Abstract
Rationale: Stress is associated with asthma morbidity in Puerto Ricans (PRs), who have reduced bronchodilator response (BDR).
Objectives: To examine whether stress and/or a gene regulating anxiety (ADCYAP1R1) is associated with BDR in PR and non-PR children with asthma.
Methods: This was a cross-sectional study of stress and BDR (percent change in FEV1 after BD) in 234 PRs ages 9–14 years with asthma. We assessed child stress using the Checklist of Children’s Distress Symptoms, and maternal stress using the Perceived Stress Scale. Replication analyses were conducted in two cohorts. Polymorphisms in ADCYAP1R1 were genotyped in our study and six replication studies. Multivariable models of stress and BDR were adjusted for age, sex, income, environmental tobacco smoke, and use of inhaled corticosteroids.
Measurements and Main Results: High child stress was associated with reduced BDR in three cohorts. PR children who were highly stressed (upper quartile, Checklist of Children’s Distress Symptoms) and whose mothers had high stress (upper quartile, Perceived Stress Scale) had a BDR that was 10.2% (95% confidence interval, 6.1–14.2%) lower than children who had neither high stress nor a highly stressed mother. A polymorphism in ADCYAP1R1 (rs34548976) was associated with reduced BDR. This single-nucleotide polymorphism is associated with reduced expression of the gene for the β2-adrenergic receptor (ADRB2) in CD4+ lymphocytes of subjects with asthma, and it affects brain connectivity of the amygdala and the insula (a biomarker of anxiety).
Conclusions: High child stress and an ADCYAP1R1 single-nucleotide polymorphism are associated with reduced BDR in children with asthma. This is likely caused by down-regulation of ADRB2 in highly stressed children.
Keywords: asthma, Puerto Ricans, bronchodilator response, stress
At a Glance Commentary
Scientific Knowledge on the Subject
Psychosocial stress is both common and associated with asthma morbidity among Puerto Ricans, who are known to have reduced responsiveness to short-acting inhaled β2-agonists (bronchodilator response [BDR]). Chronic stress has been linked to reduced expression of ADRB2 in leukocytes of children with asthma.
What This Study Adds to the Field
We found that high levels of child and household stress are associated with lower BDR in children with asthma. We also show that a polymorphism upstream of ADCYAP1R1 (a susceptibility gene for post-traumatic stress disorder and anxiety) is associated with reduced BDR in children with asthma, reduced expression of ADRB2 in CD4+ lymphocytes of subjects with asthma, and functional brain connectivity of the amygdala and insula (a biomarker of anxiety) in inner-city adults.
Asthma affects approximately 300 million people worldwide (1). Inhaled short-acting β2-agonists are the most commonly prescribed medication for asthma and relief of asthma symptoms (2, 3), regardless of disease severity. Subjects with asthma show variable response to short-acting β2-agonists (bronchodilator response [BDR]) (4). In children, increased BDR has been associated with response to inhaled corticosteroids (ICS) and is often used as a diagnostic tool for asthma (5, 6).
Psychosocial stress has been linked to dysfunction of the β-adrenergic pathway, including the effector for inhaled β2-agonists (7, 8). In particular, stress has been associated with reduced expression of the gene for the β2-adrenergic receptor (ADRB2) in leukocytes from children with asthma (8). Compared with children with asthma but no evidence of acute or chronic stress, those with simultaneous acute and chronic stress had a 9.5-fold reduction in ADRB2 expression (8). In that report, acute stress was not associated with ADRB2 expression in the absence of chronic stress, a finding suggestive of down-regulation of the β2-adrenergic receptor by persistent secretion of the hormonal products of the hypothalamic-pituitary-adrenocortical axis, including epinephrine and norepinephrine, in chronically stressed children.
Whether stress leads to reduced BDR is unknown. Yet another unanswered question is whether dysfunction of the pituitary adenylate cyclase–activating polypeptide 1 (PACAP-PAC1) pathway explains potential effects of stress on the β2-adrenergic receptor (9, 10). This is relevant, because the gene encoding the PACAP 1 receptor type 1 (ADCYAP1R1) has been previously implicated in post-traumatic stress disorder (PTSD) (9, 11), anxiety (12), and asthma (13).
Puerto Ricans (PRs) have lower BDR (14) and greater asthma morbidity (15, 16) than members of other ethnic groups, an observation partly attributed to allelic variants in ADRB2 (17). An alternative explanation for this finding is psychosocial stress, because PRs are often exposed to violence (18–20) and susceptible to its detrimental effects (21–23).
In this report, we first examine whether increased stress is associated with reduced BDR in a cohort of PR children and in two additional cohorts: one composed of children with asthma (for primary replication), and another composed of children with airflow obstruction (a reduced FEV1/FVC) but normal FEV1 and no asthma diagnosis (to further exclude nonadherence with medications or “reverse causation” as sources of bias). Next, we test whether single-nucleotide polymorphisms (SNPs) in ADCYAP1R1 are associated with BDR in seven cohorts of children with asthma. To better understand an association between an ADCYAP1R1 SNP and reduced BDR, we then test whether this SNP is associated with a biomarker of increased anxiety (functional connectivity of the amygdala and the insula, assessed by brain magnetic resonance imaging). Some results shown in this report have been previously published in the form of an abstract (24).
Methods
Further details are available in the online supplement.
Stress and BDR
Puerto Rico
From March 2009 to June 2010, 351 children (ages 6–14 yr) with asthma (defined as physician-diagnosed asthma and ≥1 episode of wheeze in the previous yr) were recruited from households in San Juan (Puerto Rico) using a multistage probability sample design (25). Study participants completed a protocol including questionnaires, spirometry, allergy skin testing, and collection of blood samples for DNA extraction. The Checklist of Children’s Distress Symptoms (CCDS) (26, 27) was administered to 234 children 9 years and older, who are included in the current analysis. This 28-item scale assesses stress symptoms in the previous 6 months as a result of exposure to violence. Answers for each question in the CCDS range from 1 to 5. An overall score is obtained by summing the scores for all 28 questions, and then dividing by the number of questions, so that the score ranges between 1 and 5 (27). This CCDS score was analyzed as binary (upper quartile [≥2.6 points] vs. lowest three quartiles [<2.6 points]). The child’s mother completed a questionnaire on the child’s demographics, family history, and respiratory and general health (28).
The mother of each participant also completed the four-item Perceived Stress Scale (PSS) questionnaire, which measures the degree to which subjects believe that their lives were unpredictable, uncontrollable, or overwhelming in the preceding month (29). Answers for each question in the maternal PSS are scored on a Likert scale from 0 to 4. A total score is obtained by summing the scores for all answers and thus ranges from 0 to 16 (29). PSS total score was analyzed as binary (upper quartile [>7 points] vs. lowest three quartiles [≤7 points]). Higher PSS scores have been associated with wheeze and upper respiratory illnesses (30–32). The CCDS and PSS have high interrater reliability and concurrent validity (29, 31, 33). Because data on maternal stress complement those for child stress, we created a composite score (hereafter referred to as “household stress”), categorized as 0 if neither the maternal stress nor the child stress score was in the upper quartile, 1 if either the maternal or child stress score was in the upper quartile, and 2 if both maternal and child stress scores were in the upper quartile.
Spirometry was conducted with an EasyOne (NDD Medical Technologies, Andover, MA) spirometer following American Thoracic Society recommendations (34). The best FEV1 and FVC from each test were selected for analysis. After completing spirometry, subjects were given 200 μg (two puffs) of an albuterol metered-dose inhaler using a spacer, and spirometry was repeated after 15 minutes.
Replication cohorts
Further details are provided in Table 1 and the online supplement. Replication of our findings for stress and BDR was attempted in 471 children with asthma (ages 7–15 yr) living in Rhode Island (n = 229; 59 PR, 81 Dominican, and 89 non-Hispanic white children) and Puerto Rico (n = 242) (Rhode Island Puerto Rico Asthma Center [RIPRAC] cohort). The DISC Predictive Scales, Youth Report instrument was used to collect information on self-reported symptoms of child anxiety and panic (35). Adherence to ICS and leukotriene inhibitors was measured in a subset of these children using electronic monitoring devices.
Table 1.
Characteristics of Participants in Our Study in Puerto Rico, RIPRAC, and NHANES*
| Cohort | Puerto Rico (n = 234) | RIPRAC (n = 471) | NHANES (n = 87) |
|---|---|---|---|
| Age, yr | 11.5 (1.9) | 11.7 (2.0) | 14.5 (0.2) |
| Male sex | 131 (56%) | 252 (53%) | 50 (57%) |
| Body mass index (z score) | 0.7 (1.1) | 0.9 (1.2) | 0.7 (0.1) |
| At least one parent graduated from high school | 144 (81%) | 388 (95%) | N/A |
| Race/ethnicity | |||
| Puerto Rican | 234 (100%) | 301 (64%) | |
| Dominican | 81 (17%) | ||
| Hispanic | 42 (48%) | ||
| Non-Hispanic white | 89 (19%) | 29 (33%) | |
| Non-Hispanic black | 12 (14%) | ||
| Others | 4 (5%) | ||
| Household income† | 63 (36%) | 194 (42%) | 71 (82%) |
| Private or employer-based health insurance | 58 (33%) | 188 (40%) | 46 (53%) |
| Maternal history of asthma | 85 (48%) | N/A | 23 (26%)‡ |
| Paternal history of asthma | 51 (31%) | N/A | N/A |
| Current exposure to environmental tobacco smoke | 78 (44%) | 183 (39%) | 13 (15%) |
| Exposure to in utero smoking | 19 (11%) | N/A | N/A |
| Use of inhaled corticosteroids in the previous 6 months | 53 (30%) | 147 (31%) | N/A |
| Prebronchodilator FEV1, L§ | 2.2 (0.6) | 2.3 (0.6) | 3.1 (0.1) |
| Post-bronchodilator FEV1, L | 2.3 (0.7) | 2.5 (0.7) | 3.4 (0.1) |
| Prebronchodilator FEV1/FVC | 0.81 (0.08) | 0.84 (0.08) | 0.75 (0.01) |
| Post-bronchodilator FEV1/FVC | 0.84 (0.09) | 0.88 (0.06) | 0.81 (0.01) |
| Bronchodilator response as a percent of baseline FEV1 | 4.0 (8.6) | 7.3 (8.4) | 8.7 (0.7) |
| ≥1 positive skin test to allergens | 136 (81%) | 294 (70%) | N/A |
| Child and maternal stress scales | |
||
| Maternal Perceived Stress Score | 4.7 (2.8) | ||
| Child Stress Score | 2.2 (0.6) | ||
| Above cutoff for YDPS generalized anxiety score | 75 (16%) | ||
| Above cutoff for YDPS panic disorder score | 27 (6%) | ||
| Number of days feeling anxious in the past 30 d | 2.2 (0.4) | ||
Definition of abbreviations: N/A = not available; NHANES = National Health and Nutrition Examination Survey; RIPRAC = Rhode Island Puerto Rico Asthma Center; YDPS = DISC Predictive Scales, Youth Report.
Values are shown as number (% of subjects with complete data) for binary variables or mean (SD) for continuous variables.
Household income greater than or equal to $15,000 per year for Puerto Rico and RIPRAC, and greater than or equal to $20,000 per year for NHANES.
Family history of asthma.
FEV1 presented as absolute values because of lack of predicted values for Puerto Ricans.
To further assess a potential role for nonadherence with treatment, we examined high child anxiety (upper quartile for the number of days feeling anxious in the prior month) and BDR in 87 children (ages 12–17 yr) in the National Health and Nutrition Examination Survey (NHANES) in 2007–2010. These children had a reduced FEV1/FVC but no previous or current diagnosis of asthma, and were on no medications for wheeze (a key asthma symptom) in the prior year.
ADCYAP1R1 and BDR
Genetic association analysis
In the Puerto Rico cohort, SNPs in ADCYAP1R1 were available as part of genome-wide genotyping conducted using the HumanOmni2.5 BeadChip platform (Illumina Inc., San Diego, CA), as previously described (36). Replication of our genetic findings was attempted through direct genotyping in two cohorts with genome-wide genotypic data: the Childhood Asthma Management Program (37), including 561 non-Hispanic white subjects; and the Genetics of Asthma in Costa Rica Study (28), including 569 Costa Ricans. Five SNPs not included in genome-wide genotyping for these studies were genotyped using the OpenArray Genotyping block (Life Technologies, Carlsbad, CA). Additional replication was attempted using imputed data from prior genome-wide genotyping in 253 PRs (189 children and 64 adults) in the Genetics of Asthma in Latino Americans study; 215 white children in the Childhood Asthma Research and Education Network; 231 African American children with asthma from the Children’s Hospital of Philadelphia; and 718 children and young adults (ages 8–21 yr) in the Study of African Americans, Genes and Environment.
Gene expression analysis
We tested whether an ADCYAP1R1 SNP found to be associated with BDR is also associated with gene expression in unstimulated CD4+ T lymphocytes from subjects in the Asthma BioRepository for Integrative Genomic Exploration, and in brain and lung tissues from subjects in the Genotype-Tissue Expression project (38) and another published dataset (39). To better understand the association between the ADCYAP1R1 SNP and reduced BDR, we then examined whether brain regional activity regulating anxiety is differentially associated with rs34548976 in a cohort of inner-city African American adults.
Statistical Analysis
In all cohorts, BDR was defined as follows: [(post-bronchodilator FEV1 − prebronchodilator FEV1)/prebronchodilator FEV1] × 100.
Because of potential correlation with stress and BDR, the following covariates were examined in bivariate analyses in the Puerto Rico cohort: age, sex, parental asthma, parental education (at least one parent completed high school vs. none), household income (< vs. ≥$15,000 [near the median household income for Puerto Rico in 2008–2009]) (40), health insurance (private or employer-based vs. others), use of ICS in the previous 6 months, current environmental tobacco smoke (ETS) (41), in utero ETS, body mass index (42) as a z score [based on CDC growth charts] [43]), and greater than or equal to one positive skin test to allergens. Bivariate analyses were conducted using Fisher exact tests for categorical variables and two tailed t tests for pairs of binary and continuous variables.
Multivariable linear regression was used to examine stress and BDR in the Puerto Rico cohort. All final models included age, sex, income, ICS use, and current ETS. Additional covariates (see above) were included in the initial models if associated with BDR at P less than 0.25 in bivariate analyses, and then subjected to removal in a step-wise fashion. At each step of model building, the variable with the largest P value was excluded; the procedure stopped when all variables not forced into the model had P less than 0.05.
Multivariable analysis of variance modeling was used to examine validated clinical cutoffs for generalized anxiety or panic disorder (44) and BDR in RIPRAC, and multivariable linear regression was used to examine anxiety and BDR in NHANES (incorporating sampling weights, stratification, and clusters provided in the dataset, to obtain proper estimates and their standard errors). These analyses were adjusted for the same covariates as in the analysis in Puerto Rico, plus race/ethnicity, and (in NHANES) family history of asthma.
Linear regression was used to test for association between SNPs within 20 kb of ADCYAP1R1 (n = 106) and BDR. All multivariable models were conducted under an additive model, and adjusted for age, sex, and the first principal component (determined by EIGENSTRAT [45], to control for population stratification). Meta-analysis of the replication cohorts was performed using effect size and standard errors, accounting for allele differences between cohorts, and weighted by the sample size of each cohort. In Childhood Asthma Research and Education Network, Children’s Hospital of Philadelphia, Study of African Americans, Genes and Environment, and Genetics of Asthma in Latino Americans study, we used imputed SNPs from the 1,000 Human Genomes Project (46). Because many of the 106 ADCYAP1R1 SNPs are in linkage disequilibrium (LD) with each other, we applied an LD-based Bonferroni correction for multiple testing (47, 48), reducing the number of effective statistical tests to 57. Statistical significance for the joint association analysis (including the original and replication cohorts) was therefore set at P less than 8.8 × 10−4 (0.05/57 independent tests). SAS version 9.2 (SAS Institute, Cary, NC) was used for all analyses except the genetic analysis, which was performed in R 2.15 (49), PLINK (50), and METAL (51).
We interrogated the relationship between an SNP associated with BDR and resting-state brain activity in 42 African American adults with available functional magnetic resonance imaging data (52). We analyzed amygdala functional connectivity, which has been repeatedly associated with increased anxiety and dyspnea perception (53–56).
All studies were approved by an institutional review board or ethics committee. All adult participants and parents of participating children gave written informed consent, and minors able to do so gave assent.
Results
The characteristics of the study participants are summarized in Table 1. Children with asthma in our study were similar to those in RIPRAC with regard to age, sex, current ETS, and lung function. Compared with children in RIPRAC, those in our study had lower parental education and BDR. As expected, children with no asthma in NHANES were slightly older and had higher FEV1 but lower FEV1/FVC than those in Puerto Rico or in RIPRAC.
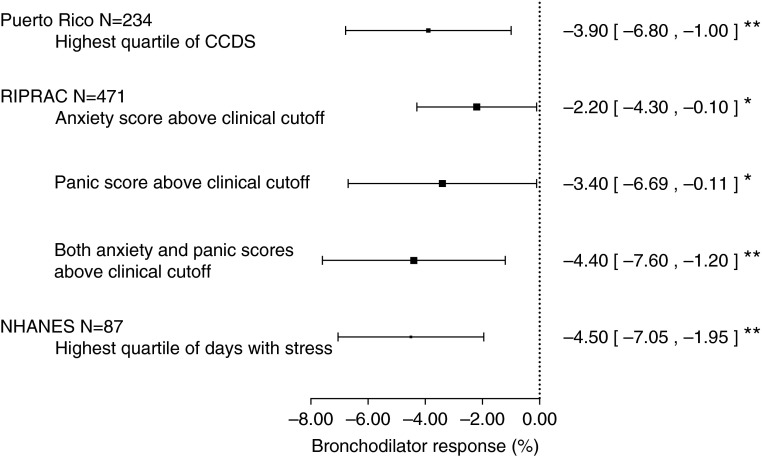
Figure 1, and Figure E1 and Table E2 in the online supplement, show the results of the analysis of child stress and BDR in PRs. In a multivariable analysis, children with a CCDS score in the upper quartile had a BDR that was 3.9% lower than that of children with a CCDS score in the lowest three quartiles. Similar results were obtained after additional adjustment for markers of asthma severity or control (≥1 positive skin test to allergens [atopy] or baseline FEV1, data not shown).
Figure 1.
Bronchodilator response among children with stress or anxiety. All multivariable models were adjusted for age, sex, household income, current environmental tobacco smoke, and use of inhaled corticosteroids; models for the Rhode Island Puerto Rico Asthma Center (RIPRAC) and the National Health and Nutrition Examination Survey (NHANES) cohorts were also adjusted for race/ethnicity. Plotted are the beta estimates and 95% confidence intervals for each cohort. Puerto Rico shows the estimate of effect for the comparison between children in the upper quartile of the Checklist of Children’s Distress Symptoms (CCDS) scores and those in the lowest three quartiles. RIPRAC shows the estimate of effect for the comparison between children with anxiety and panic scores above a clinical cutoff and those with scores below such cutoff. Additionally shown for RIPRAC is the estimate of effect for the comparison between children with scores above the clinical cutoff for both anxiety and panic, and those with scores below the clinical cutoff for anxiety and panic. NHANES shows the estimate of effect for the comparison between children in the highest quartile of anxiety in the previous month and those in the lowest three quartiles. *P < 0.05; **P < 0.01.
We replicated our findings for child stress in RIPRAC (Figure 1; see Table E2). In a multivariable analysis, children with clinically significant anxiety had a BDR that was 2.2% lower than that of children without such anxiety. In this analysis, children with clinically significant panic symptoms had a BDR that was 3.5% lower than that of those without such symptoms. Compared with children without significant symptoms of either anxiety or panic, those with significant symptoms of both panic and anxiety had a reduction in BDR of 4.4%. After adjustment for adherence to ICS or leukotriene inhibitors (available only in a subset of children), we obtained results that were similar in magnitude but nonstatistically significant (see Table E3).
To further exclude nonadherence with antiinflammatory medications or “reverse causation” (greater asthma severity leading to increased stress), we attempted additional replication among children in NHANES, who had no asthma diagnosis and were on no medications for asthma symptoms. In a multivariable analysis, the upper quartile of the child anxiety scale was significantly associated with reduced BDR (Figure 1; see Table E2). Because NHANES includes adolescents, we repeated the analysis after either excluding 16 ever-smokers or adjusting for serum cotinine level instead of parental report of ETS, obtaining very similar results (data not shown).
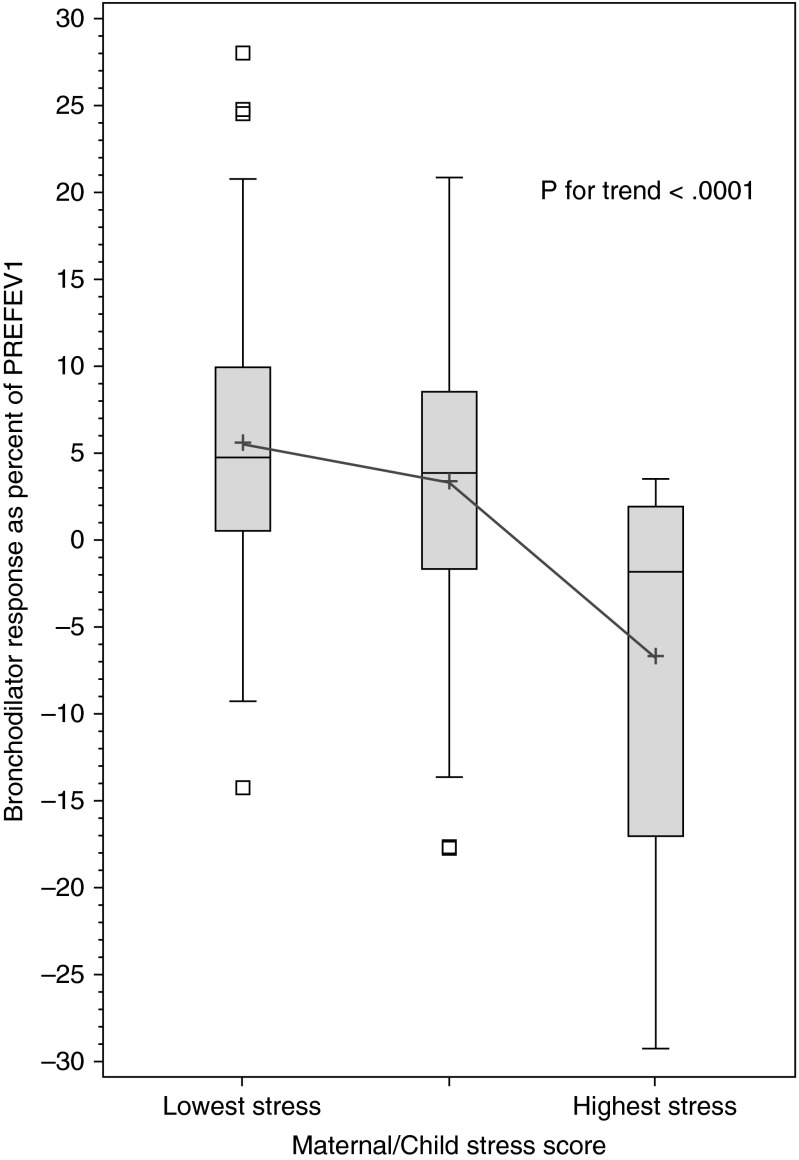
Because maternal stress is a complementary marker of stress in a child’s household, we examined whether maternal stress is associated with reduced BDR in our PR cohort. In a multivariable analysis, children whose mothers had the highest stress level had 6.3% lower BDR than those whose mothers had lower levels of stress (see Table E4). To best assess overall stress in the child’s household, we then examined combined maternal and child (“household”) stress. Compared with children who were neither highly stressed nor had a highly stressed mother, those who were highly stressed and had a highly stressed mother had the lowest BDR, with intermediate BDR values in children who were either highly stressed or had a highly stressed mother (Figure 2). In a multivariable analysis (see Table E5), each one-point increment in household stress was significantly associated with reduced BDR: children with the highest household stress (a score of 2) had a BDR that was approximately 10.2% lower than that of children with the lowest household stress (a score of 0). As change in absolute values, children with the lowest household stress had a mean increment in FEV1 of 116 ml after bronchodilator, whereas children with highest household stress had a mean decrement in FEV1 of 125 ml.
Figure 2.
Boxplots of bronchodilator responsiveness in Puerto Rican children with asthma, by household stress score. The “highest stress” group comprises children with both high personal stress (in the upper quartile of the Checklist of Children’s Distress Symptoms) and high maternal stress (in the upper quartile of the maternal Perceived Stress Score). The “lowest stress” group comprises children with neither high personal stress nor high maternal stress. The middle group is children with either high personal stress or high maternal stress, but not both. PREFEV1 = baseline (prebronchodilator) FEV1.
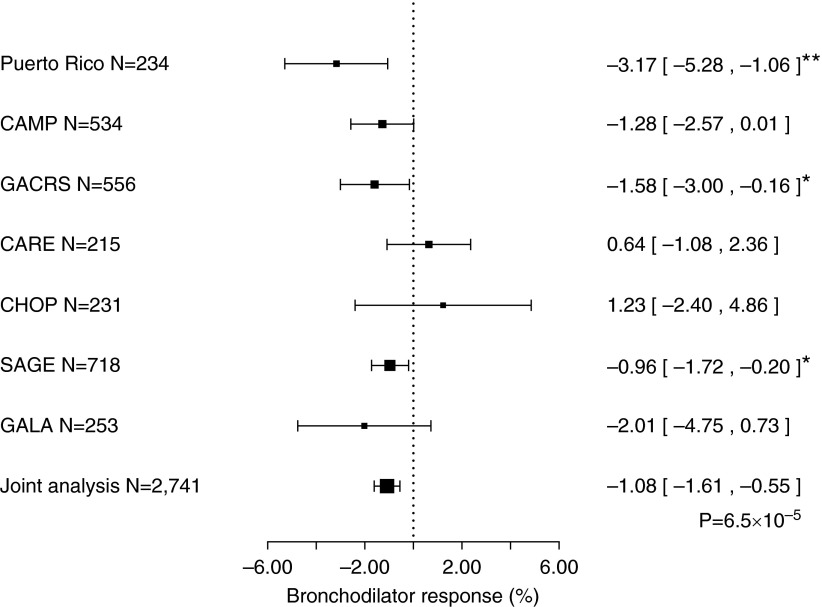
We next examined whether common SNPs (minor allele frequency ≥ 5%) in or within 20 kb of ADCYAP1R1 (n = 106) are associated with reduced BDR in our study of PR children (see Table E6). In this analysis, eight SNPs in ADCYAP1R1 were associated with BDR at P less than 0.05 in PR children, and were thus tested for replication in six cohorts of children with asthma (Figure 3; see Figure E2 and Table E7). One of the SNPs tested (rs34548976) was significantly associated with BDR in the analysis of the six replication cohorts and in the joint analysis of all seven study cohorts (including 2,741 children with asthma) (Figure 3), even after LD-adjusted correction for multiple testing (joint P < 8.8 × 104 [0.05/57] in both instances).
Figure 3.
Estimated effect of the minor allele (A) of single-nucleotide polymorphism rs34548976 on bronchodilator response (percent change in FEV1) in seven cohorts of children with asthma. CAMP = Childhood Asthma Management Program; CARE = Childhood Asthma Research and Education Network; CHOP = Children’s Hospital of Philadelphia; GACRS = Genetics of Asthma in Costa Rica Study; GALA = Genetics of Asthma in Latino Americans; SAGE = The Study of African Americans, Asthma, Genes and Environment. *P < 0.05; **P < 0.01.
Using public databases, we then found that SNP rs34548976 is associated with increased expression of ADCYAP1R1 in human brain tissue in two databases, either through direct genotyping (P = 0.01) (38) or through a surrogate SNP in complete LD (r2 = 1.0) with rs34548976 (rs10236961; P = 0.04) (39). SNP rs34548976 was also associated with increased ADCYAP1R1 expression in lung tissue (P = 0.05) (38).
Because ADCYAP1R1 could down-regulate ADRB2 through increased anxiety, we next examined whether SNP rs34548976 is associated with expression of ADCYAP1R1 or ADRB2 in unstimulated CD4+ T lymphocytes of 392 children and adults with asthma. In this linear regression analysis, rs34548976 was associated with reduced ADRB2 expression under an additive genetic model, adjusting for age, sex, race, and principal components (P = 0.009) (see Figure E3). ADCYAP1R1 was not expressed above baseline in CD4+ T lymphocytes, and thus could not be analyzed in these cells.
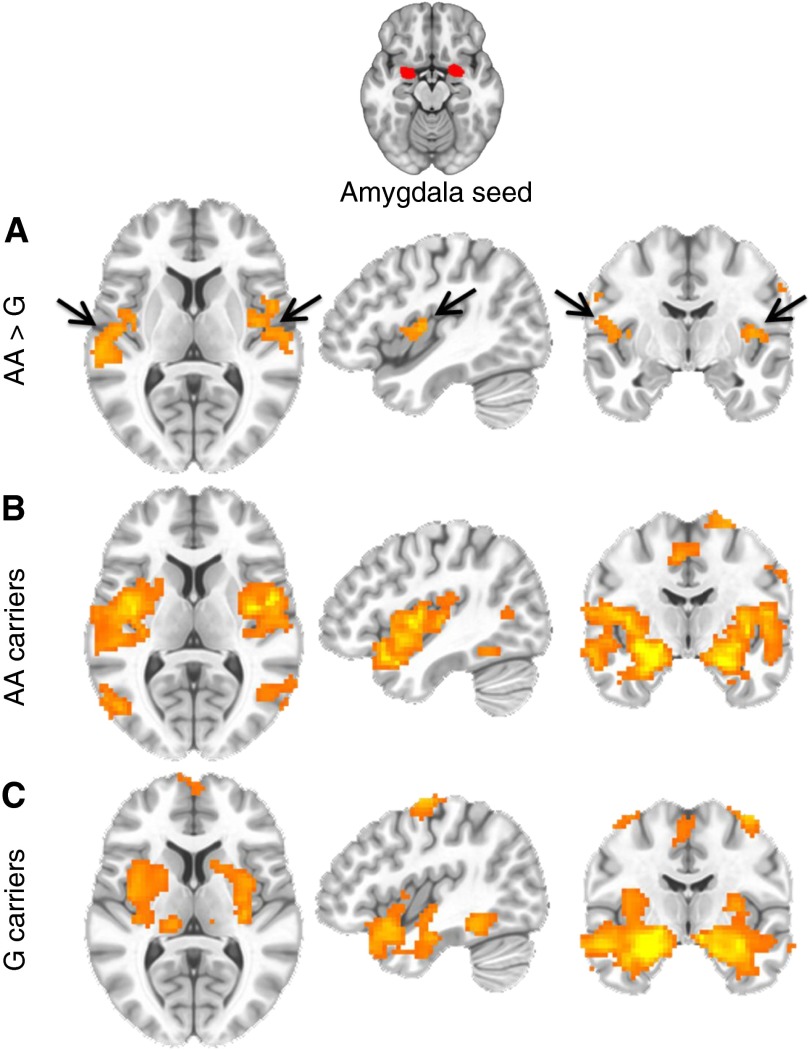
To better understand whether the ADCYAP1R1 SNP reduces BDR by increasing anxiety, we examined whether brain regional activity implicated on anxiety is differentially associated with rs34548976. Compared with carriers of the G allele, subjects with two copies of the risk (A) allele for reduced BDR had increased functional connectivity between the amygdala and the insula (whole-brain corrected, P < 0.01) (Figure 4). Moreover, we found a significant linear trend for an association between the A allele of rs34548976 and functional connectivity of the amygdala and insula (whole-brain corrected, P < 0.01).
Figure 4.
Increased functional connectivity in brain regions (amygdala–insula) previously associated with increased anxiety in at-risk adults with the rs34548976 risk allele. Resting-state functional magnetic resonance imaging was performed, and bilateral amygdala volumes were seeded, examining whole-brain corrected brain regions showing differential functional connectivity with insula (arrows) in rs3458976 AA allele carriers (n = 11) compared with GG/AG allele carriers (n = 31). (A) Group differences are shown in the horizontal (left), sagittal (middle), and coronal (right) planes between AA and G-carrier groups. (B) Whole-brain corrected (P < 0.01) regions associated with amygdala functional activity at resting state in the AA allele carriers. (C) Whole-brain corrected (P < 0.01) regions associated with amygdala functional activity at resting state in the G allele carriers. Similar results were seen with increased amygdala–insula connectivity using an additive model (AA/AG/GG) and when comparing AA with AG groups separately. All signals represent whole-brain corrected voxels at P < 0.01.
Given a plausible role for rs34548976 on baseline anxiety, we examined whether this SNP is associated with child stress, or whether it interacts with child stress on BDR among PR children. Although SNP rs34548976 was not significantly associated with CCDS, there was evidence of modification of the estimated effect of this SNP on BDR by child stress (P for interaction = 0.01). In this analysis, the estimated negative effect of the A allele of rs34548976 on BDR was significant in children with high stress (β = −5.1%; 95% confidence interval, −9.5% to −0.6%; P = 0.03) but not in those with lower stress (β = 0.05%; 95% confidence interval, −2.0 to 2.1%; P = 0.96).
Discussion
This is the first population-based study linking increased stress or ADCYAP1R1 to reduced BDR. Chance, misclassification of asthma, confounding, medication nonadherence, or “reverse causation” are unlikely to be major explanations for our findings, given consistent results in several cohorts that had different study designs and included children with asthma or subclinical airflow obstruction. The estimated negative effect of increased stress on BDR ranged from 2.2 to 10.2%, corresponding to absolute volume reductions of 44–208 ml. A BDR greater than or equal to 8% is well correlated with asthma and wheeze in school-aged children (6, 57, 58), and thus the range of the estimated effects of stress on BDR is clinically relevant (e.g., ∼28% to ∼128% of an 8% threshold).
Our results are consistent with and markedly expand previous findings implicating acute stress (superimposed on chronic stress) in down-regulation of β-adrenergic receptors in leukocytes or lymphocytes of subjects with panic disorder (7) or asthma (8). We show that an SNP in a gene implicated in anxiety (ADCYAP1R1) is associated with both functional connectivity of the amygdala and the insula, and reduced expression of ADRB2 in CD4+ lymphocytes from subjects with asthma. Moreover, we demonstrate that children who have the risk allele (and thus enhanced anxiety) are more likely to have reduced BDR when highly stressed. Our findings in three cohorts further suggest that stress leads to reduced BDR in children with asthma or reduced FEV1/FVC, regardless of their racial or ethnic group. However, we had limited statistical power to examine whether race or ethnicity modifies the estimated effects of stress on BDR, and thus this question needs to be examined in future studies.
Increased or altered connectivity between the insula and the amygdala has been reported in individuals with PTSD (59), anxiety (60), and panic attacks (61). Such connectivity may also be directly linked to airway inflammation in asthma. In a previous study, individuals with atopic asthma who were exposed to allergens had differential activation of the anterior insula by asthma-relevant emotional cues during the late phase of the dual response, and this differential activation was associated with airway inflammation (62).
ADCYAP1R1 is not expressed in human airway smooth muscle cells (see online supplement), and thus our results are most consistent with a negative effect of SNP rs34548976 on BDR through neurohormonal mechanisms leading to down-regulation of ADRB2 in highly stressed children. SNP rs34548976 lies upstream of ADCYAP1R1, and thus may affect ADRB2 expression through local (cis-) effects on ADCYAP1R1 expression. Alternatively, this SNP could have distant (trans-) effects on ADRB2 expression.
We found no association between ADCYAP1R1 SNP rs2267735 (previously associated with PTSD [9, 11, 63, 64] and asthma [13]) and BDR. SNP rs34548976 is not in LD with rs2267735, and is thus a novel risk variant for both anxiety and BDR. We did not examine rare variants in ADCYAP1R1, and thus other functional variants may be identified in future studies.
Because this study is cross-sectional, we cannot examine the temporal relationship between stress-related reductions in BDR and long-term prognosis of asthma. However, we show relevant effects of stress on the most commonly used treatment for this disease. Whether highly stressed children require larger doses of inhaled β2-agonists for bronchodilation is unknown and should be formally tested. Although direct comparison of the magnitude of the estimated effects of stress on BDR across studies is difficult (because of the different scales used), this shows that our results are robust.
Our findings are relevant to the millions of children with asthma exposed to psychosocial stressors, such as poverty and violence, worldwide. Our results further implicate dysfunction of the β-adrenergic pathway in stress-related disorders, because they are consistent with a reported association between an ADRB2 SNP and PTSD in adults (65). Studies such as this one are critical to the understanding of pleiotropic genetic effects on both stress or anxiety and cardiopulmonary diseases affected by adrenergic dysfunction, including asthma and cardiovascular conditions, such as myocardial infarction.
Acknowledgments
Acknowledgment
The authors thank all participating children and their families for their invaluable participation in the study. They also thank Dr. Amanda Myers from the Laboratory of Functional Neurogenomics at the University of Miami for providing data to complete the brain expression analysis (http://labs.med.miami.edu/myers/LFuN/data.html), and William Horne, M.S., from Children's Hospital of Pittsburgh of UPMC for technical assistance for the expression analysis in airway smooth muscle cells.
Footnotes
Supported by grants HL079966, HL073373, and HL117191 from the U.S. National Institutes of Health (NIH). The Genetics of Asthma in Costa Rica Study was supported by grants HL066289 and HL04370 from the NIH. The CAMP Genetics Ancillary Study was supported by grants U01 HL075419, U01 HL65899, P01 HL083069, and R01 HL086601 from the NIH. The RIPRAC study was supported by U01 HL 072438 from the NIH. The SAGE and GALA studies were supported by grants from the NIH (R01-ES015794, R01-HL088133, R01-HL078885, T32-GM007546, R01-HL004464, R01-HL104608, HL117004; and the National Institute on Minority Health and Health Disparities under Award Number P60MD006902), the Sandler Foundation, and the American Asthma Foundation (E.G.B.). The Single-Nucleotide Polymorphism Health Association Asthma Resource Project was funded by NHLBI grants U01 HL51510, U01 HL51834, U01 HL51831, U01 HL51845, U01 HL51843, M01 RR00079, and M01 RR03186, and was performed by researchers from the CAMP and Childhood Asthma Research and Education Network. J.M.B. was supported by grant HD052892 from the NIH. S.J.L. is supported by the Intramural Research Program of the NIH, NIEHS. K.C.B. was supported in part by the Mary Beryl Patch Turnbull Scholar Program. M.P.-Y. was funded by a postdoctoral fellowship from Fundación Ramón Areces. D.C.C.-C. was supported by grant T32 HL007427 from the NHLBI.
Author Contributions: J.M.B., S.K.R., and J.C.C. conceived of the study, participated in its design and coordination, and helped to draft the manuscript. J.M.B., S.K.R., S.M.T., D.C.C.-C., M.P.-Y., Y.-Y.H., A.L.M., Q.L.D., D.H., J.S., and N.R.N. performed statistical analysis. C.R.-S., A.A.L., B.A.R., N.B., W.C., E.F., A.L.M., N.R.N., C.E., A.C.-S., M.A., E.A.-P., M.L.S., F.D.M., L.A., S.T.W., M.S.-Q., C.O., D.L.N., K.C.B., R.F.L., R.C.S., A.L., S.J.L., F.G., P.S., M.M., H.H., J.K.K., G.K.F., N.F., J.S.S., L.M.A., E.G.B., E.L.M., K.R., and G.C. participated in the design and coordination of the study. All authors read and approved the final manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201501-0037OC on April 28, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bousquet J, Khaltaev NG, Cruz AA. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. Geneva: World Health Organization; 2007. [Google Scholar]
- 2.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(Suppl 5):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 3.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O’Byrne P, Pedersen SE, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 4.Sharma S, Litonjua AA, Tantisira KG, Fuhlbrigge AL, Szefler SJ, Strunk RC, Zeiger RS, Murphy AJ, Weiss ST. Clinical predictors and outcomes of consistent bronchodilator response in the Childhood Asthma Management Program. J Allergy Clin Immunol. 2008;122:921–928, e4. doi: 10.1016/j.jaci.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galant SP, Morphew T, Guijon O, Pham L. The bronchodilator response as a predictor of inhaled corticosteroid responsiveness in asthmatic children with normal baseline spirometry. Pediatr Pulmonol. 2014;49:1162–1169. doi: 10.1002/ppul.22957. [DOI] [PubMed] [Google Scholar]
- 6.Tse SM, Gold DR, Sordillo JE, Hoffman EB, Gillman MW, Rifas-Shiman SL, Fuhlbrigge AL, Tantisira KG, Weiss ST, Litonjua AA. Diagnostic accuracy of the bronchodilator response in children. J Allergy Clin Immunol. 2013;132:554–559, e5. doi: 10.1016/j.jaci.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee IS, Kim KJ, Kang EH, Yu BH. Beta-adrenoceptor affinity as a biological predictor of treatment response to paroxetine in patients with acute panic disorder. J Affect Disord. 2008;110:156–160. doi: 10.1016/j.jad.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Miller GE, Chen E. Life stress and diminished expression of genes encoding glucocorticoid receptor and beta2-adrenergic receptor in children with asthma. Proc Natl Acad Sci USA. 2006;103:5496–5501. doi: 10.1073/pnas.0506312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foda HD, Sharaf HH, Absood A, Said SI. Pituitary adenylate cyclase-activating peptide (PACAP), a VIP-like peptide, has prolonged airway smooth muscle relaxant activity. Peptides. 1995;16:1057–1061. doi: 10.1016/0196-9781(95)00087-z. [DOI] [PubMed] [Google Scholar]
- 11.Uddin M, Chang SC, Zhang C, Ressler K, Mercer KB, Galea S, Keyes KM, McLaughlin KA, Wildman DE, Aiello AE, et al. Adcyap1r1 genotype, posttraumatic stress disorder, and depression among women exposed to childhood maltreatment. Depress Anxiety. 2013;30:251–258. doi: 10.1002/da.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jovanovic T, Norrholm SD, Davis J, Mercer KB, Almli L, Nelson A, Cross D, Smith A, Ressler KJ, Bradley B. PAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children. Mol Psychiatry. 2013;18:742–743. doi: 10.1038/mp.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Boutaoui N, Brehm JM, Han YY, Schmitz C, Cressley A, Acosta-Pérez E, Alvarez M, Colón-Semidey A, Baccarelli AA, et al. ADCYAP1R1 and asthma in children. Am J Respir Crit Care Med. 2013;187:584–588. doi: 10.1164/rccm.201210-1789OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burchard EG, Avila PC, Nazario S, Casal J, Torres A, Rodriguez-Santana JR, Toscano M, Sylvia JS, Alioto M, Salazar M, et al. Genetics of Asthma in Latino Americans (GALA) Study. Lower bronchodilator responsiveness in Puerto Rican than in Mexican subjects with asthma. Am J Respir Crit Care Med. 2004;169:386–392. doi: 10.1164/rccm.200309-1293OC. [DOI] [PubMed] [Google Scholar]
- 15.Forno E, Celedón JC. Asthma and ethnic minorities: socioeconomic status and beyond. Curr Opin Allergy Clin Immunol. 2009;9:154–160. doi: 10.1097/aci.0b013e3283292207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012;94:1–8. [PubMed] [Google Scholar]
- 17.Choudhry S, Ung N, Avila PC, Ziv E, Nazario S, Casal J, Torres A, Gorman JD, Salari K, Rodriguez-Santana JR, et al. Pharmacogenetic differences in response to albuterol between Puerto Ricans and Mexicans with asthma. Am J Respir Crit Care Med. 2005;171:563–570. doi: 10.1164/rccm.200409-1286OC. [DOI] [PubMed] [Google Scholar]
- 18.Martínez-Taboas A, Canino G, Wang MQ, García P, Bravo M. Prevalence and victimization correlates of pathological dissociation in a community sample of youths. J Trauma Stress. 2006;19:439–448. doi: 10.1002/jts.20144. [DOI] [PubMed] [Google Scholar]
- 19.Purugganan OH, Stein RE, Silver EJ, Benenson BS. Exposure to violence among urban school-aged children: is it only on television? Pediatrics. 2000;106(Suppl 4):949–953. [PubMed] [Google Scholar]
- 20.Vermeiren R, Schwab-Stone M, Deboutte D, Leckman PE, Ruchkin V. Violence exposure and substance use in adolescents: findings from three countries. Pediatrics. 2003;111:535–540. doi: 10.1542/peds.111.3.535. [DOI] [PubMed] [Google Scholar]
- 21.Galea S, Vlahov D, Tracy M, Hoover DR, Resnick H, Kilpatrick D. Hispanic ethnicity and post-traumatic stress disorder after a disaster: evidence from a general population survey after September 11, 2001. Ann Epidemiol. 2004;14:520–531. doi: 10.1016/j.annepidem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Ortega AN, Rosenheck R. Posttraumatic stress disorder among Hispanic Vietnam veterans. Am J Psychiatry. 2000;157:615–619. doi: 10.1176/appi.ajp.157.4.615. [DOI] [PubMed] [Google Scholar]
- 23.Cohen RT, Canino GJ, Bird HR, Celedón JC. Violence, abuse, and asthma in Puerto Rican children. Am J Respir Crit Care Med. 2008;178:453–459. doi: 10.1164/rccm.200711-1629OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brehm JM, Ramratnam S, Tse SM, Rosas-Salazar C, Litonjua AA, Raby BA, Han YY, Chen W, Forno E, Marsland A, et al. ADCYAP1R1 polymorphisms and bronchodilator responsiveness in Puerto Rican children [abstract] Am J Respir Crit Care Med. 2014;189:A3817. [Google Scholar]
- 25.Brehm JM, Acosta-Pérez E, Klei L, Roeder K, Barmada M, Boutaoui N, Forno E, Kelly R, Paul K, Sylvia J, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med. 2012;186:140–146. doi: 10.1164/rccm.201203-0431OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suglia SF, Ryan L, Bellinger DC, Enlow MB, Wright RJ. Children’s exposure to violence and distress symptoms: influence of caretakers’ psychological functioning. Int J Behav Med. 2011;18:35–43. doi: 10.1007/s12529-010-9090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suglia SF, Ryan L, Wright RJ. Creation of a community violence exposure scale: accounting for what, who, where, and how often. J Trauma Stress. 2008;21:479–486. doi: 10.1002/jts.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunninghake GM, Soto-Quiros ME, Avila L, Ly NP, Liang C, Sylvia JS, Klanderman BJ, Silverman EK, Celedón JC. Sensitization to Ascaris lumbricoides and severity of childhood asthma in Costa Rica. J Allergy Clin Immunol. 2007;119:654–661. doi: 10.1016/j.jaci.2006.12.609. [DOI] [PubMed] [Google Scholar]
- 29.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 30.Wright RJ, Cohen S, Carey V, Weiss ST, Gold DR. Parental stress as a predictor of wheezing in infancy: a prospective birth-cohort study. Am J Respir Crit Care Med. 2002;165:358–365. doi: 10.1164/ajrccm.165.3.2102016. [DOI] [PubMed] [Google Scholar]
- 31.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 32.Cohen S, Tyrrell DAJ, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 33.Richters JE, Martinez P. The NIMH community violence project: I. Children as victims of and witnesses to violence. Psychiatry. 1993;56:7–21. doi: 10.1080/00332747.1993.11024617. [DOI] [PubMed] [Google Scholar]
- 34.American Thoracic Society. Standardization of spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 35.Schwab-Stone ME, Shaffer D, Dulcan MK, Jensen PS, Fisher P, Bird HR, Goodman SH, Lahey BB, Lichtman JH, Canino G, et al. Criterion validity of the NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3) J Am Acad Child Adolesc Psychiatry. 1996;35:878–888. doi: 10.1097/00004583-199607000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Brehm JM, Acosta-Perez E, Klei L, Roeder K, Barmada MM, Boutaoui N, Forno E, Cloutier MM, Datta S, Kelly R, et al. African ancestry and lung function in Puerto Rican children. J Allergy Clin Immunol. 2012;129:1484–1490, e6. doi: 10.1016/j.jaci.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Childhood Asthma Management Program Research Group. The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Control Clin Trials. 1999;20:91–120. [PubMed] [Google Scholar]
- 38.Consortium GT; GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myers AJ, Gibbs JR, Webster JA, Rohrer K, Zhao A, Marlowe L, Kaleem M, Leung D, Bryden L, Nath P, et al. A survey of genetic human cortical gene expression. Nat Genet. 2007;39:1494–1499. doi: 10.1038/ng.2007.16. [DOI] [PubMed] [Google Scholar]
- 40.U.S. CensusHousehold income for states: 2008 and 2009. 2010 [accessed 2011 Oct 4]. Available from: http://www.census.gov/prod/2010pubs/acsbr09-2.pdf
- 41.Freeman NC, Schneider D, McGarvey P. Household exposure factors, asthma, and school absenteeism in a predominantly Hispanic community. J Expo Anal Environ Epidemiol. 2003;13:169–176. doi: 10.1038/sj.jea.7500266. [DOI] [PubMed] [Google Scholar]
- 42.Pérez-Perdomo R, Pérez-Cardona C, Disdier-Flores O, Cintrón Y. Prevalence and correlates of asthma in the Puerto Rican population: Behavioral Risk Factor Surveillance System, 2000. J Asthma. 2003;40:465–474. doi: 10.1081/jas-120018713. [DOI] [PubMed] [Google Scholar]
- 43.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 44.Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, et al. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 46.Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, Kang HM, Marth GT, McVean GA 1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity (Edinb) 2005;95:221–227. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- 49.R Development Core Team. Vienna, Australia: 2012. R: A language and environment for statistical computing. [Google Scholar]
- 50.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevens JS, Almli LM, Fani N, Gutman DA, Bradley B, Norrholm SD, Reiser E, Ely TD, Dhanani R, Glover EM, et al. PACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus. Proc Natl Acad Sci USA. 2014;111:3158–3163. doi: 10.1073/pnas.1318954111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baur V, Hänggi J, Langer N, Jäncke L. Resting-state functional and structural connectivity within an insula-amygdala route specifically index state and trait anxiety. Biol Psychiatry. 2013;73:85–92. doi: 10.1016/j.biopsych.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 54.Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, Essex MJ. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci USA. 2013;110:19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liotti M, Brannan S, Egan G, Shade R, Madden L, Abplanalp B, Robillard R, Lancaster J, Zamarripa FE, Fox PT, et al. Brain responses associated with consciousness of breathlessness (air hunger) Proc Natl Acad Sci USA. 2001;98:2035–2040. doi: 10.1073/pnas.98.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Leupoldt A, Sommer T, Kegat S, Baumann HJ, Klose H, Dahme B, Büchel C. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Respir Crit Care Med. 2008;177:1026–1032. doi: 10.1164/rccm.200712-1821OC. [DOI] [PubMed] [Google Scholar]
- 57.Dundas I, Chan EY, Bridge PD, McKenzie SA. Diagnostic accuracy of bronchodilator responsiveness in wheezy children. Thorax. 2005;60:13–16. doi: 10.1136/thx.2004.029934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galant SP, Morphew T, Amaro S, Liao O. Value of the bronchodilator response in assessing controller naive asthmatic children. J Pediatr. 2007;151:457–462, e1. doi: 10.1016/j.jpeds.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, Phan KL. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry. 2011;2:62. doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 61.Dresler T, Hahn T, Plichta MM, Ernst LH, Tupak SV, Ehlis AC, Warrings B, Deckert J, Fallgatter AJ. Neural correlates of spontaneous panic attacks. J Neural Transm. 2011;118:263–269. doi: 10.1007/s00702-010-0540-2. [DOI] [PubMed] [Google Scholar]
- 62.Rosenkranz MA, Busse WW, Sheridan JF, Crisafi GM, Davidson RJ. Are there neurophenotypes for asthma? Functional brain imaging of the interaction between emotion and inflammation in asthma. PLoS One. 2012;7:e40921. doi: 10.1371/journal.pone.0040921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L, Cao C, Wang R, Qing Y, Zhang J, Zhang XY. PAC1 receptor (ADCYAP1R1) genotype is associated with PTSD’s emotional numbing symptoms in Chinese earthquake survivors. J Affect Disord. 2013;150:156–159. doi: 10.1016/j.jad.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 64.Almli LM, Mercer KB, Kerley K, Feng H, Bradley B, Conneely KN, Ressler KJ. ADCYAP1R1 genotype associates with post-traumatic stress symptoms in highly traumatized African-American females. Am J Med Genet B. 2013;162B:262–272. doi: 10.1002/ajmg.b.32145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liberzon I, King AP, Ressler KJ, Almli LM, Zhang P, Ma ST, Cohen GH, Tamburrino MB, Calabrese JR, Galea S. Interaction of the ADRB2 gene polymorphism with childhood trauma in predicting adult symptoms of posttraumatic stress disorder. JAMA Psychiatry. 2014;71:1174–1182. doi: 10.1001/jamapsychiatry.2014.999. [DOI] [PMC free article] [PubMed] [Google Scholar]