Abstract
Aims/Introduction
A low-carbohydrate diet based on animal sources is associated with higher all-cause mortality, whereas a vegetable-based low-carbohydrate diet is associated with lower cardiovascular disease mortality. It has been suggested that acid/base imbalance might play an important role in some cardiometabolic abnormalities. The aims of the present study were to evaluate whether carbohydrate intake is associated with quality of dietary protein and acid load, and whether these are related to metabolic syndrome in patients with type 2 diabetes.
Materials and Methods
The present cross-sectional study involved 149 patients with type 2 diabetes. Dietary intake was assessed using a validated self-administered diet history questionnaire. Dietary acid load was assessed by potential renal acid load and net endogenous acid production.
Results
Mean daily total energy intake, carbohydrate intake, animal protein intake and vegetable protein intake were 1821.5 kcal, 248.8 g, 36.1 g and 31.1 g, respectively. Carbohydrate energy/total energy was negatively correlated with animal protein energy/total energy, potential renal acid load or net endogenous acid production score, and was positively correlated with vegetable protein energy/total energy. Logistic regression analyses showed that the subgroup of patients with a lower vegetable protein energy/total energy or higher potential renal acid load or net endogenous acid production score was significantly associated with the prevalence of metabolic syndrome.
Conclusions
The present study showed that carbohydrate intake was associated with the quality of dietary protein and dietary acid load. Furthermore, decreased vegetable protein intake and increased dietary acid load were associated with the prevalence of metabolic syndrome.
Keywords: Low-carbohydrate diet, Metabolic syndrome, Type 2 diabetes mellitus
Introduction
Type 2 diabetes mellitus is a chronic condition that can lead to various complications over time, and it has developed into a public health issue. Type 2 diabetes mellitus is a multifactorial disease that is caused by environmental and genetic factors. In the environmental factors, medical nutrition therapy for the management of diabetes plays a crucial role in preventing diabetic complications, and especially in the management of metabolic control and optimal bodyweight1,2. Currently, controversy regarding the appropriate carbohydrate intake is in progress. The guidelines for the medical treatment of diabetes in Japan recommend a diet based on the following: carbohydrates comprising 50–60% of the total energy intake, protein 1.0–1.2 g/kg of the ideal bodyweight and fat ≤25% of the total energy intake. In the recommendations of the American Diabetes Association, which was published in 2012, it was reported that either low-fat calorie-restricted, low-carbohydrate or Mediterranean diets could be effective for bodyweight loss, and that glycemic control can be improved by regulating carbohydrate intake if followed for up to 2 years3.
A recent study showed that high intakes of total and animal protein were associated with a moderate elevated risk of type 2 diabetes in a large cohort of European adults4. An animal-based low-carbohydrate diet was also associated with higher all-cause mortality, whereas a low-carbohydrate diet based on vegetable sources was associated with lower cardiovascular disease and all-cause mortality rates5. Besides, it has been suggested that acid/base imbalance might play a crucial role in some cardiometabolic abnormalities6,7.
Nevertheless, little is known about the actual dietary habits of Japanese patients with type 2 diabetes. In particular, the association between carbohydrate intake and dietary animal or vegetable protein intake or dietary acid load still remains unclear in patients with type 2 diabetes. In addition, the association between dietary animal or vegetable protein intake or dietary acid load and metabolic syndrome (MetS), which increases the risk of developing cardiovascular disease, remains unclear. Therefore, we aimed to evaluate whether carbohydrate intake is associated with the quality of dietary protein and acid load, and whether these are related to the presence of MetS in patients with type 2 diabetes.
Materials and Methods
Patients
We carried out a cross-sectional study of 260 consecutive patients with type 2 diabetes who were recruited from the outpatient clinic at Kyoto Prefectural University of Medicine, Kyoto, Japan. The data were collected from June to September 2011. A self-administered diet history questionnaire (DHQ) was given to 260 patients (140 men and 120 women), and a total of 215 patients (112 men and 103 women) completed the questionnaire. We excluded patients due to the following criteria: under the age of 40 years (4 women), patients with type 1 diabetes (6 men and 7 women), chronic renal failure or hemodialysis patients (7 men and 4 women), incomplete information (12 men and 6 women) and patients in the ≥95 or ≤5 percentile for energy intake (10 males and 10 females). Finally, a total of 149 patients (77 men and 72 women) met the inclusion criteria. Patients were divided into two subgroups according to the median animal or vegetable protein energy/total energy. In addition, patients were divided into two subgroups according to the median dietary acid load scores, assessed by both the potential renal acid load (PRAL) or the net endogenous acid production (NEAP). Approval for the study was obtained from the local research ethics committee, and written informed consent was obtained from all patients.
Estimation and Assessment of Habitual Food and Nutrient Intake
The usual dietary habits of the patients were assessed by the validated DHQ system8. DHQ is a questionnaire that inquires about the dietary intake situation of the past 1 month, and consists of questions of the following four items: questions about eating behavior of day-to-day, questions about each amount and frequency of intake of 117 foods, questions about each amount and frequency of staple foods, and questions about each amount and frequency of intake of food other than the aforementioned. Using DHQ and the nutritional value calculation program, we calculated dietary total, carbohydrate, animal protein, vegetable protein, animal fat, vegetable fat and salt intake.
Assessment of Dietary Acid Load Scores
The PRAL and NEAP scores were derived from estimations of several nutrient intakes9. The PRAL and NEAP scores were calculated as estimates of dietary acid load using the following equation.
| 1 |
| 2 |
Data collection
All patients provided details of their demographics. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. After an overnight fast, venous blood was collected for the measurement of the levels of various factors, including fasting plasma glucose, total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, uric acid and creatinine. Hemoglobin A1c was assayed using high-performance liquid chromatography and was expressed as a National Glycohemoglobin Standardization Program unit. Glomerular filtration rate (GFR) was estimated using the Japanese Society of Nephrology equation: estimated GFR (eGFR) = 194 × Cre−1.094 × age−0.287 (mL/min/1.73 m2). For women, the eGFR was multiplied by a correction factor of 0.739. Urinary albumin and creatinine concentrations were determined using early morning spot urine. A mean value for urine albumin excretion was determined from three urine collections.
Definition of Metabolic Syndrome
The diagnosis of MetS was determined by a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; the National Heart, Lung and Blood Institute; the American Heart Association; the World Heart Federation; the International Atherosclerosis Society; and the International Association for the Study of Obesity, using the criteria for Asians10. The patients were diagnosed with the presence of MetS when three or more of the following criteria were present: elevated blood pressure (systolic blood pressure ≥130 mmHg and diastolic blood pressure ≥85 mmHg and/or medication for hypertension, in both sexes); hyperglycemia (fasting plasma glucose ≥5.6 mmol/L and/or medication for diabetes, in both sexes); hypertriglyceridemia (serum triglycerides ≥1.70 mmol/L and/or medication for dyslipidemia, in both sexes); low HDL cholesterol levels (serum HDL cholesterol <1.03 mmol/L in men and <1.29 mmol/L in women); and abdominal obesity (waist circumference ≥90 cm in men and ≥80 cm in women). Because waist measurements were not available for the entire study sample, we submitted a BMI of ≥25 kg/m2, which has been proposed as a cut-off for the diagnosis of obesity in Asian people11, for all patients as an index of obesity.
Statistical Analysis
The statistical analyses were carried out using the JMP version 10.0 software (SAS Institute Inc., Cary, NC, USA) and a P-value <0.05 was considered statistically significant. The mean or frequencies of potential confounding variables were calculated. Continuous variables were presented as the mean ± standard deviation. Because urine albumin excretion showed a skewed distribution, logarithmic transformation was carried out before carrying out unpaired Student's t-tests. The relationships between carbohydrate energy/total energy (C/E ratio) and animal protein energy/total energy (AP/E ratio), vegetable protein energy/total energy (VP/E ratio) or dietary acid load were examined by Pearson's correlation analyses. Differences in categorical and continuous variables across two subgroups according to the median of AP/E ratio, VP/E ratio or dietary acid load scores were assessed by Chi square test analyses and unpaired Student's t-tests. The associations between the two subgroups and prevalence of MetS were analyzed by logistic regression analyses. The logistic regression analyses were adjusted for age, sex, serum uric acid and creatinine, total energy intake, carbohydrate intake, and sodium intake, which were known to be risk factors of MetS. Odds ratios (OR) and 95% confidence intervals (CI) were calculated.
Results
The characteristics of patients are shown in Table1. There were 149 patients, aged 65.7 ± 9.3 years. Mean total energy intake, carbohydrate intake, animal protein intake and vegetable protein intake were 1821.5 kcal, 248.8 g, 36.1 g and 31.1 g, respectively. The median of AP/E ratio, VP/E ratio, C/E ratio and PRAL and NEAP scores were 7.7%, 6.5%, 56.9%, 6.9 mEq/day or −8.7 mEq/day, respectively. Carbohydrate energy/total energy was negatively correlated with AP/E ratio, PRAL or NEAP score, and was positively correlated with VP/E ratio (Figure1). The subgroup of patients with higher AP/E ratio was associated with age, sodium intake, C/E ratio, protein energy/total energy, AP/E ratio, fat energy/total energy, animal fat intake energy/total energy, PRAL or NEAP score (Table2). The subgroup of patients with lower VP/E ratio was associated with sex, prevalence of MetS, total energy intake, C/E ratio, VP/E ratio, fat energy/total energy, animal fat intake energy/total energy, PRAL or NEAP score. In addition, the subgroup of patients with higher PRAL score was associated with sex, LDL cholesterol, triglycerides, eGFR, prevalence of MetS, total energy intake, sodium intake, C/E ratio, AP/E ratio, VP/E ratio, fat energy/total energy, animal fat intake energy/total energy or NEAP score (Table2). The subgroup of patients with higher NEAP score was associated with sex, triglycerides, prevalence of MetS, VP/E ratio or PRAL score (Table2). Logistic regression analyses showed that the subgroup of patients with lower VP/E ratio, or higher PRAL or NEAP score was significantly associated with the prevalence of MetS (Table3). In a multivariate approach, including age, sex, serum uric acid and creatinine, total energy intake, carbohydrate intake and sodium intake, the subgroup of patients with lower VP/E ratio or higher PRAL or NEAP score showed an increased OR for the prevalence of MetS. In logistic regression analyses, animal fat energy/total energy was not a statistically significant risk factor for the prevalence of MetS.
Table 1.
Clinical characteristics of patients
| n | 149 |
| Age (years) | 65.7 ± 9.3 |
| Sex (male/female) | 77/72 |
| Body mass index (kg/m2) | 24.0 ± 4.2 |
| Systolic blood pressure (mmHg) | 129.6 ± 14.6 |
| Diastolic blood pressure (mmHg) | 72.4 ± 10.4 |
| HbA1c (%) | 6.9 ± 0.9 |
| Total cholesterol (mmol/L) | 4.8 ± 0.8 |
| High-density lipoprotein cholesterol (mmol/L) | 1.6 ± 0.4 |
| Low-density lipoprotein cholesterol (mmol/L) | 2.6 ± 0.8 |
| Triglycerides (mmol/L) | 1.5 ± 1.0 |
| Uric acid (μmol/L) | 298.4 ± 78.9 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 77.9 ± 21.7 |
| Urinary albumin excretion (mg/g creatinine) | 46.9 ± 94.1 |
| No. metabolic risk factors | 2.5 ± 2.0 |
| Metabolic syndrome (–/+) | 82/67 |
| Insulin treatment (–/+) | 116/33 |
| Antihypertensive drugs (–/+) | 79/70 |
| Statins (–/+) | 91/58 |
| Total energy intake (kcal) | 1821.5 ± 400.1 |
| Carbohydrate intake (g) | 248.8 ± 60.9 |
| Protein intake (g) | 67.2 ± 18.5 |
| Animal protein intake (g) | 36.1 ± 14.4 |
| Vegetable protein intake (g) | 31.1 ± 8.4 |
| Fat intake (g) | 53.3 ± 20.2 |
| Animal fat intake (g) | 23.3 ± 10.5 |
| Vegetable fat intake (g) | 29.9 ± 12.9 |
| Sodium intake (g) | 10.5 ± 3.5 |
| Carbohydrate energy/total energy (%) | 56.9 ± 7.7 |
| Protein energy/total energy (%) | 15.3 ± 2.4 |
| Fat energy/total energy (%) | 26.9 ± 6.4 |
| Potential renal acid load score (mEq/day) | 5.7 ± 11.8 |
| Net endogenous acid production score (mEq/day) | −8.7 ± 0.3 |
Data are number of patients or mean ± standard deviation.
HbA1c, hemoglobin A1c.
Figure 1.
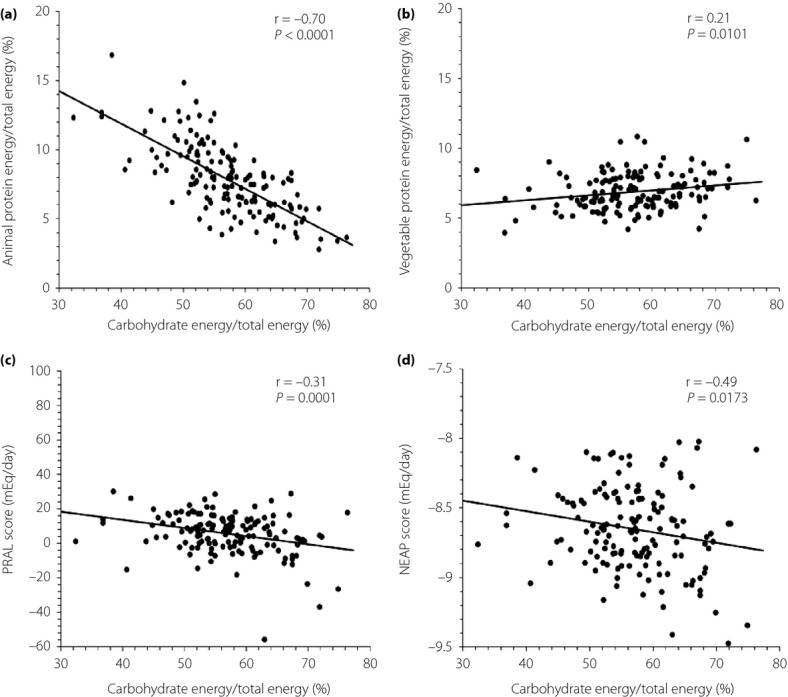
Relationships of carbohydrate energy/total energy with (a) animal or (b) vegetable protein energy/total energy, or (c) potential renal acid load (PRAL) or (d) net endogenous acid production (NEAP) score.
Table 2.
Clinical characteristics of patients divided into four subgroups according to the median of animal or vegetable protein energy/total energy or potential renal acid load or net endogenous acid production score
| Animal protein energy/total energy | Vegetable protein energy/total energy | |||||
|---|---|---|---|---|---|---|
| ≤7.7% | ≥7.8% | P | ≤6.5% | ≥6.6% | P | |
| n | 74 | 75 | – | 74 | 75 | – |
| Age (years) | 63.9 ± 8.8 | 67.5 ± 9.5 | 0.0183 | 66.4 ± 10.2 | 65.0 ± 8.4 | 0.3687 |
| Sex (male/female) | 40/34 | 37/38 | 0.5642 | 48/26 | 29/46 | 0.0014 |
| Body mass index (kg/m2) | 24.1 ± 3.9 | 23.9 ± 4.5 | 0.7010 | 24.5 ± 4.0 | 23.5 ± 4.3 | 0.1203 |
| Systolic blood pressure (mmHg) | 131.5 ± 14.4 | 127.7 ± 14.5 | 0.1107 | 130.8 ± 14.6 | 128.4 ± 14.5 | 0.3191 |
| HbA1c (%) | 7.0 ± 1.1 | 6.7 ± 0.7 | 0.0935 | 7.0 ± 1.1 | 6.8 ± 0.7 | 0.2008 |
| Low-density lipoprotein cholesterol (mmol/L) | 2.6 ± 0.7 | 2.6 ± 0.8 | 0.6041 | 2.7 ± 0.7 | 2.5 ± 0.8 | 0.3556 |
| Triglycerides (mmol/L) | 1.5 ± 0.9 | 1.5 ± 1.0 | 0.7747 | 1.7 ± 1.0 | 1.4 ± 0.9 | 0.0511 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 79.8 ± 22.6 | 75.9 ± 20.8 | 0.2718 | 75.7 ± 22.4 | 80.0 ± 20.9 | 0.2288 |
| Metabolic syndrome (–/+) | 40/34 | 42/33 | 0.8113 | 30/44 | 52/23 | 0.0004 |
| Total energy intake (kcal) | 1810.5 ± 438.6 | 1832.4 ± 360.7 | 0.7397 | 1902.2 ± 360.9 | 1741.9 ± 422.7 | 0.0139 |
| Carbohydrate energy/total energy (%) | 61.3 ± 6.2 | 52.7 ± 6.7 | <0.0001 | 55.5 ± 7.5 | 58.4 ± 7.8 | 0.0247 |
| Protein energy/total energy (%) | 13.6 ± 1.6 | 16.9 ± 1.9 | <0.0001 | 15.0 ± 2.3 | 15.6 ± 2.5 | 0.1039 |
| Animal protein energy/total energy (%) | 5.8 ± 1.2 | 9.9 ± 1.8 | <0.0001 | 8.2 ± 2.5 | 7.5 ± 2.4 | 0.0809 |
| Vegetable protein energy/total energy (%) | 7.0 ± 1.3 | 6.7 ± 1.2 | 0.1729 | 5.8 ± 0.6 | 7.9 ± 0.9 | <0.0001 |
| Fat energy/total energy (%) | 24.3 ± 5.5 | 29.5 ± 6.2 | <0.0001 | 28.2 ± 6.2 | 25.6 ± 6.4 | 0.0122 |
| Animal fat energy/total energy (%) | 8.7 ± 2.7 | 14.1 ± 3.7 | <0.0001 | 12.5 ± 4.4 | 10.3 ± 3.6 | 0.0013 |
| Vegetable fat energy/total energy (%) | 14.1 ± 4.0 | 14.9 ± 4.9 | 0.2864 | 14.1 ± 4.2 | 15.0 ± 4.7 | 0.2615 |
| Sodium intake (g) | 9.6 ± 3.2 | 11.5 ± 3.5 | 0.0009 | 10.3 ± 3.0 | 10.7 ± 3.9 | 0.4543 |
| Potential renal acid load score (mEq/day) | 3.1 ± 11.2 | 8.2 ± 11.9 | 0.0079 | 9.4 ± 8.8 | 2.0 ± 13.2 | 0.0001 |
| Net endogenous acid production score (mEq/day) | −8.7 ± 0.3 | −8.6 ± 0.3 | 0.0235 | −8.6 ± 0.3 | −8.7 ± 0.3 | 0.0049 |
| PRAL score | NEAP score | |||||
|---|---|---|---|---|---|---|
| ≤6.9 mEq/day | ≥7.0 mEq/day | P | ≤−8.7 mEq/day | ≥−8.6 mEq/day | P | |
| n | 74 | 75 | – | 74 | 75 | – |
| Age (years) | 66.2 ± 8.8 | 65.2 ± 9.8 | 0.5479 | 66.4 ± 9.1 | 65.0 ± 9.6 | 0.3324 |
| Sex (male/female) | 29/45 | 48/27 | 0.0024 | 31/44 | 46/29 | 0.0176 |
| Body mass index (kg/m2) | 23.7 ± 4.3 | 24.3 ± 4.1 | 0.3590 | 23.8 ± 4.3 | 24.2 ± 4.1 | 0.4742 |
| Systolic blood pressure (mmHg) | 130.7 ± 16.0 | 128.4 ± 13.0 | 0.3358 | 129.9 ± 15.7 | 129.2 ± 13.4 | 0.7556 |
| HbA1c (%) | 6.9 ± 0.9 | 6.8 ± 0.9 | 0.8540 | 6.9 ± 0.9 | 6.9 ± 0.9 | 0.9612 |
| Low-density lipoprotein cholesterol (mmol/L) | 2.5 ± 0.7 | 2.7 ± 0.8 | 0.0474 | 2.5 ± 0.7 | 2.7 ± 0.8 | 0.1437 |
| Triglycerides (mmol/L) | 1.3 ± 0.7 | 1.7 ± 1.1 | 0.0257 | 1.3 ± 0.7 | 1.7 ± 1.2 | 0.0046 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 81.5 ± 21.4 | 74.3 ± 21.6 | 0.0429 | 79.8 ± 19.6 | 76.0 ± 23.6 | 0.2902 |
| Metabolic syndrome (–/+) | 49/25 | 33/42 | 0.0064 | 50/24 | 32/43 | 0.0023 |
| Total energy intake (kcal) | 1706.9 ± 405.8 | 1934.5 ± 362.7 | 0.0004 | 1786.8 ± 424.2 | 1855.7 ± 374.4 | 0.2949 |
| Carbohydrate energy/total energy (%) | 59.0 ± 8.0 | 54.9 ± 6.9 | 0.0009 | 58.0 ± 7.8 | 55.9 ± 7.6 | 0.0998 |
| Protein energy/total energy (%) | 14.9 ± 2.6 | 15.6 ± 2.2 | 0.0703 | 15.4 ± 2.5 | 15.2 ± 2.4 | 0.7260 |
| Animal protein energy/total energy (%) | 7.3 ± 2.5 | 8.4 ± 2.4 | 0.0069 | 7.7 ± 2.5 | 8.0 ± 2.5 | 0.5116 |
| Vegetable protein energy/total energy (%) | 7.1 ± 1.2 | 6.5 ± 1.3 | 0.0046 | 7.1 ± 1.2 | 6.5 ± 1.3 | 0.0061 |
| Fat energy/total energy (%) | 25.6 ± 6.4 | 28.2 ± 6.2 | 0.0127 | 26.3 ± 6.3 | 27.5 ± 6.5 | 0.2689 |
| Animal fat energy/total energy (%) | 10.2 ± 3.9 | 12.6 ± 4.1 | 0.0004 | 10.9 ± 3.9 | 11.9 ± 4.4 | 0.1780 |
| Vegetable fat energy/total energy (%) | 14.6 ± 4.5 | 14.5 ± 4.4 | 0.9066 | 14.6 ± 4.6 | 14.5 ± 4.3 | 0.9866 |
| Sodium intake (g) | 9.9 ± 3.9 | 11.1 ± 2.9 | 0.0417 | 10.6 ± 3.9 | 10.4 ± 3.0 | 0.7821 |
| Potential renal acid load score (mEq/day) | −3.0 ± 10.0 | 14.2 ± 5.5 | <0.0001 | −2.7 ± 10.3 | 13.9 ± 6.0 | <0.0001 |
| Net endogenous acid production score (mEq/day) | −8.9 ± 0.2 | −8.4 ± 0.2 | <0.0001 | −8.9 ± 0.2 | −8.4 ± 0.2 | <0.0001 |
Data are number of patients or mean ± standard deviation.
HbA1c, hemoglobin A1c
NEAP, net endogenous acid production
PRAL, potential renal acid load.
Table 3.
Odds ratios for prevalence of metabolic syndrome (logistic regression) according to the median of animal or vegetable protein energy/total energy or potential renal acid load or net endogenous acid production score
| OR (95% CI) | P | ||
|---|---|---|---|
| Animal protein energy/total energy | |||
| ≤7.7% (n = 74) | ≥7.8% (n = 75) | ||
| Unadjusted OR | 1 (Reference) | 0.92 (0.48–1.76) | 0.8113 |
| Adjusted OR* | 1 (Reference) | 1.17 (0.52–2.60) | 0.6978 |
| Vegetable protein energy/total energy | |||
| ≤6.5% (n = 74) | ≥6.6% (n = 75) | ||
| Unadjusted OR | 1 (Reference) | 0.30 (0.15–0.59) | 0.0005 |
| Adjusted OR* | 1 (Reference) | 0.28 (0.12–0.66) | 0.0038 |
| PRAL score | |||
| ≤6.9 mEq/day (n = 74) | ≥7.0 mEq/day (n = 75) | ||
| Unadjusted OR | 1 (Reference) | 2.49 (1.28–4.84) | 0.0083 |
| Adjusted OR* | 1 (Reference) | 2.22 (1.04–4.83) | 0.0384 |
| NEAP score | |||
| ≤−8.7 mEq/day (n = 74) | ≥−8.6 mEq/day (n = 75) | ||
| Unadjusted OR | 1 (Reference) | 2.79 (1.43–5.46) | 0.0029 |
| Adjusted OR* | 1 (Reference) | 2.61 (1.25–5.55) | 0.0098 |
Adjusted for age, sex, serum uric acid and creatinine, total energy intake, carbohydrate intake and sodium intake.
CI, confidence interval.
NEAP; net endogenous acid production
OR, odds ratio
PRAL, potential renal acid load.
Discussion
In the present study, we found that C/E ratio was negatively correlated with AP/E ratio, PRAL or NEAP score, and was positively correlated with VP/E ratio in patients with type 2 diabetes. Furthermore, the decreased VP/E ratio and increased acid load scores, assessed by PRAL and NEAP scores, were associated with the prevalence of MetS after adjusting for confounding variables.
Evidence has been accumulating to suggest that low carbohydrate diets and their combination with high-protein diets are potent in bodyweight loss12, and might have favorable effects on the risk markers of cardiovascular disease in the short term13. However, recent reports suggested that low-carbohydrate diets were associated with a significantly higher risk of all-cause mortality in the long term14. One of the possible explanations for the association might be increased intake of protein based on animal sources and reduced intake of protein based on vegetable sources15. In addition, a low-carbohydrate diet has a tendency to result in a reduced intake of fruits and fiber, and an increased intake of protein based on cholesterol, saturated fat and animal sources16,17. The present study also provided equally suggestive evidence that low-carbohydrate intake was associated with increased animal protein intake and decreased vegetable protein intake in patients with type 2 diabetes.
MetS is defined by the clustering of cardiovascular risk factors, including hyperglycemia, hypertension, visceral obesity and dyslipidemia. MetS is also related to cardiovascular disease, which is the main cause of morbidity and mortality18. We showed that decreased vegetable protein intake was associated with the prevalence of MetS. In the PREMIER study of 810 participants, intake of vegetable protein was inversely associated with blood pressure in cross-sectional analyses19. In addition, a previous study reported that dietary vegetable protein intake improved metabolic features and reduced the pro-inflammatory status in obese subjects20. It is difficult to assess the effect of vegetable protein interventions compared with animal protein interventions, because the former includes more polyunsaturated fatty acid and fiber, whereas the latter includes more saturated fatty acid, and these of course are fundamental components of these two different protein sources. Although no studies, to our knowledge, have examined just the effect of the protein per se, this is not relevant to normal consumption of these protein sources.
In general, a high intake of fat has been shown to predict development of glucose intolerance in a group of healthy subjects21,22. Then, we analyzed the relationship between animal fat energy/total energy and the prevalence of MetS. However, in logistic regression analyses, animal fat energy/total energy was not a statistically significant risk factor for the prevalence of MetS in the present study.
The PRAL and NEAP scores are frequently used to estimate dietary acid load in epidemiological studies. The PRAL score considers the intestinal absorption rates of contributing nutrient ionic balances for protein, potassium, calcium and magnesium, and the dissociation of phosphate at pH 7.4. A positive PRAL value reflects an acid-forming potential, whereas a negative PRAL value reflects a base (or alkaline)-forming potential. The PRAL score was calculated using protein, potassium, calcium, magnesium and phosphorus intake from the diet. The NEAP score was developed by using chemical analysis of experimentally controlled diets and diet composition tables. The NEAP score was calculated from the dietary acid load from dietary intakes of protein and potassium, but did not contain the acid load of other nutrients. Therefore, dietary acid load was assessed by PRAL and NEAP scores in the present study. Our present finding, that increased dietary acid load was associated with the prevalence of MetS, was in line with the results of previous studies. Previous studies reported that high dietary acid load was associated with hypertension and type 2 diabetes6,23. This mechanism was supported by cross-sectional studies showing that dietary acid load was positively associated with insulin resistance24,25. Furthermore, chronic metabolic acidosis, which could lead to reduced insulin sensitivity, might be caused by an acidogenic diet over time26. Other possible mechanisms are as follows. A high dietary acid load causes compensatory increases in renal ammoniagenesis and acid excretion27. Although this is favorable in the short term for controlling acid-based homeostasis, it causes a decline in renal function and might elevate blood pressure in the long term28. In addition, diet-induced metabolic acidosis29–31 could elevate blood pressure32,33, probably by elevating cortisol production29, elevating calcium excretion34 or declining citrate excretion35.
The present study had several limitations that require consideration. First, this study was a cross-sectional design, which did not permit the determination of causality. Thus, further studies are required to better assess the relationships between vegetable protein intake or dietary acid load and MetS. Second, to collect dietary data, other reports used a semiquantitative dietary assessment questionnaire (i.e. DHQ). The misreporting of dietary intake, especially by obese individuals, is an important problem related to self-report dietary assessment methods36. However, at least for dietary protein, energy adjustment seems to cancel BMI-dependent misreporting37. Third, the lack of waist circumference data weakens the definition of MetS. However, BMI of ≥25 kg/m2 was reported as a cut-off for the diagnosis of obesity in Asian people11, and was validated previously38. Finally, the study population consisted of Japanese men and women, therefore, it is uncertain whether these findings are generalized in other ethnic groups.
In conclusion, the present study showed that carbohydrate intake was negatively correlated with animal protein intake or dietary acid load, and was positively correlated with vegetable protein intake in patients with type 2 diabetes. Furthermore, decreased vegetable protein intake and increased dietary acid load were associated with the prevalence of MetS. Further research to elucidate the apparent influence of vegetable protein intake and dietary acid load associated with low carbohydrate intake on metabolic risk factors is required in patients with type 2 diabetes.
Acknowledgments
We thank Akiko Matsumoto, Masami Okagaki, Hiroko Neriya (University Hospital, Kyoto Prefectural University of Medicine, Japan) for their technical support and helpful discussion. The authors declare no conflict of interest.
References
- 1.Franz MJ, Boucher JL, Green-Pastors J, et al. Evidence-based nutrition practice guidelines for diabetes and scope and standards of practice. J Am Diet Assoc. 2008;108(Suppl 1):S52–S58. doi: 10.1016/j.jada.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 2.Franz MJ, Powers MA, Leontos C, et al. The evidence for medical nutrition therapy for type 1 and type 2 diabetes in adults. J Am Diet Assoc. 2010;110:1852–1889. doi: 10.1016/j.jada.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Standards of medical care in diabetes-2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Nielen M, Feskens EJ, Mensink M, et al. Dietary protein intake and incidence of type 2 diabetes in Europe: the EPIC-InterAct Case-Cohort Study. Diabetes Care. 2014;37:1854–1862. doi: 10.2337/dc13-2627. [DOI] [PubMed] [Google Scholar]
- 5.Fung TT, van Dam RM, Hankinson SE, et al. Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Ann Intern Med. 2010;153:289–298. doi: 10.1059/0003-4819-153-5-201009070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engberink MF, Bakker SJ, Brink EJ, et al. Dietary acid load and risk of hypertension: the Rotterdam Study. Am J Clin Nutr. 2012;95:1438–1444. doi: 10.3945/ajcn.111.022343. [DOI] [PubMed] [Google Scholar]
- 7.Adeva MM, Souto G. Diet-induced metabolic acidosis. Clin Nutr. 2011;30:416–421. doi: 10.1016/j.clnu.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki S, Yanagibori R, Amano K. Self-administered diet history questionnaire developed for health education: a relative validation of the test-version by comparison with 3-day diet record in women. J Epidemiol. 1998;8:203–215. doi: 10.2188/jea.8.203. [DOI] [PubMed] [Google Scholar]
- 9.Remer T, Dimitriou T, Manz F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am J Clin Nutr. 2003;77:1255–1260. doi: 10.1093/ajcn/77.5.1255. [DOI] [PubMed] [Google Scholar]
- 10.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 11.Steering Committee of the Western Pacific Region of the World Health Organization, the International Association for the Study of Obesity, the International Obesity Task Force. 2000. The Asia-Pacific perspective: redefining obesity and its treatment. Melbourne, Australia, Health Communications Australia Pty Limited [article online]. Available from: http://www.wpro.who.int/nutrition/documents/en/
- 12.Hession M, Rolland C, Kulkarni U, et al. Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its comorbidities. Obes Rev. 2009;10:36–50. doi: 10.1111/j.1467-789X.2008.00518.x. [DOI] [PubMed] [Google Scholar]
- 13.Gögebakan O, Kohl A, Osterhoff MA, et al. Effects of weight loss and long-term weight maintenance with diets varying in protein and glycemic index on cardiovascular risk factors: the diet, obesity, and genes (DiOGenes) study: a randomized, controlled trial. Circulation. 2011;124:2829–2838. doi: 10.1161/CIRCULATIONAHA.111.033274. [DOI] [PubMed] [Google Scholar]
- 14.Noto H, Goto A, Tsujimoto T, et al. Low-carbohydrate diets and all-cause mortality: a systematic review and meta-analysis of observational studies. PLoS ONE. 2013;8:e55030. doi: 10.1371/journal.pone.0055030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagiou P, Sandin S, Lof M, et al. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ. 2012;344:e4026. doi: 10.1136/bmj.e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Floegel A, Pischon T. Low carbohydrate-high protein diets. BMJ. 2012;344:e3801. doi: 10.1136/bmj.e3801. [DOI] [PubMed] [Google Scholar]
- 17.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76:1261–1271. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 18.Sone H, Tanaka S, Iimuro S, et al. Components of metabolic syndrome and their combinations as predictors of cardiovascular disease in Japanese patients with type 2 diabetes. Implications for improved definition. Analysis from Japan Diabetes Complications Study (JDCS) J Atheroscler Thromb. 2009;16:380–387. doi: 10.5551/jat.no117. [DOI] [PubMed] [Google Scholar]
- 19.Wang YF, Yancy WS, Jr, Yu D, et al. The relationship between dietary protein intake and blood pressure: results from the PREMIER study. J Hum Hypertens. 2008;22:745–754. doi: 10.1038/jhh.2008.64. [DOI] [PubMed] [Google Scholar]
- 20.Hermsdorff HH, Zulet MÁ, Abete I, et al. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur J Nutr. 2011;50:61–69. doi: 10.1007/s00394-010-0115-x. [DOI] [PubMed] [Google Scholar]
- 21.Feskens EJM, Virtanen SM, Raäsaänen L, et al. Dietary factors determining diabetes and impaired glucose tolerance. A 20-year follow-up of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetes Care. 1995;18:1104–1112. doi: 10.2337/diacare.18.8.1104. [DOI] [PubMed] [Google Scholar]
- 22.Marshall JA, Hoag S, Shetterly S, et al. Dietary fat predicts conversion from impaired glucose tolerance to NIDDM. The San Luis Valley Diabetes Study. Diabetes Care. 1994;17:50–56. doi: 10.2337/diacare.17.1.50. [DOI] [PubMed] [Google Scholar]
- 23.Fagherazzi G, Vilier A, Bonnet F, et al. Dietary acid load and risk of type 2 diabetes: the E3N-EPIC cohort study. Diabetologia. 2014;57:313–320. doi: 10.1007/s00125-013-3100-0. [DOI] [PubMed] [Google Scholar]
- 24.Maalouf NM, Cameron MA, Moe OW, et al. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:883–888. doi: 10.2215/CJN.00670207. [DOI] [PubMed] [Google Scholar]
- 25.Souto G, Donapetry C, Calvino J, et al. Metabolic acidosis-induced insulin resistance and cardiovascular risk. Metab Syndr Relat Disord. 2011;9:247–253. doi: 10.1089/met.2010.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeFronzo RA, Beckles AD. Glucose intolerance following chronic metabolic acidosis in man. Am J Physiol. 1979;236:E328–E334. doi: 10.1152/ajpendo.1979.236.4.E328. [DOI] [PubMed] [Google Scholar]
- 27.Smogorzewski MJ. Historical perspective on the role of the kidney in acid-base regulation. J Nephrol. 2009;22(Suppl 14):108–114. [PubMed] [Google Scholar]
- 28.Nath KA, Hostetter MK, Hostetter TH. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest. 1985;76:667–675. doi: 10.1172/JCI112020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maurer M, Riesen W, Muser J, et al. Neutralization of Western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. Am J Physiol Renal Physiol. 2003;284:F32–F40. doi: 10.1152/ajprenal.00212.2002. [DOI] [PubMed] [Google Scholar]
- 30.Remer T, Pietrzik K, Manz F. Short-term impact of a lactovegetarian diet on adrenocortical activity and adrenal androgens. J Clin Endocrinol Metab. 1998;83:2132–2137. doi: 10.1210/jcem.83.6.4883. [DOI] [PubMed] [Google Scholar]
- 31.Sicuro A, Mahlbacher K, Hulter HN, et al. Effect of growth hormone on renal and systemic acid–base homeostasis in humans. Am J Physiol. 1998;274:F650–F657. doi: 10.1152/ajprenal.1998.274.4.F650. [DOI] [PubMed] [Google Scholar]
- 32.Sharma AM, Kribben A, Schattenfroh S, et al. Salt sensitivity in humans is associated with abnormal acid–base regulation. Hypertension. 1990;16:407–413. doi: 10.1161/01.hyp.16.4.407. [DOI] [PubMed] [Google Scholar]
- 33.Sharma AM, Cetto C, Schorr U, et al. Renal acid–base excretion in normotensive salt-sensitive humans. Hypertension. 1993;22:884–890. doi: 10.1161/01.hyp.22.6.884. [DOI] [PubMed] [Google Scholar]
- 34.Cappuccio FP, Kalaitzidis R, Duneclift S, et al. Unraveling the links between calcium excretion, salt intake, hypertension, kidney stones and bone metabolism. J Nephrol. 2000;13:169–177. [PubMed] [Google Scholar]
- 35.Taylor EN, Mount DB, Forman JP, et al. Association of prevalent hypertension with 24-hour urinary excretion of calcium, citrate, and other factors. Am J Kidney Dis. 2006;47:780–789. doi: 10.1053/j.ajkd.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 36.Livingstone MBE, Black AE. Markers of the validity of reported energy intake. J Nutr. 2003;133(Suppl 3):S895–S920. doi: 10.1093/jn/133.3.895S. [DOI] [PubMed] [Google Scholar]
- 37.Murakami K, Sasaki S, Takahashi Y, et al. Misreporting of dietary energy, protein, potassium and sodium in relation to body mass index in young Japanese women. Eur J Clin Nutr. 2008;62:111–118. doi: 10.1038/sj.ejcn.1602683. [DOI] [PubMed] [Google Scholar]
- 38.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]


