Abstract
Genetic inactivation of the mitochondrial self-destruction mechanism improves cognition in a mouse model of Alzheimer’s disease (pages 1097–1105).
Ample evidence suggests that a key event in the etiology of Alzheimer’s disease is the excessive production of β amyloid peptide (Aβ) and its extracellular deposition; deregulation of neuronal metabolism and signaling cascades ensues1. Large Aβ aggregates, also called fibrils, form characteristic amyloid plaques in the brains of individuals with Alzheimer’s disease. These plaques have long been viewed as highly cytotoxic, but many recent studies indicate that smaller soluble forms of Aβ may be the true culprits causing neuronal injury2.
The biochemistry of Aβ toxicity is a work in progress—however, multiple lines of evidence suggest that mitochondria are among the important or even major intracellular targets of soluble Aβ oligomers. Soluble Aβ localizes to mitochondria and interferes with their normal functioning, causing overproduction of reactive oxygen species (ROS), inhibiting respiration and ATP production and damaging the structure of mitochondria3.
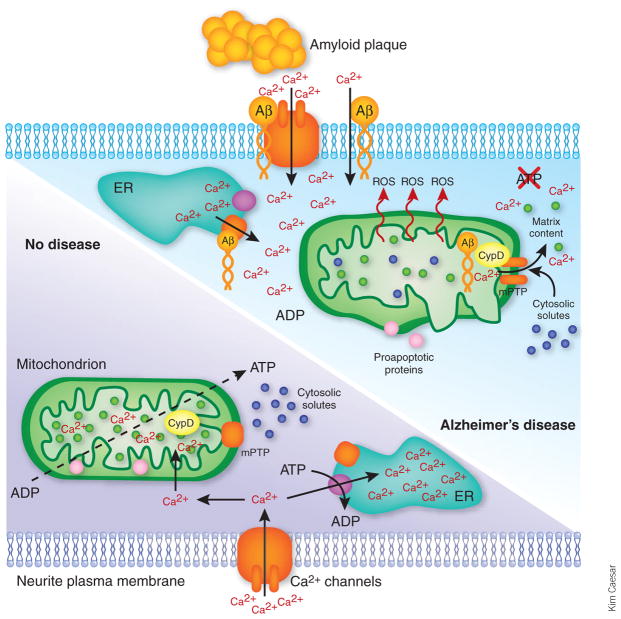
It is quite possible that the deleterious effects of Aβ on mitochondria result from disruption of intracellular calcium homeostasis and signaling (Fig. 1), which is another signature aspect of Aβ toxicity. Abnormally high cytosolic calcium levels and deregulated calcium signaling occur in neurites proximal to Aβ plaques4. Moreover, Aβ oligomers (but not fibrils) induce massive calcium entry into cultured neurons, mitochondrial calcium overload and apoptosis of neurons—all preventable if mitochondria are not allowed to accumulate excess calcium. The calcium overload of mitochondria results in the opening of the mitochondrial permeability transition pore (mPTP)5, a large channel in the inner mitochondrial membrane. Its opening allows uncontrolled bidirectional passage of large molecules, which results in the functional and structural disintegration of mitochondria—akin to an activation of a natural self-destruction facility built into the complicated mitochondrial fabric.
Figure 1.
Mitochondria in Alzheimer’s disease. In nondiseased neurons, the mPTP is closed, and mitochondria produce ATP and maintain other functions. Cytosolic calcium levels are kept low via ATP-dependent sequestration into endoplasmic reticulum (ER), ATP-dependent extrusion through the Ca2+-ATPase of the plasma membrane (not shown) and energy-dependent sequestration into mitochondria. However, in neurons affected with Alzheimer’s disease, an accumulation of soluble Aβ results in elevated cytosolic calcium levels owing to leaks from Aβ-modified calcium-conducting channels in the ER and plasma membrane4. Elevated cytosolic calcium may result in calcium overload of mitochondria. An accumulation of Aβ in mitochondria3 and its direct interaction with CypD promotes calcium-induced mPTP opening6, which damages mitochondrial ultrastructure, inhibits mitochondrial ATP production and other energy-dependent functions, and releases calcium stored in mitochondria, thereby further deregulating neuronal calcium signaling. Finally, an energy deficit and a release of proapoptotic proteins from damaged mitochondria results in neuronal injury.
In this issue of Nature Medicine, Du et al.6 present compelling evidence that Aβ targets the mPTP and amplifies calcium-induced mitochondrial damage. They find that inhibiting the mPTP ameliorates cognitive deficiencies in a transgenic mouse model of Alzheimer’s disease6. Furthermore, the authors prove that Aβ oligomers target the mitochondrial protein cyclophilin D (CypD), a well known regulator of mPTP6.
The mPTP has been studied for more than 40 years but remains enigmatic. It is drawing tremendous interest now because of its suggested role in cell death induced by ischemia, trauma and various neurodegenerative diseases7,8.
The mPTP opens upon excessive calcium accumulation by mitochondria; ROS enhance the probability of its opening. Opening of the mPTP renders mitochondria totally incapable of producing ATP, performing many other functions such as calcium accumulation and retention, and maintaining ion homeostasis and osmotic balance. Severe swelling of mitochondria and damage to their ultra-structure frequently ensues after the mPTP opening, resulting in an obligatory decrease in the membrane potential and, eventually, in a loss of matrix solutes such as nicotinamite adenine dinucleotides and glutathione, which inhibits respiration. Moreover, a release of proapoptotic proteins such as cytochrome c may accompany the swelling and rupture of the outer mitochondrial membrane evoked by mPTP9, frequently resulting in cell death by apoptosis. The protein composition of the mPTP remains unknown10,11.
Cyclosporine A is a strong inhibitor of calcium-induced mPTP opening. This compound binds CypD and inhibits its binding to the matrix side of the inner mitochondrial membrane, which is thought to be crucial for its activity as an mPTP modulator10. The mPTP in mitochondria isolated from CypD-knockout mice is insensitive to cyclosporine A and shows a much higher calcium threshold than wild-type mitochondria12,13, thereby proving the crucial role of CypD in controlling the mPTP opening probability.
Du et al.6 provide evidence in mice that the interaction between Aβ and CypD promotes the opening of the mPTP, thereby causing neuronal injury and a decline in cognitive functions. They show that Aβ forms a complex with CypD in vivo and in vitro and that formation of this complex in vitro makes mitochondria more susceptible to mPTP opening. They further show that genetic ablation of CypD in a mouse model of Alzheimer’s disease renders their brain mitochondria more resistant to mPTP opening—and also substantially improves their cognitive abilities. These findings lend strong support to the role of mitochondrial dysfunction in Alzheimer’s disease pathology, and they suggest that developing drugs targeting neuronal CypD may be a very promising therapeutic strategy to combat Alzheimer’s disease.
Unfortunately, there are currently no safe CypD inhibitors specific to the brain that could be used to treat Alzheimer’s disease in humans. CypD is ubiquitously expressed in mammalian tissues, and its functions probably extend beyond controlling the calcium sensitivity of the mitochondrial mPTP. Although cyclosporine A is very efficient in blocking the mPTP and also has a well established medical application as an immunosuppressant preventing tissue rejection in organ transplantation, it does not permeate the blood brain barrier well. It also shows severe adverse health effects such as nephrotoxicity and cardiac toxicity, although whether these effects are linked to its ability to inhibit mitochondrial CypD is not known.
Given the complexity and insufficient knowledge of mPTP composition and regulation, it is clear that some basic questions need to be answered first. Future challenges include understanding what proteins form the mPTP channel and what modulates the opening of mPTP in vivo and figuring out how to assess and modulate the mPTP in the brain. Answering these questions could lead to a safe and effective way to specifically inhibit mPTP in the brain of individuals with Alzheimer’s disease and may provide a new avenue for treating this devastating disease.
Contributor Information
Anatoly A Starkov, Email: ans2024@med.cornell.edu, Department of Neurology and Neuroscience, Weill Medical College of Cornell University, A501 525 East 68th Street, New York, New York 10065, USA.
Flint M Beal, Department of Neurology and Neuroscience, Weill Medical College of Cornell University, F610, 1300 York Avenue, New York, New York 10065, USA.
References
- 1.Hardy J, Allsop D. Trends Pharmacol Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 2.Cerpa W, Dinamarca MC, Inestrosa NC. Curr Alzheimer Res. 2008;5:233–243. doi: 10.2174/156720508784533321. [DOI] [PubMed] [Google Scholar]
- 3.Reddy PH, Beal MF. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green KN, LaFerla FM. Neuron. 2008;59:190–194. doi: 10.1016/j.neuron.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Sanz-Blasco S, Valero RA, Rodriguez-Crespo I, Villalobos C, Nunez L. PLoS ONE. 2008;3:e2718. doi: 10.1371/journal.pone.0002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du H, et al. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soane L, Kahraman S, Kristian T, Fiskum G. J Neurosci Res. 2007;85:3407–3415. doi: 10.1002/jnr.21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norenberg MD, Rao KV. Neurochem Int. 2007;50:983–997. doi: 10.1016/j.neuint.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoratti M, Szabo I. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 10.Leung AW, Halestrap AP. Biochim Biophys Acta. 2008;1777:946–952. doi: 10.1016/j.bbabio.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Bernardi P, et al. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa T, et al. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 13.Schinzel AC, et al. Proc Natl Acad Sci USA. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]