Abstract
Background
To explore the molecular mechanisms of the anti-cancer effect of curcumin in human lung squamous cell carcinoma (LSQCC) SK-MES-1 cells.
Methods
Cell viability was determined using MTT assay. Ribonucleic acid sequencing was performed to measure expression levels of transcripts in LSQCC cells treated with 15 μmol/L curcumin (treatment groups) or an equal amount of dimethylsulfoxide (control). Cuffdiff software was used to identify differentially expressed genes (DEGs) in treatment groups, followed by enrichment analysis of DEGs using the Database for Annotation, Visualization and Integration Discovery. The protein-protein interaction (PPI) networks for up and downregulated DEGs were constructed by Cytoscape software using Search Tool for the Retrieval of Interacting Genes data to identify hub nodes.
Results
Curcumin significantly reduced cell viability in LSQCC cells. In total, 380 DEGs including 154 upregulated and 126 downregulated genes were found in the treatment groups. The upregulated genes were enriched in base excision repair (BER, such as PCNA, POLL, and MUTYH) and Janus kinase-signal transducer and activator of transcription (JAT-STAT) signaling pathways (such as AKT1 and STAT5A), while the downregulated genes were enriched in nine pathways, including the vascular endothelial growth factor (VEGF) signaling pathway (such as PTK2, VEGFA, MAPK1, and MAPK14) and mitogen-activated protein kinase (MAPK) signaling pathway (ARRB2, MAPK1, MAPK14, and NFKB1). PCNA and AKT1 were the hub nodes in the PPI network of upregulated genes while MAPK1, MAPK14, VEGFA, and NFKB1 were the hub nodes in the PPI network of downregulated genes.
Conclusions
Curcumin might exert anti-cancer effects on LSQCC via regulating BER, JAT-STAT, VEGF, and MAPK signaling pathways.
Keywords: Curcumin, enrichment analysis, lung squamous cell carcinoma, protein-protein interaction, RNA-sequencing
Introduction
Lung squamous cell carcinoma (LSQCC) is a common type of non-small cell lung cancer (NSCLC), accounting for 85% of all lung cancers and causing approximately 400 000 deaths per year globally.1,2 Similar to other cancers, LSQCC is characterized by genetic mutations on a molecular level, which probably lead to the dysregulation of various signaling pathways.3 The combined effect of gene mutations in the base excision repair (BER) pathway may cause a defect in the repair of DNA damage and, thus, contribute to lung carcinogenesis.4 The aberration of Janus kinase-signal transducer and activator of transcription (JAK-STAT) regulating cell growth, differentiation, and metabolism pathways also leads to carcinogenic potential in NSCLC cells.5 The abnormal alterations reveal the changes in cell proliferation and apoptotic signals, as well as the potential of uncontrolled replication in lung cancer progression, which help develop methods for cancer therapy. Although great progress has been made in developing chemotherapy and radiation therapy methods, there is still an urgent need for therapeutic methods with fewer side effects.
Curcumin, a natural polyphenol extracted from turmeric (Curcuma longa), has been safely demonstrated to function as an antitumor, antioxidant, and anti-inflammatory substance.6 Among its various functional properties, such as cell cycle interference, apoptosis induction, and the inhibition of infiltrative potential in cancer cells, the anti-carcinogenic property, in particular, attracts a great deal of interest.7,8 The JAK-STAT signaling pathway is one of the molecular mechanisms by which curcumin suppresses invasion and migration in small cell lung cancer (SCLC) cells.7 Curcumin could also suppress cell proliferation by inducing the expression of forkhead box protein O1 in lung cancer.9 Herein, curcumin was speculated to exert an anti-cancer effect in LSQCC cells, the potential molecular mechanisms of which are clarified in this study.
Ribonucleic acid sequencing (RNA-seq), a recently developed method for transcriptome profiling by deep-sequencing technology, provides more precise measurement of expression levels of transcripts than other methods.10 In the present study, RNA-seq was performed to identify differentially expressed genes (DEGs) in SK-MES-1 cells (human LSQCC cell line) with curcumin treatment compared with cells without curcumin treatment. The involved biological pathways and protein-protein interaction (PPI) of DEGs were further investigated in an attempt to interpret the molecular mechanisms by which curcumin may exert an anti-cancer effect in SK-MES-1 cells.
Materials and methods
Cell culture
Human LSQCC cell lines SK-MES-1 were originally obtained from the Chinese Academy of Science. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM, GBICO Co. Ltd, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (FBS, GBICO) and 1% penicillin-streptomycin solution (GBICO). The cells were incubated in a 37°C incubator with humidified atmosphere of 5% CO2 (Thermo Fisher Scientific Inc., Rockford, IL, USA). Immediately after recovery, the cells were replanted in fresh medium, cultured to form colonies, and harvested in a solution of trypsin at the logarithmic growth phase for the following experiments.
Cell viability assay
Cell proliferation was determined using the conversion of MTT to formazan via mitochondrial oxidation using MTT assay kits (Shanghai Sangon Biological Engineering Technology and Service Corporation Ltd, Shanghai, China). SK-MES-1 cells were seeded in 96-well plates with an average of 5 × 106 cells/plate. Cells were incubated overnight and then treated in sextuple with 0, 5, 15, and 30 μmol/L curcumin (Sigma-Aldrich, St. Louis, Missouri, USA) for 48 hours. After treatment with curcumin for 48 hours, the medium was added with 10 μL MTT and incubated for four hours. The MTT solution was then removed from the wells and formazan crystals were dissolved in 100 μL dimethylsulfoxide (DMSO, Sigma-Aldrich). The optical density was detected by a microplate reader (Thermo Fisher Scientific Inc.) at a wavelength of 570 nm.
Cell treatment and ribonucleic acid sequencing (RNA-seq)
SK-MES-1 cells at the logarithmic growth phase were treated with 15 μmol/L curcumin dissolved in 1% DMSO in treatment groups, while in control groups cells were treated with an equal amount of DMSO for 48 hours at 37°C. After the treatment period, attached cells were harvested for RNA extraction following the manufacturer's protocol using an RNeasy kit (Invitrogen, Carlsbad, CA, USA). RNA was properly tested for quality on 2% agarose gel electrophoresis and spectrophotometer before RNA-seq was conducted using an Illumina HiSeq 2000 Genome Analyzer (San Diego, CA, USA).
RNA libraries were prepared according to the manufacturer's recommended protocol for the NEBNext Ultra RNA Library Prep Kit for Illumina (NEB E7530). Total RNA (5 μg) for the two biological replicates from the control and treatment cell populations were sheared into fragments (200 nt) and transcribed to cDNA. The cDNA were then blunt-ended and phosphorylated, and a single 3′ adenosine moiety and Illumina adapters were added to the repaired ends. The cDNA were then amplified by 12 cycles of polymerase chain reaction with New England BioLabs (NEB) Phusion polymerase (Ipswich, MA, USA) and purified to obtain RNA libraries. RNA-seq was performed using paired-end, 100 base pair reads by Hiseq 2500 v4 100PE (Illumina).
RNA-seq data pro-processing
The reads containing either N over 3% or low quality bases (<18) over 50% of the full length in any end were filtered in data processing. TopHat2 (version 2.0.8: http://tophat.cbcb.umd.edu/) was used to align the RNA-seq reads against the reference genome hg19 with default parameters, allowing for variable-length indels with respect to the reference genome.11 Two sets of matched RNA-seq data of SK-MES-1 cells with and without curcumin treatment were included in this study.
Differentially expressed gene (DEG) screening based on RNA-seq data
The expression level of each gene was quantified by fragments per kilobase of exon model per million fragments mapped (FPKM) by employing Cuffdiff software (version 2.1.0: http://cole-trapnell-lab.github.io/cufflinks/), followed by DEG screening with the criteria of |log2 fold change (FC)| >1 and P-value <0.05.12,13
Function and pathway enrichment analysis of DEGs
To further understand the underlying functions and pathways of DEGs, enrichment analysis was performed using the Database for Annotation, Visualization and Integration Discovery (DAVID).14 In this study, both the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway15 and Gene Ontology (GO) function enrichment16 analysis were performed by DAVID with a P value of less than 0.05, including at least two DEGs in each enriched term.
Protein-protein interaction (PPI) network construction
Highly complex cellular biological processes are the results of tightly regulated interactions of genes with similar functions. Therefore, PPI pairs with the combined score of >0.4 DEGs were screened according to the Search Tool for the Retrieval of Interacting Genes.17 The PPI network was constructed using Cytoscape software to identify hub nodes that regulate or influence other genes and represent vital biological essentiality in metabolic networks.18
Statistical analysis
SPSS 13.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Data were expressed as mean ± standard deviation. Student's t tests were used to determine the significance between groups. A P-value of <0.05 was considered to indicate a statistically significant difference.
Results
Curcumin induced apoptosis of SK-MES-1 cells
To establish the optimal conditions for curcumin-induced apoptosis of SK-MES-1 cells in vitro, SK-MES-1 cells were incubated with 0, 5, 15, and 30 μmol/L curcumin. After 48 hours of incubation, the SK-MES-1 cells treated with 5, 15, and 30 μmol/L curcumin exhibited significantly reduced levels of cell viability (P < 0.05), compared with the control groups (Fig 1). These data indicated that curcumin treatment induced cell growth inhibition in a concentration-dependent manner in the human SK-MES-1 cell line. The 15 μmol/L curcumin treatment induced the most significantly reduced cell viability of SK-MES-1 cells.
Figure 1.
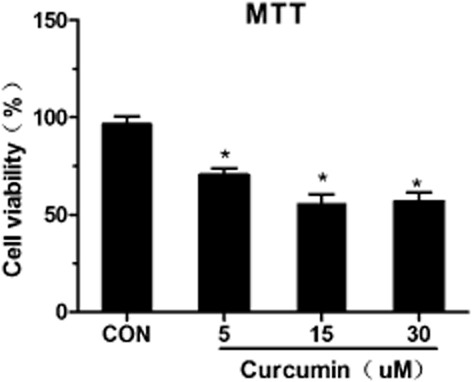
Effect of curcumin on cell viability. SK-MES-1 cells were treated with different concentrations of curcumin for 48 hours. Proliferation rates were calculated as [(ODtreated/ODcontrol) × 100%]. Experiments were performed six times. Values represent mean (± standard deviation) cell viability as a percentage of untreated control samples. Con, control.
Curcumin altered gene expression signature in SK-MES-1 cells
To identify the underlying molecular mechanisms of curcumin in SK-MES-1 cells, we applied transcriptome sequencing for SK-MES-1 cells with and without curcumin treatment. Accordingly, a total of 380 DEGs including 154 upregulated genes and 126 downregulated genes were identified in the treatment groups compared with the control groups. The DEGs are shown in the scatterplot (Fig 2).
Figure 2.
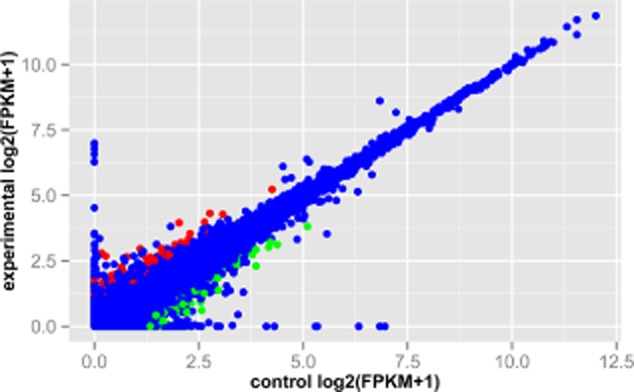
The scatterplot of gene expression levels in human squamous cell lung carcinoma SK-MES-1 cells with and without curcumin treatment. The red represents upregulated genes in SK-MES-1 cells with curcumin treatment compared with the cells without curcumin treatment; green represents downregulated genes in the treatment groups; blue represents the genes that were not significantly differentially expressed. The X-axis represents the control groups and the Y-axis represents the treatment groups.
Kyoto Encyclopedia of Genes and Genomes and Gene Ontology enrichment analysis
KEGG enrichment analysis revealed the upregulated genes were enriched in the BER (P value = 3.48E-03; such as polymerase [POLL] and poly ADP-ribose [PARP3] polymerase family) and JAT-STAT signaling pathways (P value = 4.70E-02; such as v-akt murine thymoma viral oncogene homolog 1 [AKT1] and signal transducer and activator of transcription 5A [STAT5A]). Meanwhile the downregulated genes were enriched in nine KEGG pathways, including the vascular endothelial growth factor (VEGF) (P value = 8.95E-04; such as protein tyrosine kinase 2 [PTK2], mitogen-activated protein kinase 1 [MAPK1], MAPK14, and VEGFA), and MAPK signaling pathways (P value = 1.86E-02; such as β-arrestin2 [ARRB2], MAPK1, MAPK14, and nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 [NFKB1]) (Table 1).
Table 1.
The significantly enriched Kyoto Encyclopedia of Genes and Genomes pathways of differentially expressed genes in the treatment groups compared with the control groups
| Term | Count | P-value | Genes |
|---|---|---|---|
| Upregulated genes | |||
| hsa03410:Base excision repair | 4 | 3.48E-03 | POLL, MUTYH, PCNA, PARP3 |
| hsa04630:Jak-STAT signaling pathway | 5 | 4.70E-02 | AKT1, CLCF1, STAT5A, IL15RA, STAT2 |
| Downregulated genes | |||
| hsa04150:mTOR signaling pathway | 5 | 2.19E-04 | MAPK1, HIF1A, VEGFA, PRKAA1, MLST8 |
| hsa04370:VEGF signaling pathway | 5 | 8.95E-04 | MAPK1, PTK2, MAPK14, VEGFA, PPP3CC |
| hsa05412:Arrhythmogenic right ventricular cardiomyopathy (ARVC) | 4 | 9.75E-03 | DMD, CACNG6, DAG1, TCF7L2 |
| hsa05410:Hypertrophic cardiomyopathy (HCM) | 4 | 1.32E-02 | DMD, CACNG6, DAG1, PRKAA1 |
| hsa04010:MAPK signaling pathway | 6 | 1.86E-02 | MAPK1, ARRB2, MAPK14, CACNG6, PPP3CC, NFKB1 |
| hsa04660:T cell receptor signaling pathway | 4 | 2.50E-02 | MAPK1, MAPK14, PPP3CC, NFKB1 |
| hsa04722:Neurotrophin signaling pathway | 4 | 3.56E-02 | MAPK1, MAPK14, NFKB1, SH2B1 |
| hsa05200:Pathways in cancer | 6 | 4.07E-02 | MAPK1, PTK2, HIF1A, VEGFA, NFKB1, TCF7L2 |
| hsa05221:Acute myeloid leukemia | 3 | 4.54E-02 | MAPK1, NFKB1, TCF7L2 |
Count, the number of genes enriched on the term.
AKT1, v-akt murine thymoma viral oncogene homolog 1 (ID: 207)
ARRB2, arrestin, beta 2 (ID: 409)
CACNG6, calcium channel, voltage-dependent, gamma subunit 6 (ID: 59285)
CLCF1, cardiotrophin-like cytokine factor 1 (ID: 23529)
DAG1, dystroglycan 1 (ID: 1605)
DMD, dystrophin, muscular dystrophy (ID: 1756)
HIF1A, hypoxia inducible factor 1, alpha subunit (ID: 3091)
IL15RA, interleukin 15 receptor, alpha chain (ID: 3601)
MAPK1, mitogen-activated protein kinase 1 (ID: 5594)
MAPK14, mitogen-activated protein kinase 14 (ID: 1432)
MLST8, MTOR associated protein, LST8 homolog (ID: 64223)
MUTYH, mutY homolog (ID: 4595)
NFKB1, nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 (ID: 4790)
PARP3, poly (ADP-ribose) polymerase family, member 3 (ID: 10039)
PCNA, proliferating cell nuclear antigen (ID: 5111)
POLL, polymerase (DNA directed), lambda (ID: 27343)
PRKAA1, protein kinase, AMP-activated, alpha 1 catalytic subunit (ID: 5562)
PTK2, protein tyrosine kinase 2 (ID: 5747)
PPP3CC, protein phosphatase 3, catalytic subunit, gamma isozyme (ID: 5533)
STAT2, signal transducer and activator of transcription 2, 113 kDa (ID: 6773)
STAT5A, signal transducer and activator of transcription 5A (ID: 6776)
SH2B1, SH2B adaptor protein 1 (ID: 25970)
TCF7L2, transcription factor 7-like 2 (ID: 6934)
VEGFA, vascular endothelial growth factor A (ID: 7422).
GO enrichment analysis showed the upregulated genes were associated with biological processes, such as cellular response to hormone stimulus (P value = 7.49E-04; such as AKT1 and STAT5A) (Table 2). On the other hand, the downregulated genes were related to biological processes including positive regulation of macromolecule metabolic process (P value = 1.64E-03; such as NFKB1, MAPK1, MAPK14, and VEGFA), and positive regulation of transcription from RNA polymerase II promoter (P value = 1.67E-03; such as NFKB1, MAPK14 and VEGFA) (Table 3).
Table 2.
The top 10 significantly enriched biological processes of Gene Ontology of upregulated genes in the treatment groups compared with the control groups
| Term | Count | P-value | Genes |
|---|---|---|---|
| GO:0032870∼cellular response to hormone stimulus | 7 | 7.49E-04 | HDAC5, AKT1, THRA, NUP62, STAT5A, ITGA2, GNAS |
| GO:0002520∼immune system development | 9 | 1.85E-03 | POLL, HDAC5, DHRS2, ASH2L, CLCF1, STAT5A, CASP8, ERCC1, SOD2 |
| GO:0043066∼negative regulation of apoptosis | 10 | 2.37E-03 | AKT1, DHRS2, CDH13, NUP62, CLCF1, STAT5A, PRNP, ERCC1, SOD2, BARD1 |
| GO:0030334∼regulation of cell migration | 7 | 2.55E-03 | HDAC5, AKT1, CDH13, LAMA4, SERPINE2, NF2, ITGA2 |
| GO:0043069∼negative regulation of programmed cell death | 10 | 2.60E-03 | AKT1, DHRS2, CDH13, NUP62, CLCF1, STAT5A, PRNP, ERCC1, SOD2, BARD1 |
| GO:0060548∼negative regulation of cell death | 10 | 2.65E-03 | AKT1, DHRS2, CDH13, NUP62, CLCF1, STAT5A, PRNP, ERCC1, SOD2, BARD1 |
| GO:0030097∼hemopoiesis | 8 | 3.13E-03 | HDAC5, DHRS2, ASH2L, CLCF1, STAT5A, CASP8, ERCC1, SOD2 |
| GO:0040012∼regulation of locomotion | 7 | 4.78E-03 | HDAC5, AKT1, CDH13, LAMA4, SERPINE2, NF2, ITGA2 |
| GO:0051270∼regulation of cell motion | 7 | 4.90E-03 | HDAC5, AKT1, CDH13, LAMA4, SERPINE2, NF2, ITGA2 |
| GO:0048534∼hemopoietic or lymphoid organ development | 8 | 5.30E-03 | HDAC5, DHRS2, ASH2L, CLCF1, STAT5A, CASP8, ERCC1, SOD2 |
Count, the number of genes enriched in the term.
AKT1, v-akt murine thymoma viral oncogene homolog 1 (ID: 207)
ASH2L, ash2 (absent, small, or homeotic)-like (Drosophila) (ID: 9070)
BARD1, BRCA1 associated RING domain 1 (ID: 580)
CASP8, caspase 8, apoptosis-related cysteine peptidase (ID:841)
CDH13, cadherin 13 (ID: 1012)
CLCF1, cardiotrophin-like cytokine factor 1 (ID: 23529)
DHRS2, dehydrogenase/reductase (SDR family) member 2 (ID: 10202)
ERCC1, excision repair cross-complementation group 1 (ID: 2067)
GNAS, GNAS complex locus (ID: 2778)
GO, gene ontology
HDAC5, histone deacetylase 5 (ID: 10014)
ITGA2, integrin, alpha 2 (ID: 3673)
LAMA4, laminin, alpha 4 (ID: 3910)
NF2, neurofibromin 2 (ID: 4771)
NUP62, nucleoporin 62 kDa (ID: 23636)
POLL, polymerase (DNA directed), lambda (ID: 27343)
PRNP, prion protein (ID: 5621)
SERPINE2, serpin peptidase inhibitor, clade E, member 2 (ID: 5270)
SOD2, superoxide dismutase 2 (ID: 6648)
STAT5A, signal transducer and activator of transcription 5A (ID: 6776)
THRA, thyroid hormone receptor alpha (ID: 7067).
Table 3.
The top 10 significantly enriched biological processes of Gene Ontology of downregulated genes in the treatment groups compared with the control groups
| Term | Count | P-value | Genes |
|---|---|---|---|
| GO:0010604∼positive regulation of macromolecule metabolic process | 14 | 1.64E-03 | CDK1, MORF4L2, TEAD2, NFKB1, TCF7L2, STAT6, MAPK1, HIF1A, SP1, MAPK14, VEGFA, MLST8, SMURF1, ETV4 |
| GO:0045944∼positive regulation of transcription from RNA polymerase II promoter | 9 | 1.67E-03 | STAT6, HIF1A, SP1, MAPK14, VEGFA, MORF4L2, NFKB1, TEAD2, TCF7L2 |
| GO:0045941∼positive regulation of transcription | 11 | 1.92E-03 | STAT6, MAPK1, HIF1A, SP1, MAPK14, VEGFA, MORF4L2, NFKB1, TEAD2, TCF7L2, ETV4 |
| GO:0030218∼erythrocyte differentiation | 4 | 2.15E-03 | MAEA, SP1, VEGFA, ADD1 |
| GO:0010628∼positive regulation of gene expression | 11 | 2.38E-03 | STAT6, MAPK1, HIF1A, SP1, MAPK14, VEGFA, MORF4L2, NFKB1, TEAD2, TCF7L2, ETV4 |
| GO:0009891∼positive regulation of biosynthetic process | 12 | 2.77E-03 | STAT6, MAPK1, HIF1A, SP1, MAPK14, VEGFA, MORF4L2, NFKB1, PRKAA1, TEAD2, TCF7L2, ETV4 |
| GO:0034101∼erythrocyte homeostasis | 4 | 3.12E-03 | MAEA, SP1, VEGFA, ADD1 |
| GO:0045935∼positive regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolic process | 11 | 3.97E-03 | STAT6, MAPK1, HIF1A, SP1, MAPK14, VEGFA, MORF4L2, NFKB1, TEAD2, TCF7L2, ETV4 |
| GO:0051173∼positive regulation of nitrogen compound metabolic process | 11 | 4.96E-03 | STAT6, MAPK1, HIF1A, SP1, MAPK14, VEGFA, MORF4L2, NFKB1, TEAD2, TCF7L2, ETV4 |
| GO:0010557∼positive regulation of macromolecule biosynthetic process | 11 | 5.52E-03 | STAT6, MAPK1, HIF1A, SP1, MAPK14, VEGFA, MORF4L2, NFKB1, TEAD2, TCF7L2, ETV4 |
Count, the number of genes enriched in the term.
ADD1, adducin 1 (alpha) (ID: 118)
CDK1, cyclin-dependent kinase 1 (ID: 983)
ETV4, ets variant 4 (ID: 2118)
GO, gene ontology
HIF1A, hypoxia inducible factor 1, alpha subunit (ID: 3091)
MAEA, macrophage erythroblast attacher (ID: 10296)
MAPK1, mitogen-activated protein kinase 1 (ID: 5594)
MAPK14, mitogen-activated protein kinase 14 (ID: 1432)
MLST8, MTOR associated protein, LST8 homolog (ID: 64223)
MORF4L2, mortality factor 4 like 2 (ID: 9643)
NFKB1, nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 (ID: 4790)
PRKAA1, protein kinase, AMP-activated, alpha 1 catalytic subunit (ID: 5562)
SP1, Sp1 transcription factor (ID: 6667)
SMURF1, SMAD specific E3 ubiquitin protein ligase 1 (ID: 57154)
STAT6, signal transducer and activator of transcription 6, interleukin-4 induced (ID: 6778)
TCF7L2, transcription factor 7-like 2 (ID: 6934)
TEAD2, TEA domain family member 2 (ID: 8463)
VEGFA, vascular endothelial growth factor A (ID: 7422).
PPI network analysis
PPI analysis of upregulated and downregulated genes was performed and then visualized constructing a PPI network. Proliferating cell nuclear antigen (PCNA, degree = 12) correlated with POLL, AKT1 and mutY homolog (MUTYH) and AKT1 correlated with PCNA and STAT5A (degree = 10) were hub nodes in the PPI network of upregulated genes (Fig 3). Sp1 transcription factor (SP1, degree = 9), MAPK1 (degree = 8), MAPK14 (degree = 7), VEGFA (degree = 7), and NFKB1 (degree = 6) were correlated with each other and possessed high connectivity in the PPI network of downregulated genes (Fig 3). Additionally, MAPK1, MAPK14, and NFKB1 were correlated with ARRB2, while MAPK1 and SP1 may influence PTK2 according to Figure 4.
Figure 3.
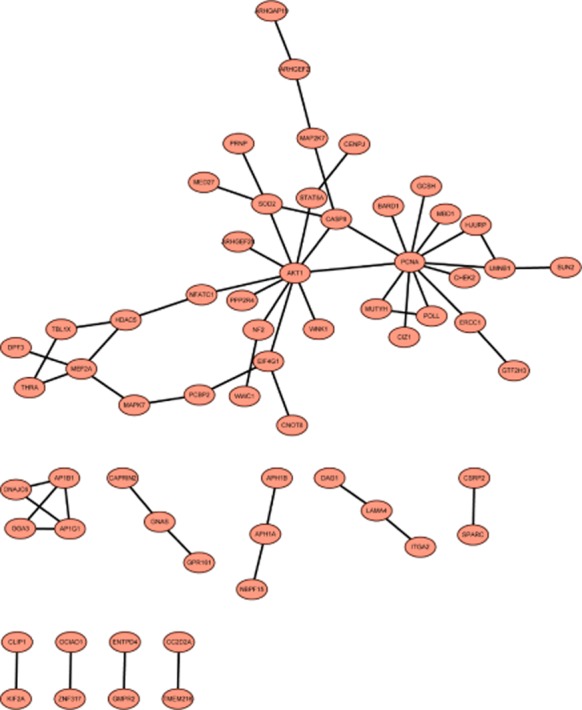
The protein-protein interaction network of upregulated genes in the treatment groups compared with the control groups.
Figure 4.
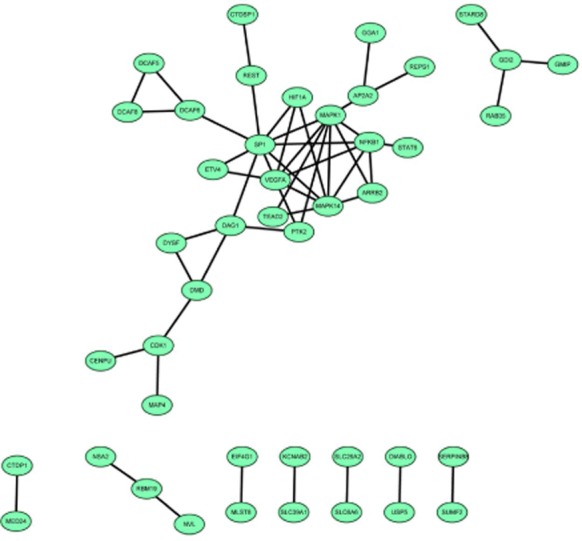
The protein-protein interaction network of downregulated genes in the treatment groups compared with the control groups.
Discussions
LSQCC, the second most prevalent type of lung cancer, is related to the accumulation of gene mutations.19 Curcumin could play a role in suppressing the cellular proliferation, invasion, and metastasis of lung cancer by regulating target gene expression.20,21 In accordance with previous studies, our study also revealed that curcumin reduced cell apoptosis and caused cell apoptosis of SK-MES-1 cells in a dose-dependent manner. Furthermore, the genome-wide transcriptional networks of SK-MES-1 (LSQCC) cells treated with and without curcumin were explored by RNA-seq to identify DEGs. The key findings of this study provided evidence that curcumin was able to upregulate the expression levels of PCNA, POLL, MUTYH, STAT5A, and AKT1, and downregulate the expressions of MAPK1, ARRB2, PTK2, MAPK14, VEGFA, and NFKB1 in SK-MES-1 cells. These DEGs were further identified to be involved in various KEGG pathways, such as BER, JAK-STAT, VEGF, and MAPK pathways, which could help elucidate the potential mechanisms of the anti-cancer effect of curcumin in LSQCC cells.
PCNA, POLL, and MUTYH were found to be upregulated by curcumin treatment and enriched in the BER pathway in this study. A previous study reported that damaged DNA could lead to cell aberrant apoptosis and cancer progression if not repaired in time.22 The BER pathway is one of the primary categories of excision repair and responsible for short patch (1–14 bp) restoration in the DNA repair system.23 The involved mutant genes in the BER pathway may be associated with an increasing susceptibility to develop lung cancer.24 The PCNA-dependent pathway is involved in base repair, in which PCNA functions as a molecular adaptor for recruiting other factors for the site of DNA repair.25 POLL is a substitute for Polβ as the main polymerase in the BER pathway, and MUTYH functions as bifunctional DNA glycosylases that could excise the damaged base.26 Curcumin intervention could prevent arsenic-induced defects of DNA repair in carcinogenesis.27 Herein, the upregulation of PCNA, POLL, and MUTYH in treatment groups compared with the control groups suggested that curcumin could improve the repair of DNA damage that exists in LSQCC cells.
Upregulated AKT1 and STAT5A were correlated with each other and enriched in the JAK-STAT pathway. An aberrantly activated JAK-STAT3 signaling pathway is involved in the oncogenesis of NSCLC.5 Curcumin could suppress SCLC cell invasion and migration via the JAK-STAT signaling pathway.7 This study identified that STAT5A, another member of the STAT family, was upregulated by curcumin treatment. The activated STAT5A may prevent STAT3 reactivation by inducing the overexpression of suppressors of cytokine signaling 2.28 AKT1, the key node in the PPI network, is an essential mediator for the biological function of STAT5 that binds to some consensus sequences within the AKT1 locus and enhances its transcription.29 Thus, the upregulation of AKT1 and STAT5A induced by curcumin treatment may jointly suggest a regulatory effect of curcumin on the JAK-STAT pathway in LSQCC cells.
On the other hand, MAPK1, MAPK14, PTK2, and VEGFA were downregulated by curcumin treatment and enriched in the VEGF pathway. VEGF could promote tumor angiogenesis in response to the increased need for the delivery of oxygen and nutrients in malignancies, including NSCLC.30 The prototype member of this family, VEGFA is regarded as a promising therapeutic target of cancer.31,32 In terms of lung cancer, VEGFA plays an important role in modulating migration and invasion of this cancer through the phosphoinositide 3 kinase/protein kinase B pathway.33 PTK2 is involved in VEGF-induced vascular permeability.34 In human intestinal microvascular endothelial cells, curcumin inhibits VEGF-induced angiogenesis via MAPKs in order to prevent cancer pathogenesis.35 Similarly, in this study, curcumin could reduce the expression of VEGFA, MAPK1, MAPK14, and PTK2 in LSQCC cells, probably suggesting an inhibition effect on the VEGF pathway in this cancer.
Meanwhile, downregulated NFKB1, ARRB2, MAPK1, and MAPK14 were involved in the MAPK pathway. NFKB1 plays an important role in controlling cell proliferation, suppressing apoptosis, and regulating tumor invasiveness and angiogenesis via various signaling pathways.36 The activated NFKB1 is involved in lung cancer pathogenesis, including LSQCC.37,38 MAPKs, known as serine-threonine kinases, are associated with cancer progression via mediating intracellular signaling.39,40 The MAPK pathway is usually activated and plays a role in the cell cycle, apoptosis, migration, and invasion of lung cancer cells.41–43 The suppression of ARRB2 could block CXCR4-induced activation of the p38 MAPK pathway.44 Dietary administration of curcumin significantly decreases the incidence of breast cancer metastasis to the lung, possibly through the suppression of NFKB1.45 Previous studies have demonstrated that there is a cross-talk between MAPK and NFKB pathways via their overlapping genes.46–48 Curcumin could also decrease the expression of MAPKs down-stream of NFKB1 in order to inhibit paclitaxel-induced upregulation of survival signaling events in HeLa cells.49 Herein, the downregulation of MAPK1, MAPK14, ARRB2, and NFKB1 induced by curcumin treatment may contribute to an inactivated MAPK pathway, suggesting the anti-cancer effect of curcumin via regulating the MAPK pathway in LSQCC cells.
Conclusion
Our study has revealed that curcumin, a functional polyphenol derived from the spice turmeric, could increase the expression levels of PCNA, POLL, MUTYH, STAT5A, and AKT1, and decrease the expression levels of PTK2, ARRB2, MAPK1, MAPK14, VEGFA, and NFKB1 in LSQCC cells. Furthermore, these genes were identified to be involved in the biological pathways of BER, JAK-STAT, VEGF, and MAPK, by which curcumin may induce cancer cell apoptosis and exert an anti-cancer effect. This study represents preliminary research for future work in exploring the anti-cancer effect of curcumin. A series of studies, both mechanical and epidemiological, are needed to validate the role of curcumin in regulating these genes and the involved pathways in LSQCC cells, with regard to its clinical application of being a novel chemotherapeutic regimen.
Disclosure
No authors report any conflict of interest.
References
- 1.Cancer Gene Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. (Published erratum appears in 2012; 288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsh F, Corrin B, Colby T. Clinical features and staging. In: Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, editors. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004. pp. 16–19. [Google Scholar]
- 3.Shi I, Hashemi Sadraei N, Duan ZH, Shi T. Aberrant signaling pathways in squamous cell lung carcinoma. Cancer Inform. 2011;10:273–285. doi: 10.4137/CIN.S8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Guan W, Li MX, et al. Genetic polymorphism of DNA base-excision repair genes (APE1, OGG1 and XRCC1) and their correlation with risk of lung cancer in a Chinese population. Arch Med Res. 2011;42:226–234. doi: 10.1016/j.arcmed.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Harada D, Takigawa N, Kiura K. The role of STAT3 in non-small cell lung cancer. Cancers. 2014;6:708–722. doi: 10.3390/cancers6020708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manju V, Nalini N. Chemopreventive efficacy of ginger, a naturally occurring anticarcinogen during the initiation, post-initiation stages of 1, 2 dimethylhydrazine-induced colon cancer. Clin Chim Acta. 2005;358:60–67. doi: 10.1016/j.cccn.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Yang CL, Liu YY, Ma YG, et al. Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3 signalling pathway. PLoS One. 2012;7(5):e37960. doi: 10.1371/journal.pone.0037960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215:129–140. doi: 10.1016/j.canlet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Li ZC, Zhang LM, Wang HB, Ma JX, Sun JZ. Curcumin inhibits lung cancer progression and metastasis through induction of FOXO1. Tumor Biol. 2014;35:111–116. doi: 10.1007/s13277-013-1013-7. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trapnell C. Cuffdiff Documentation, v7? Open Module on GenePattern Public Server [Cited 2 Aug 2014]. Available from URL: http://www.broadinstitute.org/cancer/software/genepattern/modules/docs/Cuffdiff/7.
- 13.Seyednasrollah F, Laiho A, Elo LL. Comparison of software packages for detecting differential expression in RNA-seq studies. Brief Bioinform. 2013 doi: 10.1093/bib/bbt086. doi: 10.1093/bib/bbt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dennis G, Jr, Sherman BT, Hosack DA, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 15.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2011;40:D109–114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gene Ontology Consortium. Gene ontology annotations and resources. Nucleic Acids Res. 2013;41:D530–535. doi: 10.1093/nar/gks1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franceschini A, Szklarczyk D, Frankild S, et al. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27:431–432. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y, Hammerman PS, Kim J, et al. Integrative and comparative genomic analysis of lung squamous cell carcinomas in East Asian patients. J Clin Oncol. 2014;32:121–128. doi: 10.1200/JCO.2013.50.8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha A, Kuzuhara T, Echigo N, Fujii A, Suganuma M, Fujiki H. Apoptosis of human lung cancer cells by curcumin mediated through up-regulation of “growth arrest and DNA damage inducible genes 45 and 153. Biol Pharm Bull. 2010;33:1291–1299. doi: 10.1248/bpb.33.1291. [DOI] [PubMed] [Google Scholar]
- 21.Chen HW, Lee JY, Huang JY, et al. Curcumin inhibits lung cancer cell invasion and metastasis through the tumor suppressor HLJ1. Cancer Res. 2008;68:7428–7438. doi: 10.1158/0008-5472.CAN-07-6734. [DOI] [PubMed] [Google Scholar]
- 22.Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. (Published erratum appears in 2009; 1914) [DOI] [PubMed] [Google Scholar]
- 23.Frosina G, Fortini P, Rossi O, et al. Two pathways for base excision repair in mammalian cells. J Biol Chem. 1996;271:9573–9578. doi: 10.1074/jbc.271.16.9573. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharyya N, Banerjee S. Analysis of alterations in a base-excision repair gene in lung cancer. Methods Mol Med. 2003;74:413–438. doi: 10.1385/1-59259-323-2:413. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto Y. Molecular mechanism of PCNA-dependent base excision repair. Prog Nucleic Acid Res Mol Biol. 2001;68:129–138. doi: 10.1016/s0079-6603(01)68095-4. [DOI] [PubMed] [Google Scholar]
- 26.Nemec AA, Wallace SS, Sweasy JB. Variant base excision repair proteins: Contributors to genomic instability. Semin Cancer Biol. 2010;20:320–328. doi: 10.1016/j.semcancer.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy M, Sinha D, Mukherjee S, Biswas J. Curcumin prevents DNA damage and enhances the repair potential in a chronically arsenic-exposed human population in West Bengal, India. Eur J Cancer Prev. 2011;20:123–131. doi: 10.1097/cej.0b013e328341017a. [DOI] [PubMed] [Google Scholar]
- 28.Sen B, Peng S, Woods DM, et al. STAT5A-mediated SOCS2 expression regulates Jak2 and STAT3 activity following c-Src inhibition in head and neck squamous carcinoma. Clin Cancer Res. 2012;18:127–139. doi: 10.1158/1078-0432.CCR-11-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt JW, Sakamoto K, Creamer BA, Leone G, Wagner KU. Stat5 regulates the PI3-Kinase/Akt1 pathway during mammary gland development and promotes neoplastic transformation in a mouse model for Cowden syndrome. Cancer Res. 2011;71:Abstract P4-03-01. [Google Scholar]
- 30.Das M, Wakelee H. Targeting VEGF in lung cancer. Expert Opin Ther Targets. 2012;16:395–406. doi: 10.1517/14728222.2012.669752. [DOI] [PubMed] [Google Scholar]
- 31.Ferrara N, Mass RD, Campa C, Kim R. Targeting VEGF-A to treat cancer and age-related macular degeneration. Annu Rev Med. 2007;58:491–504. doi: 10.1146/annurev.med.58.061705.145635. [DOI] [PubMed] [Google Scholar]
- 32.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 33.Chen CH, Lai JM, Chou TY, et al. VEGFA upregulates FLJ10540 and modulates migration and invasion of lung cancer via PI3K/AKT pathway. PLoS One. 2009;4(4):e5052. doi: 10.1371/journal.pone.0005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen XL, Nam JO, Jean C, et al. VEGF-induced vascular permeability is mediated by FAK. Dev Cell. 2012;22:146–157. doi: 10.1016/j.devcel.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Binion DG, Otterson MF, Rafiee P. Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut. 2008;57:1509–1517. doi: 10.1136/gut.2008.152496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 37.Tang X, Liu D, Shishodia S, et al. Nuclear factor-κB (nf-κB) is frequently expressed in lung cancer and preneoplastic lesions. Cancer. 2006;107:2637–2646. doi: 10.1002/cncr.22315. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Xu YJ, Zhang ZX, et al. Nuclear factor-kappaB activity and its correlation with cell proliferation in non-small cell lung cancer tissues. Zhonghua Jie He He Hu Xi Za Zhi. 2007;30:771–775. (In Chinese.) [PubMed] [Google Scholar]
- 39.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 40.Krens S, Spaink HP, Snaar-Jagalska BE. Functions of the MAPK family in vertebrate-development. FEBS Lett. 2006;580:4984–4990. doi: 10.1016/j.febslet.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 41.Vicent S, Garayoa M, López-Picazo JM, et al. Mitogen-activated protein kinase phosphatase-1 is overexpressed in non-small cell lung cancer and is an independent predictor of outcome in patients. Clin Cancer Res. 2004;10:3639–3649. doi: 10.1158/1078-0432.CCR-03-0771. [DOI] [PubMed] [Google Scholar]
- 42.Yan H, Zhu Y, Liu B, et al. Mitogen-activated protein kinase mediates the apoptosis of highly metastatic human non-small cell lung cancer cells induced by isothiocyanates. Br J Nutr. 2011;106:1779–1791. doi: 10.1017/S0007114511002315. [DOI] [PubMed] [Google Scholar]
- 43.Lu Z, Ding L, Hong H, Hoggard J, Lu Q, Chen YH. Claudin-7 inhibits human lung cancer cell migration and invasion through ERK/MAPK signaling pathway. Exp Cell Res. 2011;317:1935–1946. doi: 10.1016/j.yexcr.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Y, Cheng Z, Ma L, Pei G. β-Arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 45.Aggarwal BB, Shishodia S, Takada Y, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-κB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11:7490–7498. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 46.Matsuda A, Suzuki Y, Honda G, et al. Large-scale identification and characterization of human genes that activate NF-κB and MAPK signaling pathways. Oncogene. 2003;22:3307–3318. doi: 10.1038/sj.onc.1206406. [DOI] [PubMed] [Google Scholar]
- 47.Sironi L, Banfi C, Brioschi M, et al. Activation of NF-kB and ERK1/2 after permanent focal ischemia is abolished by simvastatin treatment. Neurobiol Dis. 2006;22:445–451. doi: 10.1016/j.nbd.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Mattson MP, Camandola S. NF-κB in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001;107:247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bava SV, Sreekanth CN, Thulasidasan AKT, et al. Akt is upstream and MAPKs are downstream of NF-κB in paclitaxel-induced survival signaling events, which are down-regulated by curcumin contributing to their synergism. Int J Biochem Cell Biol. 2011;43:331–341. doi: 10.1016/j.biocel.2010.09.011. [DOI] [PubMed] [Google Scholar]


