Abstract
Postural tachycardia syndrome (POTS) is characterized by exercise intolerance and sympathoactivation. To examine whether abnormal cardiac output and central blood volume changes occur during exercise in POTS, we studied 29 patients with POTS (17–29 yr) and 12 healthy subjects (18–27 yr) using impedance and venous occlusion plethysmography to assess regional blood volumes and flows during supine static handgrip to evoke the exercise pressor reflex. POTS was subgrouped into normal and low-flow groups based on calf blood flow. We examined autonomic effects with variability techniques. During handgrip, systolic blood pressure increased from 112 ± 4 to 139 ± 9 mmHg in control, from 119 ± 6 to 143 ± 9 in normal-flow POTS, but only from 117 ± 4 to 128 ± 6 in low-flow POTS. Heart rate increased from 63 ± 6 to 82 ± 4 beats/min in control, 76 ± 3 to 92 ± 6 beats/min in normal-flow POTS, and 88 ± 4 to 100 ± 6 beats/min in low-flow POTS. Heart rate variability and coherence markedly decreased in low-flow POTS, indicating uncoupling of baroreflex heart rate regulation. The increase in central blood volume with handgrip was absent in low-flow POTS and blunted in normal-flow POTS associated with abnormal splanchnic emptying. Cardiac output increased in control, was unchanged in low-flow POTS, and was attenuated in normal-flow POTS. Total peripheral resistance was increased compared with control in all POTS. The exercise pressor reflex was attenuated in low-flow POTS. While increased cardiac output and central blood volume characterizes controls, increased peripheral resistance with blunted or eliminated in central blood volume increments characterizes POTS and may contribute to exercise intolerance.
Keywords: orthostatic intolerance, mechanoreflex, metaboreflex, regional blood volume, exercise intolerance
Chronic orthostatic intolerance is identified with the postural tachycardia syndrome (POTS) (10). Reduced exercise tolerance is frequently found in POTS (22). On the one hand, it could be argued that exercise intolerance in POTS is directly related to limitations of venous return that occur in most POTS patients (44, 61); this is consistent with data showing reduced cardiac output in many POTS patients (16). On the other hand, mechanisms originating in exercising muscle could contribute to effort impairment similar to what is seen in heart failure (52).
Voluntary muscle contraction evokes central command (8) and the exercise pressor reflex (24, 40, 53), which depends on the stimulation of sensory afferents from exercising muscle (32). The exercise pressor reflex comprises the muscle mechanoreflex and muscle metaboreflex (25, 54). These produce vagal withdrawal (13) and sympathetic activation (28) and together increase heart rate (HR) and blood pressure (BP) during exercise. The absence of peripheral vasoconstriction in canine models reflects the buffering effects of baroreflexes that, while counteracting sympathetic mediated vasoconstriction, do not reduce sympathetically mediated increases in cardiac contractility (27, 40). The metaboreflex is evoked as muscle blood flow fails to keep up with metabolic demands (5, 24, 32). As shown by studies of O’Leary and associates (39, 50), graded metaboreflex stimulation in conscious dogs causes graded increases in cardiac output due to increased sympathetic stimulation of the heart and to increased central blood volume with increased ventricular preload. Systemic vasoconstriction is not an important feature of the healthy canine model, although regional vasoconstriction of skeletal muscle and renovascular tissue occur (2, 38). Similar findings have been found for the exercise pressor reflex in healthy humans, where studies often use static or intermittent handgrip contractions. Thus, central blood volume and cardiac sympathetic activation are increased, resulting in increased cardiac output (7) with, however, a small increase in total peripheral resistance due to changes in regional circulation (63).
The situation is very different during chronic states of sympathoexcitation. Best studied are canine models of heart failure in which the ability of the muscle metaboreflex to increase ventricular function by increases in contractility and filling pressure are severely limited. Here, increased peripheral resistance due to sympathetic vasoconstriction accounts for increased arterial pressure (1, 40, 41, 45), although the total BP increment is attenuated (1). This has its analog in humans with heart failure in that BP rises during static handgrip because of increased total peripheral resistance in congestive heart failure patients, while stroke volume and cardiac output are reduced (6, 51).
POTS patients often have increased baseline sympathetic activation (21, 49) but blunted responses to sympathetic stimuli (4, 10). We compared POTS patient responses with healthy control subject responses during activity evoking the exercise pressor reflex. We hypothesized that enhanced sympathetic activity in POTS would shift the mechanism mediating the pressor response during static handgrip exercise from increased central blood volume and cardiac output observed in control subjects to increased peripheral resistance and vasoconstriction similar to what is observed in other states of sympathoexcitation. This would be particularly important in those patients with clear evidence for baseline sympathoexcitation, resting peripheral vasoconstriction, and resting tachycardia in whom reduced muscle blood flow during exercise could directly contribute to early fatigue and exercise intolerance.
MATERIALS AND METHODS
Subjects
To test this hypothesis, we studied 29 patients with postural tachycardia aged 17–29 yr (median = 21.4 yr, 5 men and 24 women) and 12 healthy volunteer subjects aged 18–27 yr (median = 22.8 yr, 3 men and 9 women). Average weight (±SD) for POTS patients was 62 ± 4 kg, average height was 170 ± 6 cm, and average body mass index was 21 ± 3 kg/m2. For control subjects, average weight was 67 ± 4 kg, average height was 170 ± 7 cm, and average body mass index was 24 ± 2 kg/m2. All POTS patients and control subjects were normotensive. Physiological measurements were performed while subjects were supine.
POTS patients were referred for chronic orthostatic intolerance lasting for longer than 6 mo. Orthostatic intolerance was defined by the symptoms of lightheadedness, exercise intolerance, headache, fatigue, neurocognitive deficits, nausea, and other symptoms (48) while upright, relieved by recumbency, and with no other medical explanation. The diagnosis of POTS was confirmed on a screening upright tilt table test at 70°. POTS was diagnosed by symptoms of orthostatic intolerance during the tilt test associated with an increase in the sinus HR of >30 beats/min or to a HR of >120 beats/min during the first 10 min of tilt as defined in adult subjects in the literature (29, 47). We subgrouped POTS patients on the basis of calf blood flow measured by venous occlusion plethysmography. Occlusion cuffs were placed around the midthigh above a mercury in Silastic strain gauge (Hokanson) placed at midcalf to measure supine calf blood flow. Measurements were made in the supine position at the beginning of experiments after a 30-min resting period using standard venous occlusion methods (14). We subdivided POTS patients on the basis of calf blood flow. The stratification on calf blood flow comprised a prospective classification scheme based on a priori criteria that were obtained from calf blood flow data previously collected from >80 healthy volunteer subjects spanning prior research protocols. It was shown that calf blood flow was superior to forearm blood flow in separating POTS patients into subsets with similar physiology (64) and was consistent with norepinephrine spillover results obtained at other institutions (20). Our laboratory has described multiple groups of patients with POTS distinguished by differences in peripheral blood flow and peripheral arterial resistance (60, 61). These include a low-blood flow, high-arterial resistance group denoted as “low-flow” POTS characterized by pallor and generally decreased blood flow, most notable in the dependent parts of the body. This low-flow condition is associated with defects in local blood flow regulation and mild absolute hypovolemia. There is also a normal-blood flow, normal-arterial resistance group denoted as “normal-flow” POTS, which is characterized by a normal supine phenotype with normal peripheral resistance supine but enhanced peripheral resistance upright. There is specific venous pooling within the splanchnic vascular bed, making this a redistributive form of hypovolemia.
For the purposes of this study, normal calf blood flow was defined as >1.2 ml · min−1 · 100 ml tissue−1, which is the smallest calf blood flow we have measured in control subjects, and <3.6 ml · min−1 · 100 ml tissue−1, which is the largest calf blood flow we have measured in control subjects. We defined normal-flow POTS as those POTS patients falling between these limits. Low-flow POTS was defined by a calf blood flow of <1.2 ml · min−1 · 100 ml tissue−1.
All subjects were free from systemic illnesses. Subjects were not taking medications and were nonsmokers. All subjects refrained from caffeinated beverages for at least 24 h. No subject had evidence of cardiovascular or systemic illness. There were no competitive athletes or bedridden subjects. Informed consent was obtained. All protocols were approved by the Committee for the Protection of Human Subjects (Institutional Review Board) of New York Medical College.
Laboratory Evaluation
Arterial pressure and HR were monitored continuously. We estimated changes in thoracic, splanchnic, pelvic, and calf segmental blood volumes and corresponding blood flows by impedance plethysmography while subjects were supine and throughout static handgrip as explained below. Thoracic blood volume corresponded to central blood volume, and thoracic blood flow corresponded to cardiac output measured by impedance cardiography. We (63) have previously used these techniques to study healthy volunteer subjects. All subjects also had absolute blood volume and resting cardiac output measured by indocyanine green dye dilution methods, and results were compared between impedance measurements.
Protocol
Tests began after an overnight fast. An intravenous catheter was placed in the right antecubital fossa. Following 30 min, we measured resistance (R0) and beat-to-beat changes in resistance (ΔR) of thoracic, splanchnic, pelvic, and leg segments (terms defined below) using supine impedance plethysmography. We also measured calf blood flow by strain-gauge plethysmography. Impedance plethysmography measurements of thoracic blood flow, calf blood flow, and splanchnic blood flow have been previously standardized against indocyanine dye dilution cardiac output, venous occlusion calf blood flow, and assessments of portal blood flow (60, 63). We therefore used impedance plethysmography measurements to continuously estimate changes in segmental blood flows throughout the handgrip evaluation.
Early in the course of the experiment, each subject performed two brief maximal voluntary contractions (MVCs) with their left hand using a handgrip dynamometer (Lafayette Instruments, Lafayette, IN). Subsequently, subjects performed static handgrip while impedance, HR, and BP monitoring were continued. Handgrip was preceded by a baseline phase lasting 5 min, during which impedance, HR, and BP data were collected. Subjects then performed 120 s of sustained isometric handgrip at 30% MVC. This was typically exhaustive exercise. Handgrip exercise at equivalent %MVC resulted in hemodynamic responses that were independent of muscle mass or sedentary state (68, 69). Posthandgrip circulatory arrest was not performed because we were interested in the exercise pressor reflex rather than measurements made after exercise. A feedback system allowed subjects to maintain force near constant. BP, ECG, and impedance flow and volume measurements were made continuously but are reported at baseline, at 1 and 2 min of sustained handgrip, and during a recovery period.
Details of the Method
HR and BP monitoring
An ECG lead was recorded for cardiac rhythm. Right upper extremity BP was continuously monitored with a finger arterial plethysmograph (Finometer, FMS, Amsterdam, The Netherlands) placed on the right middle or index finger and calibrated against an oscillometric brachial cuff pressure. ECG and Finometer pressure data were interfaced to a personal computer through an analog-to-digital converter (DI-720 DataQ Ind, Milwaukee, WI). HR was derived from both ECG and arterial pressure data. All data were multiplexed with impedance data and were thereby synchronized. Continuous BP data were used to identify pulses and to compute HR variability (HRV) and BP variability (BPV) indexes and to perform and compare coherence analyses among the subject groups.
HRV, BPV, and coherence analysis
Our prior work (63) demonstrated that the parasympathetic baroreflex regulation of HR becomes less important during handgrip and showed an uncoupling of HR from BP modulation of the baroreflex during the static pressor reflex. Abnormalities in baroreflex control also typify forms of POTS, resulting in baseline sympathoexcitation (9, 36, 56). To obtain an index of sympathetic and parasympathetic activity, we used indexes of HRV and BPV to investigate the effects of handgrip on the cardiovagal baroreflex regulation of HR. We examined the transfer function between BP and HR at middle frequency (~0.1 Hz), which relates to sympathetic modulation of BP transduced via vagal efferents (31). We examined coherence and transfer function phase and amplitude (baroreflex gain) during handgrip. Baseline HR and BP data were captured during a 5-min resting period. Beats were thereafter acquired during the first minute and during the second minute of handgrip. Data were analyzed for each minute separately and for both minutes combined as a single beat sequence. Data were also collected for a 1-min period during recovery from handgrip centered on the time of minimum BP for comparison. We used custom software to collect digital sequences containing RR interval and systolic, diastolic, and mean BPs for each heart beat, as previously described (56). The coherence function was also calculated at low frequencies. A coherence of at least 0.5 is used to indicate a significant baroreceptor-mediated relationship between changes in BP and changes in HR (42). A coherence of <0.5 suggests an “uncoupling” of the baroreflex modulation of HR and BP (31).
Dye dilution measurement of blood volume and cardiac output
We used the dye dilution technique with indocyanine green to measure blood volume and cardiac output (3). This employs a spectrophotometric photosensor (DDG2000, Nihon-Kohden) validated in clinical studies (17, 19). We measured the hematocrit from antecubital venous blood and extrapolated the dye decay curve to the time of dye injection (time 0), yielding estimated blood volume. The area under the curve is simply related to blood flow using Stewart’s classic method (55).
Calf blood flow by venous occlusion strain-gauge plethysmography
We used venous occlusion strain-gauge plethysmography to measure calf blood flow. Supine measurements were made at the beginning of experiments and were later compared with impedance estimates of blood flow. We have previously employed these techniques (61, 63).
Impedance plethysmography to measure changes in segmental blood volumes and blood flows
We have used impedance plethysmography to detect blood flow changes and volume shifts during orthostatic stress (35, 61), during the Valsalva maneuver (62), and, more recently, during static handgrip testing (63). Impedance methods have been tested and calibrated against strain-gauge plethysmography for peripheral measurements, dye dilution for cardiac output, and indocyanine dilution for splanchnic blood flow in two prior publications (60, 63). Impedance cardiography is routinely used to measure changes in cardiac output (7). Our device employs a tetrapolar high-resolution impedance monitor four-channel digital impedance plethysmograph (UFI) applied to four anatomic segments defined in practice by electrode placement. These are designated the thoracic segment (equivalent to the supraclavicular area to the xyphoid process interrogated during impedance cardiography), the splanchnic segment (the xyphoid process to the iliac crest), the pelvic segment incorporating the lower pelvis to the knee (the iliac crest to the knee), and the leg or calf segment (the upper calf just below the knee to the ankle) (58, 62). Ag/AgCl ECG electrodes were attached at these segmental boundaries and also to the right foot and right hand, where they served as current injectors. The device uses a 50-kHz, 0.1-mA root mean square constant-current signal between the foot and hand electrodes. Electrical resistance values were measured using the segmental pairs as sampling electrodes. The midline distance between the sampling electrodes (L) was measured with a tape measure. We also measured the circumferences of calf, thigh, hips, waist, and chest to obtain approximate volume contents of each anatomic segment. We estimated the change in blood volume in each segment during handgrip from the following formula:
| (13) |
where ρ is the electrical conductivity of blood and estimated as 53.2 × exp(hematocrit × 0.022), as given by Geddes and Sadler (12). R0 is the resistance of a specific segment prior to handgrip, R1 is the resistance during handgrip, and ΔR is the change in resistance (R1 − R0) in a specific segment during handgrip. ρ was regarded as constant during the maneuver.
Impedance methods were also used to measure segmental blood flows. Pulsatile changes in electrical resistance were employed to compute the time derivative ∂R/∂t, which we used to obtain the blood flow responses of each body segment during handgrip.
Blood flow was estimated for an entire anatomic segment from the following formula:
| (13) |
where T is the ejection period, R is the pulsatile resistance, and R0 is the baseline resistance at a given angle of tilt. Respiratory artifacts were removed from the signal using adaptive eight-pole Butterworth filtering. Impedance flows are expressed in milliliters per minute for the anatomic segment and can be normalized by dividing by estimated segmental volume.
Statistics
Results in the text and tables are reported as means ± SD. Results in the figures are presented as means ± SE. Changes in HRV and BPV and in impedance estimates of regional blood flow and regional blood volume, HR, and mean and systolic BPs were compared by ANOVA for repeated measures at baseline before handgrip, 1 min after the start of handgrip, 2 min after the start of handgrip, and during recovery using the minimum of BP during recovery as the time of comparison. Data in the tables were also analyzed by ANOVA and corrected for multiple tests. Results were calculated using SPSS software version 11.0.
RESULTS
Subject Size and Supine Resting Hemodynamic Data
All subjects completed the protocol. Patient dimensions and resting supine hemodynamic data before handgrip testing are shown in Table 1. Weight was significantly less in low-flow POTS patients compared with normal-flow POTS and control subjects (P < 0.01). Since there were no differences in height among the subject groups, the body mass index [equal to weight (in kg)/height (in m2)] was also significantly reduced in low-flow POTS. Blood volume was decreased in low-flow POTS but was not different among the subject groups once it was normalized for body weight. Mean hematocrit was also not different. Data were similar for males and females. Pooled gender data are presented.
Table 1.
Patient dimensions and supine hemodynamic data
| Control | Postural Tachycardia Syndrome
|
||
|---|---|---|---|
| Low Flow | Normal Flow | ||
| n | 12 | 13 | 16 |
| Age, yr | 23±3 | 22±3 | 21±3 |
| Weight, kg | 68±4 | 56±6* | 67±4 |
| Height, cm | 170±4 | 167±5 | 170±6 |
| Body mass index, kg/m2 | 23.7±0.8 | 19.8±0.9* | 22.9±1.3 |
| Normalized blood volume, ml/kg | 75±1 | 73±2 | 75±1 |
| Packed cell volume, % | 41±1 | 40±1 | 41±1 |
| Impedance cardiography cardiac index, l · min−1 · m−2 | 4.2±0.3 | 3.2±0.3* | 4.6±0.6 |
| Total peripheral resistance, mmHg · l−1 · min−1 · m−2 | 26±7 | 44±9* | 27±8 |
| HR, beats/min | 63±6 | 88±4* | 76±3* |
| Systolic BP, mmHg | 112±4 | 117±4 | 119±3 |
| Mean arterial pressure, mmHg | 83±5 | 90±6 | 87±4 |
| Venous occlusion calf blood flow, ml · 100 ml−1 ·* min−1 | 2.5±0.2 | 1.04±0.1* | 2.3±0.2 |
| Calf arterial resistance, ml · 100 ml−1 · min−1 · mmHg−1 | 33±4 | 77±9* | 40±4 |
| Impedance blood flows, ml/min | |||
| Thoracic | 6,181±717 | 3,663±411* | 5,225±586 |
| Splanchnic | 1,267±233 | 1,216±260 | 2,573±406* |
| Pelvic | 1,038±148 | 522±71* | 783±111 |
| Leg | 134±18 | 88±7* | 107±13 |
| Impedance cardiac index, l/m2 | 3.7±0.3 | 2.6±0.2* | 3.8±0.4 |
| Normed impedance blood flows, ml · 100 ml−1 · min−1 | |||
| Splanchnic | 22±2 | 29±3 | 43±6* |
| Pelvic | 6.2±0.8 | 4.1±0.8 | 11±3 |
| Leg | 2.9±0.2 | 1.3±0.1* | 2.8±0.4 |
Values are means ± SD; n, no. of subjects. HR, heart rate; BP, blood pressure.
P < 0.05, significantly different from control.
The cardiac index, as measured by dye dilution, was reduced at rest in low-flow POTS patients compared with control and normal-flow POTS. Systolic BP was similar among the groups, as was mean arterial pressure (MAP), although there was a trend toward increased MAP in low-flow POTS. Calf blood flow, as measured by venous occlusion plethysmography, was decreased in low-flow POTS by definition.
Impedance flow assessments revealed decreased thoracic, pelvic, and calf blood flows in low-flow POTS patients and increased splanchnic blood flows in normal-flow POTS patients compared with control subjects, as we have shown previously.
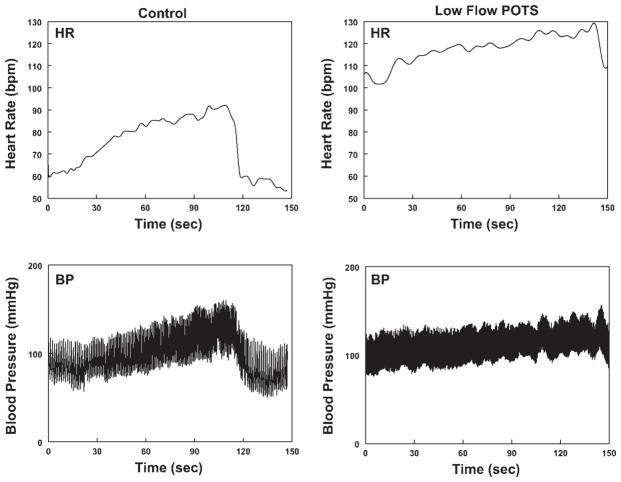
HR and BP During Handgrip
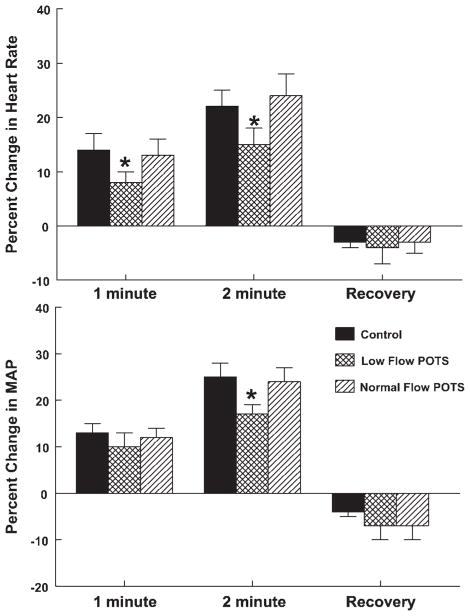
HR and BP from a representative healthy volunteer control subject and from a low-flow POTS patient are shown in Fig. 1. Both subjects show approximately linear increases in BP with time during the test. Similar changes were observed in all patients. The increases in HR and BP were blunted in low-flow POTS. Figure 2 shows HR and BP data averaged over all subjects. The percent changes in HR and MAP were decreased in low-flow POTS at 1 and 2 min of static handgrip. Although the absolute resting HR was increased in POTS, blunting of the HR increase remained significant at 2 min, even when absolute HR changes were compared (ΔHR was 15 ± 3 for low-flow POTS vs. 18 ± 4 for normal-flow POTS and 21 ± 2 in control subjects, P < 0.025 compared with control).
Fig. 1.
Left: representative heart rate [HR, in beats/min (bpm); top] and blood pressure (BP; bottom) from a control subject during static handgrip. Right: corresponding representative HR (top) and BP (bottom) from a low-flow postural tachycardia syndrome (POTS) subject. Baseline HR was increased in the POTS patient compared with control. HR and BP increases with handgrip were attenuated in the POTS patient.
Fig. 2.
Percent changes in HR (top) and BP (bottom) averaged over all subjects. Percent changes are shown after 1 and 2 min of handgrip and during the recovery period. There was an attenuation of the increases in HR and BP for low-flow POTS. *P < 0.05 compared with control subjects.
Overall, systolic BP and MAP increased significantly from baseline at 1 min of handgrip (P < 0.001) and increased further at 2 min of handgrip (P < 0.001), returning at recovery to a pressure that was slightly lower than baseline (P < 0.01) in all subgroups.
HRV, BPV, and Coherence Analysis
HRV, BPV, and coherence analysis are shown in Table 2.
Table 2.
HRV and BPV
| Baseline | Handgrip
|
Recovery | |||
|---|---|---|---|---|---|
| 1 min | 2 min | 1 and 2 min | |||
| Total HRV power (ms2/Hz) | |||||
| Control | 3,213±836 | 1,683±714 | 2,207±1156* | 2,156±895* | 3,245±906 |
| Low flow | 837±162† | 1,080±366 | 1,004±276† | 980±299† | 1,682±616*† |
| Normal flow | 1,689±380 | 2,273±565 | 1,973±463 | 2,094±275 | 2,120±503 |
| LF HRV power, ms2/Hz | |||||
| Control | 1,071±310 | 562±279* | 1,061±710 | 769±326 | 1,197±367 |
| Low flow | 198±44† | 415±169* | 294±61† | 301±108† | 593±194*† |
| Normal flow | 555±124† | 468±107 | 573±133† | 383±65† | 768±193 |
| HF HRV power, ms2/Hz | |||||
| Control | 1,476±498 | 827±417 | 847±389 | 843±377 | 1,546±423 |
| Low flow | 216±64† | 485±197* | 556±181* | 407±127* | 898±390* |
| Normal flow | 589±204 | 1,567±435*† | 1,205±338* | 845±207 | 1,093±276* |
| LF-to-HF Ratio | |||||
| Control | 0.90±.19 | 0.80±0.17 | 1.76±0.63* | 1.02±0.31 | 1.12±0.20 |
| Low flow | 1.18±0.21 | 0.87±0.16 | 1.1±0.29 | 0.81±0.11 | 1.06±0.31 |
| Normal flow | 1.33±.18 | 0.38±0.04*† | 0.83±0.26† | 0.62±0.11 | 1.09±0.17 |
| Total BPV power, mmHg2/Hz | |||||
| Control | 8.4±2.0 | 6.8±0.8* | 9.8±1.5* | 9.5±1.6 | 10.0±2.3 |
| Low flow | 10.2±2.0 | 7.1±2.0* | 6.9±1.3*† | 8.0±2.0 | 6.7±2.1† |
| Normal flow | 12.5±2.4 | 12.8±8 | 13.4±4.3† | 10.6±2.6 | 7.5±1.8 |
| Transfer coherence | |||||
| Control | 0.76±0.10 | 0.41±0.06* | 0.26±0.05* | 0.34±0.05* | 0.42±0.06* |
| Low flow | 0.36±0.05† | 0.3±0.07* | 0.30±0.07* | 0.23±0.06* | 0.17±0.04*† |
| Normal flow | 0.7±0.04 | 0.3±0.06* | 0.22±0.06* | 0.24±0.04* | 0.20±0.05*† |
| Transfer magnitude, gain · ms−1 · mmHg−1 | |||||
| Control | 18±2 | 11±2* | 8±2* | 10±2* | 11±2* |
| Low flow | 8±2† | 12±0.3 | 13±3*† | 19±10 | 18.5±9* |
| Normal flow | 13±2 | 21±0.6*† | 17±4*† | 8±1* | 12±3 |
| Transfer phase, ° | 80±17* | ||||
| Control | 24±12 | 80±46* | 90±40* | 88±42* | 92±24* |
| Low flow | 53±8† | 129±0.34* | 140±33* | 132±24*† | 210±198 |
| Normal flow | 50±5† | 169±0.32* | 171±37* | 170±25* | |
Values are means ± SD. HRV, HR variability; BPV, BP variability; LF, low frequency (0.04–0.15 Hz); HF, high frequency (0.2–0.5 Hz).
P < 0.05 compared with baseline;
P < 0.05, significantly different from control.
HRV-BPV incoherence in POTS
The squared coherence was significantly <0.5 for low-flow POTS patients. Squared coherence was decreased below 0.5 in all subjects during handgrip and therefore transfer function gain (transfer magnitude), although reported, could not be relied upon as a measure of cardiovagal baroreflex function during handgrip. Changes in transfer magnitude (i.e., baroreflex gain) may not be strictly interpretable under these circumstances. These data suggest an uncoupling of baroreflex mediation of HR and BP in low-flow POTS at all times and during handgrip in other subjects.
Decreased HRV during handgrip and varied BPV
HRV was decreased in low-flow POTS compared with control. HRV decreased during handgrip in control subjects but remained unchanged in POTS. Low-frequency HRV power was decreased (P < 0.01) in control and normal-flow patients but was unchanged in low-flow patients during handgrip. High-frequency HRV power was decreased during both 1 and 2 min of handgrip in all subjects. BPV was decreased in the first minute of handgrip (P < 0.01) in controls and POTS and was increased during the second minute (P < 0.05) in controls and normal-flow POTS compared with baseline. BPV remained reduced throughout handgrip in low-flow POTS.
Impedance Plethysmographic Changes in Segmental Blood Volumes and Blood Flows Blood volume changes during handgrip
Representative changes in segmental impedance and in the percent change in blood volume during handgrip are shown in Fig. 3. There was an increase in thoracic volume noted throughout all phases of handgrip in control subjects. Splanchnic volume changed in reciprocal fashion with smaller changes noted in pelvic and calf volumes, which both decreased significantly (P < 0.05) during the second minute of handgrip. There was no increase in central blood volume in either POTS group at the first minute of handgrip. Central blood volume increased during the second minute of handgrip in normal-flow POTS patients but remained markedly reduced compared with control in low-flow patients. Splanchnic blood volume was increased compared with control in normal-flow POTS during handgrip and also remained relatively increased in low-flow POTS at the second minute of handgrip. There was a significant (P < 0.025) relative reduction in pelvic and calf regional blood volumes in POTS patients compared with control subjects.
Fig. 3.
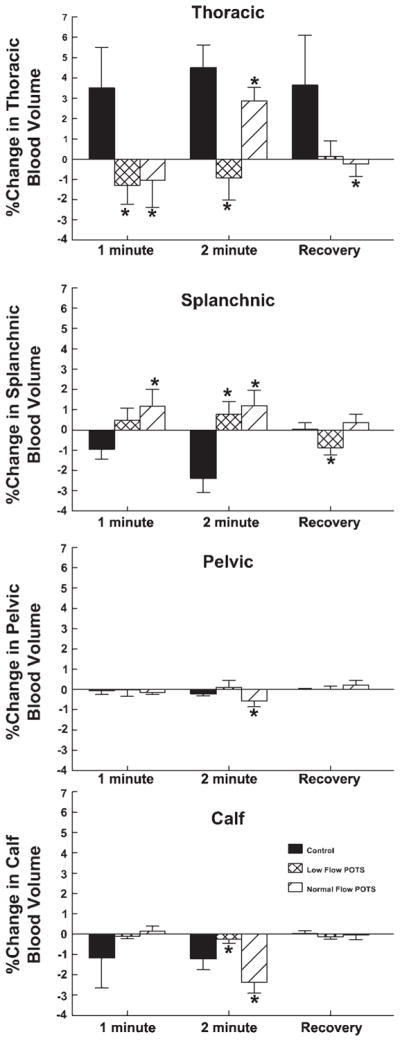
Percent changes from baseline in thoracic, splanchnic, pelvic, and calf blood volumes during handgrip averaged over all subjects at 1 and 2 min after the start of handgrip and during recovery. Central thoracic blood volume increased for control but in neither POTS group at 1 min of handgrip and remained different from control at 2 min of handgrip and during recovery. The increased cardiac volume corresponded to a decrease in splanchnic volume, which was absent in POTS. *P < 0.05 compared with control.
Segmental blood flow changes during handgrip
Changes in segmental blood flow are shown in Fig. 4. Cardiac output was increased in control subjects (P < 0.01) throughout handgrip but was not increased in low-flow POTS, where it remained unchanged throughout the handgrip maneuver. Cardiac output did increase during the second minute in normal-flow POTS but remained reduced compared with control. Splanchnic blood flow tended to be unchanged during handgrip in control subjects but was relatively increased in POTS during the second minute of handgrip and in normal-flow POTS during recovery. Pelvic and calf blood flows were increased at 2 min of handgrip in control and relatively unchanged thoroughout handgrip in low-flow patients. Pelvic and calf blood flows were increased similar to control in normal-flow POTS patients during the first minute of handgrip but then were decreased compared with control during the second minute of handgrip.
Fig. 4.
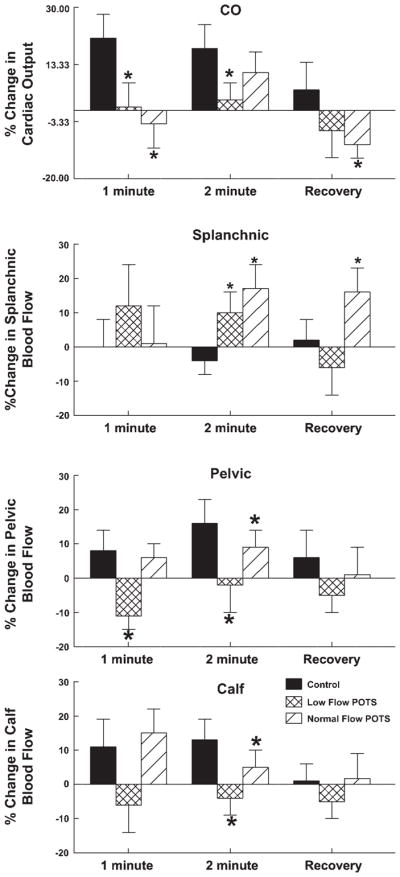
Percent changes in segmental blood flow. Changes in thoracic [cardiac output (CO)], splanchnic, pelvic, and leg (calf) blood flow are shown in order. Blood flow increased for the central thoracic blood flow (CO) in healthy controls but not in POTS at 1 min of handgrip. CO did increase in normal-flow POTS patients at the second minute of handgrip. Percent changes in splanchnic blood flows were increased in POTS, whereas pelvic and calf segments were variably affected in POTS subgroups. *P < 0.05 compared with control.
Segmental arterial resistance increases during handgrip: marked increase in total peripheral resistance in POTS
We calculated segmental vascular resistance using the following formula:
Percent changes in vascular resistance are shown in Fig. 5. Total peripheral resistance was slightly increased (P < 0.025) during the second minute of handgrip in control subjects but markedly increased in POTS patients throughout handgrip (P < 0.001). The increased splanchnic resistance seen during handgrip in control subjects was not observed in normal-flow POTS, whereas calf and pelvic resistances were increased in both POTS subgroups during handgrip.
Fig. 5.
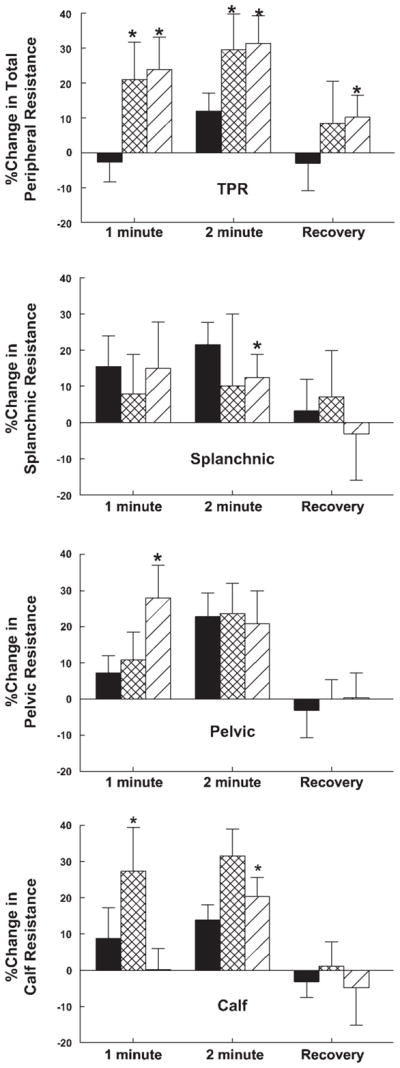
Percent changes in segmental arterial resistance. Changes in thoracic [total peripheral resistance (TPR)], splanchnic, pelvic, and calf arterial resistance are shown in order. TPR (thoracic resistance) was mildly increased in control subjects but markedly increased in POTS patients. This was generally associated with an increase in pelvic and calf resistance in POTS compared with control. *P < 0.05 compared with control.
DISCUSSION
Main Findings
Blunted HR and BP responses to static handgrip in low-flow POTS
Our results show a significantly attenuated change in BP and HR in low-flow POTS patients only. These patients have evidence for sympathetic overactivation at rest with blunted changes observed in response to sympathetic stimuli particularly affecting the distribution and redistribution of blood volume (see below). Typically, while low-flow patients are normotensive, they share many phenotypic features of circulatory insufficiency including pallor, baseline tachycardia, peripheral vasoconstriction, and reduced central blood volume with blunted responses to subsequent sympathetic stimuli (4, 10).
The increase in central blood volume is blunted in POTS
We found that the usual increase in central blood volume during the exercise pressor reflex is abolished in low-flow POTS patients and attenuated in normal-flow POTS patients. In the case of low-flow POTS, arguments related to reduced blood volume may need to be reconsidered in light of newer observations concerning the lack of difference among POTS and control blood volumes once normalized to subject weight. In general, there appears to be an overall reduction in regional blood volume redistribution in low-flow POTS.
Normal-flow POTS patients have blunting of blood volume redistribution that relates to selective pooling in the splanchnic circulation, as previously described (61). This limits the increases in central blood volume during handgrip. Similar limitation of central blood volume occurs during orthostatic stress (61).
Total peripheral resistance rather than cardiac output drives regional blood volume
The most important new finding in this study is that the exercise pressor reflex produces a significant, albeit smaller, pressor response in low-flow POTS patients and that the mechanism of the pressor response is shifted from the increased cardiac output and central blood volume observed in control subjects to increased vasoconstriction and peripheral resistance. Specifically, in low-flow POTS, the cardiac output component is essentially abolished and the pressor response is completely driven by increased peripheral resistance due to global vasoconstriction. In normal-flow POTS, vasoconstriction is more selective and is deficient within the splanchnic regional circulation, which is the largest venous reservoir in the body while supine. An increase in total peripheral resistance has been demonstrated under other conditions in low-flow POTS (61) and during orthostatic stress in normal-flow POTS patients (59) because of marked peripheral vasoconstriction in the pelvic and calf regional circulations. We have presented evidence for sympathoexcitation in POTS (59), and others have presented measurements of increased sympathetic nerve activity with blunting of responses to diverse stimuli (4, 10). We propose that the data reported here support the theories that low-flow POTS patients have inappropriate sympathetic and adrenergic activation possibly driven by central nervous system mechanisms controlling sympathetic outflow, whereas normal-flow POTS patients have reflex peripheral sympathetic activation produced by selective splanchnic blood flow deficits.
Baroreflex regulation of HR during handgrip (cardiovagal regulation)
A general discussion of the changes in baroreflex function in POTS is beyond the scope of the present investigation because only cardiovagal baroreflex is assessed by variability measures. The data do not directly convey information about baroreflex regulation of peripheral resistance or of cardiac contractility (31). However, prior work has suggested at least blunting of both sympathetic and cardiovagal baroreflex sensitivities in subsets of POTS (9, 36, 56).
As noted previously, the HRV technique alone or combined with measurement of BPV primarily estimates the parasympathetic control of HR even in low-frequency bands, although experiments using atropine have revealed a smaller direct contribution of sympathetic cardiac activity to low-frequency HRV power (67). The difficulty in interpreting sympathetic change is somewhat improved by using low-frequency to high-frequency ratios. In that regard, it is interesting that overall HRV power and low-frequency power, although decreased compared with control in low-flow POTS patients, are sustained or even increased during exercise. This is different from results from control patients, in whom HRV and, by extension, baroreflex gain are reduced by the metaboreflex (18), with similar findings in carefully executed animal models (46). Sustained sympathetic effects on the heart are consistent with sustained sympathetic cardiac contractility. In support, low-flow POTS patients have markedly increased cardiac afterload, no increase in cardiac preload, and sustained cardiac output, suggesting increased contractility. Therefore, it may be reasonable to infer that cardiac sympathetic innervation remains relatively intact in POTS even though baroreflex gain may be reduced.
On the other hand, cardiovagal coherence is inadequate in low-flow POTS at all times. This indicates an uncoupling between BP and HR regulation.
The exercise pressor reflex in POTS (central sympathetic activation)
The shift from a cardiac output driven exercise pressor response to an arterial resistance-driven pressor response is similar to observations made in congestive heart failure (6, 40, 53). In heart failure, baroreflexes are markedly impaired with reductions in both sympathetic and cardiovagal baroreflex sensitivities (30). As a result, the ability of the arterial baroreflex to buffer the muscle metaboreflex is severely attenuated (15, 26, 40). In low-flow POTS, the baroreflexes are also impaired with reductions in both sympathetic and cardiovagal baroreflex sensitivities. The arterial baroreflex buffers the vasoconstriction from the muscle metaboreflex and mechanoreflex comprising the exercise pressor reflex by reducing this peripheral vasoconstriction (27). Arguing by analogy, recent data concerning heart failure have indicated an important role for increased angiotensin II and decreased neuronal nitric oxide activity in attenuating the baroreflex (70). Increased angiotensin II and reduced nitric oxide are features of low-flow POTS (33, 57).
Limitations
Inferences concerning sympathetic activation would be more secure if there were direct assessment of sympathetic activity using microneurography. This is next on our agenda. However, changes in vasoconstriction might serve as an operational surrogate for increased sympathetic activity. While there remain issues of transduction from nerve activity to vasoconstriction, others (34) have used a similar approach. Support is offered by microneurographic measurements performed in POTS patients by other investigators (4, 9, 10). Moreover, human experimentation is limited to specific peripheral nerve recordings, which may not necessarily best reflect overall sympathetic outflow. Assessments of central mechanisms remain off limits in humans.
Impedance plethysmography is an indirect measurement of blood flow, and its accuracy and validity may be questioned. We (60, 63) have validated these methods in multiple previous experiments of similar nature using reference standards for comparison.
HRV and BPV indexes are not reference standards for autonomic or baroreflex measurements. However, measured indexes have been consistent with invasive forms of measurement such as microneurography (43). Also, reliable estimates of low-frequency power cannot be readily obtained from 1 min of HR data recording. According to the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (65, 66), data records of 5 min or longer are standard. However, 60 s of recording can theoretically estimate frequencies down to 1/60 = 0.016 Hz, although with considerable variance. Use of the average over a number of similar subjects as an ensemble average allows us to make reasonable estimates of high- and low-frequency power once averaged over an entire group.
Our aim was to assess the hemodynamic responses induced by exercise pressure reflex during exercise. Other confounding factors, such as central command, humoral changes, physical factors, and other biochemical factors, may be present during exercise. This leads many investigators to perform measures immediately after peak exercise during peripheral circulatory occlusion ischemia, which was not done here. However, central command, while responsible for much of the increase in HR and respiration during exercise, does not appear to increase sympathetic outflow unless the intensity of the exercise is near maximal (23), and humoral contributions appear to require a more sustained form of exercise to achieve importance (37). Nevertheless, central command could contribute to measured changes in HR.
Acknowledgments
We thank members of the Department of Pediatrics, especially its Chairman, Dr. Leonard Newman, and the Division of Pediatric Cardiology, especially its Director, Dr. Michael H. Gewitz, for the unflagging support. We also acknowledge an intellectual debt to our mentors, Dr. Thomas H. Hintze, Dr. Gabor Kaley, Dr. David Robertson, and Dr. Phillip Low for the constant inspiration and stimulation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants 1-RO1-HL-66007 and 1-R01-HL-074873.
References
- 1.Ansorge EJ, Augustyniak RA, Perinot ML, Hammond RL, Kim JK, Sala-Mercado JA, Rodriguez J, Rossi NF, O’Leary DS. Altered muscle metaboreflex control of coronary blood flow and ventricular function in heart failure. Am J Physiol Heart Circ Physiol. 2005;288:H1381–H1388. doi: 10.1152/ajpheart.00985.2004. [DOI] [PubMed] [Google Scholar]
- 2.Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O’Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol. 2001;280:H1645–H1652. doi: 10.1152/ajpheart.2001.280.4.H1645. [DOI] [PubMed] [Google Scholar]
- 3.Bloomfield DA. Dye Curves: the Theory and Practice of Indicator Dye Dilution. Baltimore, MD: University Park; 1974. [Google Scholar]
- 4.Bonyhay I, Freeman R. Sympathetic neural activity, sex dimorphism, and postural tachycardia syndrome. Ann Neurol. 2007;61:332–339. doi: 10.1002/ana.21090. [DOI] [PubMed] [Google Scholar]
- 5.Cornett JA, Herr MD, Gray KS, Smith MB, Yang QX, Sinoway LI. Ischemic exercise and the muscle metaboreflex. J Appl Physiol. 2000;89:1432–1436. doi: 10.1152/jappl.2000.89.4.1432. [DOI] [PubMed] [Google Scholar]
- 6.Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol. 2007;292:H2988–H2996. doi: 10.1152/ajpheart.00008.2007. [DOI] [PubMed] [Google Scholar]
- 7.Crisafulli A, Scott AC, Wensel R, Davos CH, Francis DP, Pagliaro P, Coats AJ, Concu A, Piepoli MF. Muscle metaboreflex-induced increases in stroke volume. Med Sci Sports Exerc. 2003;35:221–228. doi: 10.1249/01.MSS.0000048639.02548.24. [DOI] [PubMed] [Google Scholar]
- 8.Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG. Stimulation by central command of locomotion, respiration and circulation during exercise. Respir Physiol. 1985;59:313–337. doi: 10.1016/0034-5687(85)90136-7. [DOI] [PubMed] [Google Scholar]
- 9.Farquhar WB, Taylor JA, Darling SE, Chase KP, Freeman R. Abnormal baroreflex responses in patients with idiopathic orthostatic intolerance. Circulation. 2000;102:3086–3091. doi: 10.1161/01.cir.102.25.3086. [DOI] [PubMed] [Google Scholar]
- 10.Furlan R, Jacob G, Snell M, Robertson D, Porta A, Harris P, Mosqueda-Garcia R. Chronic orthostatic intolerance: a disorder with discordant cardiac and vascular sympathetic control. Circulation. 1998;98:2154–2159. doi: 10.1161/01.cir.98.20.2154. [DOI] [PubMed] [Google Scholar]
- 11.Geddes LA, Baker LE. Principles of Applied Biomedical Instrumentation. New York: Wiley; 1989. Detection of physiological events by impedance; pp. 594–600. [Google Scholar]
- 12.Geddes LA, Sadler C. The specific resistance of blood at body temperature. Med Biol Eng. 1973;11:336–339. doi: 10.1007/BF02475543. [DOI] [PubMed] [Google Scholar]
- 13.Gladwell VF, Fletcher J, Patel N, Elvidge LJ, Lloyd D, Chowdhary S, Coote JH. The influence of small fibre muscle mechanoreceptors on the cardiac vagus in humans. J Physiol. 2005;567:713–721. doi: 10.1113/jphysiol.2005.089243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenfield AD, Whitney RJ, Whitney RJ. Methods for the investigation of peripheral blood flow. Br Med Bull. 1963;19:101–109. doi: 10.1093/oxfordjournals.bmb.a070026. [DOI] [PubMed] [Google Scholar]
- 15.Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapa-nowski K, O’Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol. 2000;278:H818–H828. doi: 10.1152/ajpheart.2000.278.3.H818. [DOI] [PubMed] [Google Scholar]
- 16.Harms MP, van Lieshout JJ. Cerebrovascular and cardiovascular responses associated with orthostatic intolerance and tachycardia. Clin Auton Res. 2001;11:35–38. doi: 10.1007/BF02317800. [DOI] [PubMed] [Google Scholar]
- 17.He YL, Tanigami H, Ueyama H, Mashimo T, Yoshiya I. Measurement of blood volume using indocyanine green measured with pulse-spectrophotometry: its reproducibility and reliability. Crit Care Med. 1998;26:1446–1451. doi: 10.1097/00003246-199808000-00036. [DOI] [PubMed] [Google Scholar]
- 18.Iellamo F, Pizzinelli P, Massaro M, Raimondi G, Peruzzi G, Legramante JM. Muscle metaboreflex contribution to sinus node regulation during static exercise: insights from spectral analysis of heart rate variability. Circulation. 1999;100:27–32. doi: 10.1161/01.cir.100.1.27. [DOI] [PubMed] [Google Scholar]
- 19.Iijima T, Aoyagi T, Iwao Y, Masuda J, Fuse M, Kobayashi N, Sankawa H. Cardiac output and circulating blood volume analysis by pulse dye-densitometry. J Clin Monit. 1997;13:81–89. doi: 10.1023/a:1007339924083. [DOI] [PubMed] [Google Scholar]
- 20.Jacob G, Costa F, Shannon JR, Robertson RM, Wathen M, Stein M, Biaggioni I, Ertl A, Black B, Robertson D. The neuropathic postural tachycardia syndrome. N Engl J Med. 2000;343:1008–1014. doi: 10.1056/NEJM200010053431404. [DOI] [PubMed] [Google Scholar]
- 21.Jordan J, Shannon JR, Diedrich A, Black BK, Robertson D. Increased sympathetic activation in idiopathic orthostatic intolerance: role of systemic adrenoreceptor sensitivity. Hypertension. 2002;39:173–178. doi: 10.1161/hy1201.097202. [DOI] [PubMed] [Google Scholar]
- 22.Karas B, Grubb BP, Boehm K, Kip K. The postural orthostatic tachycardia syndrome: a potentially treatable cause of chronic fatigue, exercise intolerance, and cognitive impairment in adolescents. Pacing Clin Electrophysiol. 2000;23:344–351. doi: 10.1111/j.1540-8159.2000.tb06760.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman MP. Has the phoenix risen? J Physiol. 2003;548:666. doi: 10.1113/jphysiol.2003.040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology. New York: Oxford Univ. Press; 1996. pp. 381–447. [Google Scholar]
- 25.Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res. 1987;61:I60–I65. [PubMed] [Google Scholar]
- 26.Kim JK, Sala-Mercado JA, Hammond RL, Rodriguez J, Scislo TJ, O’Leary DS. Attenuated arterial baroreflex buffering of muscle metaboreflex in heart failure. Am J Physiol Heart Circ Physiol. 2005;289:H2416–H2423. doi: 10.1152/ajpheart.00654.2005. [DOI] [PubMed] [Google Scholar]
- 27.Kim JK, Sala-Mercado JA, Rodriguez J, Scislo TJ, O’Leary DS. Arterial baroreflex alters strength and mechanisms of muscle metaboreflex during dynamic exercise. Am J Physiol Heart Circ Physiol. 2005;288:H1374–H1380. doi: 10.1152/ajpheart.01040.2004. [DOI] [PubMed] [Google Scholar]
- 28.Kluess HA, Wood RH, Welsch MA. Vagal modulation of the heart and central hemodynamics during handgrip exercise. Am J Physiol Heart Circ Physiol. 2000;278:H1648–H1652. doi: 10.1152/ajpheart.2000.278.5.H1648. [DOI] [PubMed] [Google Scholar]
- 29.Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–S25. [PubMed] [Google Scholar]
- 30.Mancia G, Mark AL. Arterial baroreflexes in humans. In: Shepherd JT, Abboud FM, Geiger SR, editors. Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow. pt. 2. III. Bethesda, MD: Am. Physiol. Soc; 1983. pp. 755–793. sect. 2. chapt. 20. [Google Scholar]
- 31.Mancia G, Parati G, Castiglioni P, di Rienzo M. Effect of sinoaortic denervation on frequency-domain estimates of baroreflex sensitivity in conscious cats. Am J Physiol Heart Circ Physiol. 1999;276:H1987–H1993. doi: 10.1152/ajpheart.1999.276.6.H1987. [DOI] [PubMed] [Google Scholar]
- 32.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medow MS, Minson CT, Stewart JM. Decreased microvascular nitric oxide-dependent vasodilation in postural tachycardia syndrome. Circulation. 2005;112:2611–2618. doi: 10.1161/CIRCULATIONAHA.104.526764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Momen A, Bower D, Leuenberger UA, Boehmer J, Lerner S, Alfrey EJ, Handly B, Sinoway LI. Renal vascular response to static handgrip exercise: sympathetic vs. autoregulatory control. Am J Physiol Heart Circ Physiol. 2005;289:H1770–H1776. doi: 10.1152/ajpheart.01213.2004. [DOI] [PubMed] [Google Scholar]
- 35.Montgomery LD, Kirk PJ, Payne PA, Gerber RL, Newton SD, Williams BA. Cardiovascular responses of men and women to lower body negative pressure. Aviat Space Environ Med. 1977;48:138–145. [PubMed] [Google Scholar]
- 36.Muenter SN, Charkoudian N, Dotson RM, Suarez GA, Low PA. Baroreflex control of muscle sympathetic nerve activity in postural orthostatic tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2005;289:H1226–H1233. doi: 10.1152/ajpheart.01243.2004. [DOI] [PubMed] [Google Scholar]
- 37.Nishiyasu T, Tan N, Morimoto K, Sone R, Murakami N. Cardiovascular and humoral responses to sustained muscle metaboreflex activation in humans. J Appl Physiol. 1998;84:116–122. doi: 10.1152/jappl.1998.84.1.116. [DOI] [PubMed] [Google Scholar]
- 38.O’Leary DS. Regional vascular resistance vs. conductance: which index for baroreflex responses? Am J Physiol Heart Circ Physiol. 1991;260:H632–H637. doi: 10.1152/ajpheart.1991.260.2.H632. [DOI] [PubMed] [Google Scholar]
- 39.O’Leary DS. Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol. 1993;74:1748–1754. doi: 10.1152/jappl.1993.74.4.1748. [DOI] [PubMed] [Google Scholar]
- 40.O’Leary DS. Altered reflex cardiovascular control during exercise in heart failure: animal studies. Exp Physiol. 2006;91:73–77. doi: 10.1113/expphysiol.2005.031179. [DOI] [PubMed] [Google Scholar]
- 41.O’Leary DS, Sala-Mercado JA, Augustyniak RA, Hammond RL, Rossi NF, Ansorge EJ. Impaired muscle metaboreflex-induced increases in ventricular function in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H2612–H2618. doi: 10.1152/ajpheart.00604.2004. [DOI] [PubMed] [Google Scholar]
- 42.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympathovagal interaction in man and conscious dog. Circ Res. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 43.Pagani M, Montano N, Porta A, Malliani A, Abboud FM, Birkett C, Somers VK. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation. 1997;95:1441–1448. doi: 10.1161/01.cir.95.6.1441. [DOI] [PubMed] [Google Scholar]
- 44.Pang CC. Autonomic control of the venous system in health and disease: effects of drugs. Pharmacol Ther. 2001;90:179–230. doi: 10.1016/s0163-7258(01)00138-3. [DOI] [PubMed] [Google Scholar]
- 45.Sala-Mercado JA, Hammond RL, Kim JK, McDonald PJ, Stephenson LW, O’Leary DS. Heart failure attenuates muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol. 2007;292:H2159–H2166. doi: 10.1152/ajpheart.01240.2006. [DOI] [PubMed] [Google Scholar]
- 46.Sala-Mercado JA, Ichinose M, Hammond RL, Ichinose TK, Pallante M, Stephenson LW, O’Leary DS, Iellamo F. Muscle metaboreflex attenuates spontaneous heart rate baroreflex sensitivity during dynamic exercise. Am J Physiol Heart Circ Physiol. 2007;292:H2867–H2873. doi: 10.1152/ajpheart.00043.2007. [DOI] [PubMed] [Google Scholar]
- 47.Sandroni P, Opfer-Gehrking TL, McPhee BR, Low PA. Postural tachycardia syndrome: clinical features and follow-up study. Mayo Clin Proc. 1999;74:1106–1110. doi: 10.4065/74.11.1106. [DOI] [PubMed] [Google Scholar]
- 48.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 49.Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, Blakely RD, Robertson D. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med. 2000;342:541–549. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- 50.Sheriff DD, Augustyniak RA, O’Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol Heart Circ Physiol. 1998;275:H767–H775. doi: 10.1152/ajpheart.1998.275.3.H767. [DOI] [PubMed] [Google Scholar]
- 51.Shoemaker JK, Kunselman AR, Silber DH, Sinoway LI. Maintained exercise pressor response in heart failure. J Appl Physiol. 1998;85:1793–1799. doi: 10.1152/jappl.1998.85.5.1793. [DOI] [PubMed] [Google Scholar]
- 52.Silber DH, Sutliff G, Yang QX, Smith MB, Sinoway LI, Leuenberger UA. Altered mechanisms of sympathetic activation during rhythmic forearm exercise in heart failure. J Appl Physiol. 1998;84:1551–1559. doi: 10.1152/jappl.1998.84.5.1551. [DOI] [PubMed] [Google Scholar]
- 53.Sinoway LI, Li J. A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol. 2005;99:5–22. doi: 10.1152/japplphysiol.01405.2004. [DOI] [PubMed] [Google Scholar]
- 54.Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol. 2006;91:89–102. doi: 10.1113/expphysiol.2005.032367. [DOI] [PubMed] [Google Scholar]
- 55.Stewart GN. Researches on the circulation time and on the influences which affect it. IV. The output of the heart. J Physiol. 1897;22:159. doi: 10.1113/jphysiol.1897.sp000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stewart JM. Autonomic nervous system dysfunction in adolescents with postural orthostatic tachycardia syndrome and chronic fatigue syndrome is characterized by attenuated vagal baroreflex and potentiated sympathetic vasomotion. Pediatr Res. 2000;48:218–226. doi: 10.1203/00006450-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 57.Stewart JM, Glover JL, Medow MS. Increased plasma angiotensin II in postural tachycardia syndrome (POTS) is related to reduced blood flow and blood volume. Clin Sci (Lond) 2006;110:255–263. doi: 10.1042/CS20050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stewart JM, McLeod KJ, Sanyal S, Herzberg G, Montgomery LD. Relation of postural vasovagal syncope to splanchnic hypervolemia in adolescents. Circulation. 2004;110:2575–2581. doi: 10.1161/01.CIR.0000145543.88293.21. [DOI] [PubMed] [Google Scholar]
- 59.Stewart JM, Medow MS, Cherniack NS, Natelson BH. Postural hypocapnic hyperventilation is associated with enhanced peripheral vasoconstriction in postural tachycardia syndrome with normal supine blood flow. Am J Physiol Heart Circ Physiol. 2006;291:H904–H913. doi: 10.1152/ajpheart.01359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stewart JM, Medow MS, Glover JL, Montgomery LD. Persistent splanchnic hyperemia during upright tilt in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2006;290:H665–H673. doi: 10.1152/ajpheart.00784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stewart JM, Montgomery LD. Regional blood volume and peripheral blood flow in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2004;287:H1319–H1327. doi: 10.1152/ajpheart.00086.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stewart JM, Montgomery LD. Reciprocal splanchnic-thoracic blood volume changes during the Valsalva maneuver. Am J Physiol Heart Circ Physiol. 2005;288:H752–H758. doi: 10.1152/ajpheart.00717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stewart JM, Montgomery LD, Glover JL, Medow MS. Changes in regional blood volume and blood flow during static handgrip. Am J Physiol Heart Circ Physiol. 2007;292:H215–H223. doi: 10.1152/ajpheart.00681.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stewart JM, Weldon A. Reflex vascular defects in the orthostatic tachycardia syndrome of adolescents. J Appl Physiol. 2001;90:2025–2032. doi: 10.1152/jappl.2001.90.6.2025. [DOI] [PubMed] [Google Scholar]
- 65.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 66.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 67.Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- 68.Williams CA. Effect of muscle mass on the pressor response in man during isometric contractions. J Physiol. 1991;435:573–584. doi: 10.1113/jphysiol.1991.sp018526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wray DW, Donato AJ, Nishiyama SK, Richardson RS. Acute sympathetic vasoconstriction at rest and during dynamic exercise in cyclists and sedentary humans. J Appl Physiol. 2007;102:704–712. doi: 10.1152/japplphysiol.00984.2006. [DOI] [PubMed] [Google Scholar]
- 70.Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension. 2006;48:1005–1011. doi: 10.1161/01.HYP.0000246614.47231.25. [DOI] [PubMed] [Google Scholar]