Abstract
Background
The role of conventional bronchoscopy for peripheral pulmonary neoplasia remains controversial. We aimed to assess the diagnostic yield and the added value of non-guided bronchial aspiration, bronchoalveolar lavage (BAL), and brushing for the diagnosis of pulmonary neoplasia not visible endoscopically.
Methods
We retrospectively assessed 207 consecutive patients with a final diagnosis of peripheral lung malignancy who underwent bronchoscopy with non-guided aspiration, brushing, and BAL as their initial evaluation. The influence of clinical and radiological factors on diagnostic yield was assessed using univariate logistic regression analyses.
Results
The overall sensitivity of non-guided bronchoscopy was 25.6%, whereas sensitivities for bronchial aspiration, BAL, and brushing were 14.2%, 11.6%, and 16.5%, respectively. Younger age, larger lesion, central/intermediate distance from the hilum, presence of a bronchus sign, and higher standardized uptake value (SUV) on positron emission tomography scan were predictors of a higher diagnostic yield. Conversely, forced expiratory volume in one second, fellow implication in the procedure, and tumor histology did not influence sensitivity. The overall sensitivity of bronshoscopy was >40% for tumors >4 cm, located in the central/intermediate thirds of the lung, showing a bronchus sign, with an SUV >12 or occurring in patients <50 years of age. Conversely, the sensitivity was <10% for tumors <2 cm, located peripherally or with an SUV <4.
Conclusion
Neoplasia characteristics may help targeting situations in which conventional bronchoscopy could be used as the initial diagnostic procedure when advanced techniques are unavailable. However, advanced diagnostic tools should probably be proposed as the initial modality for the diagnosis of peripheral malignant lesions when available.
Keywords: Bronchoalveolar lavage, bronchoscopy, brushing, diagnostic yield, lung cancer
Introduction
Peripheral pulmonary lesions are common clinical problems and most are detected incidentally on chest X-rays or computed tomography (CT) scans.1 Recent data suggesting a mortality benefit with low-dose CT screening for lung cancer may eventually lead to a significant increase in patient referral for the evaluation of peripheral pulmonary nodules.2
Guidelines from the American College of Chest Physicians stress the value of risk factor assessment for guiding subsequent investigations of peripheral pulmonary lesions.3,4 Surgical resection is favored for lesions at high risk of malignancy (grade of recommendation 2C), whereas radiological follow-up is preferred for low risk lesions (grade 2C). For patients with intermediate risk lesions, additional tests are recommended, which should be selected based on nodule size, location, relation to a patent airway, risk of complications in the individual patient, and available expertise. CT scan-guided transthoracic needle aspiration (TTNA) is generally preferred for nodules located in proximity of the chest wall or for deeper lesions, provided that fissures do not need to be traversed and there is no surrounding emphysema. On the other hand, bronchoscopic techniques are favored for nodules located in proximity to a patent bronchus and in individuals who are at high risk for pneumothorax following TTNA. For peripheral nodules, radial endobronchial ultrasound (EBUS) guided biopsy and electromagnetic navigation guidance are recommended (grade 1C) if available and TTNA is also a diagnostic option (grade 1B).5 That said, because of delays in access and risks associated with TTNA, as well as unavailability of advanced diagnostic techniques in most centers, conventional bronchoscopy is still performed for many patients with peripheral pulmonary lesions. However, the role of non-guided bronchoscopy in the initial evaluation is not well established.6
Therefore, the goal of our study was to document the overall sensitivity of standard bronchoscopy without fluoroscopic/ultrasound guidance for peripheral lung malignancy not visible endoscopically and to compare the diagnostic performance of individual sampling techniques including bronchial aspiration, brushing, and bronchoalveolar lavage (BAL). We also aimed to identify clinical and radiologic factors predictive of an improved diagnostic yield.
Methods
We retrospectively reviewed medical records of all consecutive patients who underwent a conventional bronchoscopy for peripheral pulmonary lesions at the Institut universitaire de cardiologie et de pneumologie de Québec between April 2008 and December 2010, for whom the final diagnosis was a neoplasia. Criteria for inclusion in the study were: (i) presence of a circumscribed solid lung lesion not visible endoscopically (pure ground glass opacities were excluded); (ii) bronchoscopy performed with bronchial aspiration, brushing, and BAL; and (iii) a final pathologic diagnosis of lung cancer established by either bronchoscopy or any other diagnostic procedure.
Flexible bronchoscopy (BF P180, 4.9 mm, Olympus, Richmond Hill, ON, Canada) was performed as part of the initial diagnostic workup under conscious sedation using a combination of midazolam and fentanyl. Secretions present in the bronchial tree were aspirated for cytologic examination; when no secretions were present, the lobar bronchus was washed with one or two aliquots of 10 mL of saline. Based on axial CT images, blinded brushing was performed by introducing a brush (1.9 mm, Boston Scientific, Spencer, IN, USA) in the target segmental bronchus. BAL was performed by injecting three aliquots of 50 mL of saline with the bronchoscope in a wedged position in the involved segment. For bronchial brushing, the brush was smeared on two slides, which were then immediately fixed with 95% ethyl alcohol. The samples from both bronchial aspiration and BAL were preserved in 50% ethyl alcohol. The specimens were centrifuged for five minutes at 1500 revolutions per minute. Two to four slides were prepared from cell concentrate. All slides were stained with Papanicolaou stain. When malignant cells were identified, the residual specimen was used to prepare a cellblock by resuspending the cell pellet in 10% formalin for 24 hours and then in 3% agarose. A section of 5 μm thickness was then obtained. Routine hematoxylin and eosin staining was used on cellblock sections and, when necessary, immunohistochemical stainings were performed to phenotype the tumoral cells. Cytology results clearly diagnostic for lung cancer were classified as positive, while results with non-diagnostic material, benign, atypical or suspicious cells without a certain diagnosis were classified as negative. None of the patients experienced pneumothorax. All analyses were performed in accordance with the Institut Universitaire de Cardiologie et de Pneumologie de Québec biosafety and ethics committee (CER-21034).
Statistical analysis
Categorical data are expressed as proportions and numerical data as mean ± standard deviation. Differences in proportions were tested with the Chi-square or Fisher's exact tests. Continuous variables were compared with the Student t-test with the Welch correction depending on equal or unequal variances. The influence of various clinical and radiologic factors on sensitivity was first assessed using univariate logistic regression models including patient's age, forced expiratory volume in 1 second (FEV1), fellow implication in the procedure, lesion size, hypermetabolism on positron emission tomography (PET) scan, distance of the lesion from the hilum (lesions within the inner and middle third vs. outer third of the hilar-costal distance as determined on axial CT images), the presence of a bronchus sign (an air-filled bronchus leading to the lesion), and pathology. Continuous variables were checked for the assumption of linearity in the logit using quartiles of the distribution and fractional polynomials before building the model in order to obtain the correct relationships. Furthermore, in order to appreciate the appropriate functional form between predictors of a higher diagnostic yield for the three techniques and continuous variables, a generalized additive model was built using the binary distribution. Smoothing was performed by spline fitting (df = 4) to investigate inflection point, which cannot be identified using linear models. Age and lesion size had these knots for some predictors to respect the linearity in the logit before and after the inflection point. The results were considered significant with P values ≤ 0.05. Data were analyzed using the SAS v9.1.3 statistical package (SAS Institute Inc., Cary, NC, USA).
Results
Two hundred and seven patients fulfilled the inclusion criteria and were included in the study. Patients' characteristics are described in Table 1. In 20 patients, brushing was not performed or the brushing cytologic specimen was judged not satisfactory. These patients did not differ in terms of age, smoking status and FEV1, bronchoscopist, presence/absence of bronchus sign, tumor pathology, location, size or standardized uptake value (SUV) (all P > 0.20). As a conservative approach, these brushings were considered as negative.
Table 1.
Characteristics of the 207 cases
| Gender – no. (%) | |
| Men | 108 (52.2%) |
| Women | 99 (47.8%) |
| Age – mean ± SD (range), years | 66 ± 9 (40–85) |
| Smoking status – no. (%) | |
| Smokers | 190 (91.8%) |
| Non-smokers | 17 (8.2%) |
| FEV1 – mean ± SD (range), % (n = 179) | 87 ± 20 (30–132) |
| Bronchoscopist – no. (%) | |
| Respirologist | 138 (66.7%) |
| Fellow | 69 (33.3%) |
| BAL fluid return – mean ± SD (range), mL (n = 109) | 37 (1–90) |
| Lesion size – mean ± SD (range), cm | 3.3 ± 1.8 (0.8–10.2) |
| SUV on PET scan – mean ± SD (range) (n = 176) | 9.2 ± 6.5 (1.0–34.1) |
| Distance from the hilum – no. (%) (n = 205)† | |
| Central/intermediate | 94 (45.9%) |
| Peripheral | 111 (54.1%) |
| Bronchus sign – no. (%) (n = 205) | |
| Present | 49 (23.9%) |
| Absent | 156 (76.1%) |
Lesions within the inner, middle, and outer thirds of the hilar-costal distance on computed tomography scan were classified as central, intermediate or peripheral, respectively.
BAL, bronchoalveolar lavage
FEV1, forced expiratory volume in one second
PET, positron emission tomography
SD, standard deviation
SUV, standardized uptake value.
The overall sensitivity of non-guided bronchoscopy for malignancy was 25.6% (53/207 patients). In the other 154 patients, the diagnosis was established most frequently by TTNA (Table 2). The sensitivities of individual sampling techniques were 14.2% for bronchial aspiration, 11.6% for BAL, and 16.5% for brushing. Only BAL and brushing were statistically different (P = 0.003). When the individual contributions of each technique were compared to the diagnostic performance of bronchoscopy, brushing had the highest added yield, although the differences between the various techniques were not statistically significant (P = 0.08) (Fig 1).
Table 2.
Diagnostic method and final diagnosis
| n | % | |
|---|---|---|
| Final diagnostic method | ||
| Bronchoscopy | 53 | 25.6% |
| Guided transbronchial biopsy | 8 | 3.9% |
| Lymph node biopsy with EBUS | 6 | 2.9% |
| Transthoracic fine-needle aspiration biopsy | 94 | 45.4% |
| Sampling from distant metastasis | 8 | 3.9% |
| Mediastinoscopy | 3 | 1.4% |
| Thoracoscopy | 35 | 16.9% |
| Pathology | ||
| NSCLC† | 185 | 89.4% |
| Others‡ | 22 | 10.6% |
| Stage | ||
| Limited§ | 129 | 62.3% |
| Extensive¶ | 78 | 37.7% |
Adenocarcinoma (n = 142), squamous cell carcinoma (SCLC, n = 28), and non-small cell lung carcinoma (NSCLC) not otherwise specified (n = 15).
Small cell lung carcinoma (n = 10), carcinoid tumor (n = 9), sarcomatoid carcinoma (n = 1), fusiform cell sarcoma (n = 1), mucinous cystadenocarcinoma (n = 1).
Limited SCLC and stages 1a, 1b, 2a, 2b NSCLC.
Extensive SCLC and stages 3a, 3b, 4 NSCLC. EBUS, endobronchial ultrasound.
Figure 1.

A summary of the diagnostic yield of bronchial aspiration, brushing, bronchoalveolar lavage (BAL), and their combination for non-endobronchial lung neoplasia. The overall diagnostic yield of non-guided bronchoscopy using these techniques was 25.6%. Brushing tended to be associated with the highest diagnostic yield (16.4%), compared to aspiration (14.0%) and BAL (11.6%), as well as the highest added yield, although the differences between the various techniques were not statistically significant (P = 0.08).
In univariate analysis, younger age, larger lesion size, central/intermediate distance from the hilum, presence of a bronchus sign, and higher SUV on PET scan were predictors of a higher overall diagnostic yield (Table 3). Conversely, FEV1, fellow implication in the procedure, and tumor histology did not influence sensitivity. Interestingly, the influence of lesion size and patient age was not linear. In fact, an increased overall diagnostic yield was observed with increasing lesion size up to 3 cm in diameter, after which point size had no effect on diagnostic performance. Regarding the effect of age, although the yield decreased in older patients, age had no influence on diagnostic performance in patients of 70 years and older. The overall sensitivity of bronchoscopy was more than 40% for tumors >4 cm, located in the central/intermediate thirds of the lung, showing a bronchus sign, with an SUV >12 or occurring in patients <50 years of age. Conversely, the diagnostic yield was less than 10% for tumors <2 cm, located peripherally or with an SUV <4 (Fig 2).
Table 3.
Predictors of a higher diagnostic yield
| BAL OR (95% CI) | Bronchial aspiration OR (95% CI) | Brushing OR (95% CI) | Overall OR (95% CI) | |
|---|---|---|---|---|
| Age† | ||||
| Age, per year | 0.95 (0.91–0.99)‡ | |||
| ≤50, per year | 0.65 (0.45–0.94)§ | 0.65 (0.46–0.92)§ | ||
| >50, per year | 0.98 (0.93–1.04) | 1.01 (0.95–1.06) | ||
| ≤70, per year | 0.92 (0.88–0.97)‡ | |||
| >70, per year | 1.06 (0.95–1.19) | |||
| FEV1, per % | 1.02 (0.99–1.04) | 1.00 (0.98–1.02) | 1.00 (0.98–1.02) | 1.00 (0.98–1.02) |
| Bronchoscopist, fellow versus respirologist | 0.36 (0.12–1.11) | 1.07 (0.47–2.45) | 1.11 (0.51–2.43) | 1.06 (0.54–2.08) |
| Lesion size¶ | ||||
| Size, per cm | 1.37 (1.13–1.66)‡ | |||
| ≤3 cm, per cm | 4.00 (1.23–13.00) § | 3.24 (1.60–6.58)‡ | ||
| >3 cm, per cm | 1.11 (0.88–1.46) | 1.01 (0.80–1.28) | ||
| ≤4 cm, per cm | 2.10 (1.31–3.37)‡ | |||
| >4 cm, per cm | 0.75 (0.50–1.12) | |||
| Distance from the hilum, central/intermediate versus peripheral | 2.64 (1.08–6.48)§ | 2.51 (1.10–5.71)§ | 5.96 (2.43–14.6)‡ | 6.38 (3.05–13.32)‡ |
| Presence of a bronchus sign, yes versus no | 3.89 (1.62–9.37)‡ | 2.61 (1.15–5.95)§ | 2.85 (1.29–6.32)‡ | 2.95 (1.46–5.97)‡ |
| SUV, per unit | 1.06 (0.99–1.14) | 1.07 (1.00–1.14)§ | 1.07 (1.01–1.14)§ | 1.09 (1.03–1.15)§ |
| Pathology, NSCLC versus other | 2.98 (0.38–23.20) | 1.05 (0.29–3.80) | 4.16 (0.53–32.36) | 1.11 (0.38–3.25) |
The influence of patient age was not linear for bronchoalveolar lavage (BAL), aspiration, and the combination of diagnostic techniques. In fact, a decreased diagnostic yield was observed with increasing age up to 50 years for BAL and bronchial aspiration, after which point age had no effect on diagnostic performance. The overall diagnostic yield decreased with increasing age up to 70 years, after which point age had no effect on diagnostic performance.
P < 0.01
P < 0.05.
The influence of lesion size was not linear for BAL, brushing, and the combination of diagnostic techniques. In fact, an increased overall diagnostic yield was observed with increasing lesion size up to a diameter of 3 cm and 4 cm for BAL and brushing, respectively, after which point it had no effect on diagnostic performance. The overall diagnostic yield increased with increasing lesion size up to a diameter of 3 cm.
FEV1, forced expiratory volume in one second
NSCLC, non-small cell lung carcinoma; SUV, standardized uptake value.
Figure 2.
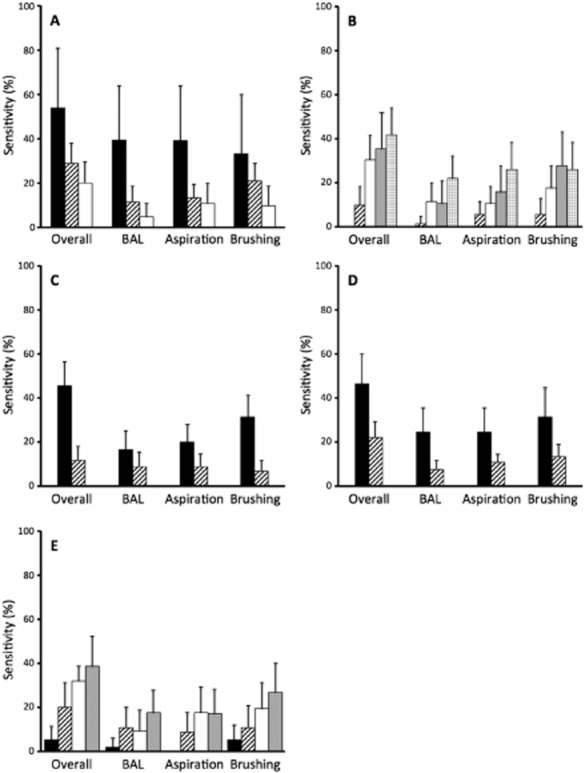
Diagnostic yield of bronchial aspiration, brushing, bronchoalveolar lavage (BAL) and their combination for non-endobronchial lung neoplasia according to: (a) patients' age; (b) tumor size; (c) distance from the hilum; (d) presence or absence of bronchus sign; and (e) standardized uptake value (SUV) on positron emission tomography. Diagnostic yield is described as sensitivity with 95% confidence interval.  , ≤50 years;
, ≤50 years;  , 51–70 years;
, 51–70 years;  , >70 years;
, >70 years;  , ≤1 cm;
, ≤1 cm;  , 1.1–2 cm;
, 1.1–2 cm;  , 2.1–3 cm;
, 2.1–3 cm;  , 3.1–4 cm;
, 3.1–4 cm;  , >4 cm;
, >4 cm;  , central-intermediate;
, central-intermediate;  , peripheral;
, peripheral;  , bronchus;
, bronchus;  , no bronchus sign;
, no bronchus sign;  , SUV ≤ 4;
, SUV ≤ 4;  , SUV 4.1–8;
, SUV 4.1–8;  , SUV 8.1–12;
, SUV 8.1–12;  , SUV > 12.
, SUV > 12.
Discussion
In this cohort of patients with pulmonary neoplasia not visible through conventional bronchoscopy, non-guided bronchoscopy without transbronchial biopsy had a sensitivity of 25.6%, with the highest yield provided by brushing. Higher diagnostic yield was also associated with younger age, larger lesions located in the central/intermediate thirds of the lung, presence of a bronchus sign, and higher SUV on PET scan.
In the present study, the overall sensitivity of bronchoscopy for the diagnosis of lung neoplasia not visible endoscopically was comparable to previous studies evaluating non-guided conventional bronchoscopy without transbronchial biopsy (6–33%).7–12 While some studies suggested a global sensitivity of 78% for bronchoscopy in the diagnosis of lung cancer,5 fluoroscopic guidance was used13–25 and transbronchial biopsies were performed26–39 in the vast majority of these studies. In addition, some of those series also included endobronchial lesions and benign lesions. In concordance with previous studies, larger lesion size, distance from the hilum, and presence of a bronchus sign were predictors of a higher sensitivity. Interestingly, the mean lesion size in the present study was higher (3.3 cm) than in previous series. Despite this, the overall sensitivity remained limited. This may be explained by the fact that beyond a certain point (3–4 cm in the current study), the sensitivity of bronchoscopy reaches a plateau, possibly because large lesions not visible endoscopically may be associated with necrotic neoplastic cells, post-obstructive pneumonia, and bronchial compression that could diminish bronchial aspiration, brushing, and BAL diagnostic yield. Another likely explanation is that the brush did not reach the lesion correctly as the lesion size was lower because of inadequate airway selection given the absence of guidance. We also identified new variables increasing the diagnostic yield of bronchoscopy, including higher SUV and younger age. Conversely, the histology of lung cancer did not influence the diagnostic yield of bronchoscopy.
While brushing tended to have more added value over aspiration and BAL, the three diagnostic modalities were complementary in improving the yield of bronchoscopy for peripheral lung cancer (Fig 1), as previously suggested.40–42 However, among patients with lung cancer and based on the added diagnostic value of each technique, the number of procedures needed to diagnose one extra patient with lung neoplasia not visible endoscopically was 15 (95% confidence interval [CI] 10–29), 29 (95% CI 17–111), and 34 (95% CI 19–167) for brushing, bronchial aspiration, and BAL, respectively. Because BAL is the most costly technique (≈$160 USD for material, specimen processing, and pathologist fees, compared with ≈$90 USD for other techniques), the minimal estimated cost for each additional diagnosis of peripheral lung cancer was ≈$1350 USD ($900-$2610 USD), $2610 USD ($1530-$9990 USD), and $5440 USD ($3040-$26 720 USD) for brushing, bronchial aspiration and BAL, respectively, in addition to the cost of flexible bronchoscopy. Therefore, the cost-effectiveness of including all three techniques for each exam is questionable, especially for lesions with features associated with low diagnostic yield (Fig 2). Sequential specimen processing might be a more cost-effective alternative, where the lab processes BAL only if other specimens are non-diagnostic.43 Moreover, patients with peripheral lesions less than 2 cm should probably be referred for more advanced diagnostic modalities as non-guided bronchoscopy is associated with a diagnostic yield of <5–10% and significant costs. This is especially important with the development of lung cancer screening strategies because most early stage cancers detected by CT-based screening programs are <2 cm.44 Prospective cost-effectiveness analyses are required to determine whether such patients should be directly referred for TTNA or advanced guided bronchoscopy techniques. Bronchocoscopy should also be delayed after PET scanning as tumor stage and SUV may dictate the most appropriate diagnostic technique. Ultimately, the development of predictive tools based on patient and lesion characteristics are necessary to accurately estimate the probability that lung nodules are malignant and for which immediate lung resection may be proposed in low-risk surgical candidates, especially if sublobar resections are proved as effective as lobectomy for early-stage lung cancer.44 Conversely, the diagnostic yield of standard bronchoscopy reaches up to 45% for larger and centrally located neoplasia not visible during endoscopy, especially when a bronchus sign is present. While advanced guided bronchoscopy techniques have been associated with increased diagnostic yield, their cost-effectiveness is yet to be determined in these circumstances.
An important limitation of our study is its size, which limited our ability to detect subtle differences between the various sampling techniques. Moreover, the low number of patients diagnosed with each technique precluded building a valid multivariate regression model to adjust the univariate analyses for potential confounders. In addition, the retrospective nature of our analysis is associated with its inherent limitations. Finally, the results of this study are difficult to apply to a population with undifferentiated peripheral pulmonary lesions because only malignant lesions were included.
Conclusion
Given the limited sensitivity of bronchoscopy without guidance for the diagnosis of peripheral malignant lesions not visible endoscopically, advanced diagnostic modalities, such as radial EBUS, TTNA or even video-assisted thoracoscopic surgery, should be favored as the initial diagnostic modality when available. However, some clinical variables are predictors of a higher diagnostic yield and could help targeting situations in which bronchoscopy could be used as the initial test when advanced techniques are unavailable, namely larger lesions showing a bronchus sign and located in the central/intermediate thirds of the lung. Finally, the routine use of BAL is questionable as it is probably not cost-effective when added to bronchial brushing and aspiration.
Acknowledgments
The authors acknowledge Serge Simard (M.Sc Stats) from the Centre de recherche de l'Institut Universitaire de cardiologie et de pneumologie de Québec, Université Laval, Québec, QC, Canada for statistical analyses. SP is clinician-scientist of the Fonds de Recherche en Santé du Québec (FRSQ).
Disclosure
No authors report any conflict of interest.
References
- 1.Winer-Muram HT. The solitary pulmonary nodule. Radiology. 2006;239:34–49. doi: 10.1148/radiol.2391050343. [DOI] [PubMed] [Google Scholar]
- 2.The National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: When is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(Suppl):e93S–120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157:849–855. [PubMed] [Google Scholar]
- 5.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(Suppl):e142S–165. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]
- 6.Hergott CA, Tremblay A. Role of bronchoscopy in the evaluation of solitary pulmonary nodules. Clin Chest Med. 2010;31:49–63. doi: 10.1016/j.ccm.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Boonsarngsuk V, Raweelert P, Sukprapruet A, Chaiprasithikul R, Kiatboonsri S. Factors affecting the diagnostic yield of flexible bronchoscopy without guidance in pulmonary nodules or masses. Singapore Med J. 2010;51:660–665. [PubMed] [Google Scholar]
- 8.Rhee CK, Kang HH, Kang JY, et al. Diagnostic yield of flexible bronchoscopy without fluoroscopic guidance in evaluating peripheral lung lesions. J Bronchology Interv Pulmonol. 2010;17:317–322. doi: 10.1097/LBR.0b013e3181f552a5. [DOI] [PubMed] [Google Scholar]
- 9.Sing A, Freudenberg N, Kortsik C, Wertzel H, Klosa B, Hasse J. Comparison of the sensitivity of sputum and brush cytology in the diagnosis of lung carcinomas. Acta Cytol. 1997;41:399–408. doi: 10.1159/000332531. [DOI] [PubMed] [Google Scholar]
- 10.de Gracia J, Bravo C, Miravitlles M, et al. Diagnostic value of bronchoalveolar lavage in peripheral lung cancer. Am Rev Respir Dis. 1993;147:649–652. doi: 10.1164/ajrccm/147.3.649. [DOI] [PubMed] [Google Scholar]
- 11.Pilotti S, Rilke F, Gribaudi G, Spinelli P. Cytologic diagnosis of pulmonary carcinoma on bronchoscopic brushing material. Acta Cytol. 1982;26:655–660. [PubMed] [Google Scholar]
- 12.Roth K, Hardie JA, Andreassen AH, Leh F, Eagan TM. Predictors of diagnostic yield in bronchoscopy: A retrospective cohort study comparing different combinations of sampling techniques. BMC Pulm Med. 2008;8:2. doi: 10.1186/1471-2466-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aristizabal JF, Young KR, Nath H. Can chest CT decrease the use of preoperative bronchoscopy in the evaluation of suspected bronchogenic carcinoma? Chest. 1998;113:1244–1249. doi: 10.1378/chest.113.5.1244. [DOI] [PubMed] [Google Scholar]
- 14.Cortese DA, McDougall JC. Biopsy and brushing of peripheral lung cancer with fluoroscopic guidance. Chest. 1979;75:141–145. doi: 10.1378/chest.75.2.141. [DOI] [PubMed] [Google Scholar]
- 15.Kawaraya M, Gemba K, Ueoka H, et al. Evaluation of various cytological examinations by bronchoscopy in the diagnosis of peripheral lung cancer. Br J Cancer. 2003;89:1885–1888. doi: 10.1038/sj.bjc.6601368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kvale PA, Bode FR, Kini S. Diagnostic accuracy in lung cancer; comparison of techniques used in association with flexible fiberoptic bronchoscopy. Chest. 1976;69:752–757. doi: 10.1378/chest.69.6.752. [DOI] [PubMed] [Google Scholar]
- 17.Mori K, Yanase N, Kaneko M, Ono R, Ikeda S. Diagnosis of peripheral lung cancer in cases of tumors 2 cm or less in size. Chest. 1989;95:304–308. doi: 10.1378/chest.95.2.304. [DOI] [PubMed] [Google Scholar]
- 18.Pirozynski M. Bronchoalveolar lavage in the diagnosis of peripheral, primary lung cancer. Chest. 1992;102:372–374. doi: 10.1378/chest.102.2.372. [DOI] [PubMed] [Google Scholar]
- 19.Popp W, Rauscher H, Ritschka L, Redtenbacher S, Zwick H, Dutz W. Diagnostic sensitivity of different techniques in the diagnosis of lung tumors with the flexible fiberoptic bronchoscope. Comparison of brush biopsy, imprint cytology of forceps biopsy, and histology of forceps biopsy. Cancer. 1991;67:72–75. doi: 10.1002/1097-0142(19910101)67:1<72::aid-cncr2820670114>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Reichenberger F, Weber J, Tamm M, et al. The value of transbronchial needle aspiration in the diagnosis of peripheral pulmonary lesions. Chest. 1999;116:704–708. doi: 10.1378/chest.116.3.704. [DOI] [PubMed] [Google Scholar]
- 21.Shiner RJ, Rosenman J, Katz I, Reichart N, Hershko E, Yellin A. Bronchoscopic evaluation of peripheral lung tumours. Thorax. 1988;43:887–889. doi: 10.1136/thx.43.11.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stringfield JT, Markowitz DJ, Bentz RR, Welch MH, Weg JG. The effect of tumor size and location on diagnosis by fiberoptic bronchoscopy. Chest. 1977;72:474–476. doi: 10.1378/chest.72.4.474. [DOI] [PubMed] [Google Scholar]
- 23.Zavala DC. Diagnostic fiberoptic bronchoscopy: Techniques and results of biopsy in 600 patients. Chest. 1975;68:12–19. doi: 10.1378/chest.68.1.12. [DOI] [PubMed] [Google Scholar]
- 24.Gasparini S, Zuccatosta L, Zitti P, Bichi Secchi E, Ferretti M, Gusella P. Integration of TBNA and TCNA in the diagnosis of peripheral lung nodules. Influence on staging. Ann Ital Chir. 1999;70:851–855. [PubMed] [Google Scholar]
- 25.Gay PC, Brutinel WM. Transbronchial needle aspiration in the practice of bronchoscopy. Mayo Clin Proc. 1989;64:158–162. doi: 10.1016/s0025-6196(12)65669-9. [DOI] [PubMed] [Google Scholar]
- 26.Baba M, Iyoda A, Yasufuku K, et al. Preoperative cytodiagnosis of very small-sized peripheral-type primary lung cancer. Lung Cancer. 2002;37:277–280. doi: 10.1016/s0169-5002(02)00105-8. [DOI] [PubMed] [Google Scholar]
- 27.Bandoh S, Fujita J, Tojo Y, et al. Diagnostic accuracy and safety of flexible bronchoscopy with multiplanar reconstruction images and ultrafast Papanicolaou stain: evaluating solitary pulmonary nodules. Chest. 2003;124:1985–1992. doi: 10.1378/chest.124.5.1985. [DOI] [PubMed] [Google Scholar]
- 28.Buccheri G, Barberis P, Delfino MS. Diagnostic, morphologic, and histopathologic correlates in bronchogenic carcinoma. A review of 1,045 bronchoscopic examinations. Chest. 1991;99:809–814. doi: 10.1378/chest.99.4.809. [DOI] [PubMed] [Google Scholar]
- 29.Castella J, Buj J, Puzo C, Antón PA, Burgués C. Diagnosis and staging of bronchogenic carcinoma by transtracheal and transbronchial needle aspiration. Ann Oncol. 1995;6(Suppl 3):S21–24. doi: 10.1093/annonc/6.suppl_3.s21. [DOI] [PubMed] [Google Scholar]
- 30.Cox ID, Bagg LR, Russell NJ, Turner MJ. Relationship of radiologic position to the diagnostic yield of fiberoptic bronchoscopy in bronchial carcinoma. Chest. 1984;85:519–522. doi: 10.1378/chest.85.4.519. [DOI] [PubMed] [Google Scholar]
- 31.Joos L, Patuto N, Chhajed PN, Tamm M. Diagnostic yield of flexible bronchoscopy in current clinical practice. Swiss Med Wkly. 2006;136:155–159. doi: 10.4414/smw.2006.11344. [DOI] [PubMed] [Google Scholar]
- 32.Mak VH, Johnston ID, Hetzel MR, Grubb C. Value of washings and brushings at fibreoptic bronchoscopy in the diagnosis of lung cancer. Thorax. 1990;45:373–376. doi: 10.1136/thx.45.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oswald NC, Hinson KF, Canti G, Miller AB. The diagnosis of primary lung cancer with special reference to sputum cytology. Thorax. 1971;26:623–627. doi: 10.1136/thx.26.6.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torrington KG, Kern JD. The utility of fiberoptic bronchoscopy in the evaluation of the solitary pulmonary nodule. Chest. 1993;104:1021–1024. doi: 10.1378/chest.104.4.1021. [DOI] [PubMed] [Google Scholar]
- 35.Trkanjec JT, Peros-Golubicic T, Grozdek D, Ivicevic A, Alilovic M. The role of transbronchial lung biopsy in the diagnosis of solitary pulmonary nodule. Coll Antropol. 2003;27:669–675. [PubMed] [Google Scholar]
- 36.Wongsurakiat P, Wongbunnate S, Dejsomritrutai W, et al. Diagnostic value of bronchoalveolar lavage and postbronchoscopic sputum cytology in peripheral lung cancer. Respirology. 1998;3:131–137. doi: 10.1111/j.1440-1843.1998.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 37.Debeljak A, Mermolja M, Sorli J, Zupancic M, Zorman M, Remskar J. Bronchoalveolar lavage in the diagnosis of peripheral primary and secondary malignant lung tumors. Respiration. 1994;61:226–230. doi: 10.1159/000196342. [DOI] [PubMed] [Google Scholar]
- 38.Lam WK, So SY, Hsu C, Yu DY. Fibreoptic bronchoscopy in the diagnosis of bronchial cancer: Comparison of washings, brushings and biopsies in central and peripheral tumours. Clin Oncol. 1983;9:35–42. [PubMed] [Google Scholar]
- 39.Wallace JM, Deutsch AL. Flexible fiberoptic bronchoscopy and percutaneous needle lung aspiration for evaluating the solitary pulmonary nodule. Chest. 1982;81:665–671. doi: 10.1378/chest.81.6.665. [DOI] [PubMed] [Google Scholar]
- 40.Herth FJ, Eberhardt R. Flexible bronchoscopy and its role in the staging of non-small cell lung cancer. Clin Chest Med. 2010;31:87–100. doi: 10.1016/j.ccm.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Rennard SI, Spurzem JR. Bronchoalveolar lavage in the diagnosis of lung cancer. Chest. 1992;102:331–332. doi: 10.1378/chest.102.2.331. [DOI] [PubMed] [Google Scholar]
- 42.Rennard SI, Albera C, Carratu L, et al. Clinical guidelines and indications for bronchoalveolar lavage (BAL): Pulmonary malignancies. Eur Respir J. 1990;3:956–957. 961–9. [PubMed] [Google Scholar]
- 43.van der Drift MA, van der Wilt GJ, Thunnissen FB, Janssen JP. A prospective study of the timing and cost-effectiveness of bronchial washing during bronchoscopy for pulmonary malignant tumors. Chest. 2005;128:394–400. doi: 10.1378/chest.128.1.394. [DOI] [PubMed] [Google Scholar]
- 44.McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369:910–919. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]


