Highlights
-
•
Asct2, an amino acid transporter, could be a target for obesity prevention and treatment.
-
•
All-trans retinoic acid suppresses upregulation of Asct2 during adipogenesis of 3T3-L1 cells.
-
•
The Asct2 inhibitor, l-γ-glutamyl-p-nitroanilide, suppresses adipogenesis at early time points.
-
•
Treatment with l-γ-glutamyl-p-nitroanilide suppresses adipogenesis more effectively than l-glutamine-deficient conditions.
Abbreviations: ATRA, all-trans retinoic acid; DIM, dexamethasone, insulin and 3-isobutylmethylxanthine; FXR, farnesoid X Receptor; Gapdh, glyceraldehyde 3-phosphate dehydrogenase; GPNA, l-γ-glutamyl-p-nitroanilide; PPARγ, peroxisome proliferator-activated receptor γ; RAR, retinoic acid receptor; RXR, retinoid X receptor
Keywords: Adipocyte, All-trans retinoic acid, Asct2, l-glutamine, Obesity
Abstract
Vitamin A has preventive effects on obesity. All-trans retinoic acid (ATRA), the active form of vitamin A, inhibits lipid accumulation in 3T3-L1 cells in an experimental adipogenesis model. We found that ATRA suppressed up-regulation of the amino acid transporter, Asct2, in adipogenerating 3T3-L1 cells. We observed that Asct2 was up-regulated at 1 day after adipogenesis stimuli. The Asct2 inhibitor l-γ-glutamyl-p-nitroanilide (GPNA) decreased lipid accumulation. Glutamine-free conditions also suppressed adipogenesis. Suppression of adipogenesis by ATRA may be through Asct2 reduction. These results indicate that Asct2 could be a target for obesity prevention and treatment.
1. Introduction
Obesity is the leading risk for the development of type 2 diabetes mellitus, hypertension and cardiovascular disease. Experiments have shown that molecular mechanisms of adipogenesis form a link between obesity and insulin resistance [1]. Vitamin A is an important nutrient that has multiple biological functions that affect vision, embryonic development, reproduction, and immune function [2–4]. The bioactive form of vitamin A is all-trans retinoic acid (ATRA); a nutrient derivative with many remarkable effects on adipocyte biology affecting preadipocyte survival, and adipogenesis in preadipocyte clonal cell lines [5,6]. ATRA treatment reduces body weight and adiposity [7,8]. ATRA is a specific ligand for retinoic acid receptors (RARs), and in part, it suppresses adipogenesis through activation of RARs. Several reports have indicated that various adipogenic signaling pathways are involved in the ATRA-dependent regulation of adipogenesis [6–12].
The mouse preadipocyte cell line, 3T3-L1 provides a model for differentiation process associated with adipogenesis, including activation of the adipogenetic signaling pathway. The temporal expression pattern of important adipogenic factors is regulated by specific adipogenous transcription factors [13]. In particular, peroxisome proliferator-activated receptor γ (PPARγ) is a transcription factor that up-regulates adipocyte-specific genes involved in lipid synthesis, and insulin signaling. It also regulates adipokine production for terminal differentiation, and PPARγ agonists, including pioglitazone, promote 3T3-L1 cell differentiation to mature adipocytes [14,15].
Asct2 (SLC1A5) is a neural amino acid transporter belonging to the solute carrier 1 family. Asct2 is expressed in many tissues, including placenta, lung, intestine and adipose-tissue [16,17]. Lane et al. have shown that Asct2 expression is activated by insulin-treatment [17]. Since Asct2 shows high affinity toward glutamine, Asct2 is essential for glutamine uptake in growing epithelial cells [18]. Bungard and McGivan have shown that Asct2 expression is regulated by farnesoid X Receptor (FXR)/retinoid X receptor (RXR) heterodimers in hepatoma cell line HepG2 cells [19,20]. Recently Palmada et al. have reported that Asct2 activity is regulated by serine-threonine kinases stimulated by insulin and insulin-like growth factor (IGF) [21]. We hypothesized that Asct2 in adipocytes may represent a new anti-obesity target.
When using differentiating 3T3-L1 cells as an experimental adipogenesis model, bio-synthesis of triacylglycerol, which is made from glucose and amino acids, is accelerated, resulting in enlarged intracellular lipid droplets. We observed that ATRA down-regulates Asct2 expression in these cells. In addition, we found in 3T3-L1 cells that the specific Asct2 inhibitor, l-γ-glutamyl-p-nitroanilide (GPNA) [22], inhibits adipogenesis in an additive fashion with ATRA. While GPNA has little effect on the expression levels of asct2 and glut4, GPNA decreases the protein levels of PPARγ. We hypothesized that inhibition of Asct2 may prevent obesity by suppression of glutamine-uptake. Inhibition of Asct2 by daily vitamin A intake may be sufficient to reduce obesity.
2. Materials and method
2.1. Cell culture
Murine preadipocyte, 3T3-L1 cells (obtained from Dr. Fumio Fukai, Department of Pharmaceutical Sciences, Tokyo University of Science), were routinely cultured in growth medium consisting of DMEM (Wako Pure Chemical Industries, Ltd., Tokyo, Japan) supplemented with 10% heat-inactivated FBS (Invitrogen, Carlsbad, CA, USA), 2 mM glutamine, 100 units/ml penicillin, and 100 units/ml streptomycin in 5% CO2 incubator at 37 °C. For glutamine free conditions, DMEM high glucose without glutamine (Wako) was supplemented with 10% heat-inactivated FBS, 100 units/ml penicillin, and 100 units/ml streptomycin.
2.2. Adipocyte differentiation
Routinely cultured 3T3-L1 cells were differentiated according to an established protocol [23]. Briefly, cells were cultured to full confluence. At 2 days post confluence (referred to as day 0), cells were stimulated with induction medium consisting of growth medium with DIM (1 μM dexamethasone, 0.5 mM isobutylmethylxanthine, and 1 μg/ml insulin). After 20 h, cells were switched to “insulin medium”, consisting of growth medium with 1 μg/ml insulin, and were maintained for 7 days. The cells normally differentiated into mature adipocytes on day 7. For examining the effects on adipogenesis of ATRA (Sigma, St. Louis, USA) and l-γ-glutamyl-p-nitroanilide (GPNA, Wako), compounds were added to the insulin medium.
2.3. Oil Red-O staining
To evaluate the extent of lipid accumulation in DIM-stimulated 3T3-L1 cells, the oil Red-O staining method was utilized as previously reported [23]. In brief, cells were fixed with 10% formalin at room temperature, and were then washed with PBS and 60% isopropyl alcohol, followed by treatment with oil Red-O (2.1 mg/ml 60% isopropyl alcohol) at room temperature. Cells were then washed in turn with 60% isopropyl alcohol and PBS. To perform quantitative measurement of the accumulated lipids, the oil Red-O stained wells were treated with 100% isopropyl alcohol to extract oil Red-O, and the solutions were then measured for absorbance at 510 nm.
2.4. Immunoblotting analysis
Whole cell lysates were prepared with NP40 lysis buffer [23,24]. The protein concentration of the whole cell lysates was determined by the Bicinchoninic Acid (BCA) Protein Assay (Piece, Rockford, IL, USA). Whole lysates were subjected to SDS–polyacrylamide gel electrophoresis (SDS–PAGE), and were transferred to Immobilon-P membranes (Millipore, Billerica, MA, USA). The membranes were probed with either polyclonal antibodies against ASCT2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), PPARγ (Cell Signaling, Danvers, MA, USA), or glyceraldehyde 3-phosphate dehydrogenase (Gapdh, Abcam, Cambridge, MA, USA). Proteins were visualized using ECL-plus (Invitrogen).
2.5. Quantitative RT-PCR
Total RNA of cultured 3T3-L1 cells was prepared with ISOGEN reagent (Nippon gene, Toyama, Japan). Complementary DNA (cDNA) of each tissue was prepared by reverse transcriptase (RT) reactions from 1 μg total RNA using the Superscript First-Strand Synthesis System for RT-PCR (Invitrogen). The sequences of the PCR primers were as follows: asct2 (forward 5′-GTC ACA GCC ACA GCA TCC A-3′, reverse 5′-CCA GCC CCA AAA GCA TCA C-3′), adipoq (forward 5′-TGG CAG AGA TGG CAC TCC TG-3′, reverse 5′-GGT CGT AGG TGA AGA GAA CG-3′), pparg1 (forward 5′-TGA AAG AAG CGG TGA AC-3′, reverse 5′-TAG TGT GGA GCA GAA AT-3′), pparg2 (forward 5′-GCT GTT ATG GGT GAA AC-3′, reverse 5′-TAG TGT GGA GCA GAA AT-3′), b-actin (forward 5′-AGC CAT GTA CGT AGC CAT CC-3′, reverse 5′-ATT ACC GAG GAC GAG CCC AGA C-3′). Quantitative mRNA expression analysis was performed by a real time PCR system (StepOneTM, Applied Biosystems Inc., Foster City, CA, USA). To estimate the level of transcripts quantitatively, b-actin transcript was used as an internal control for the each prepared sample.
2.6. Confocal microscopic observation
Intracellular Asct2 localization was visualized by immunofluorescent staining with anti-ASCT2 antibodies (Cell Signaling). In brief, harvested cells on 8-well chamber slides were treated with DIM and ATRA as previously mentioned, and the cells were fixed with methanol. The fixed cells were treated with anti-ASCT2 antibodies, and subsequently immunostained with Alexa546-conjugated anti-rabbit IgG antibody (Invitrogen). Confocal microscopy was performed using an Olympus FV1200 instrument (Olympus, Tokyo, Japan).
2.7. Statistical analysis
All data were analyzed using Student’s t-test. Differences were considered to be statistically significant if the p-value was <0.001.
3. Results
3.1. ATRA suppresses up-regulation of ASCT2 during adipogenesis
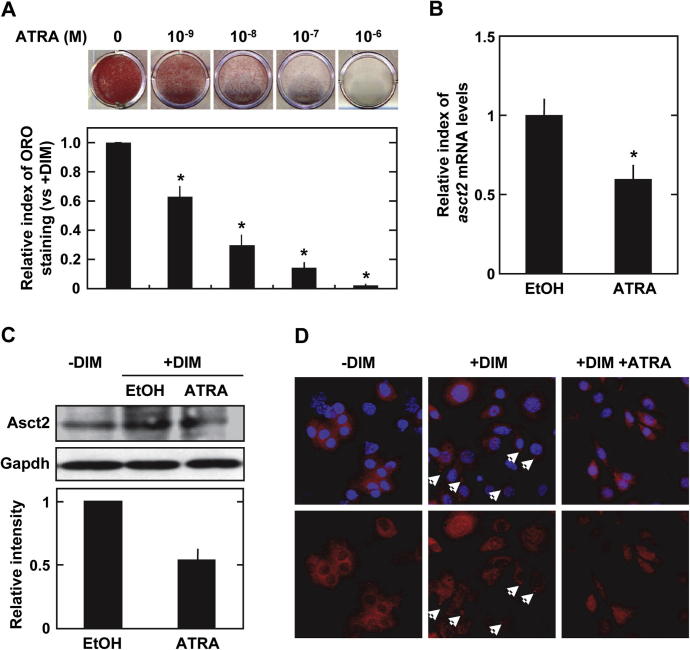
Several reports have shown that Asct2 transcripts are detected in adipose tissues and that insulin up-regulates Asct2 expression in adipogenesis-stimulated 3T3-L1 cells [16,17]. Since we hypothesized that ATRA could potentially suppress 3T3-L1 adipogenesis through Asct2 down-regulation, we added ATRA into the culture medium of DIM-stimulated 3T3-L1 cells to examine the effects of ATRA on the adipogenesis. The lipid droplets of the cultured cells were stained with oil Red-O, and a quantitative estimation of the accumulated lipid bodies was determined by measuring the oil Red-O under each culture condition.
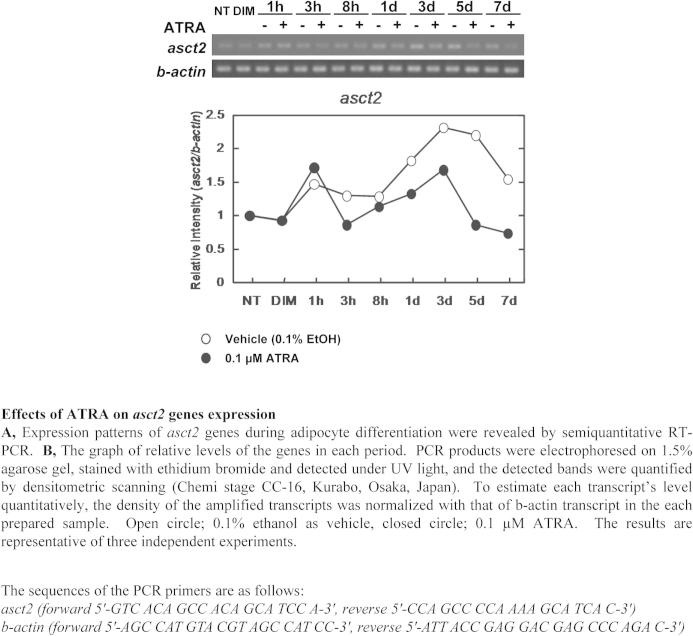
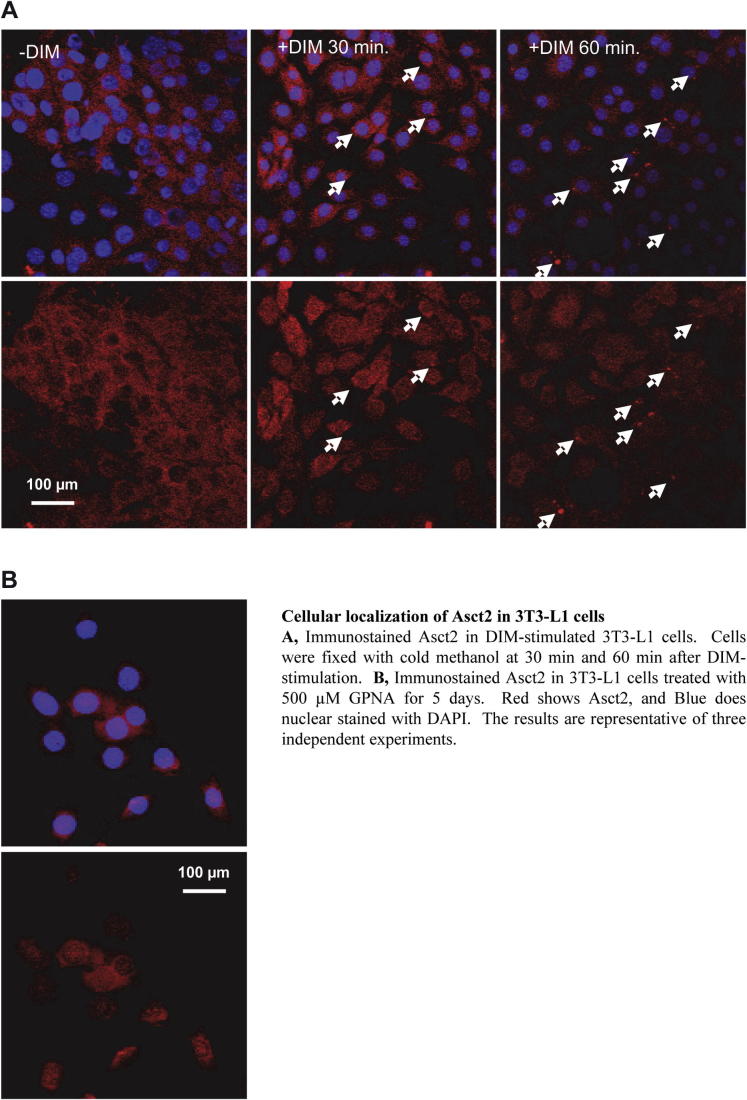
Inhibition of adipogenesis by ATRA was observed in a dose-dependent manner. The quantity of lipid droplets decreased by 63% with 1 nM, 30% with 10 nM, 15% with 100 nM, and by 2% with 1 μM ATRA during adipogenesis, respectively (Fig. 1A). In adipo-differentiating cells in vitro, the source of lipids is mainly from glucose and amino acids in the culture medium. The neutral amino acid transporter, Asct2, is an essential glutamine transporter in proliferating cells. We found that, in DIM-stimulated 3T3-L1 cells, 1 μM ATRA treatment suppressed asct2 gene expression by 59% (vs 0.1% ethanol treatment) at day 5 (Fig. 1B). The levels of asct2 genes increased until day 3, and then the levels decreased in the late stage of adipogenesis (Supplementary Fig. 1). ASCT2 protein levels in the adipogenerating 3T3-L1 were suppressed by 54% following ATRA treatment (Fig. 1C). From immunofluorescent stain analysis, DIM-stimulated 3T3-L1 cells displayed Asct2 on the surface of cells at 60 min following DIM-stimulation (Supplementary Fig. 2A). DIM-stimulated cells treated with ATRA had little Asct2 detection on cell surface (Fig. 1D). These results indicate that ATRA may suppress expression and intracellular localization of Asct2 during adipogenesis.
Fig. 1.
Effects of ATRA on adipogenesis and Asct2 expression. (A) Lipid-droplets in the cultured 3T3-L1 cells detected with oil Red-O staining in insulin medium containing various concentrations of ATRA after 7 days. Each bar represents the mean ± SD. (B) Expression levels of asct2 during adipocyte differentiation in 3T3-L1 cells treated with 0.1% ethanol (EtOH) and 0.1 μM ATRA (day 5) as revealed by quantitative RT-PCR. (C) Immunoblot detection of Asct2 and PPARγ in 3T3-L1 cells treated with 0.1% EtOH and 1 μM ATRA (day 5). Gapdh was employed as an internal control. Graph shows the density of detected Asct2 normalized with Gapdh. The results are representative of three independent experiments. (D) Immunofluorescent staining of Asct2 in DIM-stimulated 3T3-L1 cells treated with 0.1% EtOH and 1 μM ATRA (day 5). Blue shows DAPI-stained nuclei. Red shows immunostained Asct2. Arrows indicate the presence of Asct2 on the cell surface of DIM-stimulated 3T3-L1 cells during adipogenesis.
3.2. An Asct2 inhibitor suppresses adipogenesis of 3T3-L1 cells
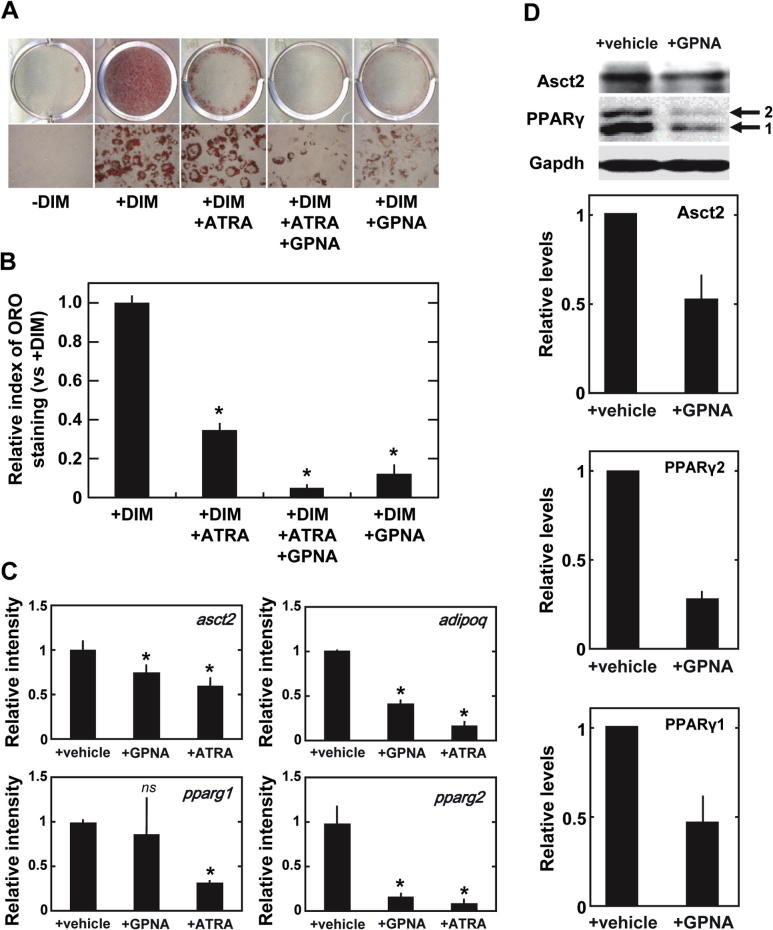
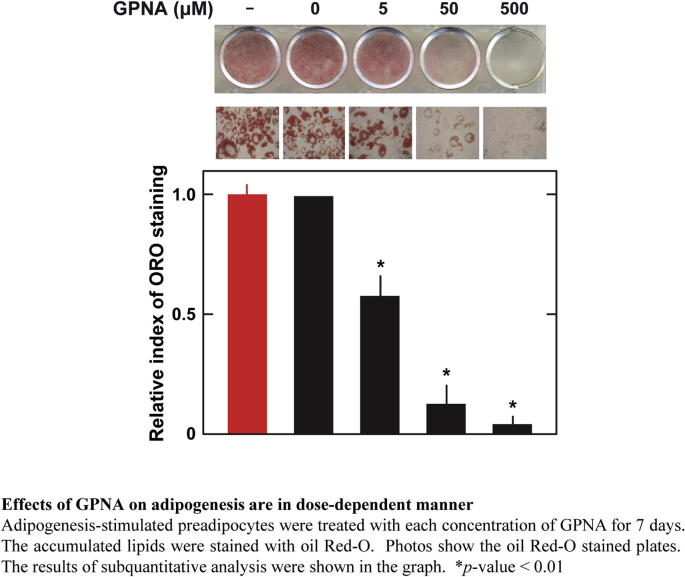
Next, we examined the role of Asct2 in adipogenesis. To measure the contribution of Asct2 to adipogenesis, we used the Asct2 inhibitor, l-γ-glutamyl-p-nitroanilide (GPNA) [22]. We performed analysis by oil Red-O staining (Fig. 2), on DIM-stimulated 3T3-L1 cells following treatment with 500 μM GPNA, which is sufficient to achieve ∼100% inhibition of glutamine uptake [22]. We found that under these conditions, lipid accumulation was reduced by approximately 12% during adipogenesis (Fig. 2A and B). Treatment with 10 nM ATRA suppressed lipid accumulation by 34%, and co-treatment of 500 μM GPNA with 10 nM ATRA resulted in 5% greater inhibitory effects on adipogenesis (Fig. 2A and B), which equaled the effects of 1 μM ATRA (Fig. 1A and B). Based on immunofluorescent stain analysis, DIM-stimulated 3T3-L1 cells treated with GPNA displayed the same level of Asct2 on the cell surface as with ATRA treatment (Supplementary Fig. 2B). GPNA suppressed adipogenesis in a dose-dependent manner (Supplementary Fig. 3).
Fig. 2.
Effects of the ASCT2 inhibitor GPNA on adipocyte-differentiation. (A) Lipid-droplets in the cultured 3T3-L1 cells detected with oil Red-O staining. Treatment conditions: –DIM, +DIM, +DIM + 10 nM ATRA, +DIM + 10 nM ATRA + 500 μM GPNA, and +DIM + 500 μM GPNA. Cells were treated with compound(s) for 7 days. (B) Graphs of relative levels of oil Red-O stained lipid-droplets in cultured 3T3-L1 cells. Relative intensities were standardized with +DIM. The results are representative of three independent experiments. Each bar represents the mean ± SD. *p < 0.001 versus +DIM condition by Student’s t-test. (C) Expressions levels of asct2, adipoq and pparg in DIM-stimulated 3T3-L1 cells treated with 500 μM GPNA or 0.1 μM ATRA for 5 days, as revealed by quantitative RT-PCR. (D) Immunoblotting analysis of Asct2 and PPARγ in 3T3-L1 cells treated with 1 μM ATRA or 500 μM GPNA for 5 days. Gapdh was employed as an internal control. The density of detected Asct2 has been normalized with Gapdh. The results are representative of three independent experiments.
We investigated the effects of 500 μM GPNA on asct2 gene expression. We found that GPNA addition down-regulated asct2 expression by 73% (Fig. 2C). GPNA also showed suppressive influence on the expression levels of adipo-marker molecules at day 5, including adipoq and pparg2. This is consistent with the fact that expression of adipoq genes is regulated by PPARγ [25,26]. The suppressive effects of GPNA were similar to those seen after treatment with 1 μM ATRA (Fig. 2C). GPNA reduced Asct2 and PPARγ2 protein levels in differentiating 3T3-L1 cells (Fig. 2D). The inhibition of amino acid intake by GPNA could potentially decrease lipid accumulation by down-regulating Asct2 and PPARγ2, which would be reflected in reduced adipogenesis.
3.3. Early effects of ATRA and an Asct2 inhibitor on adipogenesis
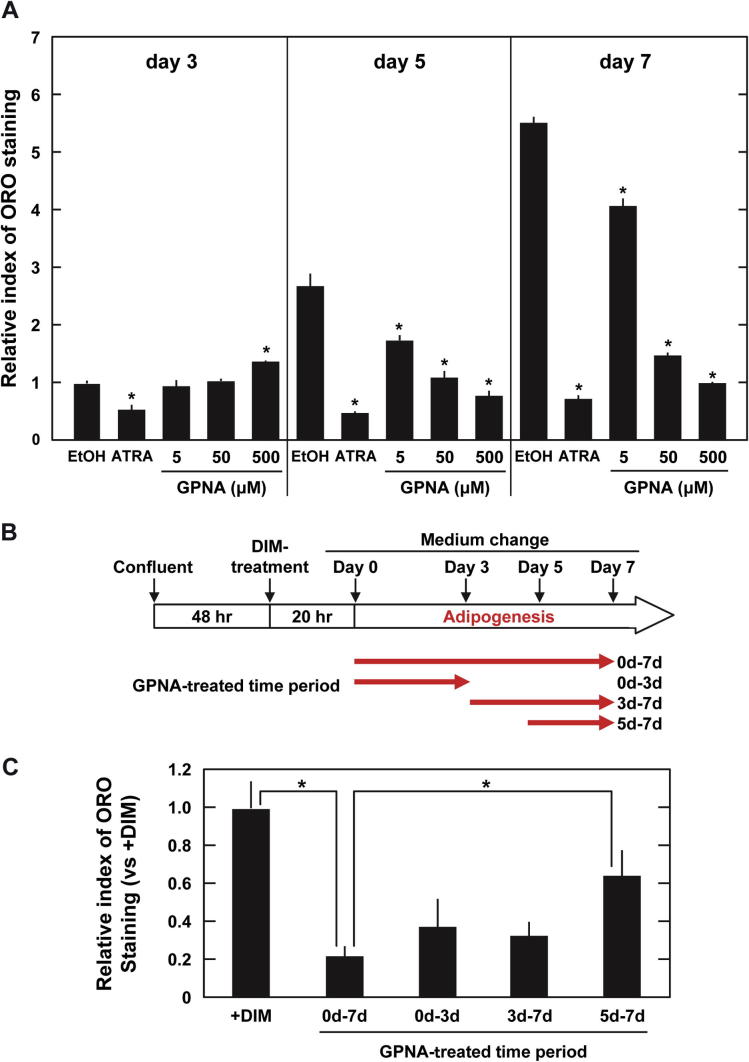
We hypothesized that suppressing the action of ATRA might be through decreasing Asct2 in DIM-stimulated 3T3-L1 cells. To identify the time course for this activity, we examined the lipid accumulation in 3T3-L1 cells treated with 1 μM ATRA at various intervals (Fig. 3A). At 3 day ATRA treatment, ATRA-treated cells already showed significantly less lipid accumulation (53%) than control cells (0.1% ethanol) (Fig. 3A). This implies that ATRA affected adipogenesis of DIM-stimulated 3T3-L1 cells in early periods, when suppression of pparg up-regulation had not yet been detected (Supplementary Fig. 1). We also investigated the time course of lipid accumulation in GPNA-treated cells. We observed that treatment with 500 μM GPNA enhanced lipid accumulation until day 3, and showed further suppressive effects in later stages (day 5 and 7) in a dose dependent manner (Fig. 3A). It is possible that during the early stages of DIM-stimulation, Asct2 might be a target of ATRA. Asct2 inhibition by GPNA might also suppress lipid accumulation in the later stages of adipogenesis. Kim et al. have shown that ATRA treatment was sufficient to significantly inhibit adipogenesis at early stages as well as later periods [27]. Accordingly, we altered GPNA-treatment time points during adipogenesis (Fig. 3B and C). The initial 3 day treatment showed suppressive effects on adipogenesis equal to the full treatment (Fig. 3B and C). This data suggested that initial amino acid uptake through Asct2 could be the primary source of the lipid droplets in mature adipocytes. Since treatment with both ATRA and GPNA indicated that adipogenesis-stimuli occurred in early stages (∼3 days), uptake of amino acids including glutamine during this time, might be indispensable for adipo-maturation and lipid droplet formation.
Fig. 3.
Effects on adipogenesis of 3T3-L1 cells of treating with ATRA or GPNA for different time intervals. (A) Effect of ATRA and GPNA on lipid accumulation in DIM-stimulated 3T3-L1 cells. Lipid droplets were stained with oil Red-O after 3 days, 5 days and 7 days of treatment with 1 μM ATRA or various concentrations of GPNA. Graphs show the relative degree of staining. Relative intensities were standardized with 0.1% EtOH on day 3. *p < 0.001 versus control (0.1% EtOH) by Student’s t-test. (B, C), Alteration of 500 μM GPNA treatment periods. (B) GPNA treatment schedules. (C) Graphs of relative levels of oil Red-O stained lipid-droplets in each condition. Relative intensities were standardized with +DIM. Each bar represents the mean ± SD.
3.4. Effects of glutamine free and GPNA treatment on 3T3-L1 cells
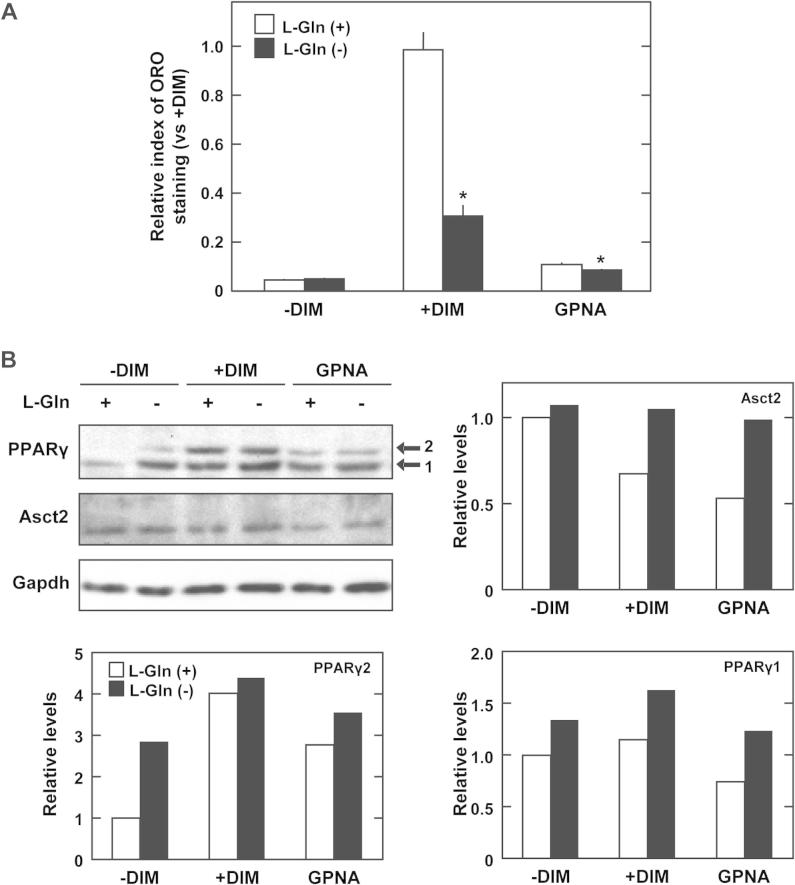
Asct2 is a neural amino acid transporter that has high affinity for glutamine. We examined the effects of GPNA treatment on adipogenesis under glutamine free conditions. Under glutamine free conditions, lipid accumulation decreased in differentiating 3T3-L1 cells by 31% (Fig. 4A, +DIM). Contrary to our expectations, glutamine free conditions did not suppress adipogenesis as great as treatment with 500 μM of GPNA (11%) (Fig. 4A, GPNA). Glutamine free conditions increased PPARγ levels in 3T3-L1 cells, and suppressed reduction of Asct2 protein levels in the DIM-treated cells (Fig. 4B). This data indicated that the degree of lipid accumulation was not concomitant with Asct2 and PPARγ levels under glutamine free condition.
Fig. 4.
Effects of glutamine free conditions on adipogenesis of 3T3-L1 cells. (A) Effect of l-glutamine (l-Gln)-free conditions and 500 μM GPNA on lipid accumulation in DIM-stimulated 3T3-L1 cells at day 5. Open columns represent treatment with 5 mM l-Gln and closed columns represent the absence of l-Gln in culture media. Relative intensities were standardized against +DIM + 5 mM l-Gln. Each bar represents the mean ± SD. Asterisk shows the statistical significance between l-Gln-free conditions and 5 mM l-Gln conditions (*p < 0.001). (B) Immunoblot detection of Asct2 and PPARγ in 3T3-L1 cells treated with 500 μM GPNA for 5 days. Gapdh was used as internal control. Graph shows the density of detected Asct2 normalized with Gapdh. The results are representative of three independent experiments.
4. Discussion
In our current work, we have investigated ATRA’s suppressive effects on adipogenesis of 3T3-L1 cells. We found that in the early stages of adipogenesis ATRA down-regulated transcripts of asct2, a neutral amino acid transporter. During adipogenesis, GPNA, an Asct2 inhibitor, enhanced the suppressive activities of ATRA. Treatment with GPNA at early time points during adipocyte differentiation, was sufficient to suppress adipogenesis at a level that was equal to full duration treatment with ATRA or GPNA. Our studies have revealed that Asct2 can effectively inhibit lipid accumulation in preadipocytes.
ATRA has long been known to be an inhibitor of adipocyte differentiation [6–12]. As shown in Fig. 3A, ATRA exhibits suppressive effects from day 3 of treatment. Since the detectable activity in early periods is concomitant with PPARγ alteration, we hypothesized a mechanism other than the nuclear receptor cascade. Based on our results, it appears that Asct2 may be an ATRA target for inhibiting adipogenesis. Asct2 is the primary amino acid transporter for glutamine uptake, which serves as major source of triacylglycerol. Accordingly, we anticipated that down-regulation of asct2 could be an important component of ATRA’s inhibitory effects on adipogenesis. In our current study, the inhibitory effects of ATRA on Asct2 expression might not be dependent on FXR/RXR (farnesoid X Receptor/retinoid X receptor). Rather these effects might arise from other mechanisms, including RA-modification [28–30], RAR activation or inhibition of glutamine influx. Kim et al. have reported that soon after treatment with ATRA, adipogenesis was significantly inhibited. In ATRA treated cells, Wnt/β-Catenin signaling components are up-regulated at 6 h after adipogenesis stimulation [27]. To our knowledge, the relationship between glutamine influx and Wnt/β-Catenin signaling on adipogenesis has not been reported. However, Olkku and Mahonen have indicated that the Wnt cascade inhibits intracellular glutamine levels by regulating glutamine synthetase [31].
Rhoderick et al. have reported that among compounds synthesized as Nγ-Aryl glutamine analogues, the Asct2 inhibitor, GPNA, had the most potent inhibitory effect on glutamine uptake [22]. Administration of GPNA to differentiating 3T3-L1 cells inhibits glutamine uptake, resulting in decreased lipid synthesis. We examined the effects of varying the treatment duration with GPNA. At early time points, GPNA suppressed adipogenesis of DIM-stimulated 3T3-L1 cells (Fig. 3C). This result is similar to Kim et al.’s results examining different time points of ATRA addition [27]. At the early time points, ATRA might inhibit adipogenesis by suppressing uptake of glucose and amino acids. GPNA might increase lipid accumulation up to day 3 by suppressing the uptake of amino acids, but not glucose. We found that under glutamine-free conditions, the formation of lipid droplets was reduced (Fig. 4A). We hypothesized that glutamine free conditions might achieve effects similar to treating differentiating 3T3-L1 cells with GPNA. Although GPNA reduced PPARγ levels in differentiating 3T3-L1 cells (Fig. 2C and D), we also found that reduction of PPARγ proteins might have occurred by mechanisms other than those arising from glutamine free conditions (Fig. 4). This indicates that glutamine is the major source for triacylglycerol. Therefore, PPARγ-upregulated 3T3-L1 cells are not able to intake insufficient glutamine to form lipid droplets under glutamine free conditions. Accordingly, PPARγ regulation and GPNA treatment are different from glutamine-free conditions in 3T3-L1 cells. Under glutamine free conditions, amino acids other than glutamine may be taken up in preadipocytes in sufficient quantities to maintain PPARγ levels, but not sufficient for forming lipid droplets.
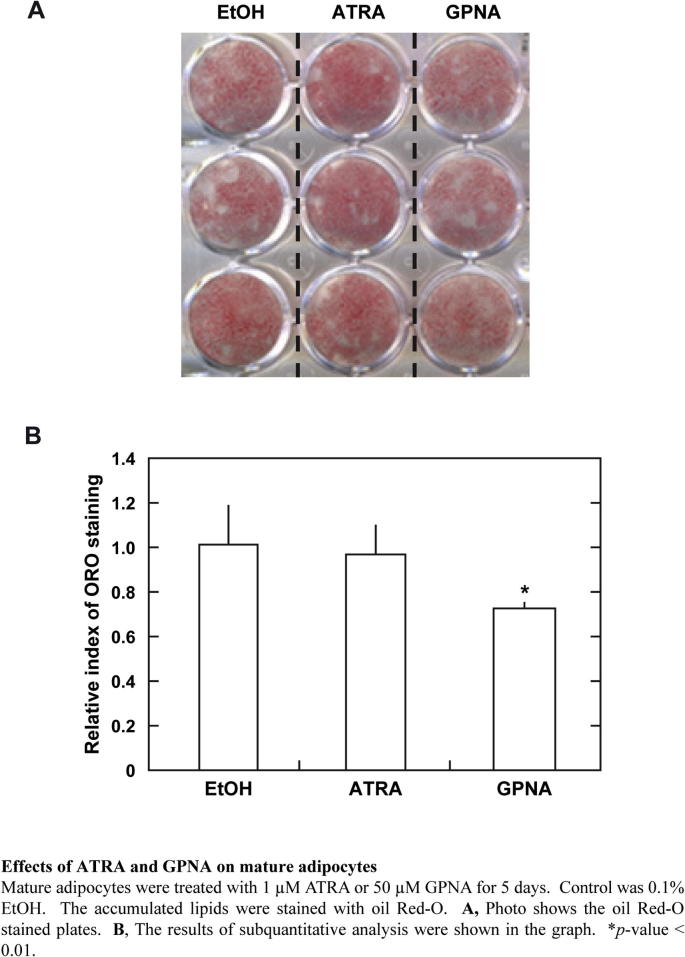
We found that treatment with GPNA had a suppressive effect not only on the adipo-generating cells, but also on mature adipocytes (Supplementary Fig. 4). Inhibition of Asct2 activities in adipo-maturated 3T3-L1 cells might be effective against lipid-accumulation. We had speculated that the GPNA might be toxic to mature adipocytes, however the number of GPNA-treated adipocytes (after 5 days) was not significantly different from the control-conditioned cultured cells. In contrast, ATRA did not decrease accumulated lipids.
Our results suggest that targeting Asct2 activity could be a means of preventing/treating obesity. Cooperation of vitamin A (or ATRA) and an Asct2 inhibitor could potentially result in further anti-obesity activity. Glutamine influx might be a novel target for the treatment/prevention of obesity.
Conflicts of interest
None declared.
Author contributions
K.T., M.I. and N.T. conceived and supervised the study; K.T., M.I. and N.T. designed experiments; K.T., N.U., C.K., and C.S. performed experiments; N.T. provided new tools and reagents; K.T., M.I. and N.T. analyzed data and wrote the manuscript.
Acknowledgments
We thank Dr. Terrence Burke, Jr. for helpful comments. We thank Takaya Matsumiya, Mieko Harada, and Yui Sato for their expert technical assistance. This investigation was supported in part by ‘the Ministry of Education, Culture, Sports, Science, and Technology, Japan’, ‘the Promotion and Mutual Aid Corporation for Private Schools of Japan’, and ‘JSPS Core-to-Core Program, A. Advanced Research Networks, Japan’.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.fob.2015.06.012.
Supplementary data
Supplementary Fig. 1.
Effects of ATRA on asct2 genes expression.
Supplementary Fig. 2.
Cellular localization of Asct2 in 3T3-L1 cells.
Supplementary Fig. 3.
Effects of GPNA on adipogenesis are in dose-dependent manner.
Supplementary Fig. 4.
Effects of ATRA and GPNA on mature adipocytes.
References
- 1.Zimmet P., Alberti K.G., Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Napoli J.L. Retinoic acid biosynthesis and metabolism. FASEB J. 1996;10:993–1001. doi: 10.1096/fasebj.10.9.8801182. [DOI] [PubMed] [Google Scholar]
- 3.Bonet M.L., Ribot J., Felipe F., Palou A. Vitamin A and the regulation of fat reserves. Cell. Mol. Life Sci. 2003;60:1311–1321. doi: 10.1007/s00018-003-2290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villarroya F., Iglesias R., Giralt M. Retinoids and retinoid receptors in the control of energy balance: novel pharmacological strategies in obesity and diabetes. Curr. Med. Chem. 2004;11:795–805. doi: 10.2174/0929867043455747. [DOI] [PubMed] [Google Scholar]
- 5.Shao D., Lazar M.A. Peroxisome proliferator activated receptor gamma, CCAAT/enhancer-binding protein alpha, and cell cycle status regulate the commitment to adipocyte differentiation. J. Biol. Chem. 1997;272:21473–21478. doi: 10.1074/jbc.272.34.21473. [DOI] [PubMed] [Google Scholar]
- 6.Marchildon F., St-Louis C., Akter R., Roodman V., Wiper-Bergeron N.L. Transcription factor Smad3 is required for the inhibition of adipogenesis by retinoic acid. J. Biol. Chem. 2010;285:13274–13284. doi: 10.1074/jbc.M109.054536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry D.C., Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol. Cell. Biol. 2009;29:3286–3296. doi: 10.1128/MCB.01742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felipe F., Mercader J., Ribot J., Palou A., Bonet M.L. Effects of retinoic acid administration and dietary vitamin A supplementation on leptin expression in mice: lack of correlation with changes of adipose tissue mass and food intake. Biochim. Biophys. Acta. 2005;1740:258–265. doi: 10.1016/j.bbadis.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Xue J.C., Schwarz E.J., Chawla A., Lazar M.A. Distinct stages in adipogenesis revealed by retinoid inhibition of differentiation after induction of PPARgamma. Mol. Cell. Biol. 1996;16:1567–1575. doi: 10.1128/mcb.16.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safonova I., Darimont C., Amri E.Z., Grimaldi P., Ailhaud G., Reichert U., Shroot B. Retinoids are positive effectors of adipose cell differentiation. Mol. Cell. Endocrinol. 1997;104:201–211. doi: 10.1016/0303-7207(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 11.Lee J.S., Park J.H., Kwon I.K., Lim J.Y. Retinoic acid inhibits BMP4-induced C3H10T1/2 stem cell commitment to adipocyte via downregulating Smad/p38MAPK signaling. Biochem. Biophys. Res. Commun. 2011;409:550–555. doi: 10.1016/j.bbrc.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 12.Kim D.M., Choi H.R., Park A., Shin S.M., Bae K.H., Lee S.C., Kim I.C., Kim W.K. Retinoic acid inhibits adipogenesis via activation of Wnt signaling pathway in 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 2013;434:455–459. doi: 10.1016/j.bbrc.2013.03.095. [DOI] [PubMed] [Google Scholar]
- 13.Rosen E.D., Spiegelman B.M. Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 14.Farmer S.R. Regulation of PPARgamma activity during adipogenesis. Int. J. Obes. (Lond.) 2005;(Suppl. 1):S13–S16. doi: 10.1038/sj.ijo.0802907. [DOI] [PubMed] [Google Scholar]
- 15.Lehrke M., Lazar M.A. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Utsunomiya-Tate N., Endou H., Kanai Y. Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J. Biol. Chem. 1996;271:14883–14890. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- 17.Liao K., Lane M.D. Expression of a novel insulin-activated amino acid transporter gene during differentiation of 3T3-L1 preadipocytes into adipocytes. Biochem. Biophys. Res. Commun. 1995;208:1008–1015. doi: 10.1006/bbrc.1995.1434. [DOI] [PubMed] [Google Scholar]
- 18.Kekuda R., Prasad P.D., Fei Y.J., Torres-Zamorano V., Sinha S., Yang-Feng T.L., Leibach F.H., Ganapathy V. Cloning of the sodium-dependent, broad-scope, neutral amino acid transporter Bo from a human placental choriocarcinoma cell line. J. Biol. Chem. 1996;271:18657–18661. doi: 10.1074/jbc.271.31.18657. [DOI] [PubMed] [Google Scholar]
- 19.Bungard C.I., McGivan J.D. Glutamine availability up-regulates expression of the amino acid transporter protein ASCT2 in HepG2 cells and stimulates the ASCT2 promoter. Biochem. J. 2004;382(Pt 1):27–32. doi: 10.1042/BJ20040487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bungard C.I., McGivan J.D. Identification of the promoter elements involved in the stimulation of ASCT2 expression by glutamine availability in HepG2 cells and the probable involvement of FXR/RXR dimers. Arch. Biochem. Biophys. 2005;443:53–59. doi: 10.1016/j.abb.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Palmada M., Speil A., Jeyaraj S., Böhmer C., Lang F. The serine/threonine kinases SGK1, 3 and PKB stimulate the amino acid transporter ASCT2. Biochem. Biophys. Res. Commun. 2005;331:272–277. doi: 10.1016/j.bbrc.2005.03.159. [DOI] [PubMed] [Google Scholar]
- 22.Esslinger C.S., Cybulski K.A., Rhoderick J.F. Ngamma-aryl glutamine analogues as probes of the ASCT2 neutral amino acid transporter binding site. Bioorg. Med. Chem. 2005;13:1111–1118. doi: 10.1016/j.bmc.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Sagara C., Takahashi K., Kagechika H., Takahashi N. Molecular mechanism of 9-cis-retinoic acid inhibition of adipogenesis in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2013;433:102–107. doi: 10.1016/j.bbrc.2013.02.057. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi K., Akiyama H., Shimazaki K., Uchida C., Akiyama-Okunuki H., Tomita M., Fukumoto M., Uchida T. Ablation of a peptidyl prolyl isomerase Pin1 from p53-null mice accelerated thymic hyperplasia by increasing the level of the intracellular form of Notch1. Oncogene. 2007;26:3835–3845. doi: 10.1038/sj.onc.1210153. [DOI] [PubMed] [Google Scholar]
- 25.Liao W., Nguyen M.T., Yoshizaki T., Favelyukis S., Patsouris D., Imamura T., Verma I.M., Olefsky J.M. Suppression of PPAR-gamma attenuates insulin-stimulated glucose uptake by affecting both GLUT1 and GLUT4 in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 2007;293:E219–E227. doi: 10.1152/ajpendo.00695.2006. [DOI] [PubMed] [Google Scholar]
- 26.Combs T.P., Wagner J.A., Berger J., Doebber T., Wang W.J., Zhang B.B., Tanen M., Berg A.H., O’Rahilly S., Savage D.B., Chatterjee K., Weiss S., Larson P.J., Gottesdiener K.M., Gertz B.J., Charron M.J., Scherer P.E., Moller D.E. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143:998–1007. doi: 10.1210/endo.143.3.8662. [DOI] [PubMed] [Google Scholar]
- 27.Kim D.M., Choi H.R., Park A., Shin S.M., Bae K.H., Lee S.C., Kim I.C., Kim W.K. Retinoic acid inhibits adipogenesis via activation of Wnt signaling pathway in 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 2013;434:455–459. doi: 10.1016/j.bbrc.2013.03.095. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi N., Breitman T.R. Retinoic acid acylation (retinoylation) of a nuclear protein in the human acute myeloid leukemia cell line HL60. J. Biol. Chem. 1989;264:5159–5163. [PubMed] [Google Scholar]
- 29.Breitman T.R., Takahashi N. Retinoylation of proteins in mammalian cells. Biochem. Soc. Trans. 1996;24:723–727. doi: 10.1042/bst0240723. [DOI] [PubMed] [Google Scholar]
- 30.Kubo Y., Ohba T., Takahashi N. Proteins in human myeloid leukemia cell line HL60 reacting with retinoic acid monoclonal antibodies. J. Biochem. 2008;144:349–355. doi: 10.1093/jb/mvn071. [DOI] [PubMed] [Google Scholar]
- 31.Olkku A., Mahonen A. Wnt and steroid pathways control glutamate signalling by regulating glutamine synthetase activity in osteoblastic cells. Bone. 2008;43:483–493. doi: 10.1016/j.bone.2008.04.016. [DOI] [PubMed] [Google Scholar]