Abstract
Aims/Introduction
The nature of the action of concomitant liraglutide to stabilize postprandial blood glucose level (PBG) in patients on intensive insulin therapy with unstable PBG remains unclear. The aim was to identify the nature of liraglutide's actions to stabilize PBGs.
Materials and Methods
The study participants consisted of 20 diabetes patients showing unstable PBGs after dinner despite undergoing intensive insulin therapy. The dose of bolus insulin was reduced by three units for each meal, and 0.9 mg/day of liraglutide was added and used in combination. We evaluated the participants' data after the first evaluation (immediately before using liraglutide in combination) and the second evaluation (16 weeks after starting concomitant therapy). PBGs after dinner were measured every day for a period of 28 days immediately before carrying out both evaluations. The mean value of the 28 sets of blood glucose data and their standard deviation (SD) values were established as PBGs after dinner, as well as the SD for each participant. The changes in the mean values of the 20 participants, as well as their SD between before and after concomitant therapy, were evaluated.
Results
The mean value of PBGs (12.0 ± 1.0 to 10.1 ± 0.9 mmol/L) and SD values (5.1 ± 0.7–3.5 ± 0.8) after dinner both declined. A multiple regression analysis showed that the combined use of liraglutide was a significant independent variable of the SD values of PBGs after dinner.
Conclusion
The treatment of reducing the dose of insulin and using liraglutide in combination not only suppresses PBGs, but also stabilizes their blood glucose fluctuations.
Keywords: Intensive insulin therapy, Liraglutide, Postprandial blood glucose fluctuations
Introduction
Recently, especially rigorous glycemic control is required to prevent diabetic complications. In contrast, the risks of hypoglycemia are attracting attention, and prevention of hypoglycemia is increasingly considered important1.
Intensive insulin therapy (IIT) is frequently carried out to reduce blood glucose levels (BGs) rigorously, but to prevent hypoglycemia at the same time. However, there are not a few patients who find it difficult to control their BGs with IIT alone. Setting the dose of insulin to be administered is especially difficult in patients who have unstable glucose levels. With the release of super-rapid-acting insulin in recent years, we now often see cases where patients develop hypoglycemia after meals. This could be in part because the content of meals varies from day to day. Super-rapid insulin has a short duration of action and reduces BG in a highly targeted fashion, if the elevation pattern of postprandial blood glucose (PBG) changes, the rise in PBG fails to synchronize with the action of insulin, frequently causing hyperglycemia and hypoglycemia2. The elevation pattern of PBG changes sharply depending on the type of carbohydrates ingested, even though the number of ingested calories might be the same3,4. However, it would not be possible to make patients eat the same meals every day. It is difficult to deal with symptoms by increasing the dose of insulin, so one possible strategy is to use a treatment drug in combination that can improve PBG levels and that is not liable to cause hypoglycemia. What we expect from this concomitant drug is PBG stabilization, rather than PBG reduction activity. The reason is that, if our aim was simply to reduce PBG levels, we could do this by increasing the dose of insulin.
Glucagon-like polypeptide (GLP)-1 analogs that have been developed recently can be expected to play this concomitant drug role. Various mechanisms are being proposed as to how GLP-1 improves PBG, although none has yet been generally accepted. The most dominant is its insulinotropic actions5.
In patients whose insulin secretion has markedly declined, however, it is unclear whether the GLP-1 analog is able to enhance insulin secretion. In addition, as GLP-1 also has the effect of reducing bodyweight and suppressing appetite6, it is very likely to have the effect of simultaneously reducing PBG and stabilizing PBG levels. However, few studies have as yet investigated these actions.
We therefore used, as our targets, type 2 diabetes patients with unstable PBG levels whose insulin secretion has markedly declined and who were undergoing IIT, and studied the PBG stabilization actions of liraglutide, a GLP-1 analog, when this drug was used concomitantly.
Material and Methods
This was a prospective, one-arm clinical study that targeted type 2 diabetes patients who, despite undergoing IIT: (i) had poorly-controlled glycemia; (ii) showed significant fluctuations in BGs, especially BGs after dinner; (iii) repeatedly developed hypoglycemia and hyperglycemia; and (iv) whose treatment had proved challenging, as the dose of insulin could neither be increased nor decreased. The participants' BG was evaluated immediately before the use of liraglutide in combination (evaluation-1), and 16 weeks after the use of the drug in combination (evaluation-2). The participants were asked to measure their BGs every day before dinner and 2 h after dinner for the 28 days immediately before evaluation-1 by self-monitoring of blood glucose (SMBG). Based on their PBG levels, we selected groups of participants that showed unstable BGs after dinner. The definition of unstable BGs after dinner is as follows: ‘Of the 28 measurements of blood glucose values 2 h after dinner, hyperglycemia exceeding 10.1 mmol/L occurred more than 10 times, and hypoglycemia below 3.9 mmol/L occurred more than three times, and the lifestyle habits that had caused these fluctuations in BGs could not be identified'7.
The dose of super-rapid insulin (bolus insulin) administered to the participants immediately before each meal was reduced by three units each time (a total of 9 units/day); and liraglutide was additionally administered in combination. Insulin's dose reduction unit used here was determined, based on a previous past retrospective survey that we carried out (unpublished) on the combination of IIT and concomitant liraglutide. The participants were asked to measure, by SMBG, their daily BGs before and after dinner every night for a period of 28 days before the additional use of liraglutide, as well as their BGs before dinner and 2 h after dinner, for 28 days immediately before evaluation-2 (16 weeks after the start of combination therapy). The mean values of BGs before and after dinner, as well as their standard deviation (SD) values, which were calculated by using the 28 blood glucose data items before dinner as well as the 28 blood glucose data items after dinner that were evaluated at evaluation-1 and evluation-2, were established as the patients' blood glucose values before and after dinner, as were their SD values of the BGs before and after dinner. We compared the BGs before and after dinner and their respective SD values for evaluation-1, and the BGs before and after dinner and their respective SD values for evaluation-2, and studied each of these changes (Table3).
Table 3.
To compare and evaluate the changes of pre- or post-dinner blood glucose concentration and SD by liraglutide combination treatment
| Liraglutide combination | (−) | P1 | (+) | P2 | ||
|---|---|---|---|---|---|---|
| Pre-dinner | Post-dinner | Pre-dinner | Post-dinner | |||
| BGC (mmol/L) | 8.2 ± 1.5 | 12.0 ± 1.0 | <0.0001 | 8.1 ± 1.3* | 10.1 ± 0.9** | <0.0001 |
| SD | 2.8 ± 1.1 | 5.1 ± 0.7 | <0.0001 | 2.7 ± 1.3§ | 3.5 ± 0.8§§ | <0.0001 |
| Pre-dinner | Post-dinner | |||||
|---|---|---|---|---|---|---|
| Change of BGC by treatment | ||||||
| Delta change (mmol/L) | −0.2 ± 0.8 | −1.9 ± 1.1 | ||||
| Percentage change (%) | −1.3 ± 9.5 | −15.3 ± 7.8 | ||||
| Change of SD by treatment | ||||||
| Delta change | −0.1 ± 0.5 | −1.6 ± 0.7 | ||||
| Percentage change (%) | −1.4 ± 14.5 | −31.2 ± 13.3 |
BGC, blood glucose concentration
SD, standard deviations.
At evaluation-1 (−) vs at evaluation-2 (+) in pre-dinner blood glucose concentration (BGC), P = 0.357.
(−) vs (+) in post-dinner BGC, P < 0.0001.
(−) vs (+) in pre-dinner SD, P = 0.422.
(−) vs (+) in post-dinner SD, P < 0.0001.
We also asked the patients to measure their BGs before and 2 h after their three daily meals within 1 week immediately before evaluation-1 and evaluation-2, and studied each of their changes (Table2). Figure1 shows the protocol of the present study.
Table 2.
Changes in blood glucose levels (mmol/L) before and after each of three meals daily as a result of concomitant liraglutide therapy
| Meal | Breakfast | Lunch | Dinner | |||
|---|---|---|---|---|---|---|
| Liraglutide | Pre | Post | Pre | Post | Pre | Post |
| (−) | 8.5 ± 2.6 | 15.2 ± 2.5 | 9.3 ± 3.2 | 15.6 ± 2.3 | 8.2 ± 3.3 | 15.9 ± 1.5 |
| (+) | 7.5 ± 1.7 | 10.2 ± 1.2 | 7.5 ± 1.2 | 9.9 ± 1.1 | 7.1 ± 1.4 | 9.8 ± 1.4 |
| P | 0.03 | <0.01 | 0.02 | <0.01 | 0.04 | <0.01 |
| Δ | −1.0 ± 2.0 | −5.0 ± 2.3 | −1.8 ± 3.2 | −5.7 ± 2.7 | −1.1 ± 3.2 | −6.2 ± 1.6 |
| % | −5.0 ± 30.3 | −31.7 ± 10.1 | −8.3 ± 39.0 | −35.2 ± 11.6 | 1.1 ± 44.4 | −38.5 ± 8.0 |
(−), At evaluation-1
(+), at evaluation-2
Δ, delta change (the value at evaluation-1 − value at Evaluation-2)
%, percentage change (Δ/the value at evaluation-1 × 100).
Figure 1.
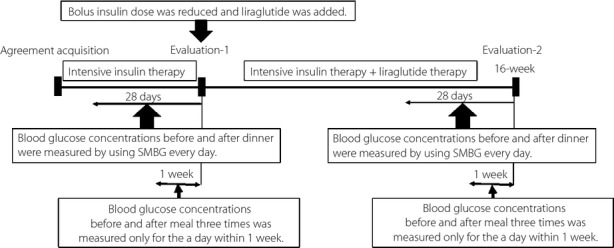
An overview of the protocol of the present study. Evaluations made when bolus insulin was reduced by 9 units/day and an added-on therapy of concomitant liraglutide used were established as evaluation-1. Evaluations made 16 weeks after the start of this concomitant therapy were established as evaluation-2. We measured the blood glucose levels before and after dinner during the 28 days immediately before each evaluation, and set their mean value and SD levels as the individual patient's blood glucose level before and after dinner, and SD level before and after the change in treatment. We evaluated the changes in these blood glucose levels before and after dinner, and the SD values of the 20 target patients. We also measured the blood glucose levels before and after three meals in an optional day within 1 week immediately before each evaluation, and evaluated the changes in various blood glucose levels between before and after the change in therapy. SMBG, self-monitoring blood glucose.
Regarding liraglutide administration, the dose was 0.3 mg/day, in principle, for 3 days immediately after the start of administration. It was then increased to 0.6 mg/day for the next 3 days, and to 0.9 mg/day, beginning with the seventh day of administration. However, the dose could be increased or decreased as required in patients with severe anorexia. All the participants enrolled in the present study increased their dose to 0.9 mg/day within 14 days, and subsequently continued their treatment with this dose until the date of evaluation. The dose of basal insulin remained unchanged during the study period, whereas the dose of bolus insulin was not changed other than when the dose was reduced by three units at the time liraglutide was added in combination.
The present study complied with the Helsinki Declaration, and was carried out with the approval of the Medical Ethics Committee of Tohoku University. All participants provided their full informed consent.
Statistical Analysis
All normally-distributed numerical figures are expressed as mean ± SD. The Shapiro–Wilk test was used as a normality test. The presence or absence of significant differences in values before and after change in therapy was examined, using Student's t-test. The chi square-test was used to compare the rate of change. A multiple regression analysis was also carried out to examine if the concomitant use of liraglutide was an independent risk factor for decreased SD values. The multiple regression analysis used the SD values before and after change in therapy as the dependent variables, and the mean BGs before and after change in therapy, body mass index (BMI) values, glycated hemoglobin (HbA1c; National Glycohemoglobin Standardization Program value) level, and use/non-use of concomitant liraglutide, as the independent variables.
Results
Table1 shows the clinical characteristics of the participants at the start of the study, and the changes in their BMI and HbA1c between evaluation-1 and at evaluation-2. Although the dose of insulin was reduced by nine units/day (3 units each immediately before each meal), the level of HbA1c decreased. BMI also decreased. The dose of insulin administered was 21.3 ± 8.6 units/day for basal insulin, and 12.2 ± 4.3, 10.0 ± 3.7 and 13.5 ± 4.5 for bolus insulin (immediately before breakfast, lunch and dinner, respectively). All the participants were diagnosed with type 2 diabetes, but had seen a marked decrease in their endogenous insulin secretion. Their postprandial 2-h plasma C-peptide levels, which were measured within the past year, were 0.33 ± 0.35 nmol/L, and the 6-min values of plasma C-peptide in a glucagon loading test implemented at the time insulin was introduced were 0.33 ± 0.21 nmol/L. Seven patients suffered diabetic retinopathy, and five suffered diabetic nephropathy, but none seriously. As concomitant drugs, five patients were using biguanide, four were using pioglitazone and six were using α-glucosidase inhibitors. However, no changes in their oral medications were made during the study period.
Table 1.
Clinical characteristics of the participants at the start of the study and the changes in their body mass index and glycated hemoglobin between before and after the change in therapy
| n | 20 | |||
| Age (years) | 58.3 ± 11.0 | |||
| Sex (M/F) | 9/11 | |||
| Duration (years) | 12.9 ± 6.1 |
| Liraglutide | At evaluation-1 | At evaluation-2 | P | |
|---|---|---|---|---|
| BMI (kg/m2) | 25.6 ± 2.5 | 25.2 ± 2.3 | <0.01 | |
| Δ BMI (kg/m2) | −0.4 ± 0.4 | |||
| %BMI (%) | −1.4 ± 1.4 | |||
| HbA1c (%) | 8.5 ± 0.7 | 7.3 ± 0.6 | <0.01 | |
| ΔHbA1c (%) | −1.2 ± 0.8 | |||
| %HbA1c (%) | −13.9 ± 8.5 |
%, Percentage change (Δ/the value at evaluation-1 × 100)
Δ, delta change (the value at evaluation-1 − the value at evaluation-2)
BMI, body mass index
F, female, M, male.
Data presented as mean ± SD.
Table2 shows the changes in BGs before and after each of three meals daily as a result of concomitant liraglutide therapy. In this particular evaluation of BGs, all the levels had declined significantly between before and after the three meals as a result of additional liraglutide therapy. However, PBG level showed a far greater range and rate of decline. Although the dose of bolus insulin was decreased, patients who used a combination of liraglutide showed that a rise in their PBG levels was suppressed more strongly than their preprandial BGs.
Table3 shows the changes between before and after concomitant therapy in the 20 participants enrolled in the present study in: (i) BG before dinner, between before and after the addition of concomitant liraglutide (mean value for the 28 measurement data items), and the SD value (calculated from the 28 measurement data items); and (ii) the BG after dinner (mean value of the 28 measurement data items) and the SD value (calculated from the 28 measurement data items). The results showed that PBG levels had elevated significantly with the intake of dinner, regardless of whether or not additional liraglutide therapy was carried out. However, the degree of elevation was smaller when liraglutide was used in combination than when it was not. The BG before dinner, as well as the SD value, remained unchanged. In contrast, the PBG level after dinner declined significantly with additional liraglutide therapy. The SD value also decreased. Additional use of liraglutide not only improved the mean value of BG after dinner; it also reduced the variation in BG after dinner.
Table4 compares the frequency of the emergence of hypoglycemia (BG < 3.9 mmol/L) and hyperglycemia (BG ≥ 10.1 mmol/L) before and after additional liraglutide administration. The incidence of hypoglycemia and hyperglycemia before dinner did not change as a result of additional administration of liraglutide. However, in terms of BG after dinner, the incidence of both hypoglycemia and hyperglycemia decreased.
Table 4.
To compare and evaluate the frequency of appearance of blood glucose concentration of over 10.8 mmol/L (blood glucose concentration ≥10.08) and under 3.92 mmol/L (blood glucose concentration <3.92)
| BGC (mg/dL) | <3.92 | ≥10.08 | ||||
|---|---|---|---|---|---|---|
| Liraglutide | Pre-dinner BGC | P1 | Pre-dinner BGC | P2 | ||
| (−) | (+) | (−) | (+) | |||
| 1.8 ± 1.6 | 1.6 ± 0.9 | 0.8149 | 1.22 ± 2.2 | 9.8 ± 3.7 | 0.2289 |
| Post-dinner BGC | Post-dinner BGC | |||||
|---|---|---|---|---|---|---|
| (−) | (+) | (−) | (+) | |||
| 4.6 ± 0.9 | 1.2 ± 1.3 | 0.0048 | 21.0 ± 1.2 | 11.6 ± 3.2 | 0.0045 |
Data presented as mean ± SD.
(−), At evaluation-1
(+), at evaluation-2
BGC, blood glucose concentration.
A multiple regression analysis was carried out, using the SD values of BG after dinner as the dependent variables, and use/non-use of concomitant liraglutide, BMI levels, HbA1c levels and BG after dinner as the independent variables. The results showed that the use/non-use of liraglutide (β = −26.9243, P < 0.0001) was the only independent risk factor. (BMI: β = −1.3999, P = 0.124, HbA1c: β = 2.4996, P = 0.457 and PBG level after dinner: β = 0.0001, P = 0.104).
To examine the influence that the target patients' residual insulin secretion properties have on PBG suppression activity of GLP-1, we therefore studied the relationship between the rate of change in PBG levels and in their SD values as a result of administration of GLP-1, and the duration of diabetes (12.9 ± 6.1 years), duration of insulin therapy (DIT; 11.1 ± 4.7 years), postprandial 2-h plasma C-peptide levels (0.33 ± 0.35 nmol/L) and the 6-min values of plasma C-peptide in a glucagon loading test (0.33 ± 0.21 nmol/L). The present results showed postprandial 2-h plasma C-peptide levels (r = −0.27, P = 0.03) and DIT (r = −0.27, P = 0.04) to be correlated to the rate of change in PBG levels. In a multiple regression analysis that used the rate of change in PBG as a dependent variable and the above four factors as independent variables, only the postprandial 2-h plasma C-peptide levels (β = −18.65, P = 0.001) was identified as an independent risk factor (DIT: β = −2.25, P = 0.06). In contrast, only the postprandial 2-h plasma C-peptide levels correlated with the rate of change in SD values of PBG (r = 0.25, P = 0.03). However, in a multiple regression analysis that used the rate of change in SD values of PBG as the dependent variable and the aforementioned four factors as the independent variables, none of the factors was identified as an independent risk factor.
We also examined the administration and non-administration of α-glucosidase inhibitors that influence PBG levels (dividing the participants into two groups; that is, the αGI [+] group taking the drugs [n = 6] and the αGI [−] group not taking the drugs [n = 14]), and compared the differences in the rate of change in PBG and the rate of change in their SD values between the two groups. The results showed that the rate of change in PBG level was −12.7 ± 5.6% for the αGI (+) group and −16.4 ± 8.5% for the αGI (−) group (P = 0.89), showing no differences. Similarly, no differences were seen in the rate of change in SD, with −29.8 ± 18.5% for the αGI (+) group and −31.7 ± 11.3% for the αGI (−) group (P = 0.90).
Discussion
Whereas the elevation pattern of PBG changes not only with the amount of food eaten, but also by type of carbohydrates eaten3,4, super-rapid insulin always acts in the same way after administration. This discrepancy between the elevation pattern of PBG level and insulin's pattern of action appears to be one reason for the variability of PBG level that results from treatment with super-rapid insulin. Many type 2 diabetes patients are believed to be correcting this mismatch by increasing or decreasing the dose of endogenous insulin. However, as in the case of the participants in the present study, in those whose endogenous insulin levels dropped (even though they were diagnosed as type 2 diabetes), there is a possibility that this correction is not enough. Most patients enrolled in the present study fell into the type 2 diabetes category, as they were unable to verify their autoantibodies. Clinically, however, they are in a state that closely resembles type 1 diabetes.
Because GLP-1 acts to increase insulin secretion5, it is important to identify whether or not the results of the present study are attributable to this increase in insulin secretion activity. In view of the present findings, to what degree the level of PBG drops with the administration of GLP-1 appears to be related to remaining food-stimulated insulin secretion capability. However, it appears that how much the variability of PBG is inhibited is unrelated to the remaining insulin secretion capability. It is believed, therefore, that improvement of PBG levels with the administration of GLP-1 depends on how much insulin-increasing action has been shown by the stimulation of GLP-1. However, its action of suppressing variability in PBG might be due not to the insulinotropic actions of GLP-1, but to ‘some other actions.’
One possible example of ‘some other actions’ could be the suppression of glucagon caused by GLP-18. GLP-1 not only modulates glucagon secretion from pancreatic α-cells through the central and peripheral nervous systems9, but also inhibits glucagon release by a direct effect on the pancreatic α-cell that is mediated by GLP-1 receptors10. Furthermore, long-term GLP-1 treatment induces increases of insulin content and secretion in β-cells, and a loss of α-cells11. In the present study, however, we did not measure the difference in pre- and postprandial glucagon levels around the time GLP-1 was being administered concomitantly, so we are unable to discuss the relationship between the results of the present study and glucagon. Furthermore, even at lower doses (9 units/day) of insulin, hypoglycemia was markedly suppressed, so it appears unlikely that glucagon had been powerfully suppressed.
One other possible example of ‘some other actions’ might be the improving of insulin resistance caused by GLP-1 therapy. Reports have been released in recent years showing evidence that GLP-1 preparations improve BG levels even in type 1 diabetes12–14, and that long-term treatment using such drugs improves insulin resistance15. It might be possible to assume from these reports that improvement of insulin resistance caused by a reduction in BMI is one reason for the observed decline in BG. In the present study, however, reduction of BMI and improvement of levels in PBG and HbA1c were not independent factors for reduction of SD values. The effect of GLP-1 on suppressing variability in PBG levels could have been caused by a factor that is not mediated by insulin reinforcements.
One other possible example of ‘some other actions’ might be the slowing of gastric emptying caused by GLP-1 therapy16–18: there is a possibility that the amount of food intake and the speed of ingestion of food had dropped by GLP-1 therapy without the patient noticing them, and had reached a constant level, leading to a reduction in the variability of PBG levels. As the participants' BMI also decreased in the present study, we cannot deny the possibility that their appetites had been suppressed, thereby improving their levels of PBG and SD. However, an interview survey carried out with the participants 16 weeks later showed the presence of three patients whose appetite dropped immediately after starting GLP-1 administration, but none after the second week. GLP-1 suppresses the rise in plasma ghrelin levels in the preprandial period19. This GLP-1-induced suppression of ghrelin secretion might be involved in not only suppressing appetite, but also suppressing variability in PBG levels. We feel that further studies are required.
It is reported that patients with strong insulin resistance and who require massive doses of insulin are able to reduce their dose of insulin by approximately 30% by concomitant liraglutide administration20,21. In the present study, only the dose of bolus insulin could be reduced by approximately 16%. The reason for this was that the protocol stipulated that the dose of insulin remain unchanged during the study period. There is the possibility, therefore, that, if we had attempted to further reduce the dose of insulin, we could have reduced the dose by a greater margin. A therapy consisting of decreased dose of insulin and the use of GLP-1 in combination is reported to reduce fluctuations in BGs, and decrease the incidence of hypoglycemia and hyperglycemia20–22. However, this suppression of the up–down swings in BG was a result of correcting the PBG level, not of suppression of the daily variability of PBG level at a set time-period. It is difficult to identify the variability of daily BGs after dinner in these patients by using continuous glucose measurement. The reason is that whereas continuous glucose measurement can only be used to measure continuously for 3–4 days, these BGs after dinner show extremely irregular hyperglycemia–hypoglycemia emergence patterns, such as hyperglycemia continuing for more than a week, then suddenly switching to hypoglycemia for several days in a row, for example. We therefore measured the BGs before and after dinner for 28 consecutive days, calculated their mean value and SD levels, and established them as the individual patient's BG before and after dinner as well as its variability.
Concomitant therapy using insulin and liraglutide has the problem of high expense, so we believe that it should be applied with caution. Nevertheless, this combination therapy that is accompanied by a reduction in the dose of insulin has the potential to be an option for treating patients who, despite undergoing ITT, frequently suffer postprandial hyperglycemia and hypoglycemia.
The present study had some limitations, such as a small number of target subjects. However, the number of individuals who fulfilled the requirements for this study, and were able to do a frequent self-measurement of their BG, was definitely not large. In addition, as this study was a one-arm trial with no comparison groups, it is not possible to strictly conclude that the results of this study were the effects of liraglutide. There also were many patients who had developed frequent hypoglycemia, making it difficult to execute a randomized control trial that included an untreated group. We also considered carrying out a cross-over, trial but could not, because of the presence of numerous patients who failed to give their consent to revert to their previous insulin-only therapy, as their incidence of hypoglycemia had decreased. Going forward, we feel it necessary to carry out a randomized control trial that uses a large number of patients in multiple medical institutions.
Acknowledgments
The authors acknowledge the editorial assistance and clinical support of Miss Manami Shimizu for her help with preparing the references, and for her expert assistance in the preparation of the tables and figures. We thank all participants for their efforts in measuring SMBG data. Our deep appreciation goes to all members of the staff who helped us in this study. This work was supported by a 21st Century Center of Excellence Program Special Research Grant from the Ministry of Education, Sports and Culture. The authors declare no conflict of interest.
References
- 1.Cryer PE. Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes. 2014;63:2188–2195. doi: 10.2337/db14-0059. [DOI] [PubMed] [Google Scholar]
- 2.Kristensen PL, Hansen LS, Jespersen MJ, et al. Insulin analogues and severe hypoglycaemia in type 1 diabetes. Diabetes Res Clin Pract. 2012;96:17–23. doi: 10.1016/j.diabres.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 3.Gannon MC, Nuttall FQ, Westphal SA, et al. Acute metabolic response to high-carbohydrate, high-starch meals compared with moderate-carbohydrate, low-starch meals in subjects with type 2 diabetes. Diabetes Care. 1998;21:1619–1626. doi: 10.2337/diacare.21.10.1619. [DOI] [PubMed] [Google Scholar]
- 4.Kawai K, Murayama Y, Okuda Y, et al. Postprandial glucose, insulin and glucagon responses to meals with different nutrient compositions in non-insulin-dependent diabetes mellitus. Endocrinol Jpn. 1987;34:745–753. doi: 10.1507/endocrj1954.34.745. [DOI] [PubMed] [Google Scholar]
- 5.Lee YS, Jun HS. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism. 2014;63:9–19. doi: 10.1016/j.metabol.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 6.van Bloemendaal L, Ten Kulve JS, la Fleur SE, et al. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol. 2014;221:T1–T16. doi: 10.1530/JOE-13-0414. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa S, Nako K, Okamura M, et al. Compared with insulin glargine, insulin degludec narrows the day-to-day variability in the glucose-lowering effect rather than lowering blood glucose levels. JDM. 2013;3:244–251. [Google Scholar]
- 8.Hare KJ, Vilsbøll T, Asmar M, et al. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes. 2010;59:1765–1770. doi: 10.2337/db09-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotoh K, Masaki T, Chiba S, et al. Hypothalamic brain-derived neurotrophic factor regulates glucagon secretion mediated by pancreatic efferent nerves. J Neuroendocrinol. 2013;25:302–311. doi: 10.1111/jne.12003. [DOI] [PubMed] [Google Scholar]
- 10.De Marinis YZ, Salehi A, Ward CE, et al. GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N- and L-type Ca2 + channel-dependent exocytosis. Cell Metab. 2010;11:543–553. doi: 10.1016/j.cmet.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwasinger-Schmidt T, Robbins DC, Williams SJ, et al. Long-term liraglutide treatment is associated with increased insulin content and secretion in β-cells, and a loss of α-cells in ZDF rats. Pharmacol Res. 2013;76:58–66. doi: 10.1016/j.phrs.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Kielgast U, Krarup T, Holst JJ, et al. Four weeks of treatment with liraglutide reduces insulin dose without loss of glycemic control in type 1 diabetic patients with and without residual beta-cell function. Diabetes Care. 2011;34:1463–1468. doi: 10.2337/dc11-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varanasi A, Bellini N, Rawal D, et al. Liraglutide as additional treatment for type 1 diabetes. Eur J Endocrinol. 2011;165:77–84. doi: 10.1530/EJE-11-0330. [DOI] [PubMed] [Google Scholar]
- 14.Behme MT, Dupré J, McDonald TJ. Glucagon-like peptide 1 improved glycemic control in type 1 diabetes. BMC Endocr Disord. 2003;3:3. doi: 10.1186/1472-6823-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkar G1, Alattar M, Brown RJ, et al. Exenatide treatment for 6 months improves insulin sensitivity in adults with type 1 diabetes. Diabetes Care. 2014;37:666–670. doi: 10.2337/dc13-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Näslund E, Bogefors J, Skogar S, et al. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol. 1999;277:R910–R916. doi: 10.1152/ajpregu.1999.277.3.R910. [DOI] [PubMed] [Google Scholar]
- 17.Imeryüz N, Yeğen BC, Bozkurt A, et al. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol. 1997;273:G920–G927. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- 18.Wettergren A, Wøjdemann M, Holst JJ. Glucagon-like peptide-1 inhibits gastropancreatic function by inhibiting central parasympathetic outflow. Am J Physiol. 1998;275:G984–G992. doi: 10.1152/ajpgi.1998.275.5.G984. [DOI] [PubMed] [Google Scholar]
- 19.Hagemann D, Holst JJ, Gethmann A, et al. Glucagon-like peptide 1 (GLP-1) suppresses ghrelin levels in humans via increased insulin secretion. Regul Pept. 2007;143:64–68. doi: 10.1016/j.regpep.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Lane W, Weinrib S, Rappaport J, et al. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16:827–832. doi: 10.1111/dom.12286. [DOI] [PubMed] [Google Scholar]
- 21.Lane W, Weinrib S, Rappaport J. The effect of liraglutide added to U-500 insulin in patients with type 2 diabetes and high insulin requirements. Diabetes Technol Ther. 2011;13:592–595. doi: 10.1089/dia.2010.0221. [DOI] [PubMed] [Google Scholar]
- 22.Li CJ, Li J, Zhang QM, et al. Efficacy and safety comparison between liraglutide as add-on therapy to insulin and insulin dose-increase in Chinese subjects with poorly controlled type 2 diabetes and abdominal obesity. Cardiovasc Diabetol. 2012;11:142. doi: 10.1186/1475-2840-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]


