Chronic orthostatic intolerance is defined by “the development of [chronic] symptoms during upright standing relieved by recumbence”1 present on a day-to-day basis. Symptoms always include dizziness or lightheadedness and are listed in Table I.
Table I.
Symptoms of orthostatic intolerance
| Lightheadedness |
| Headache |
| Fatigue |
| Neurocognitive/sleep disorders |
| Exercise intolerance |
| Weakness |
| Hyperpnea/dyspnea |
| Tremulousness |
| Nausea/abdominal pain |
| Sweating |
| Anxiety/palpitations |
The postural tachycardia syndrome (POTS) is defined by symptoms of orthostatic intolerance associated with a physical sign: an excessive increase in heart rate on orthostatic challenge. Overt fainting outside of the laboratory is relatively uncommon. POTS has been identified as a common but not the only form of chronic orthostatic intolerance.2–6 Although there are no accurate epidemiologic studies, it is estimated that affected individuals may number in the millions of Americans, mostly young women; in some cases this may result in lack of gainful employment or poor school attendance. POTS and neurally mediated hypotension are common among those with the chronic fatigue syndrome.7 POTS also affects a larger segment of the young who are less disabled but cannot perform adequately because of orthostatic intolerance.
FINDINGS IN POTS
Heart Rate and Blood Pressure in POTS
POTS was originally defined in adults by Schondorf and Low8 as an increase in heart rate by more than 30 beats per minute or an increase to a heart rate exceeding 120 beats per minute within 10 minutes when changing from supine to upright position. More recently, shorter times (eg, 5 minutes) for heart rate change have been suggested. In the young, blood pressure may decrease,9,10 whereas in older subjects blood pressure does not decrease and may even increase during orthostatic challenge.11 Sometimes vasovagal faint may be induced during upright tilt, but it is invariably preceded by severe symptoms of orthostatic intolerance and can be avoided by recumbence.12 Normal teenagers may be capable of larger orthostatic increases in heart rate than adults, and a threshold heart rate increase of 35 to 40 beats per minute may be more realistic in defining POTS in the young; there is as yet no consensus concerning this definition. Confounding matters is transient exaggerated tachycardia and hypotension, which can occur in healthy volunteer teenagers during orthostatic testing and during rapid positional changes in real life. In contrast with POTS, such changes are rapidly and spontaneously reversed within about 1 minute.13
Central Nervous System Symptoms and Hypocarbia
Many symptoms of orthostatic intolerance are related to reduced cerebral blood flow. Such symptoms had been demonstrated in adults and since by investigators in children.14,15 Symptoms are associated with inadequate systemic venous return to the right heart (ie, thoracic hypovolemia16), although precise mechanisms remain controversial. In addition, cerebral autoregulation fails in relation to the onset of dyspnea, hyperpnea, and hypocarbia. Cerebral vasoconstriction in POTS ensues in affected individuals and may be diagnosed as “hyperventilation syndrome” or “panic attack.” Interestingly, similar respiratory signs can be produced in control subjects given sufficient provocation. The underlying cause of orthostatic hyperpnea remains obscure, but postural stimulation appears to be necessary, and interactions between carotid baroreceptor and chemoreceptors are probably the root cause.
Acrocyanosis
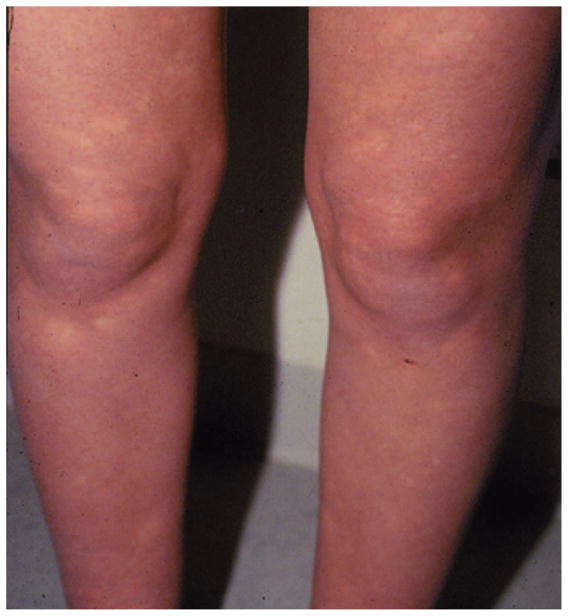
Although tachycardia and symptoms of orthostatic intolerance are necessary and sufficient conditions for POTS, peripheral acrocyanosis has been described in subsets of patients17 and is associated with decreased blood flow, especially in cyanotic skin. Acrocyanosis is also noted in many patients with chronic orthostatic intolerance. The distribution of color change does not mimic the distribution of discoloration in Raynaud phenomenon, which is confined to the hand and foot. Rather, as shown in Figure 1, there is more widespread extension, especially in dependent extremities with a mottled appearance comprising islands of pink skin with normal cutaneous blood flow interspersed among prevailing cyanosis with decreased cutaneous blood flow.
Figure 1.
Acrocyanosis is noted in many patients with POTS. There is extensive discoloration throughout the lower extremities with interspersed areas of pink skin. Photograph courtesy of Peter C. Rowe, MD, The Johns Hopkins University.
Although this appearance is often called venous pooling, recent evidence does not support excessive capacitance of dependent capacitance vessels nor enhanced collection of venous blood within the vasculature.18,19 Instead, data indicate decreased overall blood flow in the affected extremity,20,21 which can be appreciated by feeling the coolness of the limb.
ONSET, EPIDEMIOLOGY, AND NATURAL HISTORY
Onset of symptoms of orthostatic intolerance often follows an infectious disease; a relation to abnormalities in the inflammatory response have been proposed.1 Patients often slowly improve after the initial infectious illness, only to become ill again spontaneously or during an intercurrent infection.1 Approximately 75% to 80% of patients are women ranging in age from 14 to 50 years22 and therefore roughly span the ages from menarche to menopause. POTS is relatively uncommon in preadolescent children and may have a distinct pathophysiology in the very young. The reasons for sex preference are unclear, although women are known to be more vulnerable to orthostatic intolerance.23 Relations with the menstrual cycle or with altered estrogens or progestins are yet to be established.24 The illness may follow a remitting and relapsing clinical course, often enduring for years, but seems in many instances self-limited. Pregnancy may resolve abnormalities (personal communication from David Robertson, MD, Omni Shoreham Washington, July 2002). Similar findings and clinical course have been described under a number of names, including the hyperadrenergic syndrome of Streeten25,26 and idiopathic hypovolemia of Fouad.27 As currently construed, POTS was first reported in adults.1,28–31 Subsequently, pediatric cases of POTS were reported and showed that POTS is a common form of orthostatic intolerance during upright tilt in adolescents with chronic fatigue syndrome (CFS).32,33 There is evidence for POTS in adult CFS in 25% to 50% of cases.34
POTS AND RESPONSES TO ORTHOSTASIS IN HUMAN BEINGS
POTS as Thoracic Hypovolemia
POTS is heterogeneous. It represents a category of disease rather than a single distinct illness. Common to all variants is a final physiologic pathway involving excessively reduced venous return to the heart while upright. Collected evidence indicates excessive thoracic hypovolemia in all patients with POTS. The signature tachycardia may therefore result from related reflex parasympathetic withdrawal with some contribution from sympathetic activation including sympathetic cardiac effects. Thus, for example, studies of heart rate and blood pressure variability indicate vagal withdrawal and at least relative cardiac sympathetic excess.35–37 Decreases in baroreflex gain measured by the Oxford method38 or by variability techniques39 can be replicated by a model of hypovolemia in human beings.40
Response to Orthostatic Thoracic Hypovolemia
Standing up normally reduces venous return and thoracic blood volume by translocating thoracic blood to the dependent body parts. Included in these dependent structures are abdominal and pelvic regional circulations as well as lower extremities. If not for compensatory responses outlined in Table II, human beings could not maintain adequate blood pressure or essential regional blood flow while upright. Failure of compensatory mechanisms aggravates thoracic hypovolemia and produces POTS. Thus, POTS can be easily simulated by dehydration. While autonomic regulation is among the neurovascular control mechanisms that compensate for orthostatic vascular changes, other mechanisms including local forms of vascular regulation and neurohumoral factors are also involved. Humoral vasoregulation is often thought to relate to long-standing alterations in orthostatic stress and has less to do with early findings seen upon standing in otherwise normal individuals.
Table II.
Compensatory response to orthostasis
| Blood volume |
| Physical forces, |
| eg, interstitial compression |
| Neurovascular control |
| Long distance control |
| Autonomic nervous system |
| Neurohumoral: Adrenal, RAS, AVP, ANP |
| Local control |
| Autacoids, inflammatory mediators |
| Neurogenic inflammation |
RAS, Renin-Angiotensin System; AVP, Arginine Vasopressin; ANP, Atrial Natriuretic Factor.
Blood Volume
Tachycardia of POTS could represent absolute hypovolemia. This has been demonstrated in some patients with POTS41 and may relate to defective denervation of the kidneys and associated hyporeninemic hypoaldosteronism.42,43 A reflex response to fluid shifts results in the circulatory insufficiency observed in patients.44 More recent data indicate that hypovolemia tends to be modest and may not by itself explain orthostatic intolerance in these patients.45 Nevertheless, decreased blood volume further impairs venous return and is at least a contributing factor: All compensatory mechanisms for orthostasis depend on circulatory volume adequacy and all will ultimately fail during sufficiently severe hypovolemia. Conversely, repletion of blood volume is often helpful in orthostatic intolerance of whatever cause because it invariably enhances postural venous return. Thus, salt and water loading is almost always helpful for patients, treatment with fludrocortisone acetate (Florinef) is often helpful if side effects are tolerable, and erythropoietin46 has been used with limited success in patients, although effects unrelated to blood volume may play a role. Although a primary disturbance in blood volume (or renal volume regulation) has been proposed for some cases of POTS,47–49 volume loading may improve patient well-being nonspecifically as well.
Muscle Pump
As shown in Table II, physical forces comprise a primary defense against lower extremity pooling in human beings in the form of the “skeletal muscle pump” in which contractions of leg and gluteal muscles increase interstitial pressure and propel sequestered venous blood back to the heart.50 Skeletal muscle may also be involved in neurogenic compensation through chemoreceptors and through local control mechanisms.51 Recent data indicate that while the muscle pump is normal in most patients with POTS, the muscle pump is defective in a subset of patients with POTS who also have decreased resting peripheral blood flow unrelated to exercise capability but exacerbated by bed rest.52 Ambulation is essential; lower body exercise may be very helpful, but its use has not been examined in a systematic way.
Neurovascular Compensation
Neurovascular compensation limits dependent blood pooling. This includes rapid peripheral arterial vasoconstriction limiting flow to the extremities and splanchnic vascular bed while promoting passive venous emptying.53 Active venoconstriction occurs in the splanchnic circulation,54 but there is little evidence for venous beds other than splanchnic contributing to active orthostatic venoconstriction; rather, recent data suggest that other veins and venules contribute to venous return by passive elastic recoil during arterial vasoconstriction.55,56
A useful way to view vascular control systems is to view them as long distance or local control systems.
“Long Distance” Vascular Control
Long distance control is primarily autonomic. Autonomic compensatory mechanisms are mainly controlled by the high-pressure arterial baroreceptors located in the carotid sinus, aortic arch, and perhaps the proximal coronary arteries.57–59 High-pressure ventricular receptors, low-pressure cardiopulmonary receptors, and vestibularotolith systems may contribute to a lesser degree.58,60,61 These respond to a change in thoracic pressure and volume by increasing heart rate and causing peripheral and splanchnic vasoconstriction and splanchnic venoconstriction chiefly by adrenergic mechanisms. Support for a “long tract neuropathy” with a neuropathic adrenergic vasoconstrictive defect comes from Jacob et al. 62 Recent data indicate that patients are peripherally vasodilated and mildly tachycardic when supine and have relatively increased blood volume, reduced total peripheral resistance, and high resting cardiac output compared with healthy control subjects.16 These patients have been classified as having “high-flow POTS.” Peripheral vasoconstriction during orthostasis remains defective, leading to excess blood delivery to the lower limbs with enhanced microvascular filtration and edema formation.63 Acrocyanosis does not often occur. Persistent upright vasodilation responds to vasoconstrictor therapy with midodrine or similar agents. Often such patients have illness after a viral infection. A peripheral neuropathy is suspected and is often self-limited, although the clinical course may extend for months to 1 year or more.
Another recent autonomic condition producing a complex form of POTS is the norepinephrine transporter protein deficiency. This has been reported in only a single family. Investigators have identified a specific genetic defect in norepinephrine transporter protein deficiency64 exerting both central and peripheral effects on vascular regulation.65 Despite its rarity, the illness has furnished an ideal monogenetic model for autonomic illness, and appropriate animal knock-out models have been constructed and investigated.66
“Local” Vascular Control
Contributions to the compensatory response to orthostasis arising from local vasoactive responses are often less well appreciated. These include endothelial vasoactive products (ie, NO, PGI-2, endothelin, EDHF)67,68 metabolites (adenosine, Ca2+, CO2, H+ ions, lactate)69–71 autacoids (histamine, bradykinin, 5-HT, PAF, prostaglandins),72 local neurogenic mechanisms such as the axon reflex,73 and neurogenic inflammation (CGRP, substance P),74,75 especially within the cutaneous and enteric circulations. Local responses contribute to classic myogenic, metabolic, and venoarteriolar flow control, which may be important compensatory mechanisms during orthostasis.76–78 A defect in local vascular compensation to orthostatic stress is demonstrable in a subgroup of patients with chronic orthostatic intolerance.79 Patients are intensely peripherally vasoconstricted while supine and have a relatively decreased blood volume, increased total peripheral resistance, and low resting cardiac output. We have designated these patients as having “low-flow POTS.” They have a characteristic phenotype of pallor, extensive supine as well as upright acrocyanosis, cool skin and extremities, and defective skeletal muscle pump. They are usually tachycardic while supine, more so during orthostasis, and give the appearance of early circulatory insufficiency. Although blood volume tends on average to be decreased compared with control subjects, values16 fall within the normal range and fail to correlate with the degree of supine vasoconstriction. Baseline low blood flow increases while peripheral arterial resistance decreases during leg lowering and during the imposition of increased venous pressure in patients with low flow POTS. Hyperemic blood flow, a measure of endothelial cell function, is abnormal compared with either control patients or other patients with POTS, indicating abnormal local blood flow regulation in these patients. Although the precise cause of flow abnormalities is currently uncertain, it is clear that treatments that increase blood volume may help to alleviate signs and symptoms. Recent data from our laboratory showed no improvement in response to an acute infusion of a β-blocker in this subset, although we have not studied the use of β-blockers as part of combination therapy.80
POTS WITH NORMAL PERIPHERAL VASCULATURE
Some patients with POTS have neither increased nor decreased recumbent blood flow compared with control subjects. These patients with so-called “normal flow POTS” appear perfectly well when supine and make up an increasing subset of patients identified in our practice. When upright, they have excessive tachycardia, greatly enhanced peripheral vasoconstriction, and often acrocyanosis. There are no findings suggesting a local peripheral defect. Rather, excessive vasoconstriction appears to be related to thoracic hypovolemia and reciprocal splanchnic pooling, as shown in Figure 2, in which fractional changes in regional blood volumes were estimated by impedance plethysmography. A relation with hypermobility syndromes may exist81 and serves as a bridge to recent findings by Rowe et al in CFS.82,83 Vasoconstrictor therapy with midodrine is sometimes helpful. Experimental therapy with octreotide produces selective splanchnic vasoconstriction and venoconstriction and has been tested in some patients. Optimal treatments for each POTS subgroup have not yet been defined.
Figure 2.
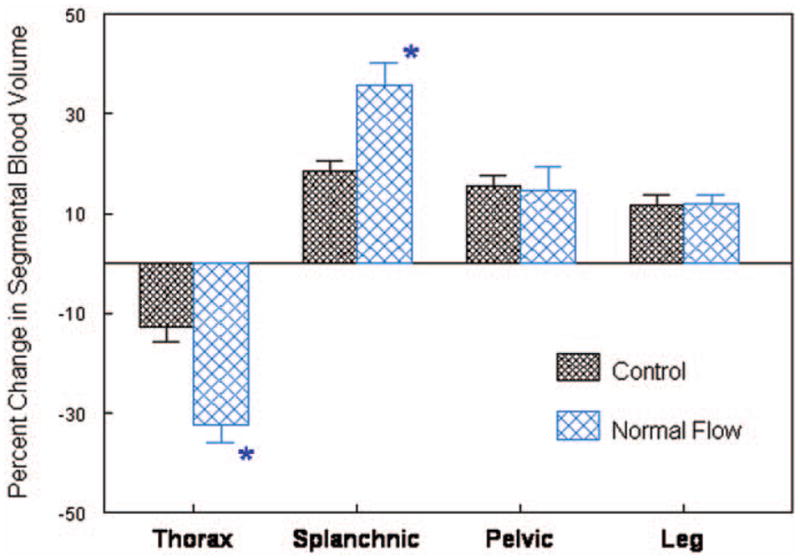
Percent change in segmental blood volume in patients with normal-flow POTS (n = 15) compared with control subjects (n = 12) during 70° upright tilt. There is enhanced thoracic hypovolemia and reciprocal splanchnic hypervolemia when upright.
At least 3 physiologic variants of POTS can be distinguished on the basis of measurements of peripheral blood flow and peripheral arterial resistance in association with characteristic changes in regional circulations: Low-flow POTS is characterized by pallor, generally decreased blood flow most notable in the dependent parts of the body. It is related to defects in local blood flow regulation and mild absolute hypovolemia. Normal-flow POTS is characterized by a normal supine phenotype, with normal peripheral resistance supine but enhanced peripheral resistance upright. There is specific venous pooling within the splanchnic vascular bed, making this a redistributive form of hypovolemia. High-flow POTS is related to a long tract neuropathy and is characterized by high cardiac output caused by inadequate peripheral vasoconstriction supine and upright. Patients typically are acyanotic, warm to touch with extensive filtration, resulting in dependent edema.
Although this physiologic classification scheme has the potential to refine treatment approaches, the classification into low-, normal-, and high-flow POTS is not made with ease in clinical laboratories. Therefore, further research will be needed to translate these research findings into clinical practice.
Acknowledgments
Supported by grants 1RO1-HL-66007 and 1R01-HL-074873 from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Glossary
- CFS
Chronic fatigue syndrome
- POTS
Postural tachycardia syndrome
References
- 1.Low PA, Schondorf R, Novak V, Sandroni P, Opfer-Gehrking TL, Novak P. Postural Tachycardia Syndrome. In: Low PA, editor. Clinical Autonomic Disorders. Philadelphia-New York: Lippincott-Raven; 1997. pp. 681–97. [Google Scholar]
- 2.Streeten DH, Anderson GH, Jr, Richardson R, Thomas FD. Abnormal orthostatic changes in blood pressure and heart rate in subjects with intact sympathetic nervous function: evidence for excessive venous pooling. J Lab Clin Med. 1988;111:326–35. [PubMed] [Google Scholar]
- 3.Streeten DH. Pathogenesis of hyperadrenergic orthostatic hypotension: evidence of disordered venous innervation exclusively in the lower limbs. J Clin Invest. 1990;86:1582–8. doi: 10.1172/JCI114878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–7. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 5.Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, et al. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–25. [PubMed] [Google Scholar]
- 6.Jacob G, Shannon JR, Black B, Biaggioni I, Mosqueda-Garcia R, Robertson RM, et al. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation. 1997;96:575–80. doi: 10.1161/01.cir.96.2.575. [DOI] [PubMed] [Google Scholar]
- 7.Stewart JM, Gewitz MH, Weldon A, Munoz J. Patterns of orthostatic intolerance: the orthostatic tachycardia syndrome and adolescent chronic fatigue. J Pediatr. 1999;135:218–25. doi: 10.1016/s0022-3476(99)70025-9. [DOI] [PubMed] [Google Scholar]
- 8.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–7. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 9.Stewart JM, Gewitz MH, Weldon A, Munoz J. Patterns of orthostatic intolerance: the orthostatic tachycardia syndrome and adolescent chronic fatigue. J Pediatr. 1999;135:218–25. doi: 10.1016/s0022-3476(99)70025-9. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka H, Yamaguchi H, Matushima R, Tamai H. Instantaneous orthostatic hypotension in children and adolescents: a new entity of orthostatic intolerance. Pediatr Res. 1999;46:691–6. doi: 10.1203/00006450-199912000-00022. [DOI] [PubMed] [Google Scholar]
- 11.Grubb BP, Kosinski DJ, Boehm K, Kip K. The postural orthostatic tachycardia syndrome: a neurocardiogenic variant identified during head-up tilt table testing. Pacing Clin Electrophysiol. 1997;20:2205–12. doi: 10.1111/j.1540-8159.1997.tb04238.x. [DOI] [PubMed] [Google Scholar]
- 12.Grubb BP, Kanjwal MY, Kosinski DJ. Review: the postural orthostatic tachycardia syndrome: current concepts in pathophysiology diagnosis and management. J Interv Card Electrophysiol. 2001;5:9–16. doi: 10.1023/a:1009845521949. [DOI] [PubMed] [Google Scholar]
- 13.Stewart JM. Transient orthostatic hypotension is common in adolescents. J Pediatr. 2002;140:418–24. doi: 10.1067/mpd.2002.122643. [DOI] [PubMed] [Google Scholar]
- 14.Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke. 1998;29:1876–81. doi: 10.1161/01.str.29.9.1876. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka H, Matsushima R, Tamai H, Kajimoto Y. Impaired postural cerebral hemodynamics in young patients with chronic fatigue with and without orthostatic intolerance. J Pediatr. 2002;140:412–7. doi: 10.1067/mpd.2002.122725. [DOI] [PubMed] [Google Scholar]
- 16.Stewart JM. A physiological classification of the postural tachycardia syndrome based on peripheral blood flow. Am J Physiol [electronic pages] 2004 Abstract. [Google Scholar]
- 17.Stewart JM. Autonomic nervous system dysfunction in adolescents with postural orthostatic tachycardia syndrome and chronic fatigue syndrome is characterized by attenuated vagal baroreflex and potentiated sympathetic vasomotion [In Process Citation] Pediatr Res. 2000;48:218–26. doi: 10.1203/00006450-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Freeman R, Lirofonis V, Farquhar WB, Risk M. Limb venous compliance in patients with idiopathic orthostatic intolerance and postural tachycardia. J Appl Physiol. 2002;93:636–44. doi: 10.1152/japplphysiol.00817.2001. [DOI] [PubMed] [Google Scholar]
- 19.Stewart JM. Pooling in chronic orthostatic intolerance: arterial vasoconstrictive but not venous compliance defects. Circulation. 2002;105:2274–81. doi: 10.1161/01.cir.0000016348.55378.c4. [DOI] [PubMed] [Google Scholar]
- 20.Freeman R, Lirofonis V, Farquhar WB, Risk M. Limb venous compliance in patients with idiopathic orthostatic intolerance and postural tachycardia. J Appl Physiol. 2002;93:636–44. doi: 10.1152/japplphysiol.00817.2001. [DOI] [PubMed] [Google Scholar]
- 21.Stewart JM. Pooling in chronic orthostatic intolerance: arterial vasoconstrictive but not venous compliance defects. Circulation. 2002;105:2274–81. doi: 10.1161/01.cir.0000016348.55378.c4. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein DS, Holmes C, Frank SM, Dendi R, Cannon RO, III, Sharabi Y, et al. Cardiac sympathetic dysautonomia in chronic orthostatic intolerance syndromes. Circulation. 2002;106:2358–65. doi: 10.1161/01.cir.0000036015.54619.b6. [DOI] [PubMed] [Google Scholar]
- 23.Fu Q, Arbab-Zadeh A, Perhonen MA, Zhang R, Zuckerman JH, Levine BD, et al. Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol Heart Circ Physiol. 2004;286:H449–57. doi: 10.1152/ajpheart.00735.2002. [DOI] [PubMed] [Google Scholar]
- 24.Hirshoren N, Tzoran I, Makrienko I, Edoute Y, Plawner MM, Itskovitz-Eldor J, et al. Menstrual cycle effects on the neurohumoral and autonomic nervous systems regulating the cardiovascular system. J Clin Endocrinol Metab. 2002;87:1569–75. doi: 10.1210/jcem.87.4.8406. [DOI] [PubMed] [Google Scholar]
- 25.Streeten DH, Anderson GH, Jr, Richardson R, Thomas FD. Abnormal orthostatic changes in blood pressure and heart rate in subjects with intact sympathetic nervous function: evidence for excessive venous pooling. J Lab Clin Med. 1988;111:326–35. [PubMed] [Google Scholar]
- 26.Streeten DH. Pathogenesis of hyperadrenergic orthostatic hypotension: evidence of disordered venous innervation exclusively in the lower limbs. J Clin Invest. 1990;86:1582–8. doi: 10.1172/JCI114878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fouad FM, Tadena-Thome L, Bravo EL, Tarazi RC. Idiopathic hypovolemia. Ann Intern Med. 1986;104:298–303. doi: 10.7326/0003-4819-104-3-298. [DOI] [PubMed] [Google Scholar]
- 28.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–7. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 29.Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999;317:75–7. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Jacob G, Biaggioni I. Idiopathic orthostatic intolerance and postural tachycardia syndromes. Am J Med Sci. 1999;317:88–101. doi: 10.1097/00000441-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Furlan R, Jacob G, Snell M, Robertson D, Porta A, Harris P, et al. Chronic orthostatic intolerance: a disorder with discordant cardiac and vascular sympathetic control. Circulation. 1998;98:2154–9. doi: 10.1161/01.cir.98.20.2154. [DOI] [PubMed] [Google Scholar]
- 32.Stewart JM, Gewitz MH, Weldon A, Munoz J. Patterns of orthostatic intolerance: the orthostatic tachycardia syndrome and adolescent chronic fatigue. J Pediatr. 1999;135:218–25. doi: 10.1016/s0022-3476(99)70025-9. [DOI] [PubMed] [Google Scholar]
- 33.Stewart JM, Gewitz MH, Weldon A, Arlievsky N, Li K, Munoz J. Orthostatic intolerance in adolescent chronic fatigue syndrome. Pediatrics. 1999;103:116–21. doi: 10.1542/peds.103.1.116. [DOI] [PubMed] [Google Scholar]
- 34.Freeman R, Komaroff AL. Does the chronic fatigue syndrome involve the autonomic nervous system? Am J Med. 1997;102:357–64. doi: 10.1016/s0002-9343(97)00087-9. [DOI] [PubMed] [Google Scholar]
- 35.Stewart JM, Weldon A. Vascular perturbations in the chronic orthostatic intolerance of the postural orthostatic tachycardia syndrome. J Appl Physiol. 2000;89:1505–12. doi: 10.1152/jappl.2000.89.4.1505. [DOI] [PubMed] [Google Scholar]
- 36.Furlan R, Jacob G, Snell M, Robertson D, Porta A, Harris P, et al. Chronic orthostatic intolerance: a disorder with discordant cardiac and vascular sympathetic control. Circulation. 1998;98:2154–9. doi: 10.1161/01.cir.98.20.2154. [DOI] [PubMed] [Google Scholar]
- 37.Goldstein DS, Holmes C, Frank SM, Dendi R, Cannon RO, III, Sharabi Y, et al. Cardiac sympathetic dysautonomia in chronic orthostatic intolerance syndromes. Circulation. 2002;106:2358–65. doi: 10.1161/01.cir.0000036015.54619.b6. [DOI] [PubMed] [Google Scholar]
- 38.Farquhar WB, Taylor JA, Darling SE, Chase KP, Freeman R. Abnormal baroreflex responses in patients with idiopathic orthostatic intolerance. Circulation. 2000;102:3086–91. doi: 10.1161/01.cir.102.25.3086. [DOI] [PubMed] [Google Scholar]
- 39.Stewart JM. Autonomic nervous system dysfunction in adolescents with postural orthostatic tachycardia syndrome and chronic fatigue syndrome is characterized by attenuated vagal baroreflex and potentiated sympathetic vasomotion [In Process Citation] Pediatr Res. 2000;48:218–26. doi: 10.1203/00006450-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 40.Iwasaki KI, Zhang R, Zuckerman JH, Pawelczyk JA, Levine BD. Effect of head-down-tilt bed rest and hypovolemia on dynamic regulation of heart rate and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2189–99. doi: 10.1152/ajpregu.2000.279.6.R2189. [DOI] [PubMed] [Google Scholar]
- 41.Fouad FM, Tadena-Thome L, Bravo EL, Tarazi RC. Idiopathic hypovolemia. Ann Intern Med. 1986;104:298–303. doi: 10.7326/0003-4819-104-3-298. [DOI] [PubMed] [Google Scholar]
- 42.Jacob G, Shannon JR, Black B, Biaggioni I, Mosqueda-Garcia R, Robertson RM, et al. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation. 1997;96:575–80. doi: 10.1161/01.cir.96.2.575. [DOI] [PubMed] [Google Scholar]
- 43.Jacob G, Biaggioni I, Mosqueda-Garcia R, Robertson RM, Robertson D. Relation of blood volume and blood pressure in orthostatic intolerance. Am J Med Sci. 1998;315:95–100. doi: 10.1097/00000441-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Brown CM, Hainsworth R. Assessment of capillary fluid shifts during orthostatic stress in normal subjects and subjects with orthostatic intolerance. Clin Auton Res. 1999;9:69–73. doi: 10.1007/BF02311762. [DOI] [PubMed] [Google Scholar]
- 45.Farquhar WB, Hunt BE, Taylor JA, Darling SE, Freeman R. Blood volume and its relation to peak O(2) consumption and physical activity in patients with chronic fatigue. Am J Physiol Heart Circ Physiol. 2002;282:H66–71. doi: 10.1152/ajpheart.2002.282.1.H66. [DOI] [PubMed] [Google Scholar]
- 46.Hoeldtke RD, Horvath GG, Bryner KD. Treatment of orthostatic tachycardia with erythropoietin. Am J Med. 1995;99:525–9. doi: 10.1016/s0002-9343(99)80230-7. [DOI] [PubMed] [Google Scholar]
- 47.Farquhar WB, Taylor JA, Darling SE, Chase KP, Freeman R. Abnormal baroreflex responses in patients with idiopathic orthostatic intolerance. Circulation. 2000;102:3086–91. doi: 10.1161/01.cir.102.25.3086. [DOI] [PubMed] [Google Scholar]
- 48.Fouad FM, Tadena-Thome L, Bravo EL, Tarazi RC. Idiopathic hypovolemia. Ann Intern Med. 1986;104:298–303. doi: 10.7326/0003-4819-104-3-298. [DOI] [PubMed] [Google Scholar]
- 49.Jacob G, Biaggioni I, Mosqueda-Garcia R, Robertson RM, Robertson D. Relation of blood volume and blood pressure in orthostatic intolerance. Am J Med Sci. 1998;315:95–100. doi: 10.1097/00000441-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Marsgall RJ, Shepherd JT. The effect of changes in posture and graded exercise on stroke volume in man. J Clin Invest. 1960;39:1051–61. doi: 10.1172/JCI104120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rowell LB, Blackmon JR. Human cardiovascular adjustments to acute hypoxaemia. Clin Physiol. 1987;7:349–76. doi: 10.1111/j.1475-097x.1987.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 52.Stewart JM, Medow MS, Montgomery LD, McLeod K. Decreased skeletal muscle pump activity in patients with postural tachycardia syndrome and low peripheral blood flow. Am J Physiol Heart Circ Physiol. 2004;286:H1216–22. doi: 10.1152/ajpheart.00738.2003. [DOI] [PubMed] [Google Scholar]
- 53.Rowell LB. Reflex control of regional circulations in humans. J Auton Nerv Syst. 1984;11:101–14. doi: 10.1016/0165-1838(84)90069-9. [DOI] [PubMed] [Google Scholar]
- 54.Hill L. The influences of the force of gravity on the circulation of the blood. J Physiol (Lond) 1951;18:15–53. doi: 10.1113/jphysiol.1895.sp000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donegan JF. The physiology of veins. J Physiol (Lond) 1921;55:226–45. doi: 10.1113/jphysiol.1921.sp001964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rothe CF. Venous system: physiology of the capacitance vessels. In: Shepherd JT, Abboud FM, Geiger SR, editors. The Cardiovascular System: Peripheral Circulation and Organ Blood Flow. Handbook of Physiology, Section 22. Part 1. III. Bethesda Md: American Physiologic Society; 1983. pp. 397–452. [Google Scholar]
- 57.Mancia G, Mark AL. Arterial baroreflexes in humans. In: Shepherd JT, Abboud FM, Geiger SR, editors. The Cardiovascular System: Peripheral Circulation and Organ Blood Flow. Bethesda, Md: American Physiological Society; 1983. pp. 755–93. [Google Scholar]
- 58.Mark AL, Mancia G. Cardiopulmonary baroreflexes in humans. In: Shepherd JT, Abboud FM, Geiger SR, editors. The Cardiovascular System: Peripheral Circulation and Organ Blood Flow. Bethesda, Md: American Physiological Society; 1983. pp. 795–813. [Google Scholar]
- 59.Wright C, Drinkhill MJ, Hainsworth R. Reflex effects of independent stimulation of coronary and left ventricular mechanoreceptors in anaesthetised dogs[In Process Citation] J Physiol. 2000;528:349–58. doi: 10.1111/j.1469-7793.2000.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biaggioni I, Costa F, Kaufmann H. Vestibular influences on autonomic cardiovascular control in humans. J Vestib Res. 1998;8:35–41. [PubMed] [Google Scholar]
- 61.Manchanda SK, Bhattarai R, Nayar U. Central nervous control of venous tone, III: Responses of capacitance and resistance vessels of skin to bulbar and hypothalamic stimulation. Ind J Physiol Pharmacol. 1975;19:105–20. [PubMed] [Google Scholar]
- 62.Jacob G, Costa F, Shannon JR, Robertson RM, Wathen M, Stein M, et al. The neuropathic postural tachycardia syndrome. N Engl J Med. 2000;343:1008–14. doi: 10.1056/NEJM200010053431404. [DOI] [PubMed] [Google Scholar]
- 63.Stewart JM. Microvascular filtration is increased in postural tachycardia syndrome. Circulation. 2003;107:2816–22. doi: 10.1161/01.CIR.0000070951.93566.FC. [DOI] [PubMed] [Google Scholar]
- 64.Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med. 2000;342:541–9. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- 65.Robertson D, Flattem N, Tellioglu T, Carson R, Garland E, Shannon JR, et al. Familial orthostatic tachycardia due to norepinephrine transporter deficiency. Ann N Y Acad Sci. 2001;940:527–43. doi: 10.1111/j.1749-6632.2001.tb03703.x. [DOI] [PubMed] [Google Scholar]
- 66.Carson RP, Diedrich A, Robertson D. Autonomic control after blockade of the norepinephrine transporter: a model of orthostatic intolerance. J Appl Physiol. 2002;93:2192–8. doi: 10.1152/japplphysiol.00033.2002. [DOI] [PubMed] [Google Scholar]
- 67.Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol. 1996;81:1807–14. doi: 10.1152/jappl.1996.81.4.1807. [DOI] [PubMed] [Google Scholar]
- 68.Kellogg DL, Jr, Liu Y, Pergola PE. Selected contribution: gender differences in the endothelin-B receptor contribution to basal cutaneous vascular tone in humans. J Appl Physiol. 2001;91:2407–11. doi: 10.1152/jappl.2001.91.5.2407. [DOI] [PubMed] [Google Scholar]
- 69.Granger DN, Korthius RJ. Physiological mechanisms of postischemic tissue injury. Annu Rev Physiol. 1995;57:311–32. doi: 10.1146/annurev.ph.57.030195.001523. [DOI] [PubMed] [Google Scholar]
- 70.Biaggioni I, Olafsson B, Robertson RM, Hollister AS, Robertson D. Cardiovascular and respiratory effects of adenosine in conscious man: evidence for chemoreceptor activation. Circ Res. 1987;61:779–86. doi: 10.1161/01.res.61.6.779. [DOI] [PubMed] [Google Scholar]
- 71.Costa F, Sulur P, Angel M, Cavalcante J, Haile V, Christman B, et al. Intravascular source of adenosine during forearm ischemia in humans: implications for reactive hyperemia. Hypertension. 1999;33:1453–7. doi: 10.1161/01.hyp.33.6.1453. [DOI] [PubMed] [Google Scholar]
- 72.Meininger GA, Davis MJ. Cellular mechanisms involved in the vascular myogenic response. Am J Physiol. 2002;263:H647–59. doi: 10.1152/ajpheart.1992.263.3.H647. [DOI] [PubMed] [Google Scholar]
- 73.Wardell K, Naver HK, Nilsson GE, Wallin BG. The cutaneous vascular axon reflex in humans characterized by laser Doppler perfusion imaging. J Physiol. 1993;460:185–99. doi: 10.1113/jphysiol.1993.sp019466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol. 1998;30:5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- 75.Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302:839–45. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- 76.Blomqvist CG, Stone HL. Cardiovascular adjustments to gravitational stress. In: Shepherd JT, Abboud FM, Geiger SR, editors. Handbook of Physiology. Bethesda, Md: American Physiological Society; 1983. pp. 1025–63. [Google Scholar]
- 77.Vissing SF, Secher NH, Victor RG. Mechanisms of cutaneous vasoconstriction during upright posture. Acta Physiol Scand. 1997;159:131–8. doi: 10.1046/j.1365-201X.1997.573344000.x. [DOI] [PubMed] [Google Scholar]
- 78.Henriksen O, Skagen K, Haxholdt O, Dyrberg V. Contribution of local blood flow regulation mechanisms to the maintenance of arterial pressure in upright position during epidural blockade. Acta Physiol Scand. 1983;118:271–80. doi: 10.1111/j.1748-1716.1983.tb07271.x. [DOI] [PubMed] [Google Scholar]
- 79.Stewart JM, Medow MS, Montgomery LD. Local vascular responses affecting blood flow in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2003;285:H2749–56. doi: 10.1152/ajpheart.00429.2003. [DOI] [PubMed] [Google Scholar]
- 80.Stewart JM, Munoz J, Weldon A. Clinical and physiological effects of an acute alpha-1 adrenergic agonist and a β-1 adrenergic antagonist in chronic orthostatic intolerance. Circulation. 2002;106:2946–54. doi: 10.1161/01.cir.0000040999.00692.f3. [DOI] [PubMed] [Google Scholar]
- 81.Gazit Y, Nahir AM, Grahame R, Jacob G. Dysautonomia in the joint hypermobility syndrome. Am J Med. 2003;115:33–40. doi: 10.1016/s0002-9343(03)00235-3. [DOI] [PubMed] [Google Scholar]
- 82.Rowe PC, Barron DF, Calkins H, Maumenee IH, Tong PY, Geraghty MT. Orthostatic intolerance and chronic fatigue syndrome associated with Ehlers-Danlos syndrome. J Pediatr. 1999;135:494–9. doi: 10.1016/s0022-3476(99)70173-3. [DOI] [PubMed] [Google Scholar]
- 83.Rowe PC, Barron DF, Calkins H, Maumenee IH, Tong PY, Geraghty MT. Ehlers-Danlos syndrome. J Pediatr. 1999;135:513. doi: 10.1016/s0022-3476(99)70176-9. [DOI] [PubMed] [Google Scholar]