Abstract
Background
In solid organ transplant patients, non-participation in all aspects of the medical regimen is a prevalent problem associated with adverse consequences particularly in the adolescent and young adult (AYA) age group. This study is the first to evaluate the feasibility, utility and impact of a text messaging (TM) intervention to improve participation in laboratory testing in adolescent liver transplant patients.
Methods
AYA patients, aged 12 to 21 years, were recruited for a prospective pilot trial evaluating a TM intervention delivered over a 1-year period. The intervention involved automated TM reminders with feedback administered according to a prescribed laboratory testing frequency. Participation rate in laboratory testing after the intervention was compared to the year prior. Patient responses and feedback by text and survey were used to assess feasibility, acceptability and use of the intervention.
Results
Forty-two patients were recruited and 33 patients remained enrolled for the study duration. Recipients of the TM intervention demonstrated a significant improvement in participation rate in laboratory testing from 58% to 78% (P<.001). This rate was also significantly higher than in non-intervention controls (P=.003). There was a high acceptability, response rate and a significant correlation with reported versus actual completion of laboratory tests by TM.
Conclusions
TM reminders significantly improved participation in laboratory testing in AYA liver transplant patients. The intervention demonstrated feasibility, acceptability, and use with a high proportion of patients who engaged in and perceived a benefit from using this technology.
Keywords: adolescents, mobile health, mHealth, HIT, Self-management, Participation, Self-care, Text-messaging, Liver Transplant
Introduction
Facilitating active participation in self-care remains an important goal in the current health care and patient community. [1]. In the pediatric literature, non-participation, commonly referred to as “non-adherence” is described as a prevalent problem that has led to poor outcomes, unreliable treatment efficacy assessments, unnecessary clinical interventions and enormous costs to American taxpayers. [2] Organ transplantation represents a life-saving procedure that requires long-term immunosuppressive medication and regular laboratory testing to ensure excellent outcomes. Frequent laboratory testing represents an important mechanism to monitor disease activity as in many patients with chronic illness. However, in adolescent transplant patients, non-participation in all aspects of the medical regimen including laboratory testing is a prevalent problem associated with adverse consequences. [3–7] The lack of proven interventions requires further studies to address this problem in this high-risk population.
Technology-based approaches represent a promising way to address non-participation in AYA patients. Cellphones, TM, and internet-based tools are widely used in the adolescent population among all socio-economic groups. [8] For many American teens, TM is the preferred method of communication and also represents a low-cost, accessible and convenient way to communicate reminders. [9] Studies in pediatric liver transplant cite forgetfulness as the primary reason for non-participation; therefore, reminder-based TM interventions could address the most common etiology for non-participation. [10] Yet, to date, there is only one study using TM reminders in pediatric liver transplant patients that targeted medication participation in patients of all ages. [11]
To our knowledge, our study is the first to investigate the impact of a TM intervention on participation in laboratory testing in the high-risk population of AYA liver transplant recipients. In a prospective pilot study, we evaluated the impact of TM reminders to improve participation in laboratory testing in adolescent liver transplant patients. Our secondary aim was to determine the feasibility, acceptability and use of an automated TM program involving feedback for test completion. We hypothesized that the TM intervention would improve participation in laboratory testing in AYA liver transplant patients.
Methods
Study population and design
This study was a non-randomized pilot feasibility and impact trial of automated TM reminders for laboratory tests. All patients 12–21 years of age and greater than 6 months post-liver transplantation were screened by chart review for eligibility. Participants with access to a mobile device with TM capability were recruited (August 2012 to December 2013) by phone or in person from the outpatient and associated outreach clinics of a quaternary care academic children’s hospital. Participants were not reimbursed for any charges associated with TM. A historical control group of patients without direct contact for consent was included in the post-hoc analysis for comparison. The institutional review board approved the study protocol; consent and assent were obtained as appropriate by age before the study procedures.
Intervention
Cell phone numbers were collected and the data were entered into a secure website with password protection. The TM intervention was fully automated and administered from January 2013 to January 2014. Patients agreed to receive messages on a predetermined schedule according to the frequency of lab tests required either monthly, bi-monthly (every 2 months) or quarterly, which was confirmed by chart review and based on clinical stability and time from transplantation.
Patients received a TM reminder the first Monday of each month in which testing was required. They also received a message the last Monday of the month to inquire about completion of laboratory testing with associated feedback responses. The messages were designed as follows: “Hi, this is a reminder to get your lab tests done this month!” End of the month reminders were structured as: “Did you get your lab tests done this month? Reply 1 for YES and 2 for NO.” If “YES,” the system responded with “Great! Please call our office at [office number] to review the results,” and if NO, they received “Please get your lab tests. It’s important to check the health of your liver!” If a non-coded response was received, the system sent the following message: “Unrecognized message. For questions or to opt out of the program, please contact [study investigator at office number].”
All responses were recorded by the system and stored on a 128-bit SSL secure server. The cost to maintain the intervention included an average of $31 per month for the website domain and phone number and $0.01 per TM. TM was not reimbursed but all patients had unlimited TM plans.
Measures
Laboratory participation
The primary outcome of participation rate in laboratory testing was calculated as the percentage of completed versus expected laboratory tests. The number of expected laboratory tests ranged from an average of 4 to 12 depending on medical complications and time from transplant. Expected frequency was defined by health care provider documentation in the chart. Completion of laboratory tests was confirmed by documented results in the electronic medical record the year prior to (2012) and during the study (2013).
Feasibility, Acceptability and Use
Feasibility considerations included the number of eligible and willing participants with continuous coverage for the study duration. Patients were asked to complete an online or paper survey regarding text messaging utilization, phone characteristics and communication preferences prior to the study. The web interface tracked errors and disconnected numbers. Rate of change in frequency of laboratory testing owing to health status was also calculated. Acceptability evaluation included survey feedback about the utility of the TM intervention. Use data included the rate of participant response as well as the overall and specific number of responses sent during the intervention. We also evaluated the correlation between reported and actual events regarding completion of laboratory tests.
Statistical analysis
Statistical analyses were performed using JMP Pro 10 statistical software (SAS Institute, Cary, NC) with significance set at P<.05. Demographic characteristics were analyzed for descriptive purposes. Paired-sample t-tests were used to compare participation rate in laboratory testing before and after the intervention. Matched paired or unpaired t tests were used as appropriate to compare secondary continuous outcomes. The Cochran-Mantel-Haenszel test was used to analyze stratified categorical data. The Shapiro-Wilk test was conducted to evaluate participation data for normality and on this basis non-parametric analyses were subsequently used.
The one-way ANOVA test was conducted to determine any effect of TM frequency on laboratory participation rate. Feasibility, acceptability and use of the intervention were also assessed using descriptive statistics. An agreement test was performed to compare the reported versus actual outcome regarding completion of laboratory testing.
Results
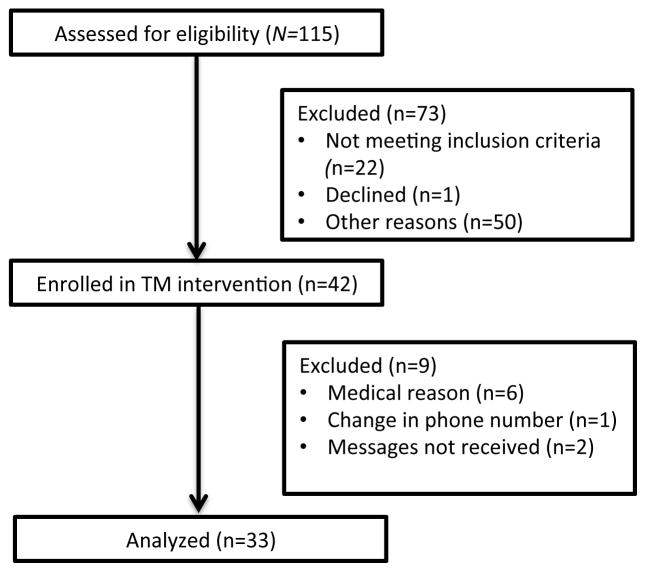
Forty-two patients (age 12–21) consented to participation and 33 (79%) completed the study. None of the patients dropped out of the study; 9 were later excluded due to various reasons (Fig. 1). Demographic data for the enrolled patients are presented in Table 1. In the intervention group, 11 patients received monthly TM (Group 1), 9 patients received bimonthly TM (Group 2), and 13 patients received quarterly TM (Group 3). There were no adverse events reported or confidentiality breaches during the study.
Figure 1.
Study diagram including patient enrollment and data analysis for the prospective TM study.
Table 1.
TM Participant Characteristics
| N | 33 |
|---|---|
| Age at study beginning, median (range), y | 16 (12–20) |
| Gender (female:male), n | 20:13 |
| Race/ethnicity, n | |
| Asian/Pacific Islander | 5 |
| Hispanic | 12 |
| White | 16 |
| Diagnosis, n | |
| Biliary atresia | 10 |
| Metabolic | 7 |
| Acute Liver Failure | 5 |
| Hepatoblastoma | 5 |
| Other | 7 |
| Age at transplant, median (range), y | 4 (1–17) |
| Years post-transplantation, median (range), y | 11 (1–19) |
| Laboratory frequency, n | |
| Monthly | 11 |
| Bi-monthly (every 2 months) | 9 |
| Quarterly | 13 |
Participation in laboratory testing
The average participation rate in laboratory testing among TM patients significantly increased from 58% (M=0.58, SD=0.31) to 78% (M=0.78, SD=0.30) after the reminder intervention by the Wilcoxon matched pairs test (p<.001). We then conducted a secondary post-hoc analysis using a comparison group of 51 patients age 12–20 not enrolled in the study (Fig 2). The average participation rate changed from 57% (M=0.57, SD=0.29) to 61% (M=.61, SD=0.33) after the intervention period, which was not a significant change (p=.38).
Figure 2.
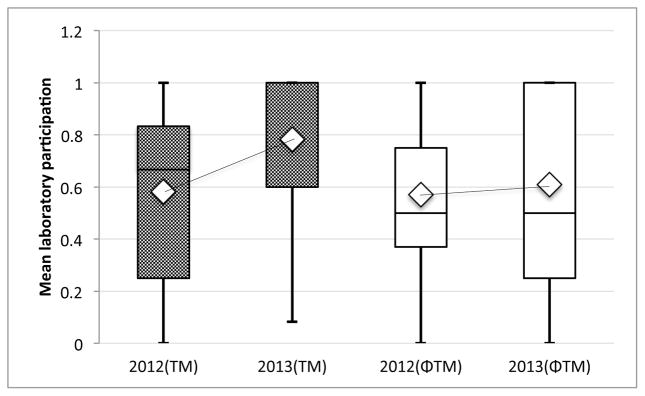
Comparison of change in mean laboratory participation within the TM group (P<0.001) and a comparison non-TM group (P=0.38), 1 year before and 1 year after the TM study period. There was a significant increase in mean participation in the TM group compared to the non-TM group (P=0.03).
Comparing the two groups, there was no significant difference in the mean laboratory participation rate at baseline (P=.80) but a significant difference was noted after the study period (P=.03). We also calculated a significant mean increase in laboratory participation in the intervention group compared to the non-intervention group after the study period (P=.003).
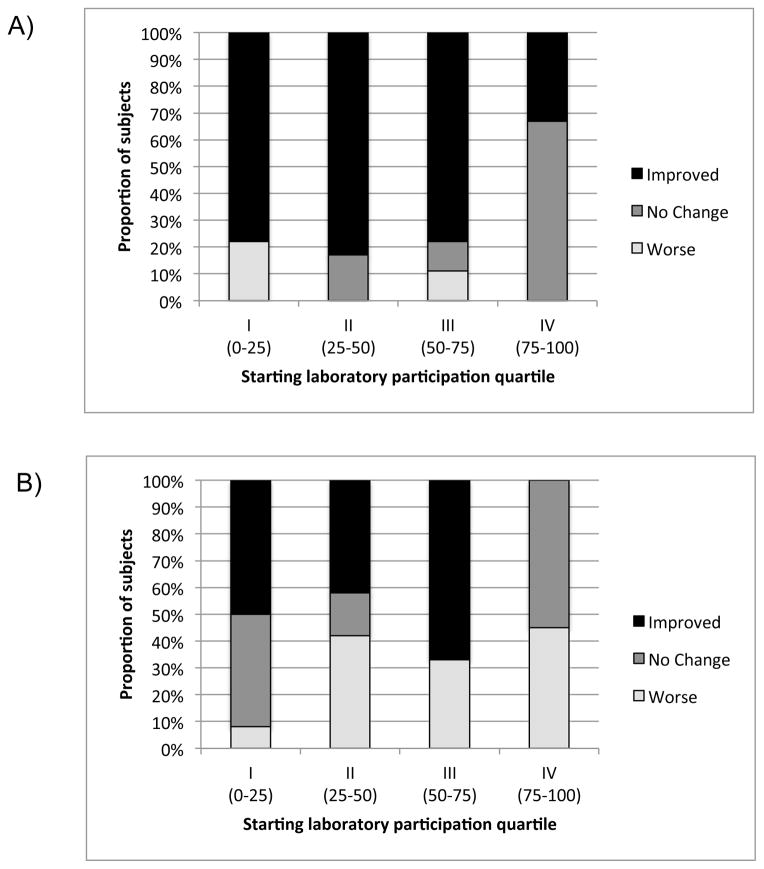
We then compared subject change in laboratory participation (improved/no change/worse) by starting participation quartile between the intervention and non-intervention groups (Fig 3). Patients were grouped accordingly: Level I=0–25%, Level II=25–50%, Level III=50–75%, Level IV=75–100%. There was a significant association between starting quartile and change in participation rate by group (text vs. no-text) using the stratified analysis (p=.002).
Figure 3.
Analysis comparing the proportional response (Improved, No Change or Worse) in participation by starting quartile of laboratory completion in the TM group (A) compared to the Non-TM (ΦTM) group (B). There was a significant association between starting quartile and change in participation by group (p=.002).
In the TM group, 22 patients (67%) improved and 3(1%) had worse laboratory participation. Eight patients did not change but 6 had 100% participation prior to TM and all maintained perfect participation post-TM. In the non-TM group, 20 patients (39%) improved and 17 (33%) had worse participation. Fourteen patients did not change participation; 11 had 100% perfect participation at baseline but only 6 of those patients maintained perfect participation in this group.
A subgroup analysis was conducted in the intervention group to determine whether there was an effect of testing frequency on participation. The one-way ANOVA test did not detect a significant effect of TM frequency on laboratory participation (p=.64).
Feasibility, Acceptability and Use
Of the 44 patients screened, only 2 patients (5%) did not enroll; 1 was excluded owing to lack of a mobile phone and the other declined. Twenty-five of the 42 enrolled patients (60%) completed pre-study questionnaires. All of the patients reported having unlimited text messaging and therefore no additional cost to participate. Thirteen patients were already using their phone for either medication or clinic reminders but none for laboratory tests. All survey participants preferred secure email or text messaging as the primary method of contact about their medical care; the majority preferred text messaging (68%) to email (32%).
Of the 42 patients initially enrolled, 9 patients (21%) were later excluded owing to medical or technical reasons (Fig 1). None of the patients discontinued the intervention. No phone numbers were disconnected according to the study log; however, it was discovered that one cellphone number changed and 2 patients did not receive messages for an unclear reason. These patients were excluded from statistical analyses. During the study, 11 patients had a change in laboratory frequency based on clinical stability; 9 had a decreased frequency and 2 required more frequent testing.
Thirty patients, (77%) of those with a working cellphone number, responded with at least one text message during the study. Twelve patients (29%) tried to communicate additional messages using the program; the majority represented clarifications regarding a revised laboratory schedule. An average of 5.7 text messages were sent per patient (SD=3.6, range 1–13). The majority of responses represented “YES” replies with a total of 95 (M=3.2, SD 2.8, range 0–11) compared to 70 “NO” replies (M=2.4, SD 2.4, range 0–11). The majority of patients who responded (83%) sent at least one “NO” message when laboratory tests were not completed.
Eighty-nine (94%) of the “YES” messages correctly corresponded to a completed laboratory test. Seventy-three (96%) of the “NO” replies corresponded to not completing a laboratory test by chart review. This excellent agreement was supported by the kappa statistic (k=0.89, p<0.001) (95% CI=0.82, 0.96). McNemar’s test failed to reject the null hypothesis (p=0.32), which supported agreement between reported and actual completion of laboratory tests by chart review.
Thirty patients completed a post-study survey. The majority of patients (80%) positively responded that the program was helpful. The remaining patients all thought it could be helpful for other patients or if better coordinated to her/his schedule. The majority of patients (70%) responded that the TM reminders definitely or possibly “made them more likely to get laboratory tests”; 1 patient was not sure and 26% did not think it impacted their care. 3 patients reported technical issues (2 who never received messages) and one reported a change in phone number after the intervention. Most of the patients (71%) wanted the reminders to be re-activated, even those who did not report a definite benefit. A larger proportion (97%) felt that TM reminders could be helpful for at least one aspect of self-management.
Discussion
Our study demonstrated a significant improvement in laboratory participation using a low cost, automated TM reminder intervention in AYA liver transplant recipients. This effect was also significant compared to the remainder of our AYA population followed during the same time period. We also showed the high rate of acceptability and communication using a TM intervention in our AYA population. This is the first study to evaluate and show a positive impact of a TM intervention specifically in a high-risk transplant population.
Active participation in medical care represents a prevalent, challenging and costly problem for pediatric and adult health care providers. [12] Adolescent transplant patients are known to represent a particularly vulnerable group, with worse long term outcomes that include rejection and death attributed to non-participation. [13]As such, there is an interest in the development and evaluation of effective solutions. Despite knowledge of the problem and its associated outcomes, few intervention studies exist in the literature specific to this population. The majority of TM studies have addressed medication and clinic appointment participation with mixed results. [14,15]
Our study is the first report to address participation in laboratory testing, which is an important element of self-management. For liver transplant patients, it represents the primary means to screen and monitor organ health. In addition, regular laboratory testing tracks the immunosuppressant drug levels, which provide as assessment of medication participation. [16] Our study did not evaluate an impact on medical outcomes. However, we hypothesize that increasing the number of recorded laboratory values may improve earlier detection of non-participation and medical complications; this could improve outcomes and potentially lower health costs through earlier intervention if studied over a longer period in a larger population. [17]
An important feature of TM interventions includes the capability for efficient implementation, increased patient engagement and an ability to address disparities in existing care. [14,17] As a primary form of communication among adolescents, mobile interventions can be used effectively across geographic and socioeconomic boundaries. [14] Our study included patients of all ages in the AYA spectrum that was demographically representative of our area. [18] Cost concerns and language barriers did not preclude participation. These are important considerations as non-participation is associated with subgroup risk factors such as low socioeconomic class. As both an effective and inclusive intervention, this TM approach represents a promising means to reach, engage and impact care among those with the greatest need. [8,10]
We noted a high rate of patients who attempted communication through the program. Our AYA patients were reliable with strong correlation between reported and actual completion of laboratory tests. Minor discrepancies may have resulted from reporting errors or information bias related to documentation in the medical record. Feedback from our patients confirmed an interest in incorporating TM for other aspects of care; this interest and engagement of AYA patients in technology-based interventions is supported by other studies. [14, 19–22] The ability of TM to offer beneficial reminders and increased communication should also promote self-management skills, which are an important element for the successful transition to adult care. [23]
This low cost, low resource-intensive intervention significantly improved rates of laboratory testing. Of note, only 3 patients had a decreased rate of testing after the intervention. On further analysis, lack of clear communication regarding expected laboratory frequency contributed to worse participation. There was also a high rate of change in required lab frequency based on clinical stability in our study. This suggests the presence of additional system factors as potential barriers to optimal participation and an opportunity to improve care. [24]
Limitations to our study include the single center recruitment, a specific disease population and a small sample size. The study was not randomized or controlled and we used the patient as her/his own historical control. Anticipating the possible impact of selection bias (non-participatory patients are less likely to enroll in studies) and regression to the mean, or the effect of time, we did utilize a comparison group. Only the TM group significantly improved participation in laboratory testing at the individual and group level. In patients with perfect participation at baseline, all who received TM maintained perfect participation compared to only 55% in the non-TM group.
Additional limitations included factors that reduced our total study population, which included many unanticipated medical events. However, the biggest technical challenge resulted from the change in laboratory frequency in a large proportion of patients. As such, the messages did not always correctly correspond to the month of required testing. Many patients felt this was a barrier to the utility and acceptability of the intervention. However, despite these technical difficulties, the majority of patients felt the reminders were beneficial and made attempts to communicate to correct their schedule. Incorporating an improved monitoring system and regularly reviewing patient responses could address these issues.
These limitations to our automated system must be weighed against the advantages. Other studies show benefits in cost, feasibility and acceptability of an automated approach, especially if supported by theory and user-specific responses. [25–27] A supplementary strategy could be to introduce more patient control, which has been found to be a well-accepted and utilized means of TM support. [20] Adding a more tailored or personalized content also appears to achieve a significant benefit. [14, 25–27] The results of our study and participant feedback provide further encouragement that mobile health applications remain important tools for our AYA populations. Continuing to modify and evaluate these approaches may achieve the most effective means to impact participation and significantly improve care. [28]
Conclusions
Our study demonstrated the feasibility, acceptability and efficacy of a TM reminder intervention to improve participation in laboratory testing in AYA liver transplant recipients. It highlights the impact of using a low-cost, fully automated means of communication that incorporated feedback to address an important aspect of self-management in a high-risk group. A future study might increase personalization, better coordination with management goals and bi-directional patient communication to achieve better results. Further longitudinal studies in larger, controlled populations should evaluate an impact on medical outcomes.
Acknowledgments
Funding Source: All phases of this study were supported by the Elizabeth and Russell Siegelman Fellowship, Child Health Research Institute, Stanford CTSA grant no. UL1 RR025744. This work was also conducted with support from a KL2 Mentored Career Development Award of the Stanford Clinical and Translational Science Award to Spectrum (NIH KL2 TR 001083).
We thank John Tamaresis, PhD for his dedicated and exceptional statistical support for the project. We all thank all of our patients and families for their participation.
Abbreviations
- AYA
adolescent and young adult
- TM
text messaging
Biographies
Rebecca B. McKenzie is Clinical Instructor of Pediatric Gastroenterology and Hepatology at Stanford Children’s Hospital. She is also currently in a Masters program in Health Services Research and a Co-Director of the Adolescent and Young Adult Liver Transplant clinic. She has a passion for improving health care delivery, long-term outcomes and quality of life for children with chronic illness with a focus in solid organ transplantation. Her current clinical and research focus is to promote a patient-centered approach to the development of self-management skills and the transition to adult care through patient-centered approaches. Dr. McKenzie conceptualized and designed the study, obtained funding for the study, acquired, analyzed and interpreted the data, drafted the initial manuscript, approved the final manuscript and oversaw the entire project.
William E. Berquist is a Professor of Pediatric Gastroenterology and Hepatology at Stanford Children's Hospital and the Medical Director of the Pediatric Liver Transplant Program. He has worked to advance quality care and research for pediatric liver disease and is the joint author of many publications involving patient participation in health care. He worked to establish the Adolescent and Young Adult Liver Transplant clinic and also enjoys his role as an instructor of the pediatric PALS program, which partners medical students with children and families to better understand the impact of living with chronic illness. Dr. Berquist conceptualized and designed the study, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Megan A. Foley obtained a Bachelors of Arts Degree from the University of California, Los Angeles. She is currently a research assistant in the Department of Pediatric Gastroenterology and Hepatology at Stanford Children's Hospital. Her work focuses on adolescent and young adult liver transplant patients. Megan Foley assisted in study design and data collection instruments, coordinated data collection, critically reviewed the manuscript and approved the final manuscript as submitted.
Jered E. Windscheimer obtained a Bachelors of Science Degree from the University of Oregon and a Master's of Science Degree in Computer Science from the University of Minnesota. He currently works as a Principal Software Engineer for Yahoo with a focus on developing scalable internet platforms. Jered was invaluable in his design and execution of the text-messaging platform and his support and maintenance throughout the trial. Jered also critically reviewed the manuscript and approved the final manuscript as submitted.
KT Park is an Assistant Professor of Pediatric Gastroenterology and member of the Child Health Research Institute. He is also the Co-Director of the Stanford Children's Inflammatory Bowel Disease Center and the Physician Lead for the ImproveCareNow Network of Pediatric Inflammatory Bowel Disease. His research interests involve optimizing the use of various diagnostic and therapeutic strategies to improve patients' health, healthcare delivery, and pharmaco-economics of chronic gastrointestinal diseases. He is also interested in applying available data and translating innovative ideas to other complex chronic diseases, especially those with child health significance, through collaborative and inter-disciplinary efforts. Dr. Park helped to conceptualized and design the study; he assisted with data analysis and interpretation, reviewed and revised the manuscript and approved the final manuscript as submitted.
Iris F. Litt: Founder and Director of The Division of Adolescent Medicine at Stanford for more than 30 years, Dr. Litt's research has focused on understanding and improving teenaged patients adherence to medical regimes. Her work has led to interventions that promote their assuming more responsibility for their own healthcare. In her role as national Director of the Robert Wood Johnson Foundations Clinical Scholars and Physician Faculty Scholar's Programs, she has supported community-based participatory research, as well. She has mentored Dr. McKenzie over the past 10 years as she has developed considerable skill in studying and intervening to improve health outcomes for teens with liver transplants. Dr. Litt helped to conceptualized and design the study; she assisted with data analysis and interpretation, reviewed and revised the manuscript and approved the final manuscript as submitted.
Footnotes
Conflict of interest: The authors have no conflicts of interest to disclose.
Clinical Trial Registration: This trial has been registered at www.clinicaltrials.gov (identifier NCT01696331).
References
- 1.Finn NB. Addressing the problem of medication non-participation. J Participat Med. 2014 Oct 10;6:e13. [Google Scholar]
- 2.Cheng J, Walter E. Non-adherence in Pediatrics. In: Bosworth HB, Oddone EZ, Weinberger M, editors. Patient treatment adherence: concepts, interventions, and measurement. Lawrence Erlbaum Associates, Inc; New Jersey: 2005. p. 239. [Google Scholar]
- 3.Berquist RK, Berquist WE, Esquivel CO, et al. Adolescent non-adherence: prevalence and consequences in adolescent liver transplant recipients. Pediatr Transplant. 2006;10(3):304–310. doi: 10.1111/j.1399-3046.2005.00451.x. [DOI] [PubMed] [Google Scholar]
- 4.Burra P, Germani G, Gnoato F, et al. Adherence in liver transplant patients. Liver Transpl. 2011;17(7):760–770. doi: 10.1002/lt.22294. [DOI] [PubMed] [Google Scholar]
- 5.Dobbels F, Van-Damme-Lombaert R, Vanhaecke J, De Geest S. Growing pains: non-adherence with the immunosuppressive regimen in adolescent transplant recipients. Pediatr Transplant. 2005;9(3):381–390. doi: 10.1111/j.1399-3046.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 6.Lurie S, Shemesh E, Sheiner PA, et al. Non-adherence in pediatric liver transplant recipients: an assessment of risk factors and natural history. Pediatr Transplant. 2000;4(3):200–206. doi: 10.1034/j.1399-3046.2000.00110.x. [DOI] [PubMed] [Google Scholar]
- 7.Shemesh E, Shneider BL, Savitzky JK, et al. Medicaiton adherence in pediatric and adolescent liver transplant recipients. Pediatrics. 2004;113(4):825–832. doi: 10.1542/peds.113.4.825. [DOI] [PubMed] [Google Scholar]
- 8.Lenhart Amanda. Pew Internet & American Life Project. Washington, DC: Pew Research Center; [Accessed July 17, 2014]. Teens, cell phones and texting: Text messagining becomes centerpiece communication. http://pewresearch.org/pubs/1572/teens-cell-phones-text-messages. [Google Scholar]
- 9.Cole-Lewis H, Kershaw T. Text Messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev. 2010;32(1):56–69. doi: 10.1093/epirev/mxq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shemesh E. Non-adherence to medicatios following pediatric liver transplantation. Pediatr Transplant. 2004;8(6):600–605. doi: 10.1111/j.1399-3046.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- 11.Miloh T, Annunziato R, Arnon R, et al. Improved Adherence and Outcomes for Pediatric Liver Transplant Recipients by Using Text Messaging. Pediatrics. 2009;124(5):844–850. doi: 10.1542/peds.2009-0415. [DOI] [PubMed] [Google Scholar]
- 12.McGrady ME, Hommel KA. Medication adherence and health care utilization in pediatric chronic illness: a systematic review. Pediatrics. 2013;132(4):730–740. doi: 10.1542/peds.2013-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly D, Wray J. The Adolescent Liver Transplant Patient. Clin Liver Dis. 2014;18(3):613–632. doi: 10.1016/j.cld.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Head KJ, Noar SM, Iannarino NT, Harrington NG. Efficacy of text messaging-based interventions for health promotion: A meta-analysis. Soc Sci Med. 2013;97:41–48. doi: 10.1016/j.socscimed.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Free C, Phillips G, Galli L, et al. The effectiveness of mobile-health technologies to improve health care service delivery processes: a systematic review and meta-analysis. PLoS Med. 2013;10(1):e1001363. doi: 10.1371/journal.pmed.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stuber ML, Shemesh E, Seacord D, et al. Evaluating non-adherence to immunosuppressant medications in pediatric liver transplant patients. Pediatr Transplant. 2008;12(3):284–288. doi: 10.1111/j.1399-3046.2008.00923.x. [DOI] [PubMed] [Google Scholar]
- 17.Huang JS, Terrones L, Tompane T, et al. Preparing adolescents with chronic disease for transition to adult care: a technology program. Pediatrics. 2014;133(6):e1639–46. doi: 10.1542/peds.2013-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Census Bureau. American Community Survey, 2008–2012 Demographic And Housing 5-Year Estimates, Table DP05 [California]; generated by Rebecca McKenzie; using American FactFinder. 2014 Apr August; http://factfinder2.census.gov.
- 19.Woolford SJ, Blake N, Clark SJ. Texting, Tweeting, and Talking: E-communicating with adolescents in primary care. [Accessed June 3, 2014];Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/news/texting-tweeting-and-facebook-e-communicating-adolescents-primary-care.
- 20.Britto MT, Munafo JK, Schoettker PJ, et al. Pilot and feasibility test of adolescent-controlled text messaging reminders. Clin Pediatr. 2012;51(2):114–121. doi: 10.1177/0009922811412950. [DOI] [PubMed] [Google Scholar]
- 21.Franklin VL, Waller A, Pagliari C, Greene SA. A randomized, controlled trial of Sweet Talk, a text-messaging system to support young people with diabetes. Diabet Med. 2006;23(12):1332–1228. doi: 10.1111/j.1464-5491.2006.01989.x. [DOI] [PubMed] [Google Scholar]
- 22.Guse K, Levine D, Martins S, et al. Interventions using new digital media to improve adolescent sexual health: a systematic review. J Adolesc Health. 2012;51(6):535–43. doi: 10.1016/j.jadohealth.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Callahan ST, Winitzer RF, Keenan P. Transition from pediatric to adult-oriented health care: a challenge for patients with chronic disease. Curr Opin Pediatr. 2001;13(4):310–316. doi: 10.1097/00008480-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Dew MA, Dabbs AD, Myaskovsky L, et al. Meta-analysis of medical regimen adherence outcomes in pediatric solid organ transplantation. Transplantation. 88(5):736–46. doi: 10.1097/TP.0b013e3181b2a0e0. 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramanathan N, Swendeman D, Comulada WS, Estrin D, Rotheram-Borus MJ. Identifyng preferences for mobile health applications for self-monitoring and self-management:focus group findings from HIV-positive persons and young mothers. Int J Med Inform. 2013;82(4):e38–46. doi: 10.1016/j.ijmedinf.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranney ML, Choo EK, Cunningham RM, et al. Acceptability, language and structure of text message-based behavioral interventions for high-risk adolescent females: a qualitative study. J Adolesc Health. 2014;55(1):33–40. doi: 10.1016/j.jadohealth.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingersoll K, Dillingham R, Reynolds G, et al. Development of a personalized bidirectional text messaging tool for HIV adherence assessment and intervention among substance abusers. J Subst Abuse Treat. 2014;46(1):66–73. doi: 10.1016/j.jsat.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu YP, Hommel KA. Using technology to assess and promote adherence to medical regimens in pediatric chronic illness. J Pediatr. 2014;164(4):922–7. doi: 10.1016/j.jpeds.2013.11.013. [DOI] [PubMed] [Google Scholar]