Abstract
Rationale: Chronic lung diseases are associated with cardiovascular disease. How these associations evolve from young adulthood forward is unknown. Understanding the preclinical history of these associations could inform prevention strategies for common heart-lung conditions.
Objectives: To use the Coronary Artery Risk Development in Young Adults (CARDIA) study to explore the development of heart-lung interactions.
Methods: We analyzed cardiac structural and functional measurements determined by echocardiography at Year 25 of CARDIA and measures of pulmonary function over 20 years in 3,000 participants.
Measurements and Main Results: Decline in FVC from peak was associated with larger left ventricular mass (β = 6.05 g per SD of FVC decline; P < 0.0001) and greater cardiac output (β = 0.109 L/min per SD of FVC decline; P = 0.001). Decline in FEV1/FVC ratio was associated with smaller left atrial internal dimension (β = −0.038 cm per SD FEV1/FVC decline; P < 0.0001) and lower cardiac output (β = −0.070 L/min per SD of FEV1/FVC decline; P = 0.03). Decline in FVC was associated with diastolic dysfunction (odds ratio, 3.39; 95% confidence interval, 1.37–8.36; P = 0.006).
Conclusions: Patterns of loss of lung health are associated with specific cardiovascular phenotypes in middle age. Decline in FEV1/FVC ratio is associated with underfilling of the left heart and low cardiac output. Decline in FVC with preserved FEV1/FVC ratio is associated with left ventricular hypertrophy and diastolic dysfunction. Cardiopulmonary interactions apparent with common complex heart and lung diseases evolve concurrently from early adulthood forward.
Keywords: lung, pulmonary heart disease, echocardiography, epidemiology
At a Glance Commentary
Scientific Knowledge on the Subject
The association between established lung disease and cardiovascular disease later in life has been well described. How heart and lung disease evolve together from health early in life to disease states later in life is unknown.
What This Study Adds to the Field
This study is unique and adds significantly to the fields of cardiovascular and pulmonary medicine. This study provides a window into the preclinical evolution of heart-lung interactions as they evolve from health in young adults to disease in middle age. This has wide ranging implications for gaining insight into understanding the strong association of comorbid heart and lung disease seen later in life.
Abnormal lung function, both obstructive physiology as occurs in chronic obstructive pulmonary disease (COPD) and restrictive physiology, is associated with cardiovascular disease and poor health outcomes (1–8). Even among individuals who do not meet spirometric criteria for COPD or restrictive lung disease, lower lung function is associated with increased risk of cardiovascular death (5–7, 9–12). An observational study of adults in the United States documented that both the extent of emphysema and degree of airflow limitation are cross-sectionally associated with reduced left ventricular (LV) filling (13). We previously found an association between decline in FVC and incident hypertension in a population of generally healthy young adults suggesting that losing lung function may present risk of future cardiovascular disease (14).
Comorbid cardiovascular disease is a common occurrence with chronic lung disease (15–18). The concurrent evolution of lung and heart structural and functional abnormalities from young adulthood into middle age, however, has not been investigated. Discovering how heart and lung disease evolve together from young adulthood to middle age could provide insight into the subclinical phase of common complex conditions, such as obstructive and restrictive lung disease, while also informing why heart and lung disease frequently occur together.
In the present study, we take advantage of longitudinal data from the Coronary Artery Risk Development in Young Adults (CARDIA) study to evaluate patterns of decline in lung health from peak lung function in generally healthy young adults and their associations with heart structure and function in middle age. We hypothesized that different patterns of decline in lung health are associated with different cardiac structural changes representing specific cardiopulmonary phenotypes.
Methods
Study Design and Sample
The CARDIA study is a prospective cohort study of the evolution of cardiovascular disease risk factors in young adults (19). Further information on the study design is available in the online supplement.
Evaluation of Lung Function
Lung function was measured at Years 0, 2, 5, and 10 using a Collins Survey 8-L water-sealed spirometer and an Eagle II Microprocessor (Warren E. Collins, Inc., Braintree, MA). At Year 20, a dry rolling-seal OMI spirometer was used (Viasys Corp, Loma Linda, CA). A comparability study performed on 25 volunteers at the LDS Hospital (Salt Lake City, UT) demonstrated excellent consistency between the old and new machines; the average difference between the Collins Survey and OMI spirometer was 6 ml for FVC and 21 ml for FEV1. Standard spirometry procedures as recommended by the American Thoracic Society were followed at all of the examinations (20, 21). Daily checks for leaks, volume calibration with a 3-L syringe, and weekly calibration in the 4- to 7-L range were undertaken to minimize methodologic artifacts between examinations. We analyzed FVC and FEV1 as the maximum of five satisfactory maneuvers. In almost all of the cases, the maximum and second highest maneuvers agreed to within 150 ml. Considering the young age of some participants at baseline, the possibility that lung function had not yet reached the maximum at Year 0, we estimated an individual's peak lung function as the maximum attained at Year 0, 2, 5, 10, or 20. A total of 4% (138 of 3,426) of participants had their highest FVC measured at the Year 20 examination and of these in only 55 participants was the Year 20 FVC measurement the greatest by more than 100 ml. Change in lung function was calculated as estimated peak lung function − Year 20 lung function (a larger positive number for the decline in lung function variables indicates greater declines in lung function).
Given the age of the cohort we were not interested in underlying lung disease but rather we were interested in patterns of loss of lung health as subjects approach potential disease states in middle age. As such we defined our two primary predictors for this analysis as a declining FEV1/FVC ratio, and declining FVC and FEV1 but a preserved FEV1/FVC ratio. Because of high correlation between FVC and FEV1 we chose to report only FVC and FEV1/FVC ratio.
Echocardiography Outcome Measures
CARDIA participants at the Year 25 examination underwent two-dimensionally guided M-mode echocardiography in a parasternal window and two-dimensional four-chamber apical views following American Society of Echocardiography recommendations (22). All studies were recorded in digital format using an Artida cardiac ultrasound scanner (Toshiba Medical Systems, Tokyo, Japan) and read at the Johns Hopkins University Echocardiography Reading Center in Baltimore, Maryland. Measurements were made by experienced analysts from digitized images using a standard software off-line image analysis system (Digisonics, Inc., Houston, TX). At Year 25, LV end-diastolic volume (LVEDV) and LV end-systolic volume were measured from apical two-dimensional four-chamber images. We chose to use M-mode imaging for assessment of LV ejection fraction and for left atrial internal dimension, LV posterior wall thickness, interventricular septal thickness, LV fractional shortening, LV stroke volume, cardiac output, and LV mass. For the purposes of defining diastolic dysfunction at Year 25 three echocardiographic parameters were used: (1) lateral e′ velocity less than 9 cm/s, (2) left atrial volume index greater than 28 ml/m2, and (3) lateral E/e′ ratio greater than 12 (23).
Statistical Analysis
Results are listed as the mean ± SD. Regression analysis was used to assess associations of lung function measures with echocardiographic parameters in two separate analyses. In the first, we used linear regression to evaluate the association between decline in lung function (both FVC and FEV1/FVC ratio), defined as estimated peak lung function − Year 20 lung function, and Year 25 echocardiographic parameters. Covariables in this analysis included race–sex group, lifetime smoking at Year 25, age at Year 25, number of years with diabetes, height at peak lung function, peak lung function (either FVC or ratio), and decline in lung function (either FVC or ratio) and years since peak lung function.
Two separate models were developed in this analysis to evaluate the impact of weight gain on these associations. In the first model, Year 5 body mass index (BMI) was included as a covariable, and in the second model, change in BMI (Year 25 BMI − Year 5 BMI) and at Year 5 BMI were included as covariables. Given discrepancies in sex distribution in quartiles of lung health we tested for sex interaction term and then performed a stratified analysis by sex (see online supplement). Logistic regression was used to determine the association between lung function and diastolic dysfunction, which was defined as meeting all three of the following criteria: (1) lateral e′ velocity less than 9 cm/s, (2) left atrial volume index greater than 28 ml/m2, and (3) lateral E/e′ ratio greater than 12. Figure 1 provides a breakdown of the number of subjects available for analysis based on available echocardiographic parameter at Year 25 by lung function.
Figure 1.
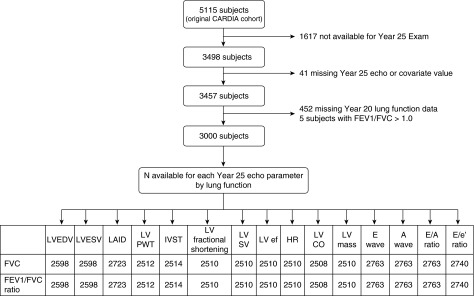
Number of participants included in the analysis in Table 3 based on available echocardiographic data and pulmonary function data. CARDIA = Coronary Artery Risk Development in Young Adults; CO = cardiac output; ef = ejection fraction; HR = heart rate; IVST = interventricular septal thickness; LAID = left atrial internal dimension; LV = left ventricular; LVEDV = left ventricular end-diastolic volume; LVESV = left ventricular end-systolic volume; PWT = posterior wall thickness; SV = stroke volume.
Results
To approximate a baseline relationship between lung function measurements and echocardiographic parameters we evaluated the association between Year 5 lung function and Year 5 echocardiograms. We found that Year 5 FVC was associated with Year 5 LVEDV, left atrial structure, and LV mass such that a larger FVC was associated with increased LVEDV, increased left atrial size, and increased LV mass. No association was noted between FVC and hypertrophy measurements or between FEV1/FVC ratio and any echocardiographic measurements (see Tables E2a and E2b in the online supplement). We focused the remainder of our analysis on patterns of loss of lung health from young adulthood onward and subsequent (Year 25) echocardiographic findings.
At the Year 25 CARDIA examination 3,000 subjects had both prior lung function and echocardiogram data and were included in the analysis. Demographic and clinical descriptions of the sample at Year 25 across quartiles of FVC decline and quartiles of FEV1/FVC ratio decline are provided in Tables 1 and 2. The mean annual decline in FVC from peak to Year 20 was 40 ml/yr in men and 33 ml/yr in women (see Table E1). Additional demographics and clinical descriptions highlighting the health of the cohort are provided in Table E1.
Table 1.
Participant Characteristics by Quartile of FVC Decline
| Quartile of Decline in FVC |
P Value | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| N | 750 | 750 | 750 | 750 | |
| FVC decline, L | 0.14 ± 0.10 | 0.40 ± 0.06 | 0.63 ± 0.07 | 1.05 ± 0.29 | |
| FEV decline, L | 0.33 ± 0.18 | 0.50 ± 0.18 | 0.63 ± 0.18 | 0.92 ± 0.32 | |
| FEV1/FVC decline, % | 7.56 ± 4.67 | 6.21 ± 4.03 | 5.28 ± 4.30 | 4.87 ± 5.00 | |
| Demographics | |||||
| Sex (male), n | 262 | 274 | 320 | 417 | |
| Age, Year 25 examination | 49.07 ± 3.60 | 50.18 ± 3.56 | 50.54 ± 3.58 | 51.10 ± 3.40 | |
| BMI, kg/m2 | 26.87 ± 5.40 | 29.25 ± 6.27 | 30.53 ± 6.94 | 33.51 ± 7.87 | |
| Height, cm | 168.85 ± 9.06 | 169.00 ± 9.21 | 170.51 ± 9.37 | 172.59 ± 9.53 | |
| Waist girth, cm | 85.48 ± 13.09 | 91.47 ± 13.60 | 95.79 ± 15.03 | 103.45 ± 15.64 | |
| Race, n | |||||
| White | 448 | 420 | 404 | 393 | |
| African American | 302 | 330 | 346 | 357 | |
| Smoking status, n | |||||
| Never | 486 | 465 | 471 | 446 | |
| Former | 165 | 159 | 154 | 166 | |
| Current | 97 | 114 | 110 | 126 | |
| Peak lung function | |||||
| Age peak FEV1, yr | 27.34 ± 5.09 | 27.52 ± 4.19 | 27.94 ± 4.40 | 28.16 ± 3.97 | |
| Peak FEV1, L | 3.57 ± 0.77 | 3.52 ± 0.75 | 3.62 ± 0.80 | 3.84 ± 0.83 | |
| Age peak FVC, yr | 31.59 ± 6.75 | 29.49 ± 4.65 | 29.19 ± 4.55 | 29.04 ± 4.35 | |
| Peak FVC, L | 4.28 ± 1.00 | 4.26 ± 0.98 | 4.46 ± 1.03 | 4.79 ± 1.06 | |
| Age peak FEV1/FVC ratio, yr | 26.33 ± 5.21 | 27.83 ± 5.74 | 29.48 ± 7.01 | 31.23 ± 7.90 | |
| Peak FEV1/FVC ratio | 86.4 ± 5.72 | 85.19 ± 6.13 | 83.96 ± 5.86 | 83.03 ± 6.19 | |
| LV size parameters | |||||
| LV end-diastolic volume, ml (2D) | 104.46 ± 28.28 | 108.00 ± 27.82 | 112.16 ± 27.84 | 120.29 ± 30.10 | <0.0001 |
| LV end-systolic volume, ml (2D) | 41.03 ± 16.42 | 41.76 ± 14.61 | 43.34 ± 15.45 | 47.49 ± 18.30 | <0.0001 |
| LA internal dimension, cm (M-mode) | 3.52 ± 0.46 | 3.69 ± 0.48 | 3.73 ± 0.48 | 3.88 ± 0.49 | <0.0001 |
| LV hypertrophy parameters | |||||
| LV posterior wall thickness, diastole, cm | 0.83 ± 0.15 | 0.88 ± 0.16 | 0.90 ± 0.16 | 0.95 ± 0.17 | <0.0001 |
| Interventricular septal wall, diastole, cm | 0.86 ± 0.15 | 0.89 ± 0.16 | 0.92 ± 0.17 | 0.97 ± 0.17 | <0.0001 |
| LV mass, g (M-mode) | 148.08 ± 43.32 | 160.44 ± 45.20 | 170.61 ± 50.34 | 191.78 ± 57.94 | <0.0001 |
| LV function parameters | |||||
| LV ejection fraction, % (M-mode) | 69.73 ± 7.65 | 70.00 ± 7.64 | 70.07 ± 8.10 | 69.07 ± 8.50 | 0.09 |
| LV stroke volume, ml (M-mode) | 83.15 ± 20.66 | 86.28 ± 20.25 | 89.76 ± 22.23 | 94.01 ± 22.54 | <0.0001 |
| Heart rate, bpm | 64.88 ± 11.03 | 65.22 ± 10.31 | 64.94 ± 10.62 | 66.44 ± 10.92 | 0.03 |
| LV cardiac output, L/min | 5.35 ± 1.43 | 5.60 ± 1.52 | 5.80 ± 1.63 | 6.20 ± 1.65 | <0.0001 |
| LV E wave, cm/s | 79.63 ± 14.93 | 79.35 ± 15.72 | 78.48 ± 16.05 | 77.49 ± 16.46 | 0.04 |
| LV A wave, cm/s | 60.74 ± 15.50 | 63.64 ± 16.02 | 64.17 ± 16.25 | 65.89 ± 17.38 | <0.0001 |
| LV E/A ratio | 1.38 ± 0.38 | 1.31 ± 0.38 | 1.28 ± 0.35 | 1.23 ± 0.33 | <0.0001 |
Definition of abbreviations: 2D = two-dimensional; BMI = body mass index; LA = left atrial; LV = left ventricular.
Data reported as mean ± SD.
Table 2.
Participant Characteristics by Quartile of FEV1/FVC Ratio Decline
| Quartile of Decline in FEV1/FVC Ratio |
P Value | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| N | 750 | 750 | 750 | 750 | |
| FEV1/FVC decline, % | 1.09 ± 0.98 | 4.08 ± 0.78 | 6.69 ± 0.81 | 12.06 ± 4.29 | |
| FEV decline, L | 0.52 ± 0.28 | 0.56 ± 0.28 | 0.59 ± 0.28 | 0.71 ± 0.38 | |
| FVC decline, L | 0.68 ± 0.36 | 0.58 ± 0.35 | 0.50 ± 0.33 | 0.45 ± 0.39 | |
| Demographics | |||||
| Sex (male), n | 408 | 342 | 274 | 249 | |
| Age, Year 25 examination | 50.66 ± 3.48 | 50.36 ± 3.60 | 50.03 ± 3.64 | 49.84 ± 3.67 | |
| BMI, kg/m2 | 32.43 ± 7.38 | 30.78 ± 7.32 | 29.24 ± 6.64 | 27.71 ± 6.09 | |
| Height, cm | 171.87 ± 9.82 | 170.41 ± 9.52 | 169.25 ± 9.28 | 169.42 ± 8.77 | |
| Waist girth, cm | 100.90 ± 15.51 | 95.76 ± 16.03 | 91.45 ± 14.70 | 88.09 ± 13.88 | |
| Race, n | |||||
| White | 402 | 420 | 443 | 400 | |
| African American | 348 | 330 | 307 | 350 | |
| Smoking status, n | |||||
| Never | 479 | 486 | 450 | 453 | |
| Former | 164 | 151 | 192 | 137 | |
| Current | 98 | 101 | 97 | 151 | |
| Peak lung function | |||||
| Age peak FEV1, yr | 28.43 ± 4.72 | 28.02 ± 4.45 | 27.47 ± 4.40 | 27.03 ± 4.06 | |
| Peak FEV1, L | 3.73 ± 0.82 | 3.68 ± 0.81 | 3.61 ± 0.78 | 3.55 ± 0.76 | |
| Age peak FVC, yr | 28.79 ± 4.70 | 29.72 ± 5.02 | 29.97 ± 5.28 | 30.83 ± 5.80 | |
| Peak FVC, L | 4.65 ± 1.08 | 4.51 ± 1.07 | 4.37 ± 1.01 | 4.25 ± 0.95 | |
| Age peak FEV1/FVC ratio, yr | 33.95 ± 9.21 | 27.78 ± 4.78 | 26.73 ± 4.54 | 26.44 ± 4.40 | |
| Peak FEV1/FVC ratio | 82.51 ± 5.68 | 83.82 ± 5.36 | 85.10 ± 5.40 | 87.17 ± 6.88 | |
| LV size parameters | |||||
| LV end-diastolic volume, ml (2D) | 117.70 ± 30.54 | 113.09 ± 29.80 | 110.01 ± 27.52 | 103.91 ± 26.75 | <0.0001 |
| LV end-systolic volume, ml (2D) | 46.49 ± 18.27 | 44.37 ± 16.77 | 42.58 ± 15.30 | 40.11 ± 14.49 | <0.0001 |
| LA internal dimension, cm (M-mode) | 3.85 ± 0.49 | 3.76 ± 0.48 | 3.64 ± 0.46 | 3.57 ± 0.49 | <0.0001 |
| LV hypertrophy parameters | |||||
| LV posterior wall thickness, diastole, cm | 0.93 ± 0.15 | 0.90 ± 0.17 | 0.87 ± 0.16 | 0.85 ± 0.17 | <0.0001 |
| Interventricular septal wall, diastole, cm | 0.95 ± 0.16 | 0.91 ± 0.17 | 0.89 ± 0.16 | 0.87 ± 0.17 | <0.0001 |
| LV mass, g (M-mode) | 183.46 ± 50.82 | 172.11 ± 55.38 | 162.54 ± 48.65 | 152.06 ± 47.11 | <0.0001 |
| LV function parameters | |||||
| LV ejection fraction, % (M-mode) | 69.48 ± 8.40 | 69.77 ± 8.23 | 69.51 ± 7.53 | 70.13 ± 7.76 | 0.40 |
| LV stroke volume, ml (M-mode) | 92.69 ± 21.81 | 90.07 ± 23.22 | 87.04 ± 20.25 | 83.28 ± 20.69 | <0.0001 |
| Heart rate, bpm | 66.12 ± 10.54 | 65.05 ± 10.83 | 64.92 ± 10.94 | 65.36 ± 10.59 | 0.17 |
| LV cardiac output, L/min | 6.09 ± 1.56 | 5.84 ± 1.75 | 5.62 ± 1.49 | 5.41 ± 1.46 | <0.0001 |
| LV E wave, cm/s | 76.85 ± 16.77 | 78.42 ± 16.01 | 79.79 ± 14.93 | 79.90 ± 15.32 | 0.0004 |
| LV A wave, cm/s | 63.97 ± 16.77 | 64.28 ± 17.03 | 62.81 ± 16.05 | 63.35 ± 15.70 | 0.32 |
| LV E/A ratio | 1.26 ± 0.36 | 1.28 ± 0.34 | 1.34 ± 0.39 | 1.32 ± 0.36 | <0.0001 |
Definition of abbreviations: 2D = two-dimensional; BMI = body mass index; LA = left atrial; LV = left ventricular.
Data reported as mean ± SD.
We evaluated if the patterns of change in lung function from young adulthood to middle age were associated with distinct echocardiographic findings at Year 25. We found that greater decline in the FEV1/FVC ratio (peak − Year 20) was associated with smaller left heart chamber size reflected by smaller left atrial internal dimension (Table 3). In contrast, we found that a greater decline in the FVC but not the FEV1/FVC ratio was associated with larger left atrial internal dimension and with markers of LV hypertrophy including greater LV mass, LV posterior wall thickness, and interventricular septal thickness (Table 3, Figure 2).
Table 3.
Association between Decline in Lung Function (Peak − Year 20) and Year 25 Cardiac Structure
| Model 1* |
Model 2† |
|||||||
|---|---|---|---|---|---|---|---|---|
| Decline FVC |
Decline FEV1/FVC Ratio |
Decline FVC |
Decline FEV1/FVC Ratio |
|||||
| β | P Value | β | P Value | β | P Value | β | P Value | |
| LV size parameters | ||||||||
| LV end-diastolic volume, ml (2D) | 0.877 | 0.10 | −0.739 | 0.15 | 0.030 | 0.96 | −0.609 | 0.23 |
| LA internal dimension, cm | 0.044 | <0.0001 | −0.038 | <0.0001 | 0.014 | 0.14 | −0.034 | 0.0001 |
| LV hypertrophy parameters | ||||||||
| LV posterior wall thickness, diastole, cm | 0.017 | <0.0001 | 0.0006 | 0.85 | 0.012 | 0.0006 | 0.002 | 0.60 |
| Interventricular septal wall, diastole, cm | 0.020 | <0.0001 | 0.001 | 0.78 | 0.014 | <0.0001 | 0.002 | 0.52 |
| LV mass, g (M-mode) | 6.05 | <0.0001 | −0.746 | 0.40 | 3.84 | <0.0001 | −0.332 | 0.70 |
| LV function parameters | ||||||||
| LV ejection fraction, % (M-mode) | −0.119‡ | 0.51 | −0.094 | 0.60 | −0.136 | 0.47 | −0.089 | 0.62 |
| LV stroke volume, ml | 0.507‡ | 0.26 | −0.830 | 0.06 | −0.055‡ | 0.90 | −0.726 | 0.09 |
| Heart rate, bpm | 0.889 | 0.0002 | −0.037§ | 0.87 | 0.589 | 0.01 | 0.020§ | 0.93 |
| LV cardiac output, L/min | 0.109 | 0.001 | −0.070‡ | 0.03 | 0.046 | 0.18 | −0.058‡ | 0.07 |
| LV E wave, cm/s | 0.251 | 0.45 | 0.575 | 0.07 | 0.183 | 0.59 | 0.584 | 0.06 |
| LV A wave, cm/s | 1.465§ | <0.0001 | 0.272 | 0.39 | 0.934‡ | 0.006 | 0.337‡ | 0.28 |
| LV E/A ratio | −0.029 | 0.0002 | 0.004 | 0.56 | −0.018 | 0.02 | 0.003 | 0.69 |
Definition of abbreviations: 2D = two-dimensional; LA = left atrial; LV = left ventricular.
Values shown are per 1 SD change.
Covariates: race–sex group, Year 5 (Y5) age, height at peak lung function, Y5 body mass index, lifetime smoking at Y25, number of years with diabetes, years since peak lung function, peak lung function (FVC or ratio), change in lung function (FVC or ratio).
Covariates: race–sex group, Y25 age, height at peak lung function, Y5 body mass index, Y25 − Y5 body mass index difference, lifetime smoking at Y25, number of years with diabetes, years since peak lung function, peak lung function (FVC or ratio), change in lung function (FVC or ratio).
Sex interaction term: P < 0.1.
Sex interaction terms: P < 0.05.
Figure 2.
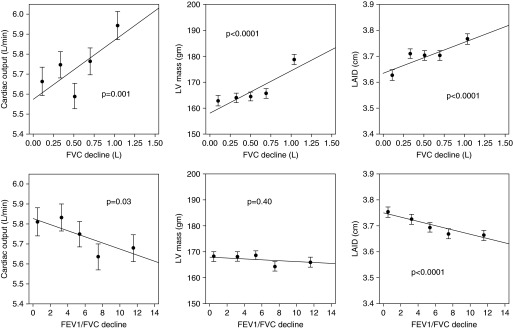
Adjusted association between decline in FVC (top three panels) or decline in FEV1/FVC ratio (bottom three panels) and selected heart structural and functional parameters. Adjusted means of each cardiac parameter by quintile of FVC or FEV1/FVC decline is plotted and a fitted regression line superimposed. The P values noted are the regression P values. LAID = left atrial internal dimension; LV = left ventricular.
We also assessed the association between decline in lung function and measures of cardiac performance. Greater decline in the FEV1/FVC ratio was associated with lower cardiac output at Year 25 (Figure 2). Greater decline in the FVC (but not the FEV1/FVC) was associated with a higher cardiac output (Figure 2) and higher mitral A wave and a lower E/A ratio at Year 25 (Table 3).
To better understand the impact obesity plays on the association between change in lung function and echocardiographic parameters a second model including the covariable change in BMI from CARDIA examination Year 5–25 was created. The association between a greater decline in FEV1/FVC ratio and smaller left atrial internal dimension persisted as did the associations of decline in FVC and markers of LV hypertrophy and higher mitral A wave and lower E/A ratio. With the addition of change in BMI the associations of both change in FVC and FEV1/FVC ratio with cardiac output were attenuated (Table 3).
To better understand the impact sex plays on the association between change in lung function and echocardiographic parameters we tested interactions between sex and lung function decline. Multiple interactions were noted and are highlighted in Table 3. We then performed a stratified analysis by sex and confirmed that the significant associations between patterns of lung function decline and echocardiographic findings were preserved in both men and women (see Tables E3a and Table E3b).
Given the observation that decline in FVC (but not FEV1/FVC ratio) was associated with cardiac hypertrophy and echocardiographic measurements of LV stiffness (mitral A wave and E/A ratio), whereas decline in the FEV1/FVC ratio was associated with smaller left heart chamber size, we evaluated if change in lung function would be associated with evidence of LV diastolic dysfunction. At the Year 25 examination when the mean subject age was about 50 years old 31 subjects met echocardiographic criteria for diastolic dysfunction. We found that greater decline in the FVC was associated with an increased likelihood of having diastolic dysfunction. This association was independent of race, sex, age, height, BMI, change in BMI, cigarette smoking, and diabetes and peak lung function. Decline in the FEV1/FVC ratio was not associated with diastolic dysfunction (Table 4).
Table 4.
Association between Decline in Lung Function (Peak − Year 20) and Left Ventricular Diastolic Dysfunction at Year 25
| OR for Diastolic Dysfunction (95% CI)* | |
|---|---|
| N | 31 |
| Peak, Y20 FVC, L | 3.39 (1.37–8.36) |
| P = 0.006 | |
| Peak, Y20 FEV1/FVC | 1.05 (0.99–1.11) |
| P = 0.11 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio; Y = Year.
Covariates: race–sex group, Y25 age, height at peak lung function, Y5 body mass index, Y25 − Y5 body mass index difference, lifetime smoking at Y25, number of years with diabetes, peak lung function (FVC or ratio).
Criteria: lateral e′ velocity < 9 cm/s, left atrial volume index > 28 ml/m2, and E/e′ ratio > 12.
Discussion
In a population-based study of generally healthy young adults followed for 25 years, we found that two distinct patterns of loss of lung health are associated with divergent cardiac changes in middle age. A decline in the FEV1/FVC ratio is associated with decreased left heart chamber size and lower cardiac output, whereas a decline in FVC but with a preserved FEV1/FVC ratio is associated with left heart hypertrophy, increased cardiac output, and diastolic dysfunction. These findings are unique because although decline in FVC has previously been associated with incident hypertension (14) and both established COPD and restriction have known associations with cardiovascular disease and poor health outcomes (1–7), how lung and heart structural and functional abnormalities evolve from young adulthood through middle age has not been investigated. We fill this gap in knowledge by reporting that two patterns of lung health loss, that is, decline in FVC (which could be a precursor of developing overt restrictive physiology) and decline in FEV1/FVC (a precursor of developing obstructive physiology), are associated with distinct cardiac structural findings. Documenting this divergence in lung-heart phenotype expands the understanding regarding lung and heart physiologic and structural changes that occur before the clinical onset of common complex conditions, such as COPD and heart failure with preserved ejection fraction.
Recently, heart and lung interactions in established COPD have been explored in some detail. Obstructive lung disease seems to have effects on the size and function of both the right and left heart (24, 25). An echocardiographic study of subjects with severe COPD found that both left atrial and LV filling was significantly reduced compared with control subjects without COPD (26). In another study evaluating subjects with varying severity of COPD, Watz and coworkers (25) found that the size of all cardiac chambers decreased with worsening lung function and that the degree of hyperinflation seemed to show the strongest correlation with heart size. The mechanistic link, however, between COPD and decreased cardiac chamber size and function is unknown. Watz and coworkers (25) postulates that the effects of lung hyperinflation on increasing intrathoracic pressure and subsequent decrease in venous return to the heart may help explain the decrease in filling pressures (25). Another study found an association between residual volume and LV mass and hypothesized that hyperinflation by augmenting LV wall stress leads to increased LV stroke work and eventually increased LV mass (27). Others have discussed the role of hemodynamic effects of hypoxia and vascular remodeling leading to pulmonary hypertension with subsequent effect on RV and LV interdependence as a cause of altered cardiac chamber size (28–30).
All of these proposed mechanisms rely on the presence of advanced lung disease to cause the cardiac changes described. However, there is growing evidence that the interaction between the heart and lungs can be seen even with nonsevere lung disease. In the Multi-Ethnic Study of Atherosclerosis Lung study, percent emphysema on computed tomography scan of the lung and FEV1/FVC ratio on spirometry were both cross-sectionally associated with lower LVEDV(13). Most participants in the Multi-Ethnic Study of Atherosclerosis Lung study had normal spirometry and the strongest associations were noted for the radiographic finding of emphysema suggesting that changes that can be interpreted as “early emphysema” in an otherwise healthy patient population are associated with left-sided cardiac changes. The idea that alterations in cardiac filling occur early in the course of the development of emphysema lends credence to the idea that the pathologic interaction between the heart and lung seen in advanced disease begins its evolution earlier in life presumably before many of the proposed mechanistic pathways in established COPD, such as hyperinflation, have been established.
Our findings provide additional insight into the complexity of the heart-lung interaction by evaluating the early stages of this interaction from healthy young adulthood onward. Decline in the FEV1/FVC ratio from young adulthood to middle age is associated with a small heart, low-output phenotype consistent with cross-sectional findings reported in an older cohort attributed to emphysema (13). However, we studied an age range that would not be expected to have emphysematous changes in the lung yet still found associations with underfilling of the left side of the heart. Alternatively, a decline in FVC from young adulthood to middle age is associated with a hypertrophic, high-output cardiovascular phenotype suggesting that the decline in FVC is linked to the risk for diastolic heart dysfunction as would be expected from our previous findings linking decline in FVC to incident hypertension. Importantly, even when controlling for changes in weight over time the associations between change in lung function and structural changes to the heart were preserved. Although the associations between change in lung function and cardiac outputs seem to be attenuated by change in BMI, our findings show that the interplay between declining lung function and structural heart changes extends beyond the mechanical effects of weight gain on chest wall compliance.
Although a large epidemiologic study like CARDIA lacks the ability to define precise mechanisms of disease, our findings suggest that those who lose lung function at an accelerated rate may be at risk of developing cardiac structural and functional alterations in the future. Animal models have shown that in cigarette smoke–exposed mice pulmonary vascular dysfunction and remodeling causing hemodynamic alterations precedes the development of emphysema (31). Our observation that a greater decline in the FEV1/FVC ratio is associated with an underfilled/low-output cardiac phenotype at Year 25 is compatible with the findings of early vascular changes seen in the animal model. Reduced pulmonary vein area has been described in individuals with established COPD suggesting an “upstream” obstruction to blood flow as the cause of reduced LV preload (32). Our findings indicate that the development of an under-filled left heart occurs concurrent with the decline in lung function from young adulthood into middle age and is not the consequence of only severe emphysema or lung hyperinflation.
Alternatively, those exhibiting symmetric reductions in FEV1 and FVC likely follow a different mechanistic pathway leading to cardiac hypertrophy and increased LV mass. Obesity and the metabolic syndrome have been linked to lung function decline. Although we have previously shown the association between decline in FVC and development of hypertension in the CARDIA cohort, others have described an association between the presence of hypertension and an accelerated rate of lung function decline, which can be attenuated with treatment of hypertension (33, 34). These findings along with our finding that the association of change in lung function and structural changes in the heart occurs independent of BMI show that the link between metabolic syndrome, hypertension, and lung function changes extends beyond the idea of obesity causing restrictive lung physiology through mechanical effects on chest wall compliance. These findings suggest an adaptive link between declining lung function and onset of hypertension, which places patients at risk for cardiac hypertrophy and the development of diastolic dysfunction.
There are several limitations and strengths to our study. A notable strength is that at the Year 25 CARDIA examination participants are still generally young and healthy, which allows us the unique opportunity to explore the heart-lung interaction from a starting point of health rather than at established disease states. As we discuss alterations in lung function it should be noted that most participants in CARDIA still fall within the normal ranges of lung function and we are studying patterns of loss of lung health and not disease states. Care should be taken in extrapolating these findings to “early” disease, such as early COPD or early restrictive lung disease, because we do not know yet who will actually go on to develop disease later in life. Although the magnitude of the actual changes noted may seem small this is also likely reflective of the fact that we are reporting changes in a young healthy population and observing subclinical changes in their lung function. Although COPD is well recognized as an obstructive lung disease there is a growing recognition that restrictive spirometric impairments are common in patients diagnosed with COPD (35–37). Despite this recognition, there is a general lack of understanding of this restrictive disease phenotype, which is highlighted in the absence of recommendations addressing it in disease-focused guidelines. This gap in knowledge makes interpretation of the full implications of our findings of decline in FVC with a preserved FEV1/FVC ratio difficult (35, 36, 38).
A major strength of our study is that it includes comprehensive echocardiography of a population-based cohort that is younger than most studies that evaluate the intersection of lung disease and cardiac disease. Although evaluation of right heart structure and function would have added value to this study, unfortunately, right heart evaluation was not a focus of the Year 25 CARDIA examination. Finally, an intrinsic limitation to large population-based epidemiologic studies is the inability to study pathobiologic mechanisms of disease. Although we describe several provocative physiologic associations we lack lung imaging by computed tomography scan to help provide more in-depth information on lung structure and intrathoracic vascular changes that could provide more insight into the mechanisms of our observations.
The differential patterns of loss of lung health, decline in FVC, and decline FEV1/FVC ratio from peak lung function attained in young adulthood are associated with divergent heart-lung phenotypes in middle age. By understanding the distinct cardiovascular changes associated with decline in FEV1/FVC ratio and decline in FVC with preserved FEV1/FVC ratio we can begin to better understand the evolution of comorbid cardiac and lung disease states. It is reasonable to hypothesize that as these divergent patterns of loss of lung health develop into identifiable heart-lung phenotypes in middle age that the groundwork is being laid for the eventual development of distinct heart failure syndromes seen later in life (Figure 3). Identifying specific subclinical heart-lung phenotypes, such as “declining FVC and cardiac hypertrophy” or “declining FEV1/FVC ratio and underfilled, hyperdynamic left heart,” and beginning to understand their preclinical natural history may eventually allow for more targeted therapy to impact the onset and progression of common pulmonary and cardiovascular diseases that manifest later in life.
Figure 3.
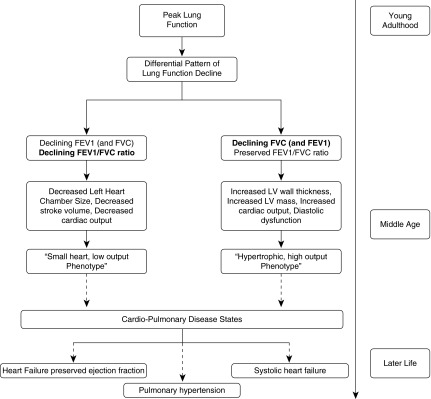
Proposed pathway of the evolution through lifecycle of the differential patterns of loss of lung health and their association with distinct cardiopulmonary phenotypes. LV = left ventricular.
Footnotes
Supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the NHLBI; the Intramural Research Program of the National Institute on Aging; and an intraagency agreement between National Institute on Aging and NHLBI (AG0005). Additional support for this project came from R01 HL122477 (R.K.).
Author Contributions: M.J.C. and R.K. had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design, M.J.C., S.J.S., and R.K. Acquisition of data, D.R.J., K.L., D.L.-J., and J.L. Analysis and interpretation of data, all authors. Drafting of the manuscript, M.J.C. and R.K. Critical revision of the manuscript for important intellectual content, all authors. Statistical analysis, M.J.C., L.A.C., D.R.J., K.L., and R.K. Obtained funding, D.R.J. and K.L. Administrative, technical, or material support, K.L. Study supervision, K.L. and D.L.-J.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201501-0116OC on April 15, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mannino DM, Holguin F, Pavlin BI, Ferdinands JM. Risk factors for prevalence of and mortality related to restriction on spirometry: findings from the First National Health and Nutrition Examination Survey and follow-up. Int J Tuberc Lung Dis. 2005;9:613–621. [PubMed] [Google Scholar]
- 2.Duprez DA, Hearst MO, Lutsey PL, Herrington DM, Ouyang P, Barr RG, Bluemke DA, McAllister D, Carr JJ, Jacobs DR., Jr Associations among lung function, arterial elasticity, and circulating endothelial and inflammation markers: the multiethnic study of atherosclerosis. Hypertension. 2013;61:542–548. doi: 10.1161/HYPERTENSIONAHA.111.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford ES, Wheaton AG, Mannino DM, Presley-Cantrell L, Li C, Croft JB. Elevated cardiovascular risk among adults with obstructive and restrictive airway functioning in the United States: a cross-sectional study of the National Health and Nutrition Examination Survey from 2007-2010. Respir Res. 2012;13:115. doi: 10.1186/1465-9921-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curkendall SM, DeLuise C, Jones JK, Lanes S, Stang MR, Goehring E, Jr, She D. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16:63–70. doi: 10.1016/j.annepidem.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 6.Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313:711–715, discussion 715–716. doi: 10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schünemann HJ, Dorn J, Grant BJ, Winkelstein W, Jr, Trevisan M. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest. 2000;118:656–664. doi: 10.1378/chest.118.3.656. [DOI] [PubMed] [Google Scholar]
- 8.McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA TORCH Clinical Endpoint Committee. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62:411–415. doi: 10.1136/thx.2006.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcus EB, Curb JD, MacLean CJ, Reed DM, Yano K. Pulmonary function as a predictor of coronary heart disease. Am J Epidemiol. 1989;129:97–104. doi: 10.1093/oxfordjournals.aje.a115128. [DOI] [PubMed] [Google Scholar]
- 10.Hospers JJ, Rijcken B, Schouten JP, Postma DS, Weiss ST. Eosinophilia and positive skin tests predict cardiovascular mortality in a general population sample followed for 30 years. Am J Epidemiol. 1999;150:482–491. doi: 10.1093/oxfordjournals.aje.a010037. [DOI] [PubMed] [Google Scholar]
- 11.Kuller LH, Ockene JK, Townsend M, Browner W, Meilahn E, Wentworth DN. The epidemiology of pulmonary function and COPD mortality in the multiple risk factor intervention trial. Am Rev Respir Dis. 1989;140:S76–S81. doi: 10.1164/ajrccm/140.3_Pt_2.S76. [DOI] [PubMed] [Google Scholar]
- 12.Speizer FE, Fay ME, Dockery DW, Ferris BG., Jr Chronic obstructive pulmonary disease mortality in six U.S. cities. Am Rev Respir Dis. 1989;140:S49–S55. doi: 10.1164/ajrccm/140.3_Pt_2.S49. [DOI] [PubMed] [Google Scholar]
- 13.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal RA, Lima JA, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs DR, Jr, Yatsuya H, Hearst MO, Thyagarajan B, Kalhan R, Rosenberg S, Smith LJ, Barr RG, Duprez DA. Rate of decline of forced vital capacity predicts future arterial hypertension: the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2012;59:219–225. doi: 10.1161/HYPERTENSIONAHA.111.184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams MC, Murchison JT, Edwards LD, Agustí A, Bakke P, Calverley PM, Celli B, Coxson HO, Crim C, Lomas DA, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators. Coronary artery calcification is increased in patients with COPD and associated with increased morbidity and mortality. Thorax. 2014;69:718–723. doi: 10.1136/thoraxjnl-2012-203151. [DOI] [PubMed] [Google Scholar]
- 16.Sin DD, Man SF. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc. 2005;2:8–11. doi: 10.1513/pats.200404-032MS. [DOI] [PubMed] [Google Scholar]
- 17.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Crim C, Willits LR, Yates JC, Vestbo J TORCH Investigators. Cardiovascular events in patients with COPD: TORCH study results. Thorax. 2010;65:719–725. doi: 10.1136/thx.2010.136077. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez LA, Wallander MA, Martín-Merino E, Johansson S. Heart failure, myocardial infarction, lung cancer and death in COPD patients: a UK primary care study. Respir Med. 2010;104:1691–1699. doi: 10.1016/j.rmed.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 20.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 21.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 22.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Rakowski H, Appleton C, Chan KL, Dumesnil JG, Honos G, Jue J, Koilpillai C, Lepage S, Martin RP, Mercier LA, et al. Canadian consensus recommendations for the measurement and reporting of diastolic dysfunction by echocardiography: from the Investigators of Consensus on Diastolic Dysfunction by Echocardiography. J Am Soc Echocardiogr. 1996;9:736–760. doi: 10.1016/s0894-7317(96)90076-0. [DOI] [PubMed] [Google Scholar]
- 24.Cuttica MJ, Shah SJ, Rosenberg SR, Orr R, Beussink L, Dematte JE, Smith LJ, Kalhan R. Right heart structural changes are independently associated with exercise capacity in non-severe COPD. PLoS One. 2011;6:e29069. doi: 10.1371/journal.pone.0029069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watz H, Waschki B, Meyer T, Kretschmar G, Kirsten A, Claussen M, Magnussen H. Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: role of hyperinflation. Chest. 2010;138:32–38. doi: 10.1378/chest.09-2810. [DOI] [PubMed] [Google Scholar]
- 26.Boussuges A, Pinet C, Molenat F, Burnet H, Ambrosi P, Badier M, Sainty JM, Orehek J. Left atrial and ventricular filling in chronic obstructive pulmonary disease. An echocardiographic and Doppler study. Am J Respir Crit Care Med. 2000;162:670–675. doi: 10.1164/ajrccm.162.2.9908056. [DOI] [PubMed] [Google Scholar]
- 27.Smith BM, Kawut SM, Bluemke DA, Basner RC, Gomes AS, Hoffman E, Kalhan R, Lima JA, Liu CY, Michos ED, et al. Pulmonary hyperinflation and left ventricular mass: the Multi-Ethnic Study of Atherosclerosis COPD Study. Circulation. 2013;127:1503–1511, e1–e6. doi: 10.1161/CIRCULATIONAHA.113.001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barberà JA, Peinado VI, Santos S. Pulmonary hypertension in chronic obstructive pulmonary disease. Eur Respir J. 2003;21:892–905. doi: 10.1183/09031936.03.00115402. [DOI] [PubMed] [Google Scholar]
- 29.Haeck ML, Höke U, Marsan NA, Holman ER, Wolterbeek R, Bax JJ, Schalij MJ, Vliegen HW, Delgado V. Impact of right ventricular dyssynchrony on left ventricular performance in patients with pulmonary hypertension. Int J Cardiovasc Imaging. 2014;30:713–720. doi: 10.1007/s10554-014-0384-1. [DOI] [PubMed] [Google Scholar]
- 30.Vizza CD, Lynch JP, Ochoa LL, Richardson G, Trulock EP. Right and left ventricular dysfunction in patients with severe pulmonary disease. Chest. 1998;113:576–583. doi: 10.1378/chest.113.3.576. [DOI] [PubMed] [Google Scholar]
- 31.Seimetz M, Parajuli N, Pichl A, Veit F, Kwapiszewska G, Weisel FC, Milger K, Egemnazarov B, Turowska A, Fuchs B, et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell. 2011;147:293–305. doi: 10.1016/j.cell.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 32.Smith BM, Prince MR, Hoffman EA, Bluemke DA, Liu CY, Rabinowitz D, Hueper K, Parikh MA, Gomes AS, Michos ED, et al. Impaired left ventricular filling in COPD and emphysema: is it the heart or the lungs? The Multi-Ethnic Study of Atherosclerosis COPD Study. Chest. 2013;144:1143–1151. doi: 10.1378/chest.13-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koo HK, Kim DK, Chung HS, Lee CH. Association between metabolic syndrome and rate of lung function decline: a longitudinal analysis. Int J Tuberc Lung Dis. 2013;17:1507–1514. doi: 10.5588/ijtld.12.0906. [DOI] [PubMed] [Google Scholar]
- 34.Petersen H, Sood A, Meek PM, Shen X, Cheng Y, Belinsky SA, Owen CA, Washko G, Pinto-Plata V, Kelly E, et al. Rapid lung function decline in smokers is a risk factor for COPD and is attenuated by angiotensin-converting enzyme inhibitor use. Chest. 2014;145:695–703. doi: 10.1378/chest.13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and markers of inflammation: data from the Third National Health and Nutrition Examination. Am J Med. 2003;114:758–762. doi: 10.1016/s0002-9343(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 36.Mannino DM, McBurnie MA, Tan W, Kocabas A, Anto J, Vollmer WM, Buist AS, Group BCR BOLD Collaborative Research Group. Restricted spirometry in the burden of lung disease study. Int J Tuberc Lung Dis. 2012;16:1405–1411. doi: 10.5588/ijtld.12.0054. [DOI] [PubMed] [Google Scholar]
- 37.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, Austin JH, Jiang R, Lovasi GS, Barr RG. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Marciniuk DD, Denberg T, Schünemann H, Wedzicha W, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]


