Abstract
Acetylcholinesterase (AChE) plays a key role in catalytic hydrolysis of cholinergic neurotransmitters. Intensive research has proven the involvement of this protein in novel functions, such as cell adhesion, differentiation, and proliferation. In addition, several recent studies have indicated that acetylcholinesterase is potentially a marker and regulator of apoptosis. Importantly, AChE is also a promising tumor suppressor. In this review, we briefly summarize the involvement of AChE in apoptosis and cancer, focusing on the role of AChE in lung cancer, as well as the therapeutic consideration of AChE for cancer therapy.
Keywords: Acetylcholinesterase (AChE), apoptosis, biomarker, lung cancer
Introduction
Lung cancer is the most commonly diagnosed cancer and is the leading cause of death among male cancer patients worldwide. Lung cancer accounts for 17% of the total new cancer cases and 23% of total cancer mortality in male patients.1 Worldwide, 80% of lung cancer diagnosed in men and at least 50% in women is caused by smoking, which is characterized as one of the main factors leading to cancer growth.2 Other risk factors contribute to lung cancer, including exposure to several occupational and environmental carcinogens, such as crystalline silica dust, asbestos, arsenic, residential radon, polycyclic aromatic hydrocarbons, passive smoking, and ambient air pollution, as well as unknown risk factors.3 Lung cancers are classified into small cell lung carcinomas (SCLC) and non-small cell lung carcinomas (NSCLC) based on morphological criteria and clinical outcome.4 SCLC, comprising 20–25% of lung cancers, are closely associated with smoking and develop and metastasize rapidly.5–7 NSCLC are categorized into adenocarcinomas (AC), squamous cell carcinomas (SCC), and large cell carcinomas (LCC). Despite innovations in diagnostic testing, surgical techniques, and chemotherapeutic treatments, the five-year survival rate of lung cancer patients remains low (13–15%).8 Because of a lack of specific biomarkers and tools for early screening, most patients are only diagnosed at advanced stage, and most die from metastasis.9,10 A better understanding of the molecular mechanism underlying lung cancer would lead to new therapies for improving patient survival and quality of life.
Acetylcholinesterase (AChE), a member of the alpha/beta-hydrolase fold superfamily of proteins, is a serine hydrolase responsible for terminating transmission at cholinergic synapses by rapidly hydrolyzing the neurotransmitter acetylcholine (ACh).11 The gene-encoding AChE is located at 7q22 in the human genome.12 There are three distinct alternative splicing isoforms.13 Tetrameric AChE-synaptic (S) isoform expressed in brain and muscle tissue contains C-terminal collagen tail or hydrophobic subunits, which can anchor to the membrane. Dimeric AChE-erythrocytic (E) isoform can generate a hydrophobic glycosylphosphatidylinositol (GPI) group near the C-terminal. This isoform is prevalent in the human erythrocytes. The third isoform AChE-read-through (R) can produce a hydrophilic monomer at the cholinergic synapse in response to stress.14–16
Although AChE is an essential enzyme primarily expressed in the central and peripheral nervous systems, different isoforms of AChE are also constitutive of various cell types, including human fibroblasts, osteoblasts, rat kidney cells, erythrocytes, vascular endothelial cells, and leukocytes, as well as tissues, such as nerve endings, and the brain, lung, and spleen.17 In these tissues, the biological role of AChE is not limited to its classical role in hydrolyzing ACh, but includes non-classical functions, such as neuritogenesis and neurodegeneration, which are related to Alzheimer's disease, and influence apoptotic sensitivity, cellular proliferation, and differentiation, suggesting a possible role of cholinesterases in tumorigenesis.16,18–24 AChE has also been reported to be associated with stress responses and related to inflammation.25–27 Furthermore, abnormal expression and structural alteration of AChE and multiple activities have been found in different types of tumors, such as brain, lung, ovarian, breast, hepatocellular, renal, and colon cancers, which indicate the involvement of AChE in regulating tumor development.14,28–34 However, the mechanism of AChE gene regulation in tumors remains unclear; therefore, its detailed functions need to be investigated. Recent reports have revealed that AChE activity in lung cancer is reduced, which may contribute to lung cancer growth. Moreover, AChE may participate in apoptosis, contributing to tumor suppression. In this review, we focus on the latest developments of AChE function in lung cancer.
Acetylcholinesterase (AChE) expression is related to apoptosis and oxidative stress
Many studies have found that the expression level of AChE increases during apoptosis in various cell types. It has been demonstrated that normal human lung fibroblast (HLF) cells have almost no expression of AChE; however the expression level of AChE is highly upregulated during apoptosis or stimulated states.20,35 During apoptosis in normal rat kidney (NRK) cells, the expression of AChE protein is upregulated, which results in reducing cell proliferation and promoting apoptosis.35 AChE-R was shown to increase germ cell apoptosis.36 Furthermore, AChE is weakly expressed in normal human retina pigment epithelial (RPE) cells; however H2O2 treatment in RPE cells stably increased AChE expression.37 It has also been demonstrated that AChE can hydrolyze lipid peroxides, which may support the possibility that a reduction in enzyme activity augments oxidative stress and cellular damage of bronchopulmonary epithelial cells.38 Stress stimuli in the cortex of thymus may also induce AChE expression.39
Park et al. discovered that AChE plays a pivotal role in the formation of apoptosome by affecting the interaction between apoptotic protease-activating factor-1 (Apaf-1) and cytochrome c, which consequently triggers cleavage of pro-caspase-9 to its active form (Fig 1).40 Inhibition of the expression of AChE by small interfering ribonucleic acids (siRNAs) impedes the interaction between Apaf-1 and cytochrome c, which results in a blockage of caspase-9 activation, leading to a decrease of cell viability, nuclear condensation, and poly (adenosine diphosphate-ribose) polymerase cleavage.40 The anti-sense oligonucleotides of AChE may also prevent apoptosis.20 A further study showed that the expression of AChE was upregulated in MCF-7 cells treated with cisplatin, an anti-tumor drug; when p53 is blocked with siRNA, cisplatin-induced AChE expression is decreased in cisplatin treated MCF-7 cells. These results imply that AChE might be a downstream component of p53, which induces apoptosis in chemotherapy.41
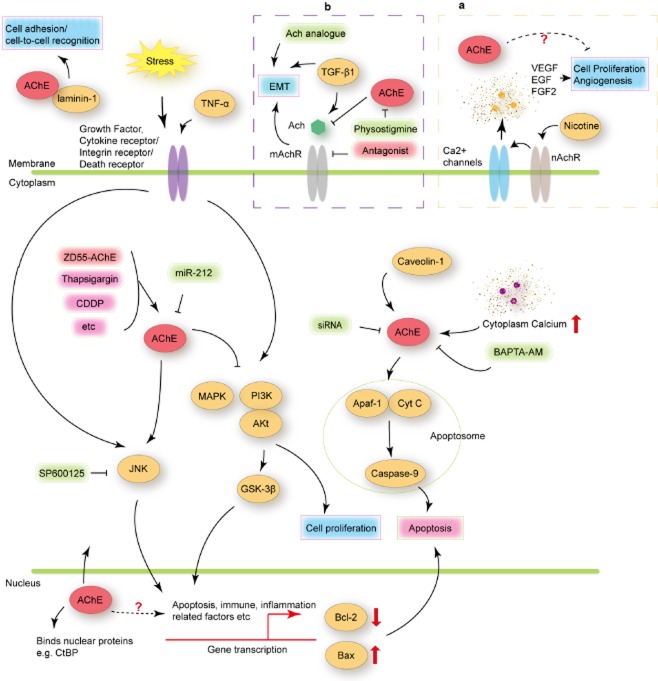
Figure 1.
Schematic diagram for signaling regulation of acetylcholinesterase (AChE). AChE may bind laminin-1 contributing to cell-to-cell recognition. During the early phase of apoptosis, AChE may translocate into the nucleus, and then shuttle to the cytoplasm. In the nucleus, AChE may interact with nuclear proteins, for example, transcriptional co-repressor C-terminal binding protein (CtBP), while in the cytoplasm, AChE may bind to caveolin-1, assisting the interaction of apoptotic protease-activating factor-1 (Apaf-1) and cytochrome C (Cyt C), promoting apoptosis. Anticancer drugs, such as thapsigargin and cisplatin, ZD55-AChE (overexpressed AChE by oncolytic adenoviral vector), could upregulate the expression of AChE, and may promote apoptosis through the c-Jun N-terminal kinase (JNK) pathway, inhibiting phosphatidylinositol-3 kinase (PI3K)/protein kinase B (Akt) and mitogen activated protein kinase (MAPK) signaling pathways while activating glycogen synthase kinase (GSK)-3β, which may suppress cell proliferation. High cytoplasm calcium concentration also upregulated AChE expression, while the intracellular Ca2+ chelator, BAPTA-AM, decreased the AChE level. (a) AChE involved in transforming growth factor (TGF)-β1-induced epithelial-mesenchymal transition (EMT). (b) AChE may associate with anti-angiogenesis. EGF, epidermal growth factor; FGF2, fibroblast growth factor 2; mAchR, muscarinic acetylcholine receptor; nAchR, nicotinic acetylcholine receptor; SP600125, specific inhibitor of stress activated kinases (SAPK)/JNK; TNF-α, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
Growing evidence suggests that AChE-R is involved in cell proliferation, whereas AChE-S is involved in apoptosis.19,20,40,42,43 Over-expression of the N-terminally extended N-AChE-S variant induces cell death and morphological impairments both in primary cortical cells and in different cell lines of various tissue and species origins, attributing to its conserved and ubiquitous role as a general trigger of apoptosis by activating an apoptotic pathway that includes activation of glycogen synthase kinase 3 (GSK3), Bax, and caspases.44,45 Silencing the expression of AChE protein by small hairpin RNA prevented the Y79 cell from entering the apoptotic pathway.46 In addition, the decrease of AChE activity in LCC and, particularly, in SCC, may contribute to tumor development by increasing cell growth or proliferation.47 In this regard, AChE may act as a pro-apoptotic gene in NSCLC cells, and attenuate the growth of xenografts in nude mice when the expression is upregulated.
However, recent reports observed an increase in AChE activity in the blood of lung cancer and acute lymphoblastic leukemia (ALL) patients. These results indicate that AChE related reduction of ACh levels in the blood may cause a consequent decrease in the anti-inflammatory activity promoted by this molecule, which contributes to the regulation of immune function, suggesting a dual role of AChE.48,49
Signaling pathways involved in regulation of AChE
With the discovery of non-classical functions of AChE, the partners that interact with AChE and the signaling pathways that regulate AChE have been intensively investigated. For example, an AChE protein localized to the nucleus has been documented. AChE may move into the nucleus in early phases of apoptosis and translocate from the nucleus to the cytosol.50 The variations of AChE cellular localization led to the detection of a variety of interaction partners for the AChE protein. AChE may localize to caveolae via interacting with caveolin-1.51 Nuclear proteins, such as the transcriptional co-repressor C-terminal binding protein or β-amyloid, and cytoplasmic proteins, such as receptors for activated C-kinase, can interact with AChE and, consequently, may contribute to its functional diversity.52–55 Recent findings have revealed that laminin-1 is an interaction partner for AChE. The binding of AChE to this extracellular matrix component may allow cell-to-cell recognition and cell signaling via membrane receptors. Recent research has suggested that several signaling pathways are involved in the regulation of AChE (Fig 1).
Recently, Deng et al. demonstrated that the mechanism underlying AChE upregulation is mediated by the c-Jun N-terminal kinase (JNK)/c-Jun pathway in colon cancer cell line SW620.56 The JNK signaling pathway can be activated under multiple forms of stress, such as growth factor withdrawal, excitotoxic stress, and ultraviolet radiation, contributing to apoptotic signal transduction and regulation of the transcription of some downstream genes and some pro-apoptotic or anti-apoptotic proteins, such as Bax and B-cell lymphoma 2.57–61 Inhibition expression of JNK1/2 by a specific inhibitor SP600125 or siRNA can block the expression of AChE, induced by anti-cancer drugs etoposide or excisanin-A in messenger (m)RNA and protein levels.56 Furthermore, an overexpression of AChE in cancer cells could inhibit critical proliferation pathways, including the phosphatidylinositol-3 kinase (PI3K)/protein kinase B (Akt) pathway.32 AChE also inactivates mitogen activated protein kinase (MAPK) and PI3K/Akt pathways and activates GSK3β in hepatocellular carcinoma (HCC) cells. Moreover, AChE is reported to restrain intestinal cell or stem cell differentiation, probably through inhibition of signal transduction of the PI3K/Akt pathway.62–64 Activation of the PI3K/Akt signaling pathway was discovered in lung epithelial cells induced by both nicotine and nicotine-derived carcinogenic nitrosamine, indicating a correlation between cholinergic stimulation and lung cancer.65
Another important factor that needs to be highlighted is that synaptic AChE may be a tumor suppressor in NSCLC, which could increase cell apoptosis and attenuate NSCLC growth. In addition, cisplatin combined with upregulated AChE effectively suppressed xenograft growth, while micro ribonucleic acid (miR)-212 that directly targeted the 3′ untranslated region (UTR) of AChE mRNA in NSCLC cells negatively regulated AChE expression and inhibited cisplatin-induced apoptosis. These results demonstrate that targeting to AChE and/or miR-212 may improve the pharmaco-toxicological profile of cisplatin in NSCLC.66 AChE inhibition also restricts inflammation in both the peripheral and central nervous systems.67 MiR-132 targeted AChE potentiates cholinergic anti-inflammatory activity, which facilitates tumor progression.27
Cytosolic calcium concentration also plays an important role in AChE regulation during apoptosis. Calcium ionophore A23187 could significantly elevate AChE expression in both mRNA and protein levels, while the intracellular Ca2+ chelator, BAPTA-AM, suppresses AChE expression.68 During thapsigargin-induced apoptosis in HeLa and MDA-MB-435 s cells, thapsigargin interfered with cellular Ca2+ homeostasis, which gave rise to the upregulation of AChE expression and AChE promoter activity.69 Recent research has indicated that nicotine binds to the nicotinic acetylcholine receptors, activates Ca2+ channels, and triggers the secretion of growth factors, including vascular endothelial growth factor, epidermal growth factor, and fibroblast growth factor 2, which may consequently promote cell proliferation and tumor angiogenesis in lung cancer (Fig 1a).70 These findings possibly indicate the role of AChE in angiogenesis and tumor development. Further investigation is required to unveil the anti-angiogenesis function of AChE in cancer therapy.
AChE may function as a potential biomarker for cancer diagnosis or prognosis
As reported previously, AChE is involved in apoptosis; thus, AChE activity is a potential marker of apoptosis. Xiao et al. proved this hypothesis, utilizing AChE with Bax, c-fos, and p53 genes as markers of apoptosis.71,72 Cholinesterases have been implicated in the development process as cellular proliferation stimulators.73,74 AChE can be considered a marker of early differentiation, while butyrylcholinesterase may be involved in cellular migration and fiber guidance.75,76 Previous studies have indicated that the AChE gene is frequently amplified, mutated or deleted in cancer patients. The AChE activity in lymphocytes from chronic lymphocytic leukemia patients was significantly lower than normal.77 Later reports have indicated that mutations in the AChE genetic locus at 7q22 are associated with leukemias and myelodysplastic syndrome, and structural alteration or aberrant expression of AChE are commonly found in a variety of tumor types, such as lung, prostate, head and neck cancers.47,78–81 AChE enzyme composition, glycosylation, and enzyme activity are altered in breast cancer.31 Amplification of the proto-oncogene ERBB2 was at a higher frequency after deletion of the AChE gene.82 All of these results suggest that AChE activity may be a potential biomarker for cancer diagnosis or prognosis.
Zhao et al. demonstrated that the expression of AChE was significantly decreased in the cancer tissues of 69.2% of HCC patients, and the low expression level of AChE in HCC was correlated with tumor aggressiveness, the increased risk of postoperative recurrence, low survival rate, and poor prognosis.32 Overexpression of AChE significantly inhibited HCC cell growth in vitro and tumorigenicity in vivo, suggesting the function of AChE as a tumor growth suppressor that acts in the regulation of cell proliferation, relevant signaling pathways, and the chemosensitivity of HCC cells. Thus, AChE is a promising independent prognostic biomarker to predict HCC recurrence and the survival of HCC patients. Gastric cancer patients with higher AChE levels also demonstrated a longer survival. AChE activity in breast cancer has been reported to be reduced in metastasis-bearing lymph nodes (MLN) when compared with normal lymph nodes (NLN).13 The different profile of AChE forms in NLN and MLN may be useful for diagnosis.
Several studies have compared AChE expression levels and activities in patients with different subtypes of lung cancers and normal controls, revealing that AChE activity was significantly lower in lung cancer.29,47 According to Song et al., acetylcholine stimulates cancerous cells growing in lung tumors.83 As a result of the abnormal expression of AChE, elevated ACh was observed in lung cancer tissues.47 The decrease in cholinesterase activity and the consequent increased level of acetylcholine could cause cholinergic overstimulation and enhance the cell proliferation in cancer, which indicates the role of AChE in the regulation of lung cancer growth. Furthermore, cholinesterases were also downregulated in human colorectal carcinoma, squamous cell carcinoma, and retinoblastoma.14,84 AChE has been reported to suppress cell proliferation via catalytic hydrolysis of acetylcholine in human colorectal carcinoma, and modulate programmed cell death in colon tumor cells, which might be consistent with the tumor suppressor role.14,20,40
Although many results have shown that AChE activities are decreased in cancer patients, the opposite variation of AChE activities have also been found.13,31,85,86 Therefore, the detailed functions of AChE in different types and stages of cancer need to be further investigated.
Increasing the expression of AChE could be used in future cancer treatment
Most of the previous studies aimed to inhibit AChE activity to prevent the development of Alzheimer's disease, as AChE was believed to play a critical role in the pathogenesis of neurodegenerative diseases.44 By contrast, the development of the therapeutic strategy of upregulating AChE activity is a growing field. To date, many studies have suggested that AChE is upregulated in a variety of apoptosis systems: human colon cancer HT-29 cells treated with etoposide, sulindac, thapsigargin, or LY294002 (a PI3K inhibitor); raw264.7 cells treated with 3-morpholinosyndnomine (a nitric oxide donor); human brain tumor TE671 and U373MG cells treated with etoposide; human melanoma SK-MEL-5 and Malme-3 M cells treated with etoposide; interleukin-3 deprived (murine) bone marrow derived mast cells and primary cultured rat articular chondrocytes treated with sodium nitroprusside (a nitric oxide donor), or infected with adenoviral TRAIL.40,51 In the promoter region of the AChE gene, there are GC rich element, activator protein-1, and specificity protein-1 transcription factor binding sites. Additionally, many factors, such as GSK3, Aurora, and G-associated kinases, the membrane integrin receptors, and the death receptor FAS, could potentially modulate N-AChE-S-induced apoptosis with possible therapeutic value for the treatment of relative diseases. Cytokines, such as tumor necrosis factor-α have also been suggested as upregulating the expression of AChE. Thus, the significant challenge will be the development of a highly specific strategy of upregulating AChE activity for clinical trials and, eventually, cancer therapies.
A recent study showed that overexpressed AChE by an oncolytic adenoviral vector (ZD55-AChE) significantly inhibited gastric cancer cell proliferation and reduced the growth of gastric tumors in mice. In addition, ZD55-AChE suppressed gastric cancer stem cell growth.70 This work demonstrated for the first time that the overexpression of AChE is effective for suppressing digestive system cancers. Elevated AChE levels induced by the inhibition of AChE-antisense transcript (AS) enhanced anticarcinogen-induced apoptosis. These observations demonstrated that AChE-AS modulates AChE expression and exerts an anti-apoptotic effect through direct repression of AChE expression in HCC cells. Thus, natural antisense RNA may play an important role in AChE regulation via affecting the epigenetic modification in the AChE promoter region.87 Therefore, introducing the AChE protein back may lead to growth inhibition of cancer cells.
Tumor necrosis factor-β1-induced epithelial-mesenchymal transition (EMT) in lung epithelial A549 cells can be blocked by muscarinic acetylcholine receptor (mAChR) antagonists and enhanced by the AChE inhibitor physostigmine, while ACh synthesis and release from lung epithelial cells can be enhanced by transforming growth factor-β1. In addition, physostigmine alone or mAChR stimulation with ACh analogue carbachol also induced lung epithelial cells to undergo EMT (Fig. 1b).88 These findings demonstrated that non-neuronal cholinergic system components, as well as AChE, were involved in EMT in lung epithelial cells. These results provided insights into novel therapeutic strategies for airway diseases in which lung remodeling occurs.
Despite the fact that AChE is a potential tumor suppressor, several results imply a dual role of AChE in colon cancer. For example, etoposide or excisanin A-induced the expression of AChE in the colon cancer SW620 cell line in both mRNA and protein levels; however, in contrast, several AChE inhibitors dose-dependently suppressed colony formation of HTB-38 cells in soft agar, suggesting a potential effect of AChE in controlling tumor cell adhesion.56 Another report suggested that the AChE-peptide has a novel, selective bioactivity on breast cancer cells and can potentiate metastatic cell behavior. Accordingly, the non-enzymatic function of AChE could be a novel target for the clinical management of metastatic breast cancer.
Although elevated AChE activity could be a promising anticancer therapeutic, there are still many challenging task and factors, such as the significant gap between in vivo and in vitro activity of AChE. Furthermore, AChE functions by binding with specific partners or by influencing other elements involved in apoptosis, thus, the detailed molecular mechanism of AChE should be further investigated for the development of therapeutics modulating AChE activity.89
Conclusions
Increasing evidence has shown that AChE may be involved in pivotal processes of carcinogenesis and tumor progression, although it is well known as the enzyme that hydrolyses acetylcholine. However, the exact role of AChE still requires deciphering. During recent years, investigations have revealed that regardless of the cellular origin, AChE gene expression is always decreased in tumor tissues, and AChE is a novel regulator in cell proliferation and cell death, allowing AChE as a potential marker for cancer diagnosis and prognosis. The application of an AChE enhancer, in the near future, as single agents or in combination with conventional cytotoxic drugs or other molecular targeted agents, would provide promising efficacy in anticancer therapeutics. Improved understanding of this complicated regulation will yield new insights into apoptosis biology and pathways that might be strategic targets for the design of anticancer therapeutics.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. (Published erratum appears in 2011;: 134) [DOI] [PubMed] [Google Scholar]
- 2.Proctor RN. Tobacco and the global lung cancer epidemic. Nat Rev Cancer. 2001;1:82–86. doi: 10.1038/35094091. [DOI] [PubMed] [Google Scholar]
- 3.Field RW, Withers BL. Occupational and environmental causes of lung cancer. Clin Chest Med. 2012;33:681–703. doi: 10.1016/j.ccm.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18:1059–1068. doi: 10.1183/09031936.01.00275301. [DOI] [PubMed] [Google Scholar]
- 5.Khuder SA. Effect of cigarette smoking on major histological types of lung cancer: A meta-analysis. Lung Cancer. 2001;31:139–148. doi: 10.1016/s0169-5002(00)00181-1. [DOI] [PubMed] [Google Scholar]
- 6.Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet. 2003;362:847–852. doi: 10.1016/S0140-6736(03)14338-3. [DOI] [PubMed] [Google Scholar]
- 7.Ezzati M, Henley SJ, Lopez AD, Thun MJ. Role of smoking in global and regional cancer epidemiology: Current patterns and data needs. Int J Cancer. 2005;116:963–971. doi: 10.1002/ijc.21100. [DOI] [PubMed] [Google Scholar]
- 8.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molina R, Auge JM, Escudero JM, et al. Mucins CA 125, CA 19.9, CA 15.3 and TAG-72.3 as tumor markers in patients with lung cancer: Comparison with CYFRA 21-1, CEA, SCC and NSE. Tumour Biol. 2008;29:371–380. doi: 10.1159/000181180. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DH, Schiller JH, Bunn PA., Jr Recent clinical advances in lung cancer management. J Clin Oncol. 2014;32:973–982. doi: 10.1200/JCO.2013.53.1228. [DOI] [PubMed] [Google Scholar]
- 11.Silman I, Sussman JL. Acetylcholinesterase: “Classical” and “non-classical” functions and pharmacology. Curr Opin Pharmacol. 2005;5:293–302. doi: 10.1016/j.coph.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Getman DK, Eubanks JH, Camp S, Evans GA, Taylor P. The human gene encoding acetylcholinesterase is located on the long arm of chromosome 7. Am J Hum Genet. 1992;51:170–177. [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz-Espejo F, Cabezas-Herrera J, Illana J, Campoy FJ, Muñoz-Delgado E, Vidal CJ. Breast cancer metastasis alters acetylcholinesterase activity and the composition of enzyme forms in axillary lymph nodes. Breast Cancer Res Treat. 2003;80:105–114. doi: 10.1023/A:1024461108704. [DOI] [PubMed] [Google Scholar]
- 14.Montenegro MF, Ruiz-Espejo F, Campoy FJ, et al. Cholinesterases are down-expressed in human colorectal carcinoma. Cell Mol Life Sci. 2006;63:2175–2182. doi: 10.1007/s00018-006-6231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massoulié J, Bon S, Perrier N, Falasca C. The C-terminal peptides of acetylcholinesterase: Cellular trafficking, oligomerization and functional anchoring. Chem Biol Interact. 2005;157–158:3–14. doi: 10.1016/j.cbi.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Soreq H, Seidman S. Acetylcholinesterase–new roles for an old actor. Nat Rev Neurosci. 2001;2:294–302. doi: 10.1038/35067589. [DOI] [PubMed] [Google Scholar]
- 17.Patocka J, Kuca K, Jun D. Acetylcholinesterase and butyrylcholinesterase–important enzymes of human body. Acta Medica (Hradec Kralove) 2004;47:215–228. [PubMed] [Google Scholar]
- 18.Small DH, Michaelson S, Sberna G. Non-classical actions of cholinesterases: Role in cellular differentiation, tumorigenesis and Alzheimer's disease. Neurochem Int. 1996;28:453–483. doi: 10.1016/0197-0186(95)00099-2. [DOI] [PubMed] [Google Scholar]
- 19.Ye W, Gong X, Xie J, et al. AChE deficiency or inhibition decreases apoptosis and p53 expression and protects renal function after ischemia/reperfusion. Apoptosis. 2010;15:474–487. doi: 10.1007/s10495-009-0438-3. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XJ, Yang L, Zhao Q, et al. Induction of acetylcholinesterase expression during apoptosis in various cell types. Cell Death Differ. 2002;9:790–800. doi: 10.1038/sj.cdd.4401034. [DOI] [PubMed] [Google Scholar]
- 21.Zhang XJ, Greenberg DS. Acetylcholinesterase involvement in apoptosis. Front Mol Neurosci. 2012;5:40. doi: 10.3389/fnmol.2012.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Layer PG. Cholinesterases during development of the avian nervous system. Cell Mol Neurobiol. 1991;11:7–33. doi: 10.1007/BF00712798. [DOI] [PubMed] [Google Scholar]
- 23.Ben Aziz-Aloya R, Sternfeld M, Soreq H. Promoter elements and alternative splicing in the human ACHE gene. Prog Brain Res. 1993;98:147–153. doi: 10.1016/s0079-6123(08)62392-4. [DOI] [PubMed] [Google Scholar]
- 24.Layer PG, Willbold E. Novel functions of cholinesterases in development, physiology and disease. Prog Histochem Cytochem. 1995;29:1–94. doi: 10.1016/s0079-6336(11)80046-x. [DOI] [PubMed] [Google Scholar]
- 25.Pick M, Perry C, Lapidot T, et al. Stress-induced cholinergic signaling promotes inflammation-associated thrombopoiesis. Blood. 2006;107:3397–3406. doi: 10.1182/blood-2005-08-3240. [DOI] [PubMed] [Google Scholar]
- 26.Sternfeld M, Shoham S, Klein O, et al. Excess “read-through” acetylcholinesterase attenuates but the “synaptic” variant intensifies neurodeterioration correlates. Proc Natl Acad Sci U S A. 2000;97:8647–8652. doi: 10.1073/pnas.140004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaked I, Meerson A, Wolf Y, et al. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity. 2009;31:965–973. doi: 10.1016/j.immuni.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Vidal CJ. Expression of cholinesterases in brain and non-brain tumours. Chem Biol Interact. 2005;157–158:227–232. doi: 10.1016/j.cbi.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 29.Martínez-López de Castro A, Nieto-Cerón S, Aurelio PC, et al. Cancer-associated differences in acetylcholinesterase activity in bronchial aspirates from patients with lung cancer. Clin Sci (Lond) 2008;115:245–253. doi: 10.1042/CS20070393. [DOI] [PubMed] [Google Scholar]
- 30.Zakut H, Ehrlich G, Ayalon A, et al. Acetylcholinesterase and butyrylcholinesterase genes coamplify in primary ovarian carcinomas. J Clin Invest. 1990;86:900–908. doi: 10.1172/JCI114791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiz-Espejo F, Cabezas-Herrera J, Illana J, Campoy FJ, Vidal CJ. Cholinesterase activity and acetylcholinesterase glycosylation are altered in human breast cancer. Breast Cancer Res Treat. 2002;72:11–22. doi: 10.1023/a:1014904701723. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Wang X, Wang T, et al. Acetylcholinesterase, a key prognostic predictor for hepatocellular carcinoma, suppresses cell growth and induces chemosensitization. Hepatology. 2011;53:493–503. doi: 10.1002/hep.24079. [DOI] [PubMed] [Google Scholar]
- 33.Muñoz-Delgado E, Montenegro MF, Campoy FJ, et al. Expression of cholinesterases in human kidney and its variation in renal cell carcinoma types. FEBS J. 2010;277:4519–4529. doi: 10.1111/j.1742-4658.2010.07861.x. [DOI] [PubMed] [Google Scholar]
- 34.Syed M, Fenoglio-Preiser C, Skau KA, Weber GF. Acetylcholinesterase supports anchorage independence in colon cancer. Clin Exp Metastasis. 2008;25:787–798. doi: 10.1007/s10585-008-9192-0. [DOI] [PubMed] [Google Scholar]
- 35.Jin QH, He HY, Shi YF, Lu H, Zhang XJ. Overexpression of acetylcholinesterase inhibited cell proliferation and promoted apoptosis in NRK cells. Acta Pharmacol Sin. 2004;25:1013–1021. [PubMed] [Google Scholar]
- 36.Mor I, Bruck T, Greenberg D, et al. Alternate AChE-R variants facilitate cellular metabolic activity and resistance to genotoxic stress through enolase and RACK1 interactions. Chem Biol Interact. 2008;175(1–3):11–21. doi: 10.1016/j.cbi.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 37.Cai L, Liao HF, Zhang XJ, Shao Y, Xu M, Yi JL. Acetylcholinesterase function in apoptotic retina pigment epithelial cells induced by H2O2. Int J Ophthalmol. 2013;6:772–777. doi: 10.3980/j.issn.2222-3959.2013.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuhrman B, Partoush A, Aviram M. Acetylcholine esterase protects LDL against oxidation. Biochem Biophys Res Commun. 2004;322:974–978. doi: 10.1016/j.bbrc.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Meshorer E, Toiber D, Zurel D, et al. Combinatorial complexity of 5′ alternative acetylcholinesterase transcripts and protein products. J Biol Chem. 2004;279:29740–29751. doi: 10.1074/jbc.M402752200. [DOI] [PubMed] [Google Scholar]
- 40.Park SE, Kim ND, Yoo YH. Acetylcholinesterase plays a pivotal role in apoptosome formation. Cancer Res. 2004;64:2652–2655. doi: 10.1158/0008-5472.can-04-0649. (Published erratum appears in 2004;: 9230) [DOI] [PubMed] [Google Scholar]
- 41.Ye X, Zhang C, Chen Y, Zhou T. Upregulation of acetylcholinesterase mediated by p53 contributes to cisplatin-induced apoptosis in human breast cancer cell. J Cancer. 2015;6:48–53. doi: 10.7150/jca.10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perry C, Sklan EH, Soreq H. CREB regulates AChE-R-induced proliferation of human glioblastoma cells. Neoplasia. 2004;6:279–286. doi: 10.1593/neo.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang H, Zhang XJ. Acetylcholinesterase and apoptosis. A novel perspective for an old enzyme. FEBS J. 2008;275:612–617. doi: 10.1111/j.1742-4658.2007.06236.x. [DOI] [PubMed] [Google Scholar]
- 44.Toiber D, Berson A, Greenberg D, Melamed-Book N, Diamant S, Soreq H. N-acetylcholinesterase-induced apoptosis in Alzheimer's disease. PLoS ONE. 2008;3(9):e3108. doi: 10.1371/journal.pone.0003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toiber D, Greenberg DS, Soreq H. Pro-apoptotic protein-protein interactions of the extended N-AChE terminus. J Neural Transm. 2009;116:1435–1442. doi: 10.1007/s00702-009-0249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masha'our RS, Heinrich R, Garzozi HJ, Perlman I. Acetylcholinesterase (AChE) is an important link in the apoptotic pathway induced by hyperglycemia in Y79 retinoblastoma cell line. Front Mol Neurosci. 2012;5:69. doi: 10.3389/fnmol.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martínez-Moreno P, Nieto-Cerón S, Torres-Lanzas J, et al. Cholinesterase activity of human lung tumours varies according to their histological classification. Carcinogenesis. 2006;27:429–436. doi: 10.1093/carcin/bgi250. [DOI] [PubMed] [Google Scholar]
- 48.Zanini D, Schmatz R, Pelinson LP, et al. Ectoenzymes and cholinesterase activity and biomarkers of oxidative stress in patients with lung cancer. Mol Cell Biochem. 2013;374:137–148. doi: 10.1007/s11010-012-1513-6. [DOI] [PubMed] [Google Scholar]
- 49.Pohanka M. Inhibitors of acetylcholinesterase and butyrylcholinesterase meet immunity. Int J Mol Sci. 2014;15:9809–9825. doi: 10.3390/ijms15069809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santos SC, Vala I, Miguel C, et al. Expression and subcellular localization of a novel nuclear acetylcholinesterase protein. J Biol Chem. 2007;282:25597–25603. doi: 10.1074/jbc.M700569200. [DOI] [PubMed] [Google Scholar]
- 51.Park SE, Jeong SH, Yee SB, et al. Interactions of acetylcholinesterase with caveolin-1 and subsequently with cytochrome c are required for apoptosome formation. Carcinogenesis. 2008;29:729–737. doi: 10.1093/carcin/bgn036. [DOI] [PubMed] [Google Scholar]
- 52.Perry C, Pick M, Podoly E, et al. Acetylcholinesterase/C terminal binding protein interactions modify Ikaros functions, causing T lymphopenia. Leukemia. 2007;21:1472–1480. doi: 10.1038/sj.leu.2404722. [DOI] [PubMed] [Google Scholar]
- 53.Carvajal FJ, Inestrosa NC. Interactions of AChE with Aβ aggregates in Alzheimer's brain: Therapeutic relevance of IDN 5706. Front Mol Neurosci. 2011;4:19. doi: 10.3389/fnmol.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Birikh KR, Sklan EH, Shoham S, Soreq H. Interaction of “readthrough” acetylcholinesterase with RACK1 and PKCbeta II correlates with intensified fear-induced conflict behavior. Proc Natl Acad Sci U S A. 2003;100:283–288. doi: 10.1073/pnas.0135647100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waiskopf N, Ofek K, Gilboa-Geffen A, et al. AChE and RACK1 promote the anti-inflammatory properties of fluoxetine. J Mol Neurosci. 2014;53:306–315. doi: 10.1007/s12031-013-0174-6. [DOI] [PubMed] [Google Scholar]
- 56.Deng R, Li W, Guan Z, et al. Acetylcholinesterase expression mediated by c-Jun-NH2-terminal kinase pathway during anticancer drug-induced apoptosis. Oncogene. 2006;25:7070–7077. doi: 10.1038/sj.onc.1209686. [DOI] [PubMed] [Google Scholar]
- 57.Kurinna SM, Tsao CC, Nica AF, Jiffar T, Ruvolo PP. Ceramide promotes apoptosis in lung cancer-derived A549 cells by a mechanism involving c-Jun NH2-terminal kinase. Cancer Res. 2004;64:7852–7856. doi: 10.1158/0008-5472.CAN-04-1552. [DOI] [PubMed] [Google Scholar]
- 58.Hamdi M, Kool J, Cornelissen-Steijer P, et al. DNA damage in transcribed genes induces apoptosis via the JNK pathway and the JNK-phosphatase MKP-1. Oncogene. 2005;24:7135–7144. doi: 10.1038/sj.onc.1208875. [DOI] [PubMed] [Google Scholar]
- 59.Khelifi AF, D'Alcontres MS, Salomoni P. Daxx is required for stress-induced cell death and JNK activation. Cell Death Differ. 2005;12:724–733. doi: 10.1038/sj.cdd.4401559. [DOI] [PubMed] [Google Scholar]
- 60.Cuadrado A, González L, Suárez Y, Martínez T, Muñoz A. JNK activation is critical for Aplidin-induced apoptosis. Oncogene. 2004;23:4673–4680. doi: 10.1038/sj.onc.1207636. [DOI] [PubMed] [Google Scholar]
- 61.Essers MA, Weijzen S, de Vries-Smits AM, et al. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23:4802–4812. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiang AC, Xie J, Zhang XJ. Acetylcholinesterase in intestinal cell differentiation involves G2/M cell cycle arrest. Cell Mol Life Sci. 2008;65:1768–1779. doi: 10.1007/s00018-008-8016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coleman BA, Taylor P. Regulation of acetylcholinesterase expression during neuronal differentiation. J Biol Chem. 1996;271:4410–4416. doi: 10.1074/jbc.271.8.4410. [DOI] [PubMed] [Google Scholar]
- 64.Bleau AM, Hambardzumyan D, Ozawa T, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.West KA, Brognard J, Clark AS, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu L, Zhang X, Zhang B, Wu J, Zhang X. Synaptic acetylcholinesterase targeted by microRNA-212 functions as a tumor suppressor in non-small cell lung cancer. Int J Biochem Cell Biol. 2013;45:2530–2540. doi: 10.1016/j.biocel.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 67.Pollak L, Hanoch T, Rabey MJ, Seger R. Infectious inflammation of the CNS involves activation of mitogen-activated protein kinase and AKT proteins in CSF in humans. Neurol Sci. 2005;26:324–329. doi: 10.1007/s10072-005-0504-8. [DOI] [PubMed] [Google Scholar]
- 68.Zhu H, Gao W, Jiang H, Wu J, Shi YF, Zhang XJ. Calcineurin mediates acetylcholinesterase expression during calcium ionophore A23187-induced HeLa cell apoptosis. Biochim Biophys Acta. 2007;1773:593–602. doi: 10.1016/j.bbamcr.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 69.Zhu H, Gao W, Jiang H, et al. Regulation of acetylcholinesterase expression by calcium signaling during calcium ionophore A23187- and thapsigargin-induced apoptosis. Int J Biochem Cell Biol. 2007;39:93–108. doi: 10.1016/j.biocel.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 70.Xu H, Shen Z, Xiao J, et al. Acetylcholinesterase overexpression mediated by oncolytic adenovirus exhibited potent anti-tumor effect. BMC Cancer. 2014;14:668. doi: 10.1186/1471-2407-14-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steinritz D, Emmler J, Hintz M, et al. Apoptosis in sulfur mustard treated A549 cell cultures. Life Sci. 2007;80:2199–2201. doi: 10.1016/j.lfs.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 72.Xiao R, Qiao JT, Zhao HF, et al. Sodium selenite induces apoptosis in cultured cortical neurons with special concomitant changes in expression of the apoptosis-related genes. Neurotoxicology. 2006;27:478–484. doi: 10.1016/j.neuro.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Layer PG, Weikert T, Alber R. Cholinesterases regulate neurite growth of chick nerve cells in vitro by means of a non-enzymatic mechanism. Cell Tissue Res. 1993;273:219–226. doi: 10.1007/BF00312823. [DOI] [PubMed] [Google Scholar]
- 74.Robitzki A, Mack A, Hoppe U, Chatonnet A, Layer PG. Butyrylcholinesterase antisense transfection increases apoptosis in differentiating retinal reaggregates of the chick embryo. J Neurochem. 1998;71:413–420. [PubMed] [Google Scholar]
- 75.Layer PG, Sporns O. Spatiotemporal relationship of embryonic cholinesterases with cell proliferation in chicken brain and eye. Proc Natl Acad Sci U S A. 1987;84:284–288. doi: 10.1073/pnas.84.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Layer PG, Alber R, Rathjen FG. Sequential activation of butyrylcholinesterase in rostral half somites and acetylcholinesterase in motoneurones and myotomes preceding growth of motor axons. Development. 1988;102:387–396. doi: 10.1242/dev.102.2.387. [DOI] [PubMed] [Google Scholar]
- 77.Bartha E, Szelényi JG, Hollán SR. Acetylcholinesterase (AchE) activity of lymphocytes in chronic lymphoid leukemia (CLL) Leuk Res. 1982;6:861–864. doi: 10.1016/0145-2126(82)90071-6. [DOI] [PubMed] [Google Scholar]
- 78.Stephenson J, Czepulkowski B, Hirst W, Mufti GJ. Deletion of the acetylcholinesterase locus at 7q22 associated with myelodysplastic syndromes (MDS) and acute myeloid leukaemia (AML) Leuk Res. 1996;20:235–241. doi: 10.1016/0145-2126(95)00146-8. [DOI] [PubMed] [Google Scholar]
- 79.Montenegro MF, Nieto-Cerón S, Ruiz-Espejo F, Páez de la Cadena M, Rodríguez-Berrocal FJ, Vidal CJ. Cholinesterase activity and enzyme components in healthy and cancerous human colorectal sections. Chem Biol Interact. 2005;157–158:429–430. doi: 10.1016/j.cbi.2005.10.091. [DOI] [PubMed] [Google Scholar]
- 80.Perry C, Sklan EH, Birikh K, et al. Complex regulation of acetylcholinesterase gene expression in human brain tumors. Oncogene. 2002;21:8428–8441. doi: 10.1038/sj.onc.1205945. [DOI] [PubMed] [Google Scholar]
- 81.Yokoi S, Yasui K, Iizasa T, Imoto I, Fukisawa T, Inazawa J. TERC identified as a probable target within the 3q26 amplicon that is detected frequently in non-small cell lung cancers. Clin Cancer Res. 2003;9:4705–4713. [PubMed] [Google Scholar]
- 82.Bernardi CC, Ribeiro Ede S, Cavalli IJ, Chautard-Freire-Maia EA, Souza RL. Amplification and deletion of the ACHE and BCHE cholinesterase genes in sporadic breast cancer. Cancer Genet Cytogenet. 2010;197:158–165. doi: 10.1016/j.cancergencyto.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 83.Song P, Sekhon HS, Fu XW, et al. Activated cholinergic signaling provides a target in squamous cell lung carcinoma. Cancer Res. 2008;68:4693–4700. doi: 10.1158/0008-5472.CAN-08-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qavi H, Al-Rajhi AA. Acetylcholinesterase and HHV-8 in squamous cell carcinoma and retinoblastoma. In Vivo. 2009;23:679–683. [PubMed] [Google Scholar]
- 85.Motamed-Khorasani A, Jurisica I, Letarte M, et al. Differentially androgen-modulated genes in ovarian epithelial cells from BRCA mutation carriers and control patients predict ovarian cancer survival and disease progression. Oncogene. 2007;26:198–214. doi: 10.1038/sj.onc.1209773. [DOI] [PubMed] [Google Scholar]
- 86.Nieto-Cerón S, Vargas-López H, Pérez-Albacete M, et al. Analysis of cholinesterases in human prostate and sperm: Implications in cancer and fertility. Chem Biol Interact. 2010;187:432–435. doi: 10.1016/j.cbi.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 87.Xi Q, Gao N, Zhang X, et al. A natural antisense transcript regulates acetylcholinesterase gene expression via epigenetic modification in hepatocellular carcinoma. Int J Biochem Cell Biol. 2014;55:242–251. doi: 10.1016/j.biocel.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 88.Yang K, Song Y, Tang YB, et al. mAChRs activation induces epithelial-mesenchymal transition on lung epithelial cells. BMC Pulm Med. 2014;14:53. doi: 10.1186/1471-2466-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ben-Ari S, Toiber D, Sas AS, Soreq H, Ben-Shaul Y. Modulated splicing-associated gene expression in P19 cells expressing distinct acetylcholinesterase splice variants. J Neurochem. 2006;97(Suppl. 1):24–34. doi: 10.1111/j.1471-4159.2006.03725.x. [DOI] [PubMed] [Google Scholar]