Abstract
Honey bees (Apis mellifera) use the dance language to symbolically convey information about the location of floral resources from within the nest. To figure out why this unique ability evolved, we need to understand the benefits it offers to the colony. Previous studies have shown that, in fact, the location information in the dance is not always beneficial. We ask, in which ecological habitats do honey bee colonies actually benefit from the dance language, and what is it about those habitats that makes communication useful? In this study, we examine the effects of floral distribution patterns on the benefits of dance communication across five different habitats. In each habitat, we manipulated colonies’ ability to communicate and measured their foraging success, while simultaneously characterizing the naturally occurring floral distribution. We find that communication is most beneficial when floral species richness is high and patches contain many flowers. These are ecological features that could have helped shape the evolution of the honey bee dance language.
Keywords: Apis mellifera, foraging, communication, species richness, spatial ecology, resource distribution
Introduction
Honeybees famously use their “dance language” to communicate with one another about resources such as food, water and nest sites. A forager returning from a flower rich with pollen or nectar may choose to advertise it to her nestmates by dancing; the details of her dance indicate the direction and distance of the resource (von Frisch, 1967). Although it is well known that honey bees also find flowers by other means — such as the floral odor carried by a successful forager (von Frisch 1967; Wenner et al. 1969) — the dance language is one of the few known examples of symbolic communication in non-human animals. Other bees such as bumble bees and stingless bees have also been shown to communicate about food sources, but none are known to convey location information from within the nest (Dornhaus and Chittka 1999; Nieh et al. 2003; Barth et al. 2008). To understand why honey bees in particular evolved a language for communicating resource location, we need to know how it improves colony foraging performance. In which ecological contexts does communication provide a benefit, and why?
Previous studies have shown that the benefit of communicating location information varies greatly depending on season and habitat. For bees on an agricultural research station in California, the ability to communicate location increased the amount of nectar a colony collected in winter, but had no effect in summer or autumn (Sherman and Visscher 2002). Comparing across habitats, Dornhaus and Chittka (2004) found a positive effect on nectar collection in a dry tropical forest in India, but not in temperate sites in Germany and Spain. The tropical forest may represent an ecological habitat more similar to the ancestral home of the honey bee clade (Apis), where the dance language is thought to have evolved (Lindauer 1956; Engel and Schultz 1997).
The question remains, however: what is it about those different habitats and seasons that makes communication more or less valuable? One hypothesis, for example, is that some tropical flowering trees provide a rich but short-lived resource, making communication particularly important (Dornhaus and Chittka 2004). Analytical models (Anderson 2001; Dechaume-Moncharmont et al. 2005) and individual-based simulations (Dornhaus et al. 2006; Beekman and Lew 2008) both suggest that communicating resource location should be most important when resources are scarce, patchy or variable in quality. However, to date there has been no study designed to empirically test these ideas.
We performed a series of field studies to test three non-exclusive hypotheses about the effects of resource distribution on the benefits of dance communication. First, if resources are difficult to find, as when the density of flower patches is low, information about where to find them should be of higher value. Second, if resources tend to be clustered together in space, resulting in many flowers per patch, many foragers could potentially take advantage of a single scout’s find, making communication more valuable. Third, if resources are highly variable in quality, the feedback mechanism inherent in dance communication may allow the colony to concentrate more foragers on the best resources, making foraging more efficient. Because the pollen and nectar rewards accessible to honey bees vary greatly among species of flowering plants, we expect that in environments where many species are in bloom, resources will tend to be more variable in quality. This may make it more important to communicate the location of the most rewarding species.
Methods
We set out to measure the benefit of communicating about resource location in honey bee colonies by measuring foraging success with and without location information across several different environments. We chose four sites located within 100 km of Tucson, Arizona, that represented three dramatically different vegetation communities. We performed a series of five experiments at different times and places over the course of one year, with the seasons and habitats chosen to maximize differences in resource distribution. For each of the five experiments, we also characterized the resource distribution present at the time, measuring patch density, number of flowers per patch and species richness of plants in bloom.
Experiment dates and locations
Experiments 1 and 2 were performed at the Sonoran Arthropod Studies Institute (SASI), a protected Upper Sonoran Desert habitat within Tuscon Mountain Park. Vegetation is primarily Sonoran desertscrub, dominated by cacti, creosote bush (Larrea tridentata), and small leguminous trees. Experiment 1 took place in late spring (April 21–May 2, 2010) when spring wildflowers and some cacti were in bloom. Experiment 2 took place during foresummer drought (May 20–June 1, 2010) when little was in bloom besides saguaro (Carnegiea gigantea) and two species of leguminous trees (Parkinsonia microphylla and Olneya tesota.)
Experiment 3 was performed at the Appleton-Whittell Research Ranch (AWRR), an Audobon Society preserve near Elgin, Arizona. The vegetation community is mesquite-oak grassland. The experiment took place in foresummer drought (June 18 30, 2010) when a variety of scattered annual and perennial herbs were blooming.
Experiment 4 was performed at the Santa Rita Experimental Range Headquarters in Florida Canyon (SRER.) The headquarters is located at the mouth of a riparian canyon leading into the Santa Rita Mountains. The experiment took place during the monsoon season (August 9–20, 2010); rainfall was frequent, and the wash was in full flow. A wide variety of summer wildflowers, perennial herbs and shrubs were in bloom, the most common being a prolific leguminous shrub (Mimosa dysocarpa.)
Experiment 5 was performed at Desert Station, a University of Arizona preserve in the Tucson Mountains (UADS.) The vegetation community at Desert Station is Sonoran desertscrub, similar to that at SASI. Experiment 5 took place in the fall (October 9–20, 2010) when few plants were flowering besides one highly abundant species of flowering vine (Janusia gracilis.)
Effect of communication on foraging success
To measure the effect of communication about resource location on colony success, we used an established technique to interfere with the location information in the dance (von Frisch 1967; Visscher and Seeley 1982; Dornhaus and Chittka 2004). The waggle dance normally takes place inside a dark hive, on vertically oriented honeycomb; the angle of the dance relative to vertical indicates the angle of the resource relative to the sun’s azimuth (von Frisch 1967). When combs are oriented horizontally, there is no reference point for the dance, and bees dance in random directions without conveying location information. However, if a polarized light source such as the sun is available, dancers will use it as an alternative reference point, and can successfully communicate resource location (von Frisch 1967).
Six colonies of about 10,000 domestic Italian honey bees (Apis mellifera ligustica) were placed in modified 10-frame Langstroth hives. The hives were custom built according to the design used by Dornhaus and Chittka (2004). When the hives were turned on their sides, so that the frames were oriented horizontally, a window cut into one side allowed sunlight to hit the top frame. An entrance led directly onto that top frame, and bees were forced to walk across the frame before they could reach other parts of the hive, ensuring that most dances occurred on the top frame. We compared two treatments: one without location information, where the window was covered and no reference point was available, and one with location information, where the window was uncovered and directional light could be used as a reference point. Even when the window was uncovered, we ensured that it was shaded, so that direct sunlight would not overheat the exposed frame. Previous research using the same hive design has demonstrated that dances do become disoriented (with waggle runs in random directions) when directional light is removed (Sherman and Visscher 2002; Dornhaus and Chittka 2004), resulting in decreased recruitment to artificial feeders (Sherman and Visscher 2002).
We measured colony success by weighing the hives every evening at sunset, when most foragers had returned to the hive; the daily weight change largely reflects the amount of nectar collected by the colony (Seeley 1995). We also measured pollen collection by weighing pollen collected in front-mounted pollen traps (Better bee, Greenwich, NY). Pollen was only collected every third day from each colony, so as not to discourage foraging activity. Each experiment lasted 12 days, with each colony spending two three-day blocks in each treatment, with and without location information. A typical experimental schedule is shown in Table 1.
Table 1. A typical experimental schedule.
Each experiment lasted 12 days, with each colony spending staggered blocks of 3 days in each treatment: window open(communication with location information intact) and window closed(communication with location information removed.) Pollen was collected from each colony for one day every third day.
| Day |
 window closed window closed |
□ window open | P pollen trap | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colony | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| A | P | P | P | P | ||||||||
| B | P | P | P | P | ||||||||
| C | P | P | P | P | ||||||||
| D | P | P | P | P | ||||||||
| E | P | P | P | P | ||||||||
| F | P | P | P | P | ||||||||
Besides pollen and nectar, honey bees may also use the dance to communicate about water resources when they are scarce. Because water availability is often a problem in the desert, particularly in the foresummer drought in June, we made sure that water was available close to the hives so that any benefits of communicating about water would not influence our results.
Characterization of resource distribution
For each experiment, we measured the spatial distribution of blooming plants using censored T-square sampling (Diggle 1983; Zimmerman 1991). Random sampling points were chosen along trails or roads radiating outwards from the central hive location, within a distance of 0.5 km. We generated pairs of random numbers, walked along the trail the number of meters indicated by the first number, and walked perpendicularly off the trail the number of meters indicated by the second number. Then we measured the distance from the random point i to the closest blooming plant (xi), and the distance from the closest blooming plant to the next closest blooming plant in the half-plane away from the random point (yi). If there was no plant blooming within a specified radius (Experiment 1: 10 m; Experiments 2–5: 25 m) of the random point, the first distance (xi) was set to that maximum radius, and similarly for the second distance (yi) – this is the censoring. The number of closest plants actually found is n, and the number of next closest plants actually found is m. We also estimated the number of flowers per plant for both the closest blooming plant (ki) and the next closest blooming plant (li).
To test our first hypothesis, we needed to measure the difficulty of finding resources when searching independently. On average, how much area would a forager have to search in order to find a blooming plant, when starting from a random point? We call the reciprocal of this the patch density, and define an estimate of it as follows:
This is simply the number of closest blooming plants found, divided by the total area searched for those closest blooming plants.
To test our second hypothesis, we needed a measure of how clustered the resources were in space. Once a single flower had been located, how many more flowers could a forager expect to find nearby, either on the same plant or on other plants close by? First of all, we define an estimate of the overall flower density based on Diggle’s compound density estimator λ̂D (Diggle 1983):
This is the geometric mean of two estimates of flower density: the number of flowers found divided by the area searched, for both the closest and next closest blooming plants. Then, to estimate the number of flowers per patch, we simply divide the flower density by the patch density:
We call this the number of flowers per patch, and it increases both for high numbers of flowers per plant, as for flowering trees or shrubs, and when plants are clustered together in space.
Finally, to test our third hypothesis, we needed a measure of how variable in quality the resources were. Quantifying nectar volume and sugar content from a wide variety of species, many with miniscule amounts of nectar, proved impractical. We therefore used species richness as a proxy for variation in quality. At each random point, a survey was done to identify all species of plants that were blooming within a 10-meter radius. Because different numbers of random points were sampled in each experiment (14, 15, 18, 16, and 16 sites for Experiments 1–5, respectively) we standardized on 18sites. For each experiment, we fitted a Monod function to species accumulation over sites using the R packages vegan and mmSAR, and used the predicted number of species at 18 sites as our measure of species richness (Monod 1950; Guilhaumon 2011; Oksanen et al. 2011).
Effect of resource distribution on benefits of communication
Colony foraging success is known to vary dramatically from day to day, changing with weather conditions and resource availability (Seeley 1995). To control for these factors, we compared the daily weight change of each colony only to weight changes measured for other colonies on the same day, For each colony on each day, we asked, was its daily weight change above or below the median weight change for all six colonies on that day? (The median was used because the distribution of daily weight changes was heavy-tailed, with frequent large gains or large losses that unduly influenced the mean.) If the weight change was exactly equal to the median, no response was scored. The same procedure was performed for the pollen weights. Since only two colonies had pollen traps each day, one for each treatment, we were in effect asking which of the two colonies collected more pollen: the one with or without location information?
We then performed two logistic regressions, one for each of these binary response variables, using the R software package (Dorai-Raj 2009; R Development Core Team 2009). We regressed each response variable on communication treatment crossed with the three resource distribution parameters: patch density, flowers per patch, and species richness. To identify effects of resource distribution on the benefits of communication, we looked specifically for interaction effects between communication treatment (with and without location information) and each characteristic of the resource distribution. Thus, for the first hypothesis, we predicted that if communication is more important when resources are difficult to find, we should see a significant negative interaction between communication treatment and patch density. Similarly, for the second hypothesis, we predicted that if communication is more important when resources are clustered, we should see a significant positive interaction between communication treatment and the number of flowers per patch. Finally, for the third hypothesis, we predicted that if communication is more important when resources are highly variable in quality, and species vary in their floral rewards, we might see a significant positive interaction between communication treatment and floral species richness.
Honey bees are also known to show consistent differences between colonies in the amount of nectar and pollen they collect (Page et al. 1995). The balanced experimental design controlled for potential differences by subjecting each colony to both experimental treatments (communication with location information and without; see Table 1.) However, to ensure that the statistical model accounted for any differences between colonies, we performed a mixed-effects logistic regression with colony identity included as a random effect. Results for the regression with and without these random effects were not qualitatively different; here we report the results from the mixed-effects model.
Results
The five experiments each showed a different combination of our three resource distribution variables: patch density, number of flowers per patch, and species richness (see Fig. 1.) For example, Experiments 2, 4 and 5 all had many flowers per patch, but Experiment 5 was distinguished from the others by its high patch density, while Experiment 4 was distinguished by its high species richness. This allowed us to statistically disentangle the effects of the three different resource distribution variables.
Fig 1. Resource distributions.
At left, the patch density (λ̂p) and number of flowers per patch (k̂p) are shown for each of the five experiments; contour lines indicate equal flower density (λ̂f = k̂p λ̂p). At right, the species accumulation curves are extrapolated to 18 sites (dashed line) to calculate estimated species richness for each experiment (see text.)
The daily weight change of colonies varied quite a bit in some experiments and very little in others (see Fig 2a.) For example, in Experiment 1, it varied from −1.14 kg to +0.92 kg (colonies weighed between 15.18 and 23.80 kg), while in Experiment 5 it varied from −0.30 kg to +0.08 kg (colonies weighed between 14.90 and 19.30 kg.)Such dramatic changes are consistent with results from previous experiments (Seeley 1995; Sherman and Visscher 2002; Dornhaus and Chittka 2004). Similarly, the amount of pollen collected varied dramatically from day to day and between colonies (see Fig 2b), with the largest amounts collected by the same colony in Experiments 1 (39 g) and 2 (35 g). Again, this high level of variation is consistent with previous reports (e.g. O’Neal and Waller 1984). Because both daily weight change and pollen weight departed significantly from normality (Shapiro-Wilk test; p = 1 × 10−9 for daily weight change, p = 1 × 10−14 for pollen weight) we used a non-parametric test, as described in the Methods section, to evaluate the effects of environmental characteristics on the benefits of communication for each measure of foraging success.
Fig 2. Foraging success.
Foraging success was highly variable both with time and across colonies. (a) The amount of nectar collected by each colony per day is measured by the daily weight change. (b) The amount of pollen collected by each colony per day is measured by weighing pollen collected in a pollen trap. Horizontal lines indicate the median for each experiment.
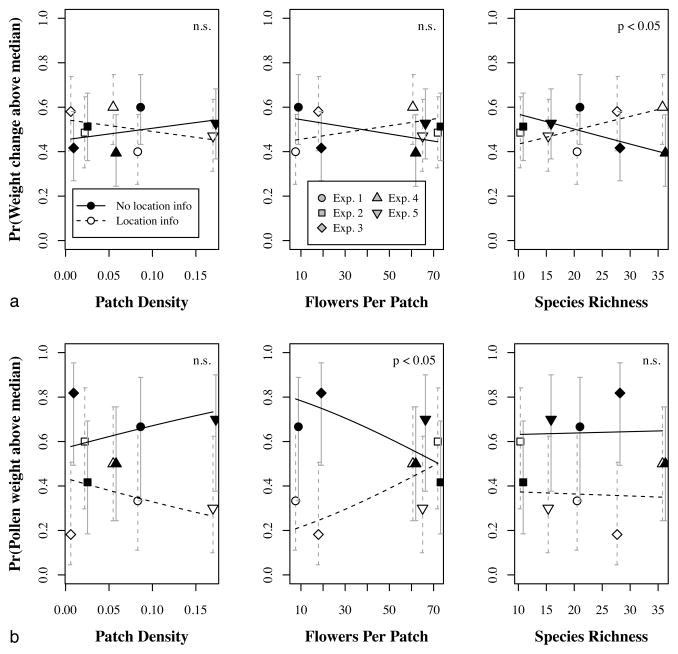
For all experiments taken together, there was no benefit of communication to the amount of nectar collected: whether a colony collected more nectar than others on the same day was not affected by its ability to communicate location information (main effect of communication treatment on weight change was not significant at the 5% level, Table 2.) However, the benefit of communication varied markedly from one experiment to the next, and greater benefit was significantly associated with higher floral species richness (interaction effect of communication treatment and species richness on weight change, Table 2 and Fig 3a.) In fact, the two experiments with the highest benefit of communication were the two with the highest species richness (Experiments 3 and 4, Fig 3a.) This supports our third hypothesis: high species richness should make communicating about resource location more valuable.
Table 2. Logistic regression for colony weight change.
Estimated effects and marginal p-values for each term specified in the full logistic regression model for daily weight change (see text.) Across all experiments, there was no significant effect of the ability to communicate location on the probability of a colony’s weight change being above the median for the day. However, there was a significant interaction effect for communication and species richness: in experiments with higher species richness, the benefit of communicating location information was greater.
| Nectar Collection (Daily weight change) | Estimated Coefficient | Standard Error | Z value | p-value |
|---|---|---|---|---|
| (Intercept) | 0.697 | 0.619 | 1.126 | 0.260 |
| Communication | −1.341 | 0.856 | −1.566 | 0.117 |
| Patch Density | 2.063 | 2.712 | 0.761 | 0.447 |
| Flowers Per Patch | −0.006 | 0.006 | −1.054 | 0.292 |
| Species Richness | −0.027 | 0.018 | −1.465 | 0.143 |
| Communication × Patch Density | −4.197 | 3.919 | −1.071 | 0.284 |
| Communication × Flowers Per Patch | 0.013 | 0.009 | 1.438 | 0.151 |
| Communication × Species Richness | 0.052 | 0.026 | 2.000 | 0.046 |
Fig 3. Effect of resource distribution and location information on foraging success.
(a) As species richness increases, location information significantly improves the rate of nectar collection. (b) As the number of flowers per patch increases, location information significantly improves the rate of pollen collection. Lines show predicted values from the overall logistic regression (see text for details); p-values shown are for the interaction between communication and each resource distribution characteristic (see Table 2 and Table 3.) Error bars are 95% confidence intervals calculated for the observed proportions. The x axis values for the treatment without location information are slightly offset, so that the error bars do not overlap.
Similarly, the ability to communicate location information did not increase the amount of pollen collected overall (main effect of communication treatment on pollen weight, Table 3.) For pollen foraging, high species richness did not significantly increase the value of communication, but having more flowers per patch did (interaction effect of communication treatment and flowers per patch on pollen weight, Table 3 and Fig 3b.) Of the three experiments with more than 50 flowers per patch on average, two showed the highest benefit of communication to pollen collection (Experiments 2 and 4, Fig 3b.) In the third one, Experiment 5, communication was not beneficial; however, the high number of flowers per patch was driven primarily by one highly abundant species of flowering vine (Janusia gracilis), which produces oil instead of nectar and is pollinated by specialist oil-collecting bees in the genus Centris (Buchmann 1987). Honey bees are not known to visit this species and thus are unlikely to have been greatly affected by its distribution. This supports our second hypothesis: communicating resource location should be more important when patches have many flowers.
Table 3. Logistic regression for pollen weight.
Estimated effects and marginal p-values for each term specified in the full logistic regression model for pollen collected (see text.) As for the overall colony weight change, there was no consistent effect of the ability to communicate resource location on the probability of collecting more pollen than another colony on the same day. However, there was a significant interaction effect for communication and the number of flowers per patch: when patches contained more flowers, the benefit of communicating location was higher.
| Pollen Collection (Pollen weight) | Estimated Coefficient | Standard Error | Z value | p-value |
|---|---|---|---|---|
| (Intercept) | 1.144 | 1.198 | 0.954 | 0.340 |
| Communication | −2.252 | 1.670 | −1.349 | 0.177 |
| Patch Density | 4.277 | 5.363 | 0.798 | 0.425 |
| Flowers Per Patch | −0.021 | 0.013 | −1.605 | 0.109 |
| Species Richness | 0.003 | 0.032 | 0.085 | 0.932 |
| Communication × Patch Density | −8.763 | 7.704 | −1.137 | 0.255 |
| Communication × Flowers Per Patch | 0.042 | 0.018 | 2.271 | 0.023 |
| Communication × Species Richness | −0.007 | 0.047 | −0.145 | 0.885 |
Discussion
To understand why honey bees evolved their unique dance language, we need to know how ecological context shapes the benefits of the dance. Previous theoretical work suggested that resource distribution could play an important role, with communication being most important when resources are (1) sparse, (2) clustered in space, or (3) variable in quality (Anderson 2001; Dechaume-Moncharmont et al. 2005; Dornhaus et al. 2006; Beekman and Lew 2008). The only existing empirical support for any of these hypotheses comes from Dornhaus and Chittka (2004), in which it was found that communication is more beneficial in a tropical Indian site than two temperate European ones. By decoding dance information a posteriori, the authors showed that advertised resources in tropical sites tended to be more clustered together than those in temperate sites, lending support to the second hypothesis.
In this study, we looked at the impacts of patch density, the number of flowers per patch, and species richness on the value of dance communication by comparing foraging success with and without communication across several natural environments. This is the first study designed to empirically assess the influence of several different aspects of resource distribution on the value of dance communication. We found support for two of our hypotheses: first of all, that communication should be more valuable in species-rich environments, and secondly, that it should be more valuable when there are many flowers per patch. The result for species richness is the first empirical support for the theoretical prediction that communication is more valuable when resources vary in quality. The result for flower number per patch greatly strengthens the existing evidence that communication is more valuable when resources are patchily distributed. It improves upon previous work by directly evaluating the distribution of all potential floral resources, not just the ones that were actually found and advertised by the bees (Dornhaus and Chittka 2004). We did not find any evidence for an influence of the sparseness of resources, as measured with patch density. This fits with Sherman and Visscher’s observation that the benefits of dance communication were greatest in the season with intermediate weight gains; they suggested that spatial and temporal heterogeneity might play a greater role than overall resource abundance (Sherman and Visscher 2002).
Our motivation for looking at species richness was that environments with many species of flowers in bloom may also tend to have high variation in resource profitability, because flower species vary in the quality, quantity and accessibility of the pollen and nectar rewards they offer. When resources vary in profitability, dance communication allows the colony to focus more of its foraging effort on more profitable resources (Seeley 1986). Thus we predicted that in environments with many species in bloom, communication might help colonies by allowing them to concentrate on the most rewarding species. Although there may be substantial variation in resource value within plant species as well, the fact that we did see a significant effect of species richness on the benefits of communication to nectar collection suggests that colonies may indeed benefit from communication by preferentially recruiting to the most profitable species. Though information about the species of flower being advertised is available in odor cues even when location information is not present, odor information alone appears to be effective only at shorter distances; location information significantly improves recruitment as long as the resource is at least 100 meters away (von Frisch 1967; Wenner et al. 1969; Kirchner and Grasser 1998). The observed effect of species richness on the value of location information may thus reflect a communicating colony’s improved ability to allocate foragers to high quality flower species that are not located near the hive.
We suggested that patches of many flowers, like flowering trees or large clumps of annual wildflowers, should increase the value of communicating location information because many individual foragers could take advantage of a single bee’s discovery. It could also be that high variation in the number of flowers per patch makes communication more valuable because the dance language allows the colony to allocate more foragers to patches with more flowers. Since environments with a high average number of flowers per patch also have high variation in flower number, we could not distinguish these two potential effects. Yet another possibility is that large patches of flowers are more conspicuous and thus easier to find given only the rather imprecise location information in the dance, which could potentially make communication more effective. Further research will be required to distinguish between these reasons for the positive effect of flower number on the benefits of communication.
We found that species richness affected the value of communication for nectar collection, while the number of flowers per patch did the same for pollen collection. The fact that different aspects of the resource distribution were important for our two different measures of foraging success underscores the importance of measuring both. It also raises the question: why did we see different results for these two measures? First of all, our measures of foraging success for pollen and nectar may not equally reflect resource quality. The value of nectar lies in its sugar content; while floral nectars vary greatly in sugar concentration, stored honey has been reduced to about 16–20% water and thus its weight is a good indicator of the amount of sugar collected (Seeley 1995). On the other hand, pollen is valued for the protein, vitamins, and lipids it contains, which bear little relation to the overall weight of the pollen (for example, pollen protein content ranges from 2.5% to 61% by weight, depending on species (Roulston et al. 2000)). Though research has suggested that foragers cannot directly assess the protein content of pollen, they do show preferences for some types of pollen over others, which could be related to the nutritional value (Pernal and Currie 2001, 2002; Cook et al. 2003; Liu et al. 2005). Communication in species-rich environments might therefore allow colonies to concentrate more on high-quality pollen, without necessarily increasing the amount of pollen collected. Alternatively, it may be that for both pollen and nectar foragers, the dance language benefits the colony by helping to allocate foragers to more profitable resources, but that profitability of nectar and pollen sources is associated with different characteristics in the floral communities we examined. Pollen source profitability might be more closely associated with flower number, while nectar source profitability might be more closely associated with species identity.
We chose to study the effects of natural resource distributions for this work because we wanted to know not just how resource distribution per se affects the value of communication, but also how ecologically relevant different aspects of the resource distribution are. However, the drawback to this approach is that the resource distributions were not the only factors that varied between experiments. Although habitat and season certainly affect resource distribution, they may also affect other variables such as visual complexity of the environment, foraging motivation and foraging competition.
Visual complexity of the environment could make flowers harder to find, even at the same patch density, potentially making communication more important. Although we did not quantitatively assess the visual complexity of the environment, Experiment 4 stands out as the most visually complex, being in the mouth of a canyon in the mountains, with relatively large trees along the banks of the wash. In contrast, Experiment 3, done in the grasslands, is certainly the least visually complex. These two experiments were the ones that showed the highest benefit of communication for nectar collection, suggesting that visual complexity does not play a major role in determining the benefits of communication.
Another potential difference between experiments is a change in foraging motivation over the course of the season. Overall foraging rate as well as the balance between pollen and nectar foraging changes during the year, due to changes in resource availability and changing colony needs over the course of its annual cycle (Percival 1947; Synge 1947; McLellan 1976; Hellmich and Rothenbuhler 1986). A previous study conducted in the Tucson area found that pollen is primarily collected during two periods of the year, in response to the biennial pattern of rainfall: February-June and August-October (O’Neal and Waller 1984). All five of our experiments were conducted during these two time periods. Although we did see more pollen collected in the first two experiments (during April and May) than the others, this is more likely due to a particularly dry monsoon season (July-August 2010) reducing flower availability later in the year rather than to a decrease in motivation to forage for pollen. We saw the greatest benefit of communication to pollen collection in May and August, so the value of communication to pollen collection was not primarily determined by the amount of pollen collected overall.
It has been suggested that competition with other pollen and nectar foragers could make dance communication particularly valuable; communicating colonies might be able to quickly recruit to rich resources, out competing other foragers (Seeley and Visscher 1988). If this is true, we might expect this effect to be strongest in environments where resource patches are particularly valuable and/or vary greatly in quality, either because they contain many flowers or because the flowers themselves vary in the amount of reward offered. This could have enhanced the hypothesized effects of both the number of flowers per patch and species richness on the benefits of communication. However, it is also possible that natural variation in the amount of competition between sites and habitats could have affected our measures of the benefits of communication, independently of resource distribution. Since we did not compare pollinator visitation rates across experiments, we cannot rule this out. Feral honey bee colonies were known to exist at all our sites, but could have varied in number and proximity to the experimental hives. Still, the close proximity of our six experimental hives to one another in each experiment likely created a fairly high level of competition in all five experiments, making differences in the resource distribution more important.
We have shown that species richness and the number of flowers per patch are both ecologically relevant variables that affect the benefits of honey bee communication. The honey bee dance language is thought to have evolved in tropical forests of south or southeast Asia, where all species of Apis except A. mellifera are currently found (Lindauer 1956; Engel and Schultz 1997). Tropical forests in general are known for their incredible species richness (MacArthur 1972). Furthermore, unlike temperate forests, in which the vast majority of trees are wind-pollinated, tropical forests consist mainly of animal-pollinated tree species, which may provide patches of many rewarding flowers (Janzen 1975). For example, in the tropical forests of Malaysia, Apis dorsata forages at the highest level of the canopy, where synchronously flowering trees such as some of the bee-pollinated dipterocarps present crowns full of highly rewarding flowers (Ashton 1988; Bawa 1990a; b). Our work therefore suggests two important characteristics of honey bees’ ancestral ecological habitat that could have selected for their unique ability to symbolically communicate resource location.
Acknowledgments
M.C.D was funded by the University of Arizona’s Center for Insect Science through NIH Training Grant #1K12GM000708. A.D. was funded by NSF grants #IOS-0921280 and IOS-0841756. Honey bee colonies were kindly provided by R. Page at Arizona State University and G. DeGrandi-Hoffman at the USDA Carl Hayden Bee Research Center, and housed at the latter facility with assistance from M. Chambers and T. Deeby. We thank E. Francis (SASI), L. Kennedy (AWRR), M. Heitlinger (SRER) and R. Smith (UADS) for their cooperation and support at our field sites. P. Jenkins of the UA Herbarium and L. Kennedy helped with plant species identification. We gratefully acknowledge the field work assistance of N. Matasci, G. Barraza, J. Brown, J. Chappell, M. Hughes, J. Icely, N. Narkhede, S. Williams and Y. Zhu.
References
- Anderson C. The adaptive value of inactive foragers and the scout-recruit system in honey bee (Apis mellifera) colonies. Behav Ecol. 2001;12:111–119. [Google Scholar]
- Ashton PS. Dipterocarp Biology as a Window to the Understanding of Tropical Forest Structure. Annu Rev Ecol Syst. 1988;19:347–370. doi: 10.1146/annurev.es.19.110188.002023. [DOI] [Google Scholar]
- Barth FG, Hrncir M, Jarau S. Signals and cues in the recruitment behavior of stingless bees (Meliponini) J Comp Physiol A. 2008;194:313–327. doi: 10.1007/s00359-008-0321-7. [DOI] [PubMed] [Google Scholar]
- Bawa KS. Reproductive ecology of tropical forest plants. UNESCO; Paris: 1990a. [Google Scholar]
- Bawa KS. Plant-Pollinator Interactions in Tropical Rain Forests. Annu Rev Ecol Syst. 1990b;21:399–422. doi: 10.1146/annurev.es.21.110190.002151. [DOI] [Google Scholar]
- Beekman M, Lew J. Foraging in honeybees--when does it pay to dance? Behav Ecol. 2008;19:255–261. doi: 10.1093/beheco/arm117. [DOI] [Google Scholar]
- Buchmann SL. The Ecology of Oil Flowers and their Bees. Annu Rev Ecol Syst. 1987;18:343–369. [Google Scholar]
- Cook SM, Awmack CS, Murray DA, Williams IH. Are honey bees’ foraging preferences affected by pollen amino acid composition? Ecol Entomol. 2003;28:622–627. doi: 10.1046/j.1365-2311.2003.00548.x. [DOI] [Google Scholar]
- Dechaume-Moncharmont FX, Dornhaus A, Houston AI, McNamara JM, Collins EJ, Franks NR. The hidden cost of information in collective foraging. P Roy Soc B-Biol Sci. 2005;272:1689. doi: 10.1098/rspb.2005.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle P. Statistical analysis of spatial point patterns. Academic Press; London; New York: 1983. [Google Scholar]
- Dorai-Raj S. binom: Binomial Confidence Intervals For Several Parameterizations 2009 [Google Scholar]
- Dornhaus A, Chittka L. Insect behaviour: Evolutionary origins of bee dances. Nature. 1999;401:38. [Google Scholar]
- Dornhaus A, Chittka L. Why do honey bees dance? Behav Ecol Sociobiol. 2004;55:395–401. [Google Scholar]
- Dornhaus A, Klugl F, Oechslein C, Puppe F, Chittka L. Benefits of recruitment in honey bees: effects of ecology and colony size in an individual-based model. Behav Ecol. 2006;17:336–344. doi: 10.1093/beheco/arj036. [DOI] [Google Scholar]
- Engel MS, Schultz TR. Phylogeny and Behavior in Honey Bees (Hymenoptera: Apidae) Ann Entomol Soc Am. 1997;90:43–53. [Google Scholar]
- Guilhaumon F. mmSAR: mmSAR is an R package for the modelling of the Species-Area Relationship (SAR) 2011 [Google Scholar]
- Hellmich RL, Rothenbuhler WC. Relationship between different amounts of brood and the collection and use of pollen by the honey bee (Apis mellifera) Apidologie. 1986;17:8. doi: 10.1051/apido:19860102. [DOI] [Google Scholar]
- Janzen D. Ecology of plants in the tropics. Edward Arnold; London: 1975. [Google Scholar]
- Kirchner WH, Grasser A. The significance of odor cues and dance language information for the food search behavior of honeybees (Hymenoptera: Apidae) J Insect Behav. 1998;11:169–178. [Google Scholar]
- Lindauer M. Uber die Verstandigung bei indischen Bienen. Z Vergl Physiol. 1956;38:521–557. doi: 10.1007/BF00341108. [DOI] [Google Scholar]
- Liu F-L, Zhang X-W, Chai J-P, Yang D-R. Pollen phenolics and regulation of pollen foraging in honeybee colony. Behav Ecol Sociobiol. 2005;59:582–588. doi: 10.1007/s00265-005-0084-x. [DOI] [Google Scholar]
- MacArthur R. Geographical ecology : patterns in the distribution of species. Harper & Row; New York: 1972. [Google Scholar]
- McLellan AR. Factors Affecting Pollen Harvesting by the Honeybee. J Appl Ecol. 1976;13:801–811. doi: 10.2307/2402256. [DOI] [Google Scholar]
- Monod J. La technique de culture continue theorie et applications. Ann I Pasteur Paris. 1950;79:390–410. [Google Scholar]
- Nieh JC, Contrera FAL, Rangel J, Imperatriz-Fonseca VL. Effect of food location and quality on recruitment sounds and success in two stingless bees, Melipona mandacaia and Melipona bicolor. Behav Ecol Sociobiol. 2003;55:87–94. doi: 10.1007/s00265-003-0680-6. [DOI] [Google Scholar]
- O’Neal RJ, Waller GD. On the Pollen Harvest by the Honey Bee (Apis mellifera L.) near Tucson, Arizona (1976–1981) Desert Plants. 1984;6:81–109. [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H. vegan: Community Ecology Package 2011 [Google Scholar]
- Page RE, Waddington KD, Hunt GJ, Kim Fondrk M. Genetic determinants of honey bee foraging behaviour. Animal Behaviour. 1995;50:1617–1625. doi: 10.1016/0003-3472(95)80015-8. [DOI] [Google Scholar]
- Percival M. Pollen collection by Apis mellifera. New Phytol. 1947;46:142–165. doi: 10.1111/j.1469-8137.1947.tb05076.x. [DOI] [Google Scholar]
- Pernal SF, Currie RW. The Influence of Pollen Quality on Foraging Behavior in Honeybees (Apis mellifera L.) Behav Ecol Sociobiol. 2001;51:53–68. [Google Scholar]
- Pernal SF, Currie RW. Discrimination and preferences for pollen-based cues by foraging honeybees, Apis mellifera L. Anim Behav. 2002;63:369–390. 06/anbe.2001.1904. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: 2009. [Google Scholar]
- Roulston TH, Cane JH, Buchmann SL. What governs protein content of pollen: pollinator preferences, pollen-pistil interactions, or phylogeny? Ecol Monogr. 2000;70:617–643. [Google Scholar]
- Seeley TD. Social foraging by honeybees: how colonies allocate foragers among patches of flowers. Behav Ecol Sociobiol. 1986;19:343–354. [Google Scholar]
- Seeley TD. The wisdom of the hive : the social physiology of honey bee colonies. Harvard University Press; Cambridge Mass: 1995. [Google Scholar]
- Seeley TD, Visscher PK. Assessing the benefits of cooperation in honeybee foraging: search costs, forage quality, and competitive ability. Behav Ecol Sociobiol. 1988;22:229–237. [Google Scholar]
- Sherman G, Visscher PK. Honeybee colonies achieve fitness through dancing. Nature. 2002;419:920–922. doi: 10.1038/nature01127. [DOI] [PubMed] [Google Scholar]
- Synge AD. Pollen Collection by Honeybees (Apis mellifera) J Anim Ecol. 1947;16:122–138. doi: 10.2307/1492. [DOI] [Google Scholar]
- Visscher PK, Seeley TD. Foraging Strategy of Honeybee Colonies in a Temperate Deciduous Forest. Ecology. 1982;63:1790–1801. [Google Scholar]
- von Frisch K. The dance language and orientation of bees. Belknap Press of Harvard University Press; Cambridge Mass: 1967. [Google Scholar]
- Wenner AM, Wells PH, Johnson DL. Honey bee recruitment to food sources: olfaction or language? Science. 1969;164:84–86. doi: 10.1126/science.164.3875.84. [DOI] [PubMed] [Google Scholar]
- Zimmerman D. Censored distance-based intensity estimation of spatial point processes. Biometrika. 1991;78:287. doi: 10.1093/biomet/78.2.287. [DOI] [Google Scholar]