined circulating free BCAAs, not whole-body storage of BCAAs, which are largely present as muscle proteins. The pool size of free BCAAs is very small (accounting for ∽0.1 g/kg muscle), and this free amino acid pool is quite constant because of the continuous supply from muscle when decreased and rapid degradation when increased23. Taken together, it is unlikely that circulating BCAAs are dominantly regulated by dietary intake, at least in the steady state.
Contrary to BCAAs, the relationships of other amino acids with insulin resistance remain to be elucidated. The aromatic amino acids, tyrosine and phenylalanine, have been reported to be elevated together with BCAAs in obesity-related insulin resistance7–10,13, although the reason is not fully discussed in the literature. Phenylalanine is metabolized to catecholamines via tyrosine through a completely different pathway from that of BCAAs. Newgard et al. 7 explained in their report that the aromatic acids, phenylalanine and tyrosine, are elevated in obese subjects compared with lean subjects, because the ‘large neutral amino acids’ (valine, isoleucine and leucine, tyrosine, phenylalanine and tryptophan) compete for transport into mammalian cells by the large neutral amino acid transporter, assuming that chronic elevations in BCAAs might impair the transport of aromatic amino acids into cells and tissues. Alanine and glutamic acid also appeared to be associated with insulin resistance. A report from the Framingham offspring study showed that alanine and glutamic acid were positively associated with the HOMA index24, which was perhaps observed in the present study. Alanine is metabolized to pyruvate and glutamic acid is derived from BCAAs catalyzed by BCAT in hepatic gluconeogenic pathways, suggesting some role in glucose homeostasis.
There were some limitations in the present study. First, the sample size was not large enough for conclusions regarding marginal insignificant P-values. Second, because of the cross-sectional nature of the study, the cause–effect relationship of our findings is uncertain. We conjecture that insulin resistance causes alteration of amino acid profile; however, other controversial evidence indicates that increased amino acid levels might cause insulin resistance7,25–27. Third, we used HOMA-IR as an index of insulin resistance, which reflects the balance between hepatic glucose output and insulin secretion in the basal state and sometimes fails to show a close relationship with whole-body insulin resistance assessed by the hyperinsulinemic-euglycemic clamp technique. HOMA-IR has been validated by the gold standard method including Japanese subjects whose BMI range was close to that of the present study (BMI 22–24)28,29, and therefore we consider it reasonable to use HOMA-IR as an alternative to the glucose clamp in this study. Finally, we simply carried out univariate analysis between HOMA-IR and the clinical parameters. Further investigations are necessary to examine the interrelations and also to elucidate the causal relationships among these factors.
In conclusion, the present study shows that the insulin resistance-related changes in amino acid profile are also observed in non-diabetic Japanese men and women. These amino acids include BCAAs (valine, isoleucine and leucine) and aromatic amino acids (tyrosine and phenylalanine), in agreement with previous studies carried out using different ethnic groups with different degrees of obesity and insulin resistance. Fasting measurement of these amino acids is expected to provide additional information on standard diabetes risk factors.
Acknowledgments
This study was supported in part by a grant-in-aid for the promotion of local healthcare business from the Ministry of Economy, Trade and Industry of Japan, and a Grant-in-Aid for Scientific Research (C) to YN (25350855) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. The authors declare no conflict of interest.
References
- 1.Felig P, Marliss E, Cahill GF., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281:811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- 2.Bain JR, Stevens RD, Wenner BR, et al. Metabolomics applied to diabetes research: moving from information to knowledge. Diabetes. 2009;58:2429–2443. doi: 10.2337/db09-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Würtz P, Tiainen M, Mäkinen VP, et al. Circulating metabolite predictors of glycemia in middle-aged men and women. Diabetes Care. 2012;35:1749–1756. doi: 10.2337/dc11-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee EP, Cheng S, Larson MG, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest. 2011;121:1402–1411. doi: 10.1172/JCI44442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu J, Xie G, Jia W, et al. Insulin resistance and the metabolism of branched-chain amino acids. Front Med. 2013;7:53–59. doi: 10.1007/s11684-013-0255-5. [DOI] [PubMed] [Google Scholar]
- 7.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huffman KM, Shah SH, Stevens RD, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32:1678–1683. doi: 10.2337/dc08-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tai ES, Tan ML, Stevens RD, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53:757–767. doi: 10.1007/s00125-009-1637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Würtz P, Mäkinen VP, Soininen P, et al. Metabolic signatures of insulin resistance in 7,098 young adults. Diabetes. 2012;61:1372–1780. doi: 10.2337/db11-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah SH, Crosslin DR, Haynes CS, et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012;55:321–330. doi: 10.1007/s00125-011-2356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCormack SE, Shaham O, McCarthy MA, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes. 2013;8:52–61. doi: 10.1111/j.2047-6310.2012.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Würtz P, Soininen P, Kangas AJ, et al. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care. 2013;36:648–655. doi: 10.2337/dc12-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costeas PA, Chinsky JM. Effects of insulin on the regulation of branched-chain alpha-keto acid dehydrogenase E1 alpha subunit gene expression. Biochem J. 1996;318:85–92. doi: 10.1042/bj3180085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimomura Y, Obayashi M, Murakami T, et al. Regulation of branched-chain amino acid catabolism: nutritional and hormonal regulation of activity and expression of the branched-chain alpha-keto acid dehydrogenase kinase. Curr Opin Clin Nutr Metab Care. 2001;4:419–423. doi: 10.1097/00075197-200109000-00013. [DOI] [PubMed] [Google Scholar]
- 16.She P, Van Horn C, Reid T, et al. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. 2007;293:E1552–E1563. doi: 10.1152/ajpendo.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuzuya T, Katano Y, Nakano I, et al. Regulation of branched-chain amino acid catabolism in rat models for spontaneous type 2 diabetes mellitus. Biochem Biophys Res Commun. 2008;373:94–98. doi: 10.1016/j.bbrc.2008.05.167. [DOI] [PubMed] [Google Scholar]
- 18.Doisaki M, Katano Y, Nakano I, et al. Regulation of hepatic branched-chain alpha-keto acid dehydrogenase kinase in a rat model for type 2 diabetes mellitus at different stages of the disease. Biochem Biophys Res Commun. 2010;393:303–307. doi: 10.1016/j.bbrc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Lackey DE, Lynch CJ, Olson KC, et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab. 2013;304:E1175–E1187. doi: 10.1152/ajpendo.00630.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Japan Diabetes Society, Treatment Guide for Diabetes Editorial Committee. Tokyo: Bunkodo; 2014. Treatment Guide for Diabetes 2014–2015; pp. 11–12. In: (ed). (Japanese) [Google Scholar]
- 22.Yamada C, Mitsuhashi T, Hiratsuka N, et al. Optimal reference interval for homeostasis model assessment of insulin resistance in a Japanese population. J Diabetes Investig. 2011;2:373–376. doi: 10.1111/j.2040-1124.2011.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimomura Y, Honda T, Shiraki M, et al. Branched-chain amino acid catabolism in exercise and liver disease. J Nutr. 2006;136:250S–253S. doi: 10.1093/jn/136.1.250S. [DOI] [PubMed] [Google Scholar]
- 24.Cheng S, Rhee EP, Larson MG, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125:2222–2231. doi: 10.1161/CIRCULATIONAHA.111.067827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krebs M, Krssak M, Bernroider E, et al. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes. 2002;51:599–605. doi: 10.2337/diabetes.51.3.599. [DOI] [PubMed] [Google Scholar]
- 26.Tremblay F, Krebs M, Dombrowski L, et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005;54:2674–2684. doi: 10.2337/diabetes.54.9.2674. [DOI] [PubMed] [Google Scholar]
- 27.Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Bonora E, Targher G, Alberiche M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 29.Emoto M, Nishizawa Y, Maekawa K, et al. Homeostasis model assessment as a clinical index of insulin resistance in type 2 diabetic patients treated with sulfonylureas. Diabetes Care. 1999;22:818–822. doi: 10.2337/diacare.22.5.818. [DOI] [PubMed] [Google Scholar]


