Abstract
A major virulence factor of Plasmodium falciparum is the adherence of parasitized erythrocytes to the wall of postcapillary venules via a specific interaction between parasite-derived erythrocyte surface ligands and receptors on endothelial cells. To study this phenomenon in vitro, we selected a parasite population that expressed at least two different ligands and demonstrated that parasitized cells may coexpress ligands with specificity for multiple receptors. This selected parasite line had several antigenic and cytoadherence characteristics that were different from those of the parent line. Single parasitized erythrocytes were able to adhere to three distinct receptors via at least two separate ligands; a trypsin-sensitive molecule mediated cytoadherence to CD36 and intercellular adhesion molecule 1 and a trypsin-insensitive molecule(s) was responsible for adherence to a third receptor on the surface of melanoma cells. We present evidence that this newly discovered receptor for cytoadherence is an N-linked glycosaminoglycan, as treatment of melanoma cells with endoglycosidase H abolished cytoadherence. These observations emphasize the adaptability of P. falciparum and the complexity of the cytoadherence phenomenon.
Full text
PDF
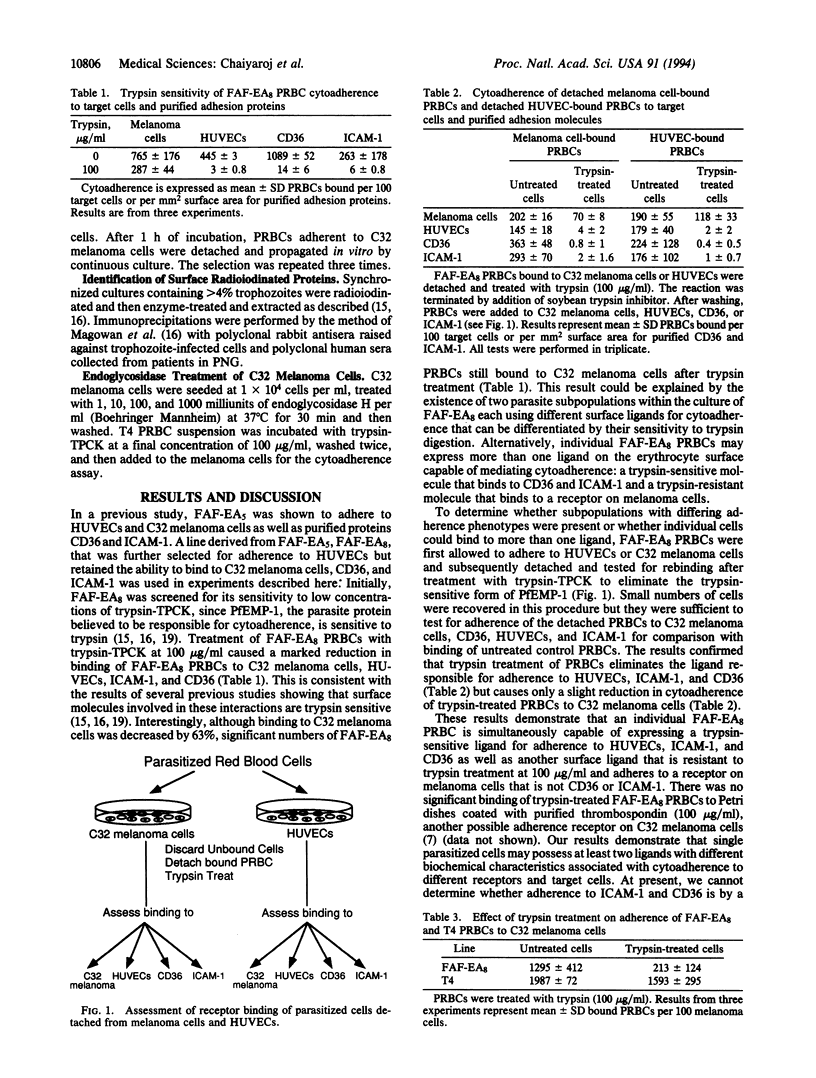
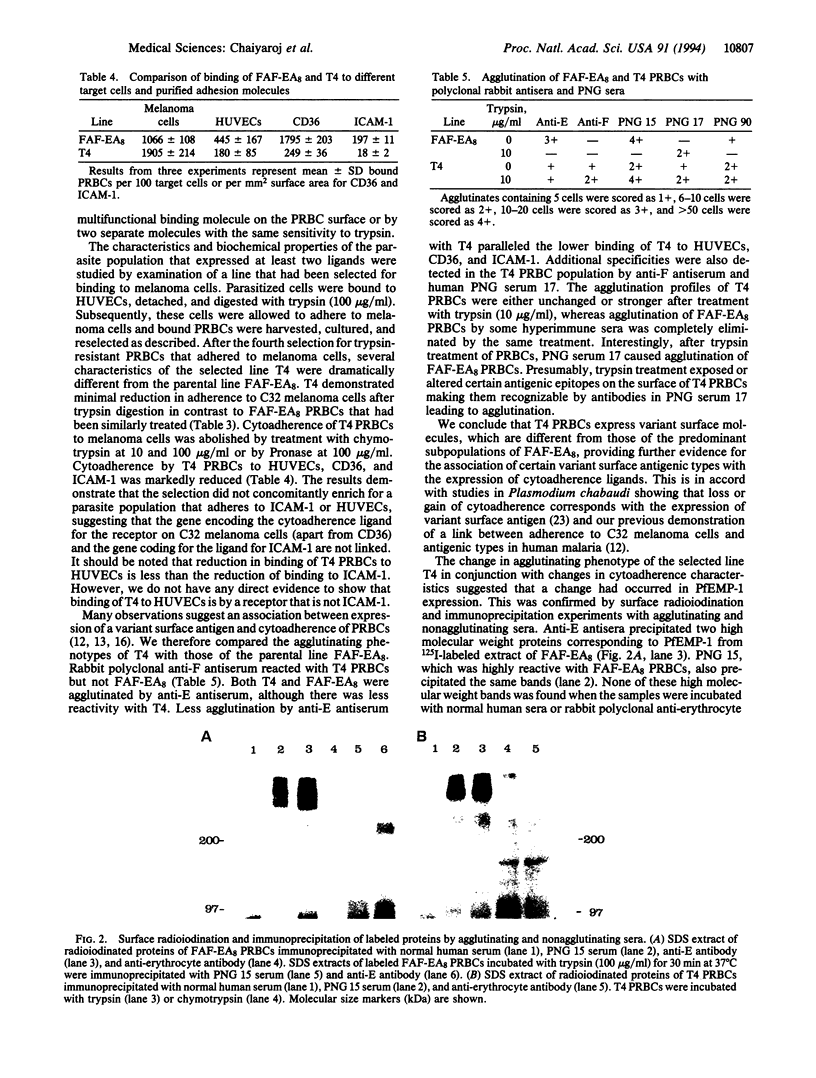
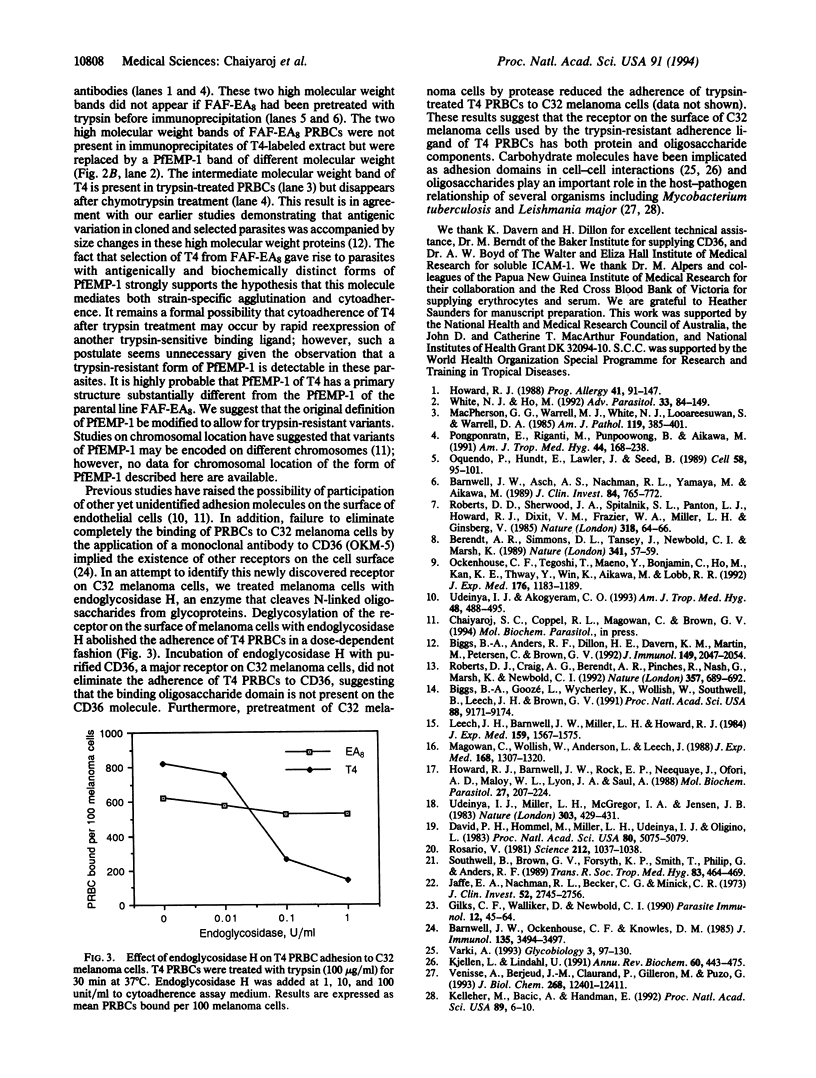
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnwell J. W., Asch A. S., Nachman R. L., Yamaya M., Aikawa M., Ingravallo P. A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J Clin Invest. 1989 Sep;84(3):765–772. doi: 10.1172/JCI114234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnwell J. W., Ockenhouse C. F., Knowles D. M., 2nd Monoclonal antibody OKM5 inhibits the in vitro binding of Plasmodium falciparum-infected erythrocytes to monocytes, endothelial, and C32 melanoma cells. J Immunol. 1985 Nov;135(5):3494–3497. [PubMed] [Google Scholar]
- Berendt A. R., Simmons D. L., Tansey J., Newbold C. I., Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989 Sep 7;341(6237):57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- Biggs B. A., Anders R. F., Dillon H. E., Davern K. M., Martin M., Petersen C., Brown G. V. Adherence of infected erythrocytes to venular endothelium selects for antigenic variants of Plasmodium falciparum. J Immunol. 1992 Sep 15;149(6):2047–2054. [PubMed] [Google Scholar]
- Biggs B. A., Goozé L., Wycherley K., Wollish W., Southwell B., Leech J. H., Brown G. V. Antigenic variation in Plasmodium falciparum. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9171–9174. doi: 10.1073/pnas.88.20.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David P. H., Hommel M., Miller L. H., Udeinya I. J., Oligino L. D. Parasite sequestration in Plasmodium falciparum malaria: spleen and antibody modulation of cytoadherence of infected erythrocytes. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5075–5079. doi: 10.1073/pnas.80.16.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilks C. F., Walliker D., Newbold C. I. Relationships between sequestration, antigenic variation and chronic parasitism in Plasmodium chabaudi chabaudi--a rodent malaria model. Parasite Immunol. 1990 Jan;12(1):45–64. doi: 10.1111/j.1365-3024.1990.tb00935.x. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Barnwell J. W., Rock E. P., Neequaye J., Ofori-Adjei D., Maloy W. L., Lyon J. A., Saul A. Two approximately 300 kilodalton Plasmodium falciparum proteins at the surface membrane of infected erythrocytes. Mol Biochem Parasitol. 1988 Jan 15;27(2-3):207–223. doi: 10.1016/0166-6851(88)90040-0. [DOI] [PubMed] [Google Scholar]
- Howard R. J. Malarial proteins at the membrane of Plasmodium falciparum-infected erythrocytes and their involvement in cytoadherence to endothelial cells. Prog Allergy. 1988;41:98–147. doi: 10.1159/000415221. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher M., Bacic A., Handman E. Identification of a macrophage-binding determinant on lipophosphoglycan from Leishmania major promastigotes. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):6–10. doi: 10.1073/pnas.89.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Leech J. H., Barnwell J. W., Miller L. H., Howard R. J. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J Exp Med. 1984 Jun 1;159(6):1567–1575. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson G. G., Warrell M. J., White N. J., Looareesuwan S., Warrell D. A. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985 Jun;119(3):385–401. [PMC free article] [PubMed] [Google Scholar]
- Magowan C., Wollish W., Anderson L., Leech J. Cytoadherence by Plasmodium falciparum-infected erythrocytes is correlated with the expression of a family of variable proteins on infected erythrocytes. J Exp Med. 1988 Oct 1;168(4):1307–1320. doi: 10.1084/jem.168.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse C. F., Tegoshi T., Maeno Y., Benjamin C., Ho M., Kan K. E., Thway Y., Win K., Aikawa M., Lobb R. R. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med. 1992 Oct 1;176(4):1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo P., Hundt E., Lawler J., Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell. 1989 Jul 14;58(1):95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- Pongponratn E., Riganti M., Punpoowong B., Aikawa M. Microvascular sequestration of parasitized erythrocytes in human falciparum malaria: a pathological study. Am J Trop Med Hyg. 1991 Feb;44(2):168–175. doi: 10.4269/ajtmh.1991.44.168. [DOI] [PubMed] [Google Scholar]
- Roberts D. D., Sherwood J. A., Spitalnik S. L., Panton L. J., Howard R. J., Dixit V. M., Frazier W. A., Miller L. H., Ginsburg V. Thrombospondin binds falciparum malaria parasitized erythrocytes and may mediate cytoadherence. Nature. 1985 Nov 7;318(6041):64–66. doi: 10.1038/318064a0. [DOI] [PubMed] [Google Scholar]
- Roberts D. J., Craig A. G., Berendt A. R., Pinches R., Nash G., Marsh K., Newbold C. I. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992 Jun 25;357(6380):689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario V. Cloning of naturally occurring mixed infections of malaria parasites. Science. 1981 May 29;212(4498):1037–1038. doi: 10.1126/science.7015505. [DOI] [PubMed] [Google Scholar]
- Southwell B. R., Brown G. V., Forsyth K. P., Smith T., Philip G., Anders R. Field applications of agglutination and cytoadherence assays with Plasmodium falciparum from Papua New Guinea. Trans R Soc Trop Med Hyg. 1989 Jul-Aug;83(4):464–469. doi: 10.1016/0035-9203(89)90248-4. [DOI] [PubMed] [Google Scholar]
- Udeinya I. J., Akogyeram C. O. Induction of adhesiveness in human endothelial cells by Plasmodium falciparum-infected erythrocytes. Am J Trop Med Hyg. 1993 Apr;48(4):488–495. doi: 10.4269/ajtmh.1993.48.488. [DOI] [PubMed] [Google Scholar]
- Udeinya I. J., Miller L. H., McGregor I. A., Jensen J. B. Plasmodium falciparum strain-specific antibody blocks binding of infected erythrocytes to amelanotic melanoma cells. Nature. 1983 Jun 2;303(5916):429–431. doi: 10.1038/303429a0. [DOI] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993 Apr;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venisse A., Berjeaud J. M., Chaurand P., Gilleron M., Puzo G. Structural features of lipoarabinomannan from Mycobacterium bovis BCG. Determination of molecular mass by laser desorption mass spectrometry. J Biol Chem. 1993 Jun 15;268(17):12401–12411. [PubMed] [Google Scholar]




