Abstract
Chronic alcohol (ethanol) abuse causes neuroinflammation and brain damage that can give rise to alcoholic dementia. Insightfully, Dr. Albert Sun was an early proponent of oxidative stress as a key factor in alcoholism-related brain deterioration. In fact, oxidative stress has proven to be critical to the hippocampal and temporal cortical neurodamage resulting from repetitive or intermittent “binge” alcohol (ethanol) exposure in adult rat models. Although the underlying mechanisms are not certain, our immunoelectrophoretic and related assays in binge alcohol experiments in vivo (adult male rats) and in vitro (rat organotypic hippocampal-entorhinal cortical slice cultures) have implicated phospholipase A2 (PLA2)-activated neuroinflammatory pathways. The results further indicated that concomitant with PLA2 activation, binge alcohol elevated oxidative stress adducts (“oxidative footprints”) and, interestingly, poly(ADP-ribose) polymerase-1 (PARP-1), an oxidative stress-responsive, DNA repair enzyme linked to a non-apoptotic neuronal death process termed parthanatos. Also significantly increased by the alcohol treatments was aquaporin-4 (AQP4), a water channel enriched in astrocytes that, when augmented, can trigger brain edema and neuroinflammation. We further have found that supplementation of brain slice cultures with docosahexaenoic acid (DHA; 22:6 ω3) exerted potent protection against binge alcohol neurotoxicity, while precluding induced changes in PLA2 isoforms, AQP4, PARP-1 and oxidative stress footprints. The DHA results lend support to recommendations of ω3 “fish oil” supplementation in alcoholism withdrawal therapies.
Keywords: ethanol, PLA2, PARP-1, neurodegeneration, neuroprotection, DHA, organotypic
According to the UN-WHO, alcohol (ethanol) misuse/abuse ranks just below depressive illness as a leading cause of lost human potential worldwide. Moderate-to-severe brain damage—synaptic and neuronal degeneration—is a key pathological outcome of alcohol (ethanol) misuse/abuse that contributes significantly to lost human potential. The neurobiological mechanisms responsible for neurodegeneration are currently equivocal, although they appear to involve oxidative stress and reactive oxygen species (ROS) [1]. Presciently, Dr. Albert Sun considered early that oxidative stress could be a major factor in ethanol’s neurobiological effects [2,3]. Studying possible brain neurodamaging mechanisms triggered by intermittent (binge) alcohol intoxication in adult rats, we and others initially anticipated that ROS would primarily be a consequence of excitotoxicity—increased neuronal calcium entry via overactivated or excess extrasynaptic glutamate/N-methyl-D-aspartate receptors (NMDAR). However, NMDAR antagonists known to exert neuroprotection in animal models of stroke, ischemia, and trauma were largely ineffective against chronic intermittent alcohol treatment—specifically, the Majchrowicz binge intoxication/withdrawal model [4–6].
Here we review experimental evidence supporting the possibility that in the Majchrowicz binge intoxication model (~12 gavage doses over 4 days, 8–10 g alcohol/kg/d; reaching blood alcohol levels of ~400+ mg/dl [7]) and in less severe modifications, as well as in alcohol-binged organotypic rat brain slice cultures, activation of phospholipase A2 (PLA2) isoforms and excessive arachidonic acid (ARA) mobilization may be a significant ROS source underlying neuroinflammatory oxidative stress and neurodegeneration. Two other key neuroinflammatory-associated proteins, aquaporin-4 (AQP4) and poly [ADP-ribose]polymerase-1 (PARP-1), a DNA repair protein known to cause a necrotic-like death cascade called parthanatos [8], are increased and could have roles in alcohol’s neurodeleterious effects. Furthermore, results with an adult-age organotypic brain slice model showing the marked neuroprotective, anti-inflammatory effects of supplemented ω3-docosahexaneoic acid (DHA) against binge ethanol.
Modest but significant brain edema consistent with reported cell swelling in primary astrocyte cultures exposed to alcohol and withdrawal [9] was first noted in vivo in a once-daily binge adaptation of the Majchrowicz intoxication/withdrawal model [5]. Prevention of the brain edema with furosemide diuretic was significantly neuroprotective, as it was in binge alcohol-treated organotypic brain slice cultures; subsequent studies found that selected other diuretics—e.g., acetazolamide (AZT), torasemide—were similarly neuroprotective in vivo (AZT) and/or in vitro [10]. Concurrently, other laboratories reported that AZT was an effective inhibitor of aquaporin-4 (AQP4) water channels enriched in brain astroglia [11,12]. Known connections between increased AQP4 and cytotoxic (cellular) edema [13] led to our considering that alcohol binges were precipitating cerebral edema via potentiation of the water channel, and indeed, initial immunoblots indicated higher AQP4 levels in alcohol-treated brain slices in culture [10]. Other reports have shown that increased AQP4 is important in alcohol’s augmentation of traumatic brain injury and edema [14] and in pro-inflammatory cytokine elevations in lipopolysaccharide-induced neuroinflammation [15]. Thus AQP4, like PLA2 below, can function as a potential neuroinflammatory stimulus.
A considerable body of evidence mainly from ischemia and stroke experiments has established that PLA2 activation causes elevated brain ROS, and that such activation is linked to brain edema [16,17]. In the nervous system, Ca+2-dependent PLA2 (cPLA2), and particularly the cPLA2 IVA isoform, is a dominant activity releasing ARA during a range of neuroinflammatory insults. Also, in an increasing number of neurodegeneration studies, low molecular weight isoforms of secreted PLA2 such as sPLA2 IIA are important in the release of ARA [18,19], perhaps even acting upstream of cPLA2. Alternatively, a neuroinflammatory role of Ca+2-independent PLA2 (iPLA2) is somewhat indistinct; indeed, its activity, and especially that of the iPLA2 VIA/B isoforms, has been associated with such favorable outcomes as mitochondrial viability, synaptic stability and cell homeostasis, as well as turnover of endogenous DHA [20,21]. Thus, decreased iPLA2 activity from insults or diseases could be unfavorable for the brain overall, whereas increased activity might be associated with (or promote) beneficial or even pro-survival pathways.
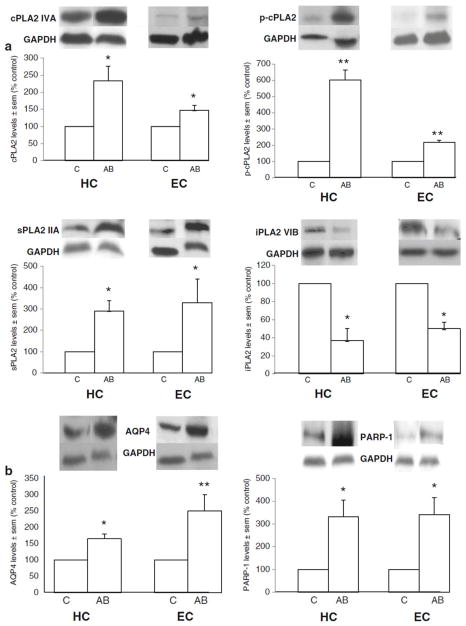
With two adult rat/binge alcohol intoxication models we have found evidence supporting the stimulation of brain neuroinflammatory pathways involving elevated AQP4, cPLA2 IVA, and sPLA2 IIA, and the possible loss of iPLA2 VIA; furthermore, the pathways could be related to neurotoxic activation of PARP-1—if so, this is a previously unrecognized association. The first model utilized relatively moderate binge ethanol treatments of Sprague-Dawley female rats at juvenile and/or young adult ages. The sequential binge treatment at both ages consisted of six 3 g/kg intraperitoneal injections over eight days beginning at 37 days of age and again at day 68. The immunoblot results of the juvenile+adult treatments have been published [22]; here we report estimated levels of the above-mentioned proteins after the adult binge only (d 68 to d 75) in two brain regions that incur definite neurodegeneration following more severe binge alcohol treatments, i.e., hippocampus (HC) and entorhinal cortex (EC) (Figure 1)—however, whether neurodegeneration transpired in this relatively moderate intoxication model was not established. Rats were sacrificed one hour after the last alcohol dose (blood levels ~210 mg/dl), but because of insufficient control (saline-injected) samples, it was necessary to use as controls the same brain regions from rats treated with a single 3 g/kg ip alcohol injection one hour pre-sacrifice. This was judged valid because the single alcohol treatment was found by comparisons with previous saline samples to have no quantitative effect on levels of proteins being examined here. The results in Fig. 1 are consistent overall with the results from doubly-binged (juvenile+ adult age) rats [23] in substantiating that binge alcohol intoxication in young adult rats (a) significantly augments HC and EC levels of cPLA2, sPLA2, AQP4 and PARP-1 while reducing iPLA2; and (b) promotes activation (phosphorylation) of the key ARA-mobilizing cPLA2 (Fig. 1A). The results also provide the first inference that parthanatos, a PARP-1-mediated necrotic process, could be a component of binge alcohol-induced brain neuroinflammation.
Figure 1.
Effect of six alcohol treatments of young adult rats (3 g/kg ip daily over 8 days with 2 intermittent withdrawal days) on brain levels of neuroinflammatory proteins. Fig. 1A: Significantly increased immunoblot levels of cPLA2 IVA, p-cPLA2 IVA and sPLA2 IIA and decreased levels of iPLA2 VIA in hippocampus (HC) and entorhinal cortex (EC) of alcohol-binged (AB) rats compared to control (C) rats. Fig. 1B: Significantly increased immunoblot levels of AQP4 and PARP-1 in hippocampus (HC) and entorhinal cortex (EC) of alcohol-binged (AB) rats compared to control (C) rats. Immunoblot levels normalized to GAPDH immunoblot levels as reported [23]. Data are means ± sem (n = 3–6 per group); *P < 0.05 vs. control; **P < 0.01 vs. control.
The results motivated us to determine the PLA2 isoforms in conjunction with PARP-1 and AQP4 in a model of binge intoxication with evident neurodegeneration. Accordingly, the original severe Majchrowicz procedure, which generates relatively selective neurodamage (cupric silver-stained neurons) in the HC (dentate gyrus), EC and olfactory bulb (OB), but not in other regions such as the frontal cortex (FC) or cerebellum (CB) [24], was used. Following sacrifice 1½ hours after the last alcohol dose (blood levels, ~ 375 mg/dl), immunoblot results showed that the same neuroinflammatory proteins which were increased by relatively moderate binge treatment in Figure 1 were likewise elevated in this neurotoxic binge intoxication model; importantly, with exception of iPLA2, significant changes occurred only in the neurovulnerable regions of HC, EC and OB and not the FC and CB (Table 1). Further immunoblot assays not shown confirmed that alcohol-dependent oxidative stress was a significant event in the HC and EC, in that “fingerprints” ultimately derived from excessive ARA release/peroxidation—4-hydroxynonenal-protein adducts—were significantly increased (Tajuddin et al., submitted).
TABLE 1.
Effect of intermittent severe binge alcohol intoxication on levels of neuroinflammatory proteins and oxidative stress footprints (4-hydroxynonenal-protein adducts) in brain regions of adult male rats: Majchrowicz protocol (% of gavage control values ± sem)
| Brain Region | cPLA2 | p-cPLA2 | sPLA2 | iPLA2 | AQP4 | PARP-1 | HNE-adducts |
|---|---|---|---|---|---|---|---|
| HC¶ | *225±45 | *155±10 | *180± | *65±10 | *170±16 | *235±40 | *150±26 |
| EC¶ | *180±20 | *165±15 | *255± | nc | *210±25 | *230±35 | *195±22 |
| OB¶ | *240±65 | *225±50 | **340± | nc | *175±27 | *250±45 | nd |
| FC¶ | nc | nc | nc | nc | nc | nc | nd |
| CB¶ | nc | nc | nc | nc | nc | nc | nd |
Values from immunoblots with GAPDH loading controls (Tajuddin et al., PLoS ONE, accepted for publication).
HC=hippocampus; EC=entorhinal cortex; OB=olfactory bulb; FC=frontal cortex; CB=cerebellum; HNE=4-hydroxynonenal.
Brain region incurring established and measurable neurodegeneration in this model; (n=4–7 rats/group).
p<0.05 vs. control;
p<0.01 vs. control;
nc, no significant change vs. control; nd, not determined.
Preceding and parallel with the above in vivo studies, binge alcohol-induced neurodamage was reproduced in an in vitro model of relatively intact rat brain—organotypic HC-EC slices in long-term culture. Such experiments in adolescent-age slice cultures (from 7 day-old rat pups and cultured ~3 weeks prior to intermittent binge 100 mM alcohol) indicated with inhibitors that increased ARA release and PLA2 activity were involved in neurodegeneration [25]. Most recently, the results with young adult-age brain slice cultures (from 40 day-old rats; cultured to provide 60+ day-old HC-EC slices [26]) that are summarized in Table 2 replicated the in vivo changes with respect to the PLA2 isoforms, AQP4 and PARP1 caused by binge alcohol exposure (Tajuddin et al., submitted).
TABLE 2.
Effect of repetitive binge alcohol treatment (100 mM), with and without supplemental docosahexaenoic acid (DHA), on levels of neuroinflammatory proteins and an oxidative stress footprint (nitrosylated tyrosine-protein adducts) in adult-age rat HC-EC slice cultures (% of medium/vehicle control ± sem)
| TREATMENT | cPLA2 | p-cPLA2 | sPLA2 | iPLA2 | AQP4 | PARP-1 | Nitrosyl- tyrosine- adducts |
|---|---|---|---|---|---|---|---|
| Binge alcohol+ vehicle | *265±25 | *240±20 | *230±30 | *35±8 | *215±20 | *220±45 | *188±50 |
| Binge alcohol+DHA | nsd | nsd | nsd | nsd | nsd | nsd | nsd |
Slice cultures treated ~16 hr (overnight) with 100 mM alcohol and ~8 hr no alcohol (media only) for 4 days. DHA= 50 uM throughout 4 days. Values from immunoblots with GAPDH loading control (Tajuddin et al., PLoS ONE, accepted for publication, except for AQP4 values, which are from reference [29]).
p<0.05 vs. respective medium/vehicle control (n= 3–5/group).
nsd=not significantly different from control+DHA. Results analyzed for significant differences usingTukey’s t-test and ANOVA with completely randomized design.
With these in vivo and in vitro rat brain experiments shedding light on the crucial role of PLA2-ARA-based oxidative stress routes in binge alcohol neuroinflammation and neurotoxicity, we examined the effects of supplementation with an anti-oxidative, pro-survival ω3 fatty acid, docosahexaenoic acid (DHA). In both ages of HC-EC slices in culture, DHA (25–50 μM), added throughout alcohol exposure and withdrawal, significantly reduced or prevented neurodegeneration [25,26]. As shown in Table 2, concurrent with neuroprotection and the suppression of ARA release as well as of oxidative stress indicators (nitrotyrosinated proteins), supplemental DHA also countered the changes in cPLA2, p-cPLA2, sPLA2, iPLA2, AQP4 and PARP-1 by normalizing their values to respective control levels. These potent effects of DHA were wholly consistent with its known anti-neuroinflammatory actions [27], and had not previously been demonstrated in the context of alcohol neurotoxicity.
Other neuroinflammatory mechanisms certainly might contribute to alcohol-induced ROS in these binge alcohol models. Alcohol metabolism itself, which occurs in the central nervous system (albeit slowly) and particularly in astrocytes [28], could be important. Specifically, alcohol oxidation by brain cytosolic catalase is a possible ROS-producing route, as is inducible cytochrome P4502E1 in endoplasmic reticulum ([29]. Also, alcohol-dependent microglial activation leading to increased brain NADPH oxidase activity and resultant ROS generation has been implicated in prolonged alcohol exposure [30]. However, the above alcohol-ROS generating possibilities have been examined largely in acute and chronic alcohol rodent models that utilize non-binge alcohol intake or administration (liquid diets, water, vapor chamber)—models that do not generally entail the repetitive, highly elevated blood alcohol levels and nadirs of binge administration/intoxication. This difference in models could give rise to considerable variations in the sources of oxidative stress, regional neuroinflammation, and ultimately, neurodegeneration.
Summarizing, these results specifically encompass the theme of this satellite collection, lipid signaling in neurodegeneration and neuroprotection, as well as harken back to and expand the pioneering alcohol and oxidative stress research of Dr. Albert Sun. They indicate that increased cPLA2 and sPLA2 activities/levels and possibly decreased iPLA2 are features of pro-oxidative neuroinflammation in adult rat brain during neurotoxic alcohol exposures which mirror binge alcoholism. The significant elevations in AQP4 and PARP-1 which accompany the PLA2 changes require in-depth investigations to understand their full importance. It is notable that DHA emerges as a remarkably potent neuroprotective, anti-neuroinflammatory molecule in the in vitro binge alcohol studies by mechanisms yet to be ascertained. These mechanisms could involve DHA’s increased incorporation into membrane phospholipids, especially phosphatidylserine, which could promote neuroprotective Akt signaling [31], as well as conversion of the non-esterified molecule to pro-survival derivatives such as neuroprotectin-1 and/or synaptamide [32,33]. The ω3 fatty acid has been suggested as a therapeutic adjuvant for hepatic protection in alcohol withdrawal treatment [34], and our studies add to the scope of its potential neuroprotective and neurorestorative effects during withdrawal in severe alcoholics.
Acknowledgments
The research was supported by the National Institutes of Health U01AA018279, R01AA0016959, and T32DA016176, and Loyola University Chicago Research Funding Committee and the Alcohol Research Program. The technical support of S. Alex Marshall is acknowledged. Drs. Toni Pak and Magdalena Szymanska, Department of Physiology at Loyola University Medical Center, are recognized for providing brain regions from alcohol-binged young adult rats generated in their neuroendocrine studies previously reported [35].
Abbreviations
- AQP4
aquaporin-4
- ARA
arachidonic acid
- AZT
acetazolamide
- DHA
docosahexaenoic acid
- PARP-1
poly(ADP-ribose) polymerase-1
- PLA2
phospholipase A2
- ROS
reactive oxygen species
Footnotes
Based on the Albert Y. Sun Memorial Lecture by MAC at the ISN-ASN satellite symposium: ‘Unveiling the Significance of Lipid Signaling in Neurodegeneration and Neuroprotection’, Cancun MX, April 2013.
The authors declare that they have no conflict of interest.
References
- 1.Collins MA, Neafsey EJ. Ethanol and adult CNS neurodamage: oxidative stress, but possibly not excitotoxicity. Frontiers in Bioscience. 2012;4:1358–67. doi: 10.2741/465. [DOI] [PubMed] [Google Scholar]
- 2.Sun GY, Rudeen PK, Wood WG, Wei YH, Sun AY. Molecular Mechanisms of Alcohol: Neurobiology and Metabolism. Humana Press; Clifton NJ: 1989. p. 397. [Google Scholar]
- 3.Sun AY, Ingelman-Sundberg M, Neve E, Matsumoto H, Nishitani Y, Minowa Y, Fukui Y, Bailey SM, Patel VB, Cunningham CC, Zima T, Fialova L, Mikulikova L, Popov P, Malbohan I, Janebova M, Nespor K, Sun GY. Ethanol and oxidative stress. Alcoholism: Clinical & Experimental Research. 2001;25:237S–243S. doi: 10.1097/00000374-200105051-00038. [DOI] [PubMed] [Google Scholar]
- 4.Corso TD, Mostafa HM, Collins MA, Neafsey EJ. Brain neuronal degeneration caused by episodic alcohol intoxication in rats: effects of nimodipine, 6,7-dinitro-quinoxaline-2,3-dione, and MK-801. Alcohol Clin Exp Res. 1998;22:217–24. [PubMed] [Google Scholar]
- 5.Collins MA, Zou JY, Neafsey EJ. Brain damage due to episodic alcohol exposure in vivo and in vitro: furosemide neuroprotection implicates edema-based mechanism. Faseb J. 1998;12:221–30. doi: 10.1096/fasebj.12.2.221. [DOI] [PubMed] [Google Scholar]
- 6.Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. Journal of Pharmacology & Experimental Therapeutics. 2005;314:780–8. doi: 10.1124/jpet.105.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–54. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- 8.David KK, Andrabi SA, Dawson TM, Dawson VL. Parthanatos, a messenger of death. Frontiers in Bioscience. 2009;14:1116–28. doi: 10.2741/3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aschner M, Allen JW, Mutkus LA, Cao C. Ethanol-induced swelling in neonatal rat primary astrocyte cultures. Brain Res. 2001;900:219–226. doi: 10.1016/s0006-8993(01)02314-9. [DOI] [PubMed] [Google Scholar]
- 10.Sripathirathan K, Brown J, Neafsey EJ, Collins MA. Linking binge alcohol-induced neurodamage to brain edema and potential aquaporin-4 upregulation: evidence in rat organotypic brain slice cultures and in vivo. Journal of Neurotrauma. 2009;26:261–73. doi: 10.1089/neu.2008.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber VJ, Tsujita M, Yamazaki M, Sakimura K, Nakada T. Identification of arylsulfonamides as Aquaporin 4 inhibitors. Bioorganic & Medicinal Chemistry Letters. 2007;17:1270–3. doi: 10.1016/j.bmcl.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Tanimura Y, Hiroaki Y, Fujiyoshi Y. Acetazolamide reversibly inhibits water conduction by aquaporin-4. Journal of Structural Biology. 2009;166:16–21. doi: 10.1016/j.jsb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda AM, Badaut J. Aquaporin 4: a player in cerebral edema and neuroinflammation. Journal of neuroinflammation. 2012;9:279. doi: 10.1186/1742-2094-9-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katada R, Nishitani Y, Honmou O, Mizuo K, Okazaki S, Tateda K, Watanabe S, Matsumoto H. Expression of aquaporin-4 augments cytotoxic brain edema after traumatic brain injury during acute ethanol exposure. Am J Pathol. 2012;180:17–23. doi: 10.1016/j.ajpath.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Zhang H, Varrin-Doyer M, Zamvil SS, Verkman AS. Proinflammatory role of aquaporin-4 in autoimmune neuroinflammation. FASEB Journal. 2011;25:1556–66. doi: 10.1096/fj.10-177279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun GY, Shelat PB, Jensen MB, He Y, Sun AY, Simonyi A. Phospholipases A2 and inflammatory responses in the central nervous system. NeuroMolecular Medicine. 2010;12:133–48. doi: 10.1007/s12017-009-8092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adibhatla RM, Hatcher JF. Phospholipase A(2), reactive oxygen species, and lipid peroxidation in CNS pathologies. BMB reports. 2008;41:560–7. doi: 10.5483/bmbrep.2008.41.8.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yagami T, Yamamoto Y, Koma H. The Role of Secretory Phospholipase A2 in the Central Nervous System and Neurological Diseases. Mol Neurobiol. 2013:1–14. doi: 10.1007/s12035-013-8565-9. [DOI] [PubMed] [Google Scholar]
- 19.Moses GS, Jensen MD, Lue LF, Walker DG, Sun AY, Simonyi A, Sun GY. Secretory PLA2-IIA: a new inflammatory factor for Alzheimer’s disease. Journal of Neuroinflammation. 2006;3 doi: 10.1186/1742-2094-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green JT, Orr SK, Bazinet RP. The emerging role of group VI calcium-independent phospholipase A2 in releasing docosahexaenoic acid from brain phospholipids. Journal of Lipid Research. 2008;49:939–44. doi: 10.1194/jlr.R700017-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Allyson J, Bi X, Baudry M, Massicotte G. Maintenance of Synaptic Stability Requires Calcium-Independent Phospholipase A 2 Activity. Neural Plast. 2012;2012 doi: 10.1155/2012/569149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tajuddin N, Przybycien-Szymanska MM, Mitchell RM, Pak TR, Neafsey EJ, Collins MA. Adult rats subjected to repetitive binge-pattern ethanol exposure show evidence of neuroinflammation involving elevated aquaporin-4, PLA2 and PARP-1. Alcoholism: Clinical & Experimental Research. 2012;36:35A. [Google Scholar]
- 23.Tajuddin N, Przybycien-Szymanska MM, Pak TR, Neafsey EJ, Collins MA. Effect of repetitive daily ethanol intoxication on adult rat brain: Significant changes in phospholipase A2 enzyme levels in association with increased PARP-1 indicate neuroinflammatory pathway activation. Alcohol. 2013;47:39–45. doi: 10.1016/j.alcohol.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins MA, Corso TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcoholism: Clinical & Experimental Research. 1996;20:284–92. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 25.Brown J, 3rd, Achille N, Neafsey EJ, Collins MA. Binge ethanol-induced neurodegeneration in rat organotypic brain slice cultures: effects of PLA2 inhibitor mepacrine and docosahexaenoic acid (DHA) Neurochemical Research. 2009;34:260–7. doi: 10.1007/s11064-008-9765-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins MA, Moon KH, Tajuddin N, Neafsey EJ, Kim HY. Docosahexaenoic acid (DHA) prevents binge ethanol-dependent aquaporin-4 elevations while inhibiting neurodegeneration: experiments in rat adult-age entorhino-hippocampal slice cultures. Neurotoxicity research. 2013;23:105–110. doi: 10.1007/s12640-012-9360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bazan NG, Molina MF, Gordon WC. Docosahexaenoic Acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annual Review of Nutrition. 2011;31:321–51. doi: 10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Du H, Jiang L, Ma X, de Graaf RA, Behar KL, Mason GF. Oxidation of ethanol in the rat brain and effects associated with chronic ethanol exposure. Proceedings of the National Academy of Sciences. 2013;110:14444–14449. doi: 10.1073/pnas.1306011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heit C, Dong H, Chen Y, Thompson DC, Deitrich RA, Vasiliou VK. The Role of CYP2E1 in Alcohol Metabolism and Sensitivity in the Central Nervous System. Subcell Biochem. 2013;67:235–247. doi: 10.1007/978-94-007-5881-0_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alikunju S, Abdul Muneer PM, Zhang Y, Szlachetka AM, Haorah J. The inflammatory footprints of alcohol-induced oxidative damage in neurovascular components. Brain, Behavior, & Immunity. 2011;25:S129–36. doi: 10.1016/j.bbi.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10858–63. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, Lukiw WJ, Bazan NG. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPAR-mediated mechanisms in Alzheimer’s disease models. PLoS ONE [Electronic Resource] 2011;6:e15816. doi: 10.1371/journal.pone.0015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HY, Spector AA. Synaptamide, endocannabinoid-like derivative of docosahexaenoic acid with cannabinoid-independent function. Prostaglandins Leukotrienes & Essential Fatty Acids. 2013;88:121–125. doi: 10.1016/j.plefa.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song BJ, Moon KH, Olsson NU, Salem N., Jr Prevention of alcoholic fatty liver and mitochondrial dysfunction in the rat by long-chain polyunsaturated fatty acids. J Hepatol. 2008;49:262–273. doi: 10.1016/j.jhep.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Przybycien-Szymanska MM, Mott NN, Paul CR, Gillespie RA, Pak TR. Binge-pattern alcohol exposure during puberty induces long-term changes in HPA axis reactivity. PLoS ONE. 2011;6:e18350. doi: 10.1371/journal.pone.0018350. [DOI] [PMC free article] [PubMed] [Google Scholar]