Abstract
Background
Leptomeningeal metastases (LM) from non-small cell lung cancer (NSCLC) are associated with poor prognosis and optimal treatment for this subgroup of NSCLC patients is controversial. The purpose of this study is to evaluate treatment options and prognostic factors of NSCLC patients with LM.
Methods
We retrospectively analyzed data of 108 patients who had been diagnosed with LM from NSCLC between May 2006 and August 2013.
Results
The median survival time (MST) of the 108 patients was 5.3 months, and the one-year survival rate was 23.7%. Forty-nine patients received whole brain radiotherapy (WBRT) and the MST of patients who received WBRT and those who did not were 6.4 and 4.3 months, respectively. Forty-two patients were treated with epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) after being diagnosed with LM. These patients had prolonged survival (11.1 vs. 4.4 months). Patients who received concomitant WBRT and EGFR-TKIs had the longest MST (12.3 months). Eastern Cooperative Oncology Group performance status, whether patients received WBRT, and/or EGFR-TKIs were independent prognostic factors for patients with LM from NSCLC.
Conclusion
WBRT, EGFR-TKIs or combined therapy, could lead to better clinical outcomes for NSCLC patients with LM. EGFR-TKIs plus WBRT has the potential to be the standard strategy for LM in NSCLC patients.
Keywords: EGFR-TKIs, leptomeningeal metastases, non- small cell lung cancer, prognostic factors, WBRT
Introduction
Lung cancer is still the most frequently diagnosed cancer, as well as the leading cause of cancer-related death globally.1 Leptomeningeal metastases (LM) occur in almost 5% of non-small cell lung cancer (NSCLC) patients.2 The median survival of patients without treatment is between four to six weeks.3 LM typically presents a devastating complication associated with poor survival and often represents a terminal event.4
The diagnosis of LM is based on clinical features, the detection of malignant cells in cerebrospinal fluid (CNF), and findings of magnetic resonance imaging (MRI).5 Once diagnosed, the prognosis for LM is poor. In clinical work, treatments for LM include systemic chemotherapy (SC), intrathecal chemotherapy (IC), whole brain radiotherapy (WBRT), and epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs). However, because of the lack of evidence from randomized trials, the optimal strategy for LM in NSCLC patients is controversial.
Previous studies have reported that performance status (PS), MRI-proven LM, age, the interval between diagnosis of primary tumor and LM, and EGFR mutation status are important prognostic factors in cancer patients with LM. In this study, we retrospectively analyze the efficacy of different treatments, in an attempt to determine prognostic factors in patients with LM and identify the optimal treatment for this subgroup of NSCLC patients.
Materials and methods
Patients and treatments
Between May 2006 and August 2013, 108 patients who had been diagnosed with LM from NSCLC were considered eligible and enrolled in this cohort. The inclusion criteria were as follows: (i) histology of NSCLC, confirmed by pathological or cytological examination; and (ii) gadolinium enhancement of MRI or the detection of malignant cells in cerebrospinal fluid, or both. Patients with other tumors were excluded. Medical records were reviewed to obtain clinical characteristics, such as age, gender, histological types, performance status according to the Eastern Cooperative Oncology Group (ECOG), and the interval between diagnosis of primary tumor and LM.
The options for treatment were based on patients’ age, ECOG PS, and organ function. All treatment modalities including SC, WBRT, EGFR-TKIs, and best supportive care, were evaluated and correlated with patients’ treatment outcome and survival time. Subgroup analyses were used to observe differences in prognostic factors and treatment efficacy.
Statistical analyses
Statistical analyses were conducted using SPSS software (version 13.0, SPSS Inc., Chicago, IL, USA). Overall survival (OS) was calculated from the date of diagnosis of LM to the date of death, or the last clinical follow-up. Survival curves were estimated using the Kaplan–Meier curve, and comparison of differences between groups using the log-rank test. Cox proportional hazard models, with confidence intervals (CIs) calculated at a 95% confidence level, were used for multivariate analysis to identify the association between prognostic factors and survival. P < 0.05 was considered to indicate statistical significance.
Results
Clinical characteristics
Table 1 summarizes the characteristics of the 108 patients, including 53 men and 55 women. All patients were diagnosed by pathological or cytological examination and enhanced MRI. Of the 108 patients, nine patients received lumbar puncture and two had malignant cells detected in their cerebrospinal fluid. The median age of the diagnosis of LM was 61 (range: 32-85); 43 patients were aged over 65 years (39.8%). The majority of patients had adenocarcinoma (78.7%), while the remaining patients had adenosquamous carcinoma (n = 9), squamous cell carcinoma (n = 7), large cell carcinoma (n = 3), alveolar cell carcinoma (n = 1) and undefined pathological type (n = 3). Twenty-one patients (19.3%) had a poor ECOG PS (≥ 3). LM was detected in 44 patients (40.7%) at the initial diagnosis of NSCLC; in 34 patients (31.5%) during the interval between diagnosis of primary tumor and LM, which was less than 12 months; and in the remaining 30 patients (27.8%) after 12 months (Table 1). In addition, the EGFR mutation status was evaluated in 17 patients. Of these 17 cases, 11 patients harbored EGFR mutations: four had exon 19 deletions and seven had a mutation in exon 21.
Table 1.
Clinical characteristics of the 108 patients
| Characteristics | No. of Patients (%) |
|---|---|
| Age (years) | |
| <65 | 65 (60.2%) |
| ≥65 | 43 (39.8%) |
| Gender | |
| Male | 53 (49.1%) |
| Female | 55 (50.9%) |
| Histology | |
| Adenocarcinoma | 85 (78.7%) |
| Non adenocarcinoma | 23 (21.3%) |
| ECOG PS | |
| 1–2 | 87 (80.7%) |
| 3–4 | 21 (19.3%) |
| Time between NSCLC and LM | |
| ≤12 months | 78 (72.2%) |
| >12 months | 30 (27.8%) |
ECOG PS, Eastern Cooperative Oncology Group performance status
LM, leptomeningeal metastases; NSCLC, non-small cell lung cancer.
Clinical treatments
Of the 108 patients, 42 (38.9%) received either Gefitinib (n = 19 250 mg/d) or Erlotinib (n = 23 150 mg/d). A total of 49 (45.4%) patients received WBRT (30Gy/10fx) and 59 (54.6) received SC (27 received a platinum-based double drug regimen and 32 received second/third line chemotherapy regimens). Twelve (11.1%) received best supportive care only. Additional details are shown in Table 2.
Table 2.
Treatment characteristics
| Characteristics | No. of Patients (%) | ||
|---|---|---|---|
| WBRT (n = 49) | SC (n = 59) | EGFR-TKIs (n = 42) | |
| Age (years) | |||
| <65 | 30 | 34 | 26 |
| ≥65 | 19 | 25 | 16 |
| Gender | |||
| Male | 25 | 32 | 17 |
| Female | 24 | 27 | 25 |
| Histology | |||
| Adenocarcinoma | 36 | 45 | 36 |
| Non adenocarcinoma | 13 | 14 | 6 |
| ECOG PS | |||
| 1–2 | 44 | 59 | 34 |
| 3–4 | 5 | 0 | 8 |
| Time between NSCLC and LM | |||
| ≤12 months | 39 | 48 | 29 |
| >12 months | 10 | 11 | 13 |
ECOG PS, Eastern Cooperative Oncology Group performance status
EGFR-TKIs, epidermal growth factor receptor tyrosine kinase inhibitor
LM, leptomeningeal metastases
NSCLC, non-small cell lung cancer
SC, systemic chemotherapy
WBRT, whole brain radiotherapy.
Median survival time of leptomeningeal metastases (LM)
The median survival time (MST) of the 108 patients, from the time of first diagnosis of LM, was 5.3 months, and the one-year survival rate was 23.7% (Fig 1).
Figure 1.
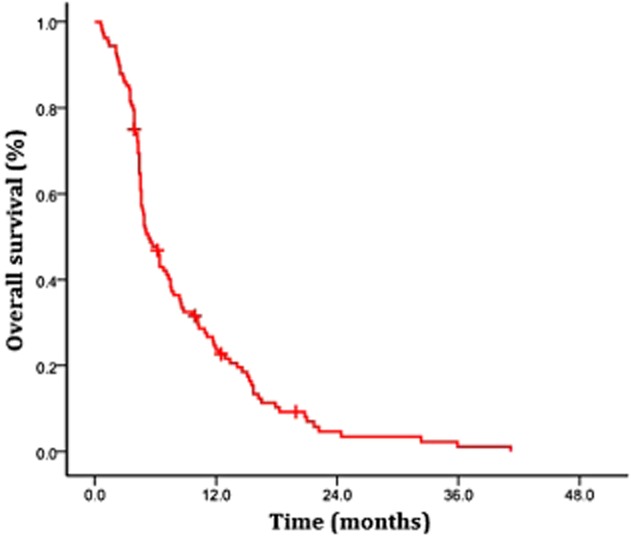
Overall survival for entire cohort.  , overall population (n = 108).
, overall population (n = 108).
Table 3 summarizes the prognostic factors analyzed by univariate analysis. Gender, age, histology type, ECOG PS, and the time between NSCLC and LM were significant factors. MSTs were 4.7 months for men and 6.4 months for women (P = 0.532). MSTs for patients younger than 65 years were 5.7 months and 4.9 months for patients older than 65 years (P = 0.494). MSTs for adenocarcinoma patients were 6.3 months and 4.6 months for non-adenocarcinoma patients (P = 0.879). MSTs in patients with an ECOG PS of 1–2 were longer compared to patients with an ECOG PS of 3–4 (6.8 vs. 2.8 months, P < 0.001). MSTs in patients with an interval between NSCLC and LM>12 months were longer than patients with an interval between NSCLC and LM≤12 months (7.5 vs. 4.9 months, P = 0.281). In conclusion, only ECOG PS had a positive impact on the MST of LM.
Table 3.
Median survival time for LM
| Characteristics | Median Survival (months) | P-value |
|---|---|---|
| Age (years) | ||
| <65 | 5.7 | 0.494 |
| ≥65 | 4.9 | |
| Gender | ||
| Male | 4.7 | 0.532 |
| Female | 6.4 | |
| Histology | ||
| Adenocarcinoma | 6.3 | 0.879 |
| Non adenocarcinoma | 4.6 | |
| ECOG PS | ||
| 1–2 | 6.8 | 0.001 |
| 3–4 | 2.8 | |
| Time between NSCLC and LM | ||
| ≤12 months | 4.9 | 0.281 |
| >12 months | 7.5 |
ECOG PS, Eastern Cooperative Oncology Group performance status
LM, leptomeningeal metastases; NSCLC, non-small cell lung cancer.
Survival time for patients with different treatments
Forty-nine patients received WBRT and the MSTs of patients who had received WBRT and those who did not were 6.4 and 4.3 months, respectively (P = 0.022, Fig 2a). There was no significant difference between the MSTs of patients who had received SC and those who did not (4.7 vs. 6.5 months, P = 0.590, Fig 2b). Forty-two patients underwent EGFR-TKIs after being diagnosed with LM. These patients had prolonged survival (11.1 vs. 4.4 months, P < 0.01, Fig 2c). Furthermore, patients who received concomitant WBRT and EGFR-TKIs had the longest MST among all of the patients (12.3 months). MST was not prolonged in patients treated with EGFR-TKIs combined with SC or with SC and WBRT, compared with those treated by EGFR-TKIs alone (Table 4).
Figure 2.
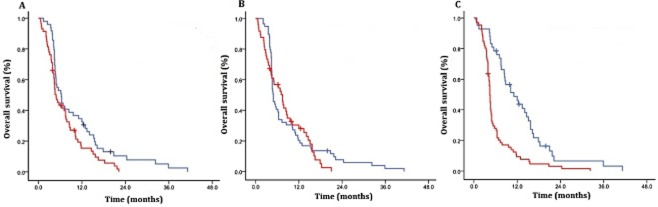
(a) Survival of patients who received whole brain radiotherapy (WBRT) compared with those who did not in entire cohort. (b) Survival of patients who received systemic chemotherapy compared with those who did not. (c) Survival of patients who received epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) compared with those who did not. (a)  , WBRT (N = 49);
, WBRT (N = 49);  , No WBRT (N = 59) P = 0.037; (b)
, No WBRT (N = 59) P = 0.037; (b)  , Chemotherapy (n = 59);
, Chemotherapy (n = 59);  , No Chemotherapy (n = 49) P = 0.59 (c)
, No Chemotherapy (n = 49) P = 0.59 (c)  , EGFR-TKIs (n = 42);
, EGFR-TKIs (n = 42);  , No EGFR-TKIs (n = 66) P = 0.001.
, No EGFR-TKIs (n = 66) P = 0.001.
Table 4.
Survival time for patients with different treatments
| Treatment | No. of Patients (%) | Median Survival (months) |
|---|---|---|
| WBRT | 49 (45.4%) | 6.4 |
| SC | 59 (54.6%) | 4.7 |
| EGFR-TKIs | 42 (38.9%) | 11.1 |
| WBRT+SC | 32 (29.6%) | 5.2 |
| WBRT+EGFR-TKIs | 19 (17.6%) | 12.3 |
| SC+EGFR-TKIs | 13 (12.0%) | 11.1 |
| WBRT+SC+EGFR-TKIs | 9 (8.3%) | 11.1 |
| BSC | 12 (11.1%) | 2.4 |
BSC, best support care
EGFR-TKIs, epidermal growth factor receptor tyrosine kinase inhibitors
SC, systemic chemotherapy
WBRT, whole brain radiotherapy.
Multivariate analysis of prognostic factors
In multivariate analysis, the Cox regression model was used to evaluate which factors could contribute to patients’ prognosis. In this study, we found that ECOG PS, whether patients received WBRT, and whether patients received EGFR-TKIs had a significant effect on patient prognosis, while gender, age, histological type, whether patients’ received SC, and the interval between NSCLC and LM had no significant influence on patients’ prognosis. More details are presented in Table 5.
Table 5.
Multivariate analysis of prognostic factors
| Predictor factor | RR | 95% CI of RR | P value |
|---|---|---|---|
| Age | 0.986 | 0.965–1.007 | 0.188 |
| Gender | 1.040 | 0.683–1.585 | 0.854 |
| Histology | 1.048 | 0.604–1.818 | 0.867 |
| ECOG PS | 0.210 | 0.111–0.399 | 0.000 |
| Time between NSCLC and LM | 0.808 | 0.596–1.097 | 0.172 |
| WBRT | 0.588 | 0.375–0.920 | 0.020 |
| SC | 0.842 | 0.574–1.392 | 0.273 |
| EGFR-TKIs | 0.215 | 0.125–0.371 | 0.000 |
ECOG PS, Eastern Cooperative Oncology Group performance status
EGFR-TKIs, epidermal growth factor receptor tyrosine kinase inhibitors
LM, leptomeningeal metastases
NSCLC, non-small cell lung cancer
RR, relative risk
SC, systemic chemotherapy
WBRT, whole brain radiotherapy.
Discussion
LM is a devastating complication of NSCLC, and although treatment methods for LM have improved in the last decades, prognosis is still poor. Even with active treatments, such as SC, IC, and WBRT, the MST of LM is only eight to 16 months.6 Few randomized trials have proved the survival benefit of a specific treatment modality, and some results of previous reports are contradictory, thus, optimal treatment modalities for LM patients are still poorly defined.
SC remains the primary treatment for NSCLC patients; however, the efficacy of SC in LM patients is controversial. Park et al. analyzed 50 NSCLC patients who were diagnosed with LM and found that the patients who received SC had improved survival compared to those who did not receive SC (11.5 vs. 2.1 months).7 Oechsle et al. similarly reported that LM patients who received SC had a longer survival time. They proposed that the significant impact of SC was that it not only treated LM, but also all other extra leptomeningeal tumor lesions.8 However, SC is not always the optimum treatment, as demonstrated by another study in LM patients, which suggested that adding SC to IC or radiotherapy would not improve patients’ survival time.9 Our data showed that SC was not a significant prognostic factor for LM patients. We attributed the failure of SC to the presence of the blood-brain barrier in the central nervous system, which resulted in limited concentration of chemotherapeutic drugs in cerebrospinal fluid.
WBRT is usually used as the first treatment choice for diffuse-type brain metastases. Reports have demonstrated that NSCLC patients with LM would benefit from WBRT.7,10 Nevertheless, WBRT is not always effective. For example, in a retrospective study by Morris et al., there was no significant difference in MST between patients who received WBRT and those who did not.11 In this study, patients who were treated by WBRT had a longer survival. After the failure of WBRT, further analysis determined that EGFR-TKIs were a viable alternative for LM patients.
EGFR-TKIs have been widely used for the treatment of NSCLC patients in clinical work. The clinical benefit of EGFR-TKIs is related to the activation of mutations in exons 19 or 21 of the EGFR gene.12,13 Several reports have indicated that EGFR-TKIs are the key factor to improve survival of NSCLC patients with LM. A case report of a 40-year-old NSCLC patient with carcinomatous meningitis showed that his neurological symptoms disappeared within two weeks after gefitinib treatment.14 A phaseIIstudy of 48 patients reported an MST of patients treated with erlotinib of 18.9 months.15 Katayama et al. found that low-dose (150 mg/d) erlotinib had a similar efficacy to high-dose (1250 mg/d) gefitinib for the treatment of NSCLC patients with LM.16 Masuda et al. also proved that erlotinib therapy could improve the clinical symptoms of LM patients with EGFR mutations.17 In this study, we found that when NSCLC patients with LM were treated with EGFR-TKIs (erlotinib or gefitinib), MST was prolonged up to 11.1 months. In addition, while there were only 11 patients harbouring EGFR mutations, 42 patients received EGFR-TKI treatment, and results indicated that patients without EGFR mutations could benefit from EGFR-TKI treatment.
Recent reports have suggested that EGFR-TKIs plus WBRT therapy may be a more effective treatment for NSCLC patients with LM. A phase II trial of erlotinib plus WBRT therapy in a patient with brain metastases from NSCLC indicated that MST was improved to 11.8 months, and patients with EGFR mutations had a longer MST compared to those with wild-type EGFR.18 Our data, along with others’ evidence, strongly demonstrates that EGFR-TKIs plus WBRT could be considered as the first choice for NSCLC patients with LM. One reason for the greater effectiveness of EGFR-TKIs plus WBRT is that WBRT can injure the blood-brain barrier, thereby increasing the concentration of EGFR-TKIs in cerebrospinal fluid.
Several studies have examined the impact on prognosis of NSCLC patients with LM. Waki et al. reported that poor PS and MRI-proven LM were associated with a poor prognosis.19 Oechsle et al. demonstrated that age >50, PS ≤ 70%, an interval between diagnosis of primary tumor and LM≤12 months all presented negative prognostic factors.8 LEELLee et al. reported that mutant EGFR in NSCLC patients was an independent prognostic factor for a better treatment response.20
Our study analyzed varieties of prognostic factors of NSCLC patients with LM. ECOG PS and whether patients received WBRT and/or EGFR-TKIs were independent prognostic factors for NSCLC patients with LM, while gender, age, histological type, SC, and the interval between NSCLC and LM had no significant influence on patients’ prognosis.
Conclusion
In conclusion, WBRT, EGFR-TKIs, or combined therapy could lead to better clinical outcomes for NSCLC patients with LM. EGFR-TKIs plus WBRT has the potential to become the standard strategy for NSCLC patients with LM.
Disclosure
No authors report any conflict of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. (Published erratum appears in 2011;: 134.) [DOI] [PubMed] [Google Scholar]
- 2.Choong NW, Dietrich S, Seiwert TY, et al. Gefitinib response of erlotinib-refractory lung cancer involving meninges – role of EGFR mutation. Nat Clin Pract Oncol. 2006;3:50–57. doi: 10.1038/ncponc0400. [DOI] [PubMed] [Google Scholar]
- 3.Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;25:103–119. doi: 10.1053/ctrv.1999.0119. [DOI] [PubMed] [Google Scholar]
- 4.Omuro AM, Kris MG, Miller VA, et al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer. 2005;103:2344–2348. doi: 10.1002/cncr.21033. [DOI] [PubMed] [Google Scholar]
- 5.Herrlinger U, Förschler H, Küker W, et al. Leptomeningeal metastasis: survival and prognostic factors in 155 patients. J Neurol Sci. 2004;223:167–178. doi: 10.1016/j.jns.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Groves MD. New strategies in the management of leptomeningeal metastases. Arch Neurol. 2010;67:305–312. doi: 10.1001/archneurol.2010.18. [DOI] [PubMed] [Google Scholar]
- 7.Park JH, Kim YJ, Lee JO, et al. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer. 2012;76:387–392. doi: 10.1016/j.lungcan.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Oechsle K, Lange-Brock V, Kruell A, Bokemeyer C, de Wit M. Prognostic factors and treatment options in patients with leptomeningeal metastases of different primary tumors: a retrospective analysis. J Cancer Res Clin Oncol. 2010;136:1729–1735. doi: 10.1007/s00432-010-0831-x. [DOI] [PubMed] [Google Scholar]
- 9.Chamberlain MC, Kormanik P. Carcinoma meningitis secondary to non-small cell lung cancer: combined modality therapy. Arch Neurol. 1998;55:506–512. doi: 10.1001/archneur.55.4.506. [DOI] [PubMed] [Google Scholar]
- 10.Verger E, Gil M, Yaya R, et al. Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: a phase II randomized trial. Int J Radiat Oncol Biol Phys. 2005;61:185–191. doi: 10.1016/j.ijrobp.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 11.Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7:382–385. doi: 10.1097/JTO.0b013e3182398e4f. [DOI] [PubMed] [Google Scholar]
- 12.Porta R, Sánchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 2011;37:624–631. doi: 10.1183/09031936.00195609. [DOI] [PubMed] [Google Scholar]
- 13.Sekine A, Kato T, Hagiwara E, et al. Metastatic brain tumors from non-small cell lung cancer with EGFR mutations: distinguishing influence of exon 19 deletion on radiographic features. Lung Cancer. 2012;77:64–69. doi: 10.1016/j.lungcan.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Sakai M, Ishikawa S, Ito H, et al. Carcinomatous meningitis from non-small-cell lung cancer responding to gefitinib. Int J Clin Oncol. 2006;11:243–245. doi: 10.1007/s10147-005-0558-x. [DOI] [PubMed] [Google Scholar]
- 15.Li T. Erlotinib as the second-line treatment for NSCLC patients of stage IIIb/IV.] Zhongguo Fei Ai Za Zhi. 2009;12:1297–1300. doi: 10.3779/j.issn.1009-3419.2009.12.15. (In Chinese.) [DOI] [PubMed] [Google Scholar]
- 16.Katayama T, Shimizu J, Suda K, et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol. 2009;4:1415–1419. doi: 10.1097/JTO.0b013e3181b62572. [DOI] [PubMed] [Google Scholar]
- 17.Masuda T, Hattori N, Hamada A, et al. Erlotinib efficacy and cerebrospinal fluid concentration in patients with lung adenocarcinoma developing leptomeningeal metastases during gefitinib therapy. Cancer Chemother Pharmacol. 2011;67:1465–1469. doi: 10.1007/s00280-011-1555-6. [DOI] [PubMed] [Google Scholar]
- 18.Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31:895–902. doi: 10.1200/JCO.2011.40.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waki F, Ando M, Takashima A, et al. Prognostic factors and clinical outcomes in patients with leptomeningeal metastasis from solid tumors. J Neurooncol. 2009;93:205–212. doi: 10.1007/s11060-008-9758-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee HL, Chung TS, Ting LL, et al. EGFR mutations are associated with favorable intracranial response and progression-free survival following brain irradiation in non-small cell lung cancer patients with brain metastases. Radiat Oncol. 2012;7:181. doi: 10.1186/1748-717X-7-181. [DOI] [PMC free article] [PubMed] [Google Scholar]


