Abstract
Background
One variant of postural tachycardia syndrome (POTS), designated low-flow POTS, is associated with decreased peripheral blood flow related to impaired local vascular regulation.
Methods and Results
To investigate the hypothesis that microvascular endothelial dysfunction produces decreased peripheral blood flow in low-flow POTS, we performed experiments using laser-Doppler flowmetry (LDF) combined with iontophoresis in 15 low-flow POTS patients, 17 normal-flow POTS patients, and 13 healthy reference volunteers varying in age from 14 to 22 years. We tested whether α-adrenergic vasoregulation was impaired using iontophoretic delivery of tyramine, phentolamine, and bretylium followed by a norepinephrine dose response. We tested endothelial-dependent and -independent receptor-mediated vasodilation by measuring acetylcholine and sodium nitroprusside dose responses. We tested whether nitric oxide–dependent vasodilation was different in these groups by testing the local thermal hyperemic response to saline used as a reference compared with the nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME). Adrenergic and receptor-dependent cutaneous vasoregulation was similar for low-flow POTS, normal-flow POTS, and reference subjects. Thermal hyperemia produced distinctly different findings: there was marked attenuation of the nitric oxide–sensitive plateau during prolonged heating, which was insensitive to L-NAME in low-flow POTS subjects. The pattern of thermal hyperemia response in low-flow POTS subjects during saline administration resembled the pattern in reference subjects during L-NAME administration and was minimally affected by L-NAME.
Conclusions
The data suggest that flow-dependent nitric oxide release is reduced in low-flow POTS. This may account for local flow regulation abnormalities.
Keywords: blood flow, lasers, nitric oxide synthase, syncope, tachycardia
Chronic orthostatic intolerance is often identified with postural tachycardia syndrome (POTS),1 in which symptoms of orthostatic intolerance are combined with findings of excessive upright tachycardia. Although some POTS patients have normal or even increased resting peripheral blood flow,2 we have previously described a subset of “low-flow” POTS patients characterized by mild hypovolemia and decreased regional blood flows3 related to defects in local blood flow regulation notable in the dependent parts of the body and in the cutaneous circulation.4 There is a characteristic phenotype distinguished by generalized pallor, cool skin, and often marked resting tachycardia (a shocklike appearance). Although patients may correspond to patients with reduced blood volume contributing to orthostatic intolerance5,6 described by other investigators, decreased blood volume cannot completely explain observations showing an increase in leg blood flow during orthostatic challenge when both local and reflex-mediated vasoconstriction should further diminish blood flow.7 In prior work we found data consistent with defective local regulation of blood flow related to the myogenic response, the venoarteriolar reflex, and the ischemic (reactive hyperemic) response in low-flow POTS patients only. Abnormalities were found in the skin as well as the lower extremities.4
Recent evidence suggests that the myogenic response and venoarteriolar reflex are linked and may result from microvascular endothelial cell dysfunction.8–11 In addition, the venoarteriolar reflex seems not to be related to adrenergic mechanisms, as previously believed,12 but may be particularly important to the cutaneous response to orthostatic stress.13 For this reason we chose to use the cutaneous circulation to investigate the hypothesis that microvascular endothelial dysfunction decreases peripheral blood flow in patients with low-flow POTS, further ruling out altered sympathetic regulation in these patients.
Methods
Three experimental studies were performed. In the first we examined the effects of adrenergic agonists and antagonists on cutaneous blood flow in low-flow POTS and in normal-flow POTS compared with reference control subjects to demonstrate that vasoconstriction is unrelated to impaired α-adrenergic mechanisms. Next, we determined whether receptor-mediated nitric oxide (NO) release was impaired in low-flow POTS by comparing the response to acetylcholine, an endothelial-dependent receptor-mediated vasodilator, with the response to sodium nitroprusside, an endothelial-independent vasodilator. Finally, we examined vasodilation produced by localized heating or “thermal hyperemia” in the presence and absence of NO synthase (NOS) blockade.
Subjects: Patient and Reference Subject Screening
We screened consecutive patients aged 14 to 22 years referred for symptoms of chronic orthostatic intolerance lasting >3 months to identify those with low-flow POTS and normal-flow POTS. Orthostatic intolerance was defined by the presence of dizziness, fatigue, exercise intolerance, headache, memory problems, nausea, pallor, and abnormal sweating while upright that improved with recumbence and that had no other medical explanation.
Diagnosis of POTS by Upright Tilt Table Testing
The diagnosis of POTS was made in patients during a screening visit. Recordings of supine blood pressure and heart rate were obtained near the end of a 30-minute resting period. Patients were then tilted upright to 70° with the use of an electrically driven tilt table (Cardiosystems 600) with a footboard. POTS was diagnosed by symptoms of orthostatic intolerance associated with an increase in sinus heart rate >30 bpm or to a rate >120 bpm during the first 10 minutes of tilt.14,15 During the same visit, POTS patients were partitioned on the basis of supine lower limb (calf) blood flow into patients with decreased blood flow (<1.0 mL/100 mL tissue per minute) and those with normal blood flow (1.0 to 3.9 mL/100 mL tissue per minute). Prior and current studies indicate that 1.0 mL/100 mL tissue per minute was the minimum and 3.9 mL/100 mL tissue per minute was the maximum blood flow that we found in healthy volunteers during experiments. We measured calf blood flow by venous occlusion plethysmography16 while the patient was supine. In brief, occlusion cuffs were placed around the midthigh 15 cm above a strain gauge attached to a Whitney-type plethysmograph (Hokanson, Inc). We rapidly inflated the cuff to 45 mm Hg to prevent venous egress. Arterial inflow in units of milliliter per 100 mL tissue per minute was estimated as the rate of change of the rapid increase in calf cross-sectional area. For the present study, patients were retained only if they belonged to the normal-flow or low-flow groups.
Using these methods, we recruited 15 low-flow patients (14 girls, 1 boy, aged 14 to 21 years; median age, 17.6 years) and 17 normal-flow patients (11 girls, 6 boys, aged 15 to 22 years; median age, 17.8 years). All patients were white.
Thirteen normal white reference subjects aged 14 to 20 years (10 girls, 3 boys, aged 15 to 20 years; median age, 17.0 years) were also studied after a screening upright tilt demonstrated normal orthostatic response. Reference subjects served as a control group and were recruited from among adolescents referred for innocent heart murmur. Subjects with a history of syncope or orthostatic intolerance were specifically excluded.
Only subjects found to be free from systemic and heart disease were deemed eligible. No subjects were taking neurally active or vasoactive medications. There were no smokers, trained competitive athletes, or completely bedridden patients. Informed consent was obtained, and the Committee for the Protection of Human Subjects (institutional review board) of New York Medical College approved all protocols.
Protocol
Testing was conducted in a temperature-controlled room after an overnight fast on a day other than the screening day. Experiments began after a 30-minute acclimatization period. All experiments were performed while the subject remained supine, ie, there were no tilt components. Measurements were made in the leg because prior experiments from our laboratory have indicated more significant findings in the calf than in the forearm in POTS.2,4,17
Monitoring
Heart rate was monitored by ECG, and upper and lower extremity blood pressures were measured intermittently by oscillometry on the right arm and right calf.
Laser-Doppler Flowmetry and Iontophoresis
We used laser-Doppler flowmeters (LDF) (Perimed) placed on the lateral aspect of the left or right calf to measure cutaneous blood flow.18 When comparisons were made (eg, between acetylcholine and sodium nitroprusside), we placed probes at similar locations of opposite legs. Baseline resting flows and experimental flows were measured in arbitrary perfusion units (perfusion units [pfu]). We used iontophoresis, an established method in which an applied electric field is used to facilitate the cutaneous penetration of drugs,19,20 in combination with LDF measurements to deliver vasoactive medications and to measure their effects on cutaneous blood flow. For iontophoretic purposes, LDF probes were positioned on the skin in specialized holders that control local temperature (Perimed). All drugs for iontophoretic delivery were placed in a 1-cm2 filter forming part of the iontophoretic drug-delivery electrode, which also contained a single-element laser-Doppler probe at its center. A battery-driven power supply provided a constant current at polarities for drug delivery, as indicated below. A second electrode of opposite polarity was placed at least 15 cm distant from the iontophoretic injection site. A saline “blank” was also tested at a separate site to control for current-mediated vasodilation. Each medication delivered by iontophoresis, with the exception of norepinephrine, was administered at a separate site. Norepinephrine was delivered at the site previously iontophoresed with bretylium tosylate.21
Comparisons were made between patients and reference subjects in the supine position. LDF was continuously measured throughout all experiments. Data were sampled at 200 Hz, multiplexed, and interfaced to a personal computer through an analog/digital converter (DI-720, DATAQ Industries) generating binary files and computer displays of simultaneously collected data from all lasers for a given patient.
Testing for Cutaneous Adrenergic Dysfunction
We tested the hypothesis that microvascular cutaneous α-adrenergic vasoregulation is impaired in low-flow POTS by using LDF in combination with α-adrenergic agonists and antagonists.
Tyramine
We used tyramine to test for a defect of norepinephrine release.22 This required predilation. Therefore, we preheated the iontophoresis site to achieve maximum vasodilation. Tyramine 1 mmol/L was then delivered at the heated site by iontophoresis at the positive electrode (anode) with a current of 200 μA for 2 minutes (24 millicoulomb [mC]).
Phentolamine
At a site separate from tyramine, we used phentolamine hydrochloride to test the response to α-adrenergic blockade.23 If excessive sympathetic activation and binding of norepinephrine at the vascular synapses were to occur in low-flow POTS, then increased vasodilation to phentolamine would be expected. To accomplish this, we delivered phentolamine hydrochloride, 0.5 mmol/L, at the negative electrode (cathode) using a current of 100 μA for 20 minutes (120 mC).
Bretylium and Norepinephrine
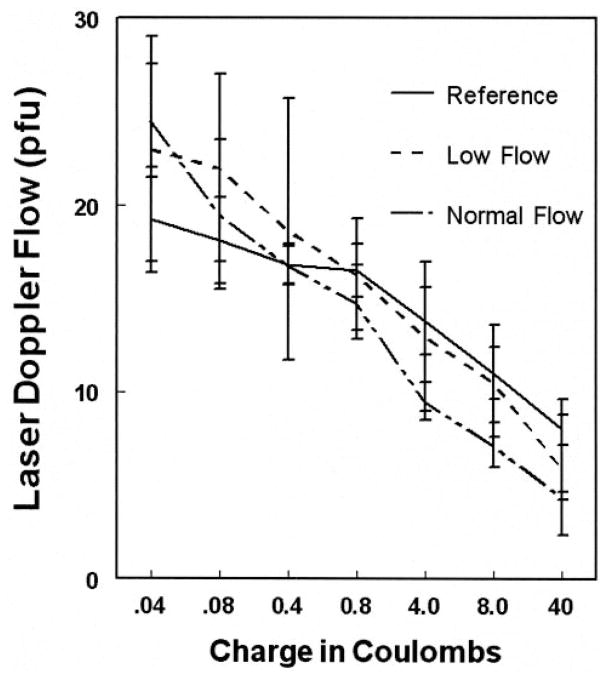
We performed norepinephrine dose-response curves by first pretreating a separate iontophoretic site with bretylium tosylate 10 mmol/L at the anode using a current of 200 μA for 20 minutes (240 mC). This produced release of endogenous norepinephrine stores, which thereafter could not contribute to the α-adrenergic response. This was followed by a 45-minute hiatus to allow for the initial norepinephrine release to subside and to be replaced by bretylium-induced norepinephrine blockade.21 Blockade was followed by escalating norepinephrine iontophoresis (dose response) to test for a defect in norepinephrine binding or response.24 Norepinephrine 1% solution was iontophoresed at the anode in an ascending dose schedule of 20 μA for 2 seconds (0.04 mC), 20 μA for 4 seconds (0.08 mC), 20 μA for 20 seconds (0.4 mC), 40 μA for 20 seconds (0.8 mC), 200 μA for 20 seconds (4.0 mC), 200 μA for 40 seconds (8.0 mC), and 200 μA for 200 seconds (40 mC), with a wait of 3 minutes between dose applications.
Testing for Cutaneous Receptor-Mediated Endothelial Dysfunction Versus Endothelial-Independent Vasodilation: Acetylcholine and Sodium Nitroprusside Iontophoresis
To test whether receptor-mediated endothelial dysfunction occurs in low-flow POTS, we used acetylcholine (Penta International; 1%, dissolved in sterile water) with anodal current to a site on the calf chosen at random. After a baseline recording, we used 7 increasing iontophoretic stimuli of varying intensity and duration (20 μA for 3 seconds [0.06 mC], 20 μA for 6 seconds [0.12 mC], 20 μA for 28 seconds [0.56 mC], 40 μA for 28 seconds [1.12 mC], 200 μA for 28 seconds [5.6 mC], 400 μA for 28 seconds [11.2 mC], and 400 μA for 140 seconds [56 mC]), waiting 3 minutes between each new dose application to generate a dose-response curve for flow with acetylcholine.25 We compared this with the same escalating iontophoretic currents and times of sodium nitroprusside 2% (Nipride, Roche) administered to the contralateral calf to examine the endothelial-independent vasodilator.
Testing for Cutaneous Endothelial Dysfunction
To investigate flow-related endothelial function, we performed experiments using prolonged (>30 minutes) local heating to 43°C. We tested whether flow-related NOS-dependent vasodilation was intact. Heating was performed at 2 separate similar sites on both legs. One site received saline iontophoresed at the cathode, at 100 μA for 20 minutes. This site also served as the saline cathode “blank.” The other site received NG-nitro-L-arginine methyl ester (L-NAME) 2% delivered by the cathode, at 100 μA for 20 minutes. Thereafter, both sites were gradually heated (1°C/10 s) to a maximum of 43°C.
A rationale for this testing is required. Local heating of nonglabrous skin such as the forearm or calf evokes vasodilation that is mediated by both local neurogenic reflexes and locally released substances.26–29 Local heating produces an initial dilator response that peaks in a few minutes, followed by a brief nadir and then a secondary dilation to a sustained plateau (Figure 1). Generally, increased rather than decreased NO blunts sympathetic activity. The thermal hyperemic response is highly repeatable.
Figure 1.
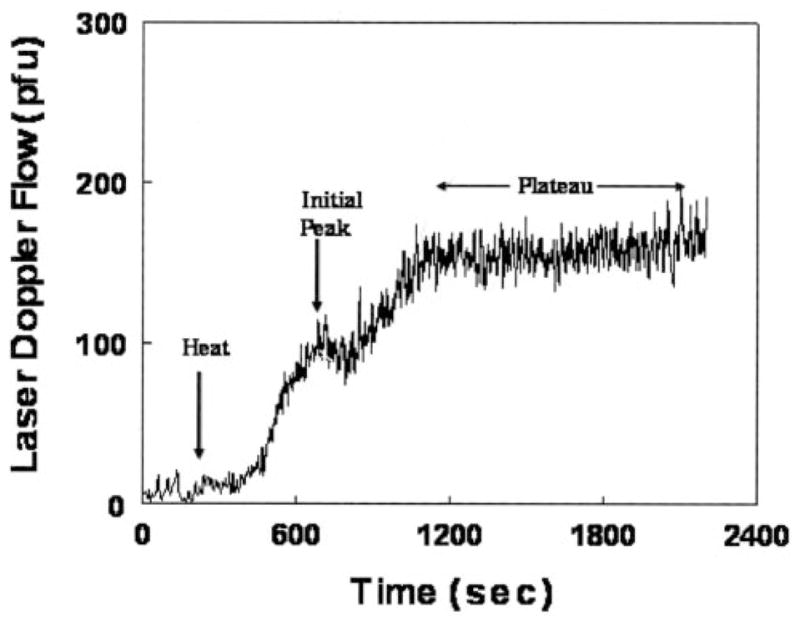
Representative tracings of the response to local heating to 43°C. The tracing is biphasic. There is an initial peak, thought to be related to the neurogenic inflammatory response, followed by a higher plateau, which is believed to be related to flow-mediated NO-dependent vasodilation.
Kellogg, Johnson, and colleagues29,30 first showed and Minson et al corroborated28,31 that the dilator response to local heating is related to NO, which plays a major role in the sustained vasodilation of prolonged heating (ie, the secondary plateau). The first peak is blocked by local anesthetics28 and may be mediated by neurogenic inflammation (nociceptors), related to the release of calcitonin gene–related peptide and substance P.32,33 The plateau is highly attenuated by NOS inhibition with L-NAME. This has been found to reduce the initial peak by ≈30% and the height of the plateau by ≈60% and implicates NO in the thermal hyperemia response.28
Patient Dimensions and Resting Hemodynamic Data
| Reference | POTS
|
||
|---|---|---|---|
| Low Flow | Normal Flow | ||
| Body surface area, m2 | 1.72±0.08 | 1.73±0.13 | 1.76±0.08 |
| Weight, kg | 62±3 | 57±4 | 64±5 |
| Height, cm | 167±8 | 180±12 | 172±7 |
| Heart rate, bpm | 67±5 | 82±9*† | 62±8 |
| MAP right arm, mm Hg | 78±6 | 84±7 | 79±4 |
| MAP right leg, mm Hg | 75±5 | 78±6 | 72±3 |
| Venous occlusion plethysmography blood flow | 2.6±0.7 | 0.7±0.2*† | 2.2±0.4 |
| LDF, pfu | 6.5±1.0 | 2.2±1.1*† | 4.9±1.3 |
MAP indicates mean arterial pressure.
P<0.05 compared with reference subjects;
P<0.05 compared with normal-flow subjects. A simple Bonferroni correction was applied throughout, and results are regarded as significant only if they are <0.05.
Statistical Analysis
Data in the Table were compared by 1-way ANOVA comparing reference, low-flow POTS, and normal-flow POTS groups. We used 2-way ANOVA with repeated measures to compare iontophoretic dose responses to norepinephrine, acetylcholine, and sodium nitroprusside using polynomial contrasts. We also used 2-way ANOVA with repeated measures and simple contrasts for the response to heat followed by tyramine and for tests of cutaneous endothelial function in response to thermal hyperemia in the presence of saline or L-NAME. We used 1-way ANOVA to compare changes in LDF during the iontophoretic administration of bretylium and phentolamine. Results were calculated with the use of SPSS (Statistical Package for the Social Sciences) software version 11.0. All text and Table results are reported as mean±SD. A maximum P<0.05 was required within comparisons to declare statistical significance.
Results
Patient Dimensions and Supine Data
Resting supine data are shown in the Table. Subjects were similar in size and in arm and leg blood pressure. Supine heart rate was increased (P=0.002) in low-flow POTS patients only. Basal LDF was significantly decreased (P=0.0001) in low-flow patients compared with normal-flow POTS patients or with reference subjects. Resting LDF data of the Table comprise an average of resting baseline flows obtained over all iontophoresis sites and all experiments yielding significant differences between low-flow POTS patients and reference subjects. Because LDF is measured in arbitrary units, investigators often choose to compute relative changes in cutaneous flow as a ratio of experimental to resting LDF. Because overall peripheral supine blood flow is reduced by definition in low-flow POTS, it is reasonable to assume that low LDF actually reflects reduced cutaneous blood flow. Normalization to this low initial value would therefore produce an artifactually increased relative LDF in our low-flow POTS patients simply because flow is low at rest. We therefore chose to present data in plaque-forming units rather than in relative units.
Iontophoresis had no effect on systemic hemodynamics (heart rate, arm and leg blood pressure, plethysmographically measured blood flow) in any patient.
Cutaneous Adrenergic Function
Dose-response curves to norepinephrine after bretylium administration are shown in Figure 2. There was no significant difference among groups (P=0.63), and LDF decreased with increasing norepinephrine dose (P=0.0052).
Figure 2.
Dose response to norepinephrine iontophoresis after bretylium had been administered to the same site. There are no significant differences among POTS patients or reference subjects.
Maximum LDF values with phentolamine and bretylium are shown in Figure 3. There were no significant differences among reference subjects or POTS patients (P=0.79 for bretylium, P=0.96 for phentolamine for between-group differences).
Figure 3.
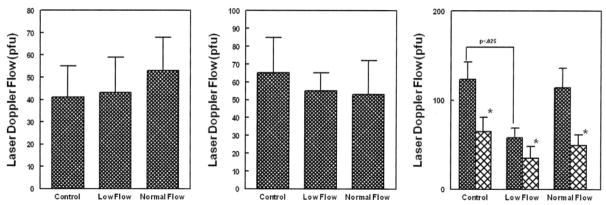
Response to additional α-adrenergic agonists and antagonists. Left panel shows the maximal LDF with bretylium; middle, maximum LDF response to phentolamine; right, response to preheating (dark bars) followed by tyramine (lighter bars). The only significant difference is a decrease in the pretyramine heating response in low-flow POTS. *P<0.05 difference from baseline for each group.
Postheating flow and minimum tyramine LDF values are also shown in Figure 3. Tyramine significantly reduced LDF in all subjects P=0.0033). There was blunted LDF response to preheating in low-flow POTS (P=0.027). This is similar to blunting during thermal hyperemia experiments (see below).
Acetylcholine and Sodium Nitroprusside Iontophoresis
Dose responses for acetylcholine and sodium nitroprusside are depicted in Figure 4. There were no differences (P=0.94 for acetylcholine, P=0.79 for sodium nitroprusside) noted among low-flow POTS, normal-flow POTS, or reference subjects for either acetylcholine or sodium nitroprusside.
Figure 4.
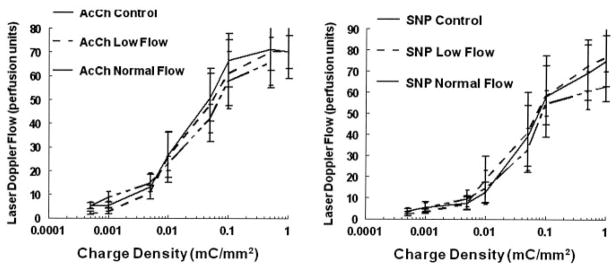
Responses to different doses of acetylcholine (AcCh), an endothelial-dependent vasodilator (left), and responses to sodium nitroprusside (SNP), an endothelial-independent vasodilator (right). There are no differences among the groups except that baseline low-flow POTS LDF is decreased in both panels.
Cutaneous Hyperemia and Endothelial Dysfunction
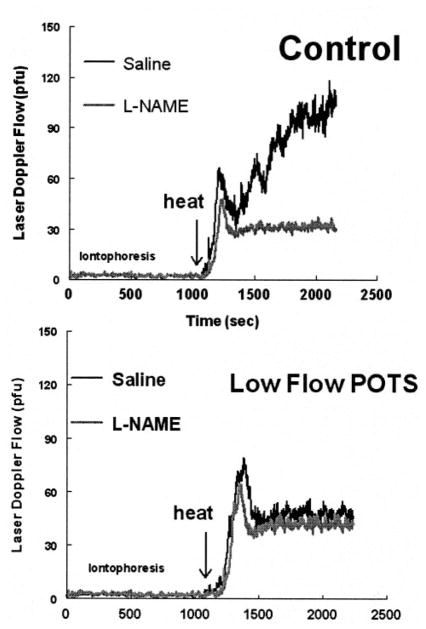
Cutaneous thermal hyperemia experiments were performed in reference subjects and POTS patients. Representative tracings for a low-flow POTS patient and for a reference subject are shown in Figure 5. Iontophoresis of saline or L-NAME did not produce significant nonspecific current effects in these experiments, nor was significant vasoconstriction produced by L-NAME during the period of unheated iontophoresis because skin is normally vasoconstricted under thermal neutral conditions. With heating, there was a similar initial peak at the saline site for both the reference and the low-flow POTS subject. However, there was a greatly attenuated secondary plateau in the low-flow POTS patient compared with the reference subject.
Figure 5.
Top, Saline or L-NAME administered to a representative reference subject by iontophoresis followed by local heating to 43°C. An initial peak occurs and is followed by a higher plateau. Iontophoretic administration of L-NAME slightly blunts the initial peak and markedly decreases the plateau phase. Bottom, Local heating followed by saline or L-NAME in a representative low-flow POTS patient. The initial peak is present, but there is marked attenuation of the NO-dependent plateau, even after saline administration, which resembles blunting by the NO inhibitor L-NAME. Iontophoretic administration of L-NAME minimally blunts the initial peak but has no additional effect on the plateau phase because of preexistent impairment of NO release.
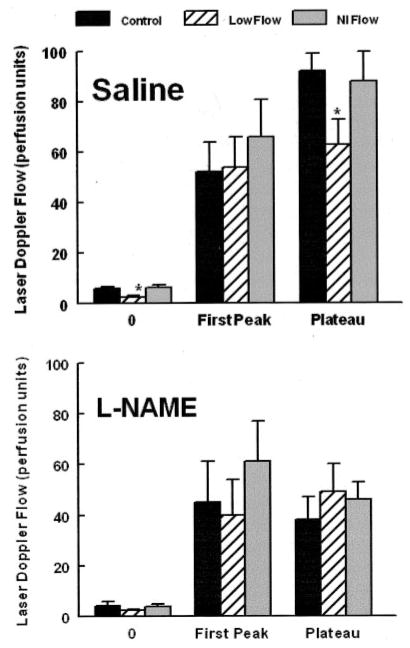
Administration of L-NAME blunted the plateau phase of thermal hyperemia in the reference subject and mildly reduced the initial peak. The low-flow POTS patient had mild reduction of the initial peak but no change in the plateau phase, which remained severely attenuated and was not substantially affected by L-NAME. Grouped data for all subjects are shown in Figure 6 and confirm these findings. Thermal hyperemia results for normal-flow POTS subjects were similar to those of reference subjects (P=0.93).
Figure 6.
Averaged data for local heating experiments. Data for saline administration (top) and L-NAME administration (bottom) are shown. The data indicate greater perfusion in heated saline reference subjects and normal-flow (Nl Flow) POTS patients. L-NAME results are similar for all groups and are not different from data obtained during saline administration in low-flow POTS patients.
Discussion
Our main findings are decreased cutaneous blood flow in low-flow POTS subjects measured by LDF as well as decreased calf blood flow measured by standard venous occlusion strain gauge plethysmography. Cutaneous blood flow is decreased in approximate proportion to overall calf blood flow. In addition, we found that cutaneous adrenergic iontophoretic response is not different among reference, low-flow POTS, and normal-flow POTS subjects. We showed that receptor-mediated endothelial-dependent vasodilation is not different among reference, low-flow POTS, and normal-flow POTS subjects and that there are comparable changes in response to sodium nitroprusside given as an endothelial-independent vasodilator. Finally, we demonstrated that thermal hyperemia produced NOS-inhibitor–sensitive vasodilation in reference subjects, which was markedly attenuated in low-flow POTS but not in normal-flow POTS subjects. The data imply that NO release is already reduced in low-flow POTS subjects (compared with reference subjects) before L-NAME administration.
Present data suggest that previously demonstrated local blood flow dysregulation in low-flow POTS characterized by impaired venoarteriolar reflex, reduced myogenic response, and abbreviated reactive hyperemia may be explained by impaired bioavailable NO.
The venoarteriolar reflex comprises arterial vasoconstriction in response to venous hypertension. It is present in both skeletal muscle and skin34 and may be important to orthostatic tolerance.13 Although dependent on local nerve integrity and independent of the central nervous system, the reflex was originally attributed to an α-adrenergic mechanism. However, recent experiments of Crandall et al12 have demonstrated that adrenergic mechanisms are not involved under physiological conditions. Rather, nonadrenergic local processes, possibly implicating NO and the myogenic response, have been proposed. Impaired venoarteriolar reflex in low-flow POTS is therefore consistent with cutaneous adrenergic competence.
Similarly, prior abnormalities of the myogenic response in low-flow POTS implicate mechanisms dominated by the actions of NO.35 It is now clear that arteriolar myogenic tone can be affected by mechanisms that modify Ca2+-dependent smooth muscle contraction, including NO, as demonstrated by Ungvari and Koller11 using local flow changes or NO donors to decrease myogenic tone. Koller and Bagi36 also showed an intimate relationship among myogenic response, reactive hyperemia, and NO-dependent vasodilation confirmed in vitro.
Receptor-Mediated Vasodilation
We used sodium nitroprusside as an endothelial-independent vasodilator. There was no difference among POTS groups or reference subjects, suggesting that smooth muscle vasodilator capability and cutaneous resistance vessel architecture in low-flow POTS are likely to be similar to those in reference subjects. We used acetylcholine as a receptor-mediated agonist for NO release and also found no difference among groups. This may not be altogether surprising. Although acetylcholine produces dilation in conduit vessels such as large coronary arteries37,38 and brachial artery39 by NO-dependent mechanisms, the microvascular response to acetylcholine is more complex. Evidence suggests that the receptor-mediated response also involves prostaglandins and endothelium-dependent hyperpolarizing factor (EDHF), as well as NO40,41; microvascular dilation in response to acetylcholine is not solely dependent on NO. Investigators have shown that compensatory EDHF-dependent mechanisms are suppressed while NO is present but may be sufficiently evoked in the absence of NO to leave acetylcholine-mediated vasodilation unchanged.40,41 Other work suggests that cutaneous acetylcholine-mediated vasodilation may be relatively independent of NO or prostaglandins in humans.42
Local Heating- and Flow-Mediated Dilation
Investigations of heat-induced NO release have shown that NO release may be evoked in the skin in a relatively minor way during passive whole-body heating.43,44 However, the NO response to local heating is far less equivocal.28,45 Unlike whole-body heating, the majority of the dilation evoked by local heating is NO dependent and seems to be directly related to the magnitude of increase in blood flow during the plateau phase of the local heating response. Our data therefore strongly suggest a defect in NO-mediated vasodilation in the low-flow POTS subset. We further tested this hypothesis by showing that thermal hyperemia can distinguish among populations of renal failure patients with and without endothelial cell dysfunction.46
The present data indicate that localized heating, rather than acetylcholine stimulation, may be the most accurate means to assess NO-dependent flow-mediated vasodilation in patient groups.
Limitations
We only studied skin. Although cutaneous circulation is unique in its autonomic control, evidence from our recent work indicates that flow regulation abnormalities in low-flow POTS occur throughout the circulatory system and that local flow abnormalities occur within the skin. There is a paucity of quantitative comparisons of local regulatory mechanisms in humans. However, it is clear that the myogenic response, venoarteriolar reflex, and reactive hyperemia occur in the skin and are abnormal in low-flow POTS patients.
We only studied leg cutaneous circulation. Our previous data indicate that flow abnormalities are widespread in low-flow POTS.3 However, whenever peripheral blood flows are compared, the most significant findings have always been demonstrated in the lower extremities. Pilot data investigating the forearm response showed findings qualitatively similar to leg responses but less marked. It may be that dependence and gravitational exposure are important to observed changes.
There was no direct assessment of skin sympathetic nerve traffic. This implies that the lack of adrenergic alterations only refers to receptor responsiveness and that no information is available concerning events that occur above the receptor level. Thus, nerve traffic may indeed be increased, as noted generally in POTS.
Reproducibility of adrenergic functional testing in the patients of the present study was not assessed. Unfortunately, the adrenergic testing has not been repeated in those individuals. However, whole-limb and cutaneous blood flow are fairly well replicated with r2=0.78 during studies over a 2-week period.
Age limitations to generalizability may exist. However, cardiovascular structure and function are essentially mature by puberty, and therefore results can be regarded as at least similar to those in older age groups. Adolescents often have the advantage of absence of confounding illness such as heart disease, renal disease, hypertension, and diabetes.
LDF measurements are somewhat arbitrary. LDF does not assess absolute blood flow but rather measurements of perfusion units (plaque-forming units) which include contributions related to factors such as age, skin density, gender, skin color, and temperature. However, all subjects were of similar age, gender, and skin color. Moreover, expression of data in normalized units, often obtained by dividing the stimulated LDF signal by the baseline signal, seems clearly incorrect when baseline flow is truly reduced, as in low-flow POTS. Therefore, we chose to express results in plaque-forming units. The relative consistency of results supports this choice.
Evidence for nonspecific current effects or current-induced hyperemia during iontophoresis has been well known since at least 1995.47 This is particularly problematic for iontophoresis at a cathode48 and is dependent on the magnitude of the current and on subject age. Thus, we strove wherever possible to limit the amount of current and to use anodal agents wherever possible. These “artifactual” currents probably represent real flow changes induced via neurogenic inflammation or a similar mechanism. In practice, they did not appreciably affect our results.
Acknowledgments
This study was supported by grants 5RO1HL66007 and 1RO1HL074873 from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
References
- 1.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 2.Jacob G, Costa F, Shannon JR, Robertson RM, Wathen M, Stein M, Biaggioni I, Ertl A, Black B, Robertson D. The neuropathic postural tachycardia syndrome. N Engl J Med. 2000;343:1008–1014. doi: 10.1056/NEJM200010053431404. [DOI] [PubMed] [Google Scholar]
- 3.Stewart JM, Montgomery LD. Regional blood volume and peripheral blood flow in postural tachycardia syndrome. Am J Physiol. 2004;287:H1319–H1327. doi: 10.1152/ajpheart.00086.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart JM, Medow MS, Montgomery LD. Local vascular responses affecting blood flow in postural tachycardia syndrome. Am J Physiol. 2003;285:H2749–H2756. doi: 10.1152/ajpheart.00429.2003. [DOI] [PubMed] [Google Scholar]
- 5.Fouad FM, Tadena-Thome L, Bravo EL, Tarazi RC. Idiopathic hypovolemia. Ann Intern Med. 1986;104:298–303. doi: 10.7326/0003-4819-104-3-298. [DOI] [PubMed] [Google Scholar]
- 6.Jacob G, Robertson D, Mosqueda-Garcia R, Ertl AC, Robertson RM, Biaggioni I. Hypovolemia in syncope and orthostatic intolerance role of the renin-angiotensin system. Am J Med. 1997;103:128–133. doi: 10.1016/s0002-9343(97)00133-2. [DOI] [PubMed] [Google Scholar]
- 7.Stewart JM, Weldon A. Reflex vascular defects in the orthostatic tachycardia syndrome of adolescents. J Appl Physiol. 2001;90:2025–2032. doi: 10.1152/jappl.2001.90.6.2025. [DOI] [PubMed] [Google Scholar]
- 8.Cooke WH, Zhang R, Zuckerman JH, Cui J, Wilson TE, Crandall CG, Levine BD. Does nitric oxide buffer arterial blood pressure variability in humans? J Appl Physiol. 2002;93:1466–1470. doi: 10.1152/japplphysiol.00287.2002. [DOI] [PubMed] [Google Scholar]
- 9.Koller A, Messina EJ, Wolin MS, Kaley G. Effects of endothelial impairment on arteriolar dilator responses in vivo. Am J Physiol. 1989;257:H1485–H1489. doi: 10.1152/ajpheart.1989.257.5.H1485. [DOI] [PubMed] [Google Scholar]
- 10.Sun D, Messina EJ, Kaley G, Koller A. Characteristics and origin of myogenic response in isolated mesenteric arterioles. Am J Physiol. 1992;263:H1486–H1491. doi: 10.1152/ajpheart.1992.263.5.H1486. [DOI] [PubMed] [Google Scholar]
- 11.Ungvari Z, Koller A. Selected contribution: NO released to flow reduces myogenic tone of skeletal muscle arterioles by decreasing smooth muscle Ca(2+) sensitivity. J Appl Physiol. 2001;91:522–527. doi: 10.1152/jappl.2001.91.1.522. [DOI] [PubMed] [Google Scholar]
- 12.Crandall CG, Shibasaki M, Yen TC. Evidence that the human cutaneous venoarteriolar response is not mediated by adrenergic mechanisms. J Physiol. 2002;538:599–605. doi: 10.1113/jphysiol.2001.013060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vissing SF, Secher NH, Victor RG. Mechanisms of cutaneous vasoconstriction during upright posture. Acta Physiol Scand. 1997;159:131–138. doi: 10.1046/j.1365-201X.1997.573344000.x. [DOI] [PubMed] [Google Scholar]
- 14.Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–S25. [PubMed] [Google Scholar]
- 15.Sandroni P, Opfer-Gehrking TL, McPhee BR, Low PA. Postural tachycardia syndrome: clinical features and follow-up study. Mayo Clin Proc. 1999;74:1106–1110. doi: 10.4065/74.11.1106. [DOI] [PubMed] [Google Scholar]
- 16.Greenfield AD, Whitney RJ, Whitney RJ. Methods for the investigation of peripheral blood flow. Br Med Bull. 1963;19:101–109. doi: 10.1093/oxfordjournals.bmb.a070026. [DOI] [PubMed] [Google Scholar]
- 17.Stewart JM, Weldon A. Contrasting neurovascular findings in chronic orthostatic intolerance and neurocardiogenic syncope. Clinical Science. 2003;104:329–340. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JM, Taylor WF, Shepherd AP, Park MK. Laser-Doppler measurement of skin blood flow: comparison with plethysmography. J Appl Physiol. 1984;56:798–803. doi: 10.1152/jappl.1984.56.3.798. [DOI] [PubMed] [Google Scholar]
- 19.Hirvonen J, Murtomaki L, Kontturi K. Experimental verification of the mechanistic model for transdermal transport including iontophoresis. J Control Release. 1998;56:169–174. doi: 10.1016/s0168-3659(98)00088-1. [DOI] [PubMed] [Google Scholar]
- 20.Singh P, Maibach HI. Iontophoresis in drug delivery: basic principles and applications. Crit Rev Ther Drug Carrier Syst. 1994;11:161–213. [PubMed] [Google Scholar]
- 21.Kellogg DL, Jr, Johnson JM, Kosiba WA. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am J Physiol. 1989;257:H1599–H1606. doi: 10.1152/ajpheart.1989.257.5.H1599. [DOI] [PubMed] [Google Scholar]
- 22.Jacob G, Shannon JR, Costa F, Furlan R, Biaggioni I, Mosqueda-Garcia R, Robertson RM, Robertson D. Abnormal norepinephrine clearance and adrenergic receptor sensitivity in idiopathic orthostatic intolerance. Circulation. 1999;99:1706–1712. doi: 10.1161/01.cir.99.13.1706. [DOI] [PubMed] [Google Scholar]
- 23.Hornqvist R, Back O, Henriksson R. Adrenoceptor-mediated responses in human skin studied by iontophoresis. Br J Dermatol. 1984;111:561–566. doi: 10.1111/j.1365-2133.1984.tb06625.x. [DOI] [PubMed] [Google Scholar]
- 24.Wilson TE, Monahan KD, Short DS, Ray CA. Effect of age on cutaneous vasoconstrictor responses to norepinephrine in humans. Am J Physiol. 2004;287:R1230–R1234. doi: 10.1152/ajpregu.00467.2004. [DOI] [PubMed] [Google Scholar]
- 25.Berghoff M, Kathpal M, Kilo S, Hilz MJ, Freeman R. Vascular and neural mechanisms of ACh-mediated vasodilation in the forearm cutaneous microcirculation. J Appl Physiol. 2002;92:780–788. doi: 10.1152/japplphysiol.01167.2000. [DOI] [PubMed] [Google Scholar]
- 26.Charkoudian N, Eisenach JH, Atkinson JL, Fealey RD, Joyner MJ. Effects of chronic sympathectomy on locally mediated cutaneous vasodilation in humans. J Appl Physiol. 2002;92:685–690. doi: 10.1152/japplphysiol.00758.2001. [DOI] [PubMed] [Google Scholar]
- 27.Johnson JM, O’Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol. 1986;6:337–346. doi: 10.1111/j.1475-097x.1986.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 28.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 29.Pergola PE, Kellogg DL, Jr, Johnson JM, Kosiba WA, Solomon DE. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am J Physiol. 1993;265:H785–H792. doi: 10.1152/ajpheart.1993.265.3.H785. [DOI] [PubMed] [Google Scholar]
- 30.Kellogg DL, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998;85:824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- 31.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 2002;93:1644–1649. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- 32.Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302:839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- 33.Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol. 1998;30:5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- 34.Henriksen O, Amtorp O, Faris I, Agerskov K. Evidence for a local sympathetic venoarteriolar “reflex” in the dog hindleg. Circ Res. 1983;52:534–542. doi: 10.1161/01.res.52.5.534. [DOI] [PubMed] [Google Scholar]
- 35.Sun D, Huang A, Koller A, Kaley G. Flow-dependent dilation and myogenic constriction interact to establish the resistance of skeletal muscle arterioles. Microcirculation. 1995;2:289–295. doi: 10.3109/10739689509146775. [DOI] [PubMed] [Google Scholar]
- 36.Koller A, Bagi Z. On the role of mechanosensitive mechanisms eliciting reactive hyperemia. Am J Physiol. 2002;283:H2250–H2259. doi: 10.1152/ajpheart.00545.2002. [DOI] [PubMed] [Google Scholar]
- 37.Bugiardini R, Manfrini O, Pizzi C, Fontana F, Morgagni G. Endothelial function predicts future development of coronary artery disease: a study of women with chest pain and normal coronary angiograms. Circulation. 2004;109:2518–2523. doi: 10.1161/01.CIR.0000128208.22378.E3. [DOI] [PubMed] [Google Scholar]
- 38.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 39.Katz SD, Schwarz M, Yuen J, LeJemtel TH. Impaired acetylcholine-mediated vasodilation in patients with congestive heart failure: role of endothelium-derived vasodilating and vasoconstricting factors. Circulation. 1993;88:55–61. doi: 10.1161/01.cir.88.1.55. [DOI] [PubMed] [Google Scholar]
- 40.Nishikawa Y, Stepp DW, Chilian WM. In vivo location and mechanism of EDHF-mediated vasodilation in canine coronary microcirculation. Am J Physiol. 1999;277:H1252–H1259. doi: 10.1152/ajpheart.1999.277.3.H1252. [DOI] [PubMed] [Google Scholar]
- 41.Boutsiouki P, Georgiou S, Clough GF. Recovery of nitric oxide from acetylcholine-mediated vasodilatation in human skin in vivo. Microcirculation. 2004;11:249–259. doi: 10.1080/10739680490425958. [DOI] [PubMed] [Google Scholar]
- 42.Buus NH, Simonsen U, Pilegaard HK, Mulvany MJ. Nitric oxide, prostanoid and non-NO, non-prostanoid involvement in acetylcholine relaxation of isolated human small arteries. Br J Pharmacol. 2000;129:184–192. doi: 10.1038/sj.bjp.0703041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shastry S, Minson CT, Wilson SA, Dietz NM, Joyner MJ. Effects of atropine and L-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol. 2000;88:467–472. doi: 10.1152/jappl.2000.88.2.467. [DOI] [PubMed] [Google Scholar]
- 44.Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol. 1998;85:830–834. doi: 10.1152/jappl.1998.85.3.830. [DOI] [PubMed] [Google Scholar]
- 45.Kellogg DL, Jr, Liu Y, Kosiba IF, O’Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86:1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- 46.Stewart J, Kohen A, Brouder D, Rahim F, Adler S, Garrick R, Goligorsky MS. Noninvasive interrogation of microvasculature for signs of endothelial dysfunction in patients with chronic renal failure. Am J Physiol. 2004;287:H2687–H2696. doi: 10.1152/ajpheart.00287.2004. [DOI] [PubMed] [Google Scholar]
- 47.Berliner MN. Skin microcirculation during tapwater iontophoresis in humans: cathode stimulates more than anode. Microvasc Res. 1997;54:74–80. doi: 10.1006/mvre.1997.2025. [DOI] [PubMed] [Google Scholar]
- 48.Grossmann M, Jamieson MJ, Kellogg DL, Jr, Kosiba WA, Pergola PE, Crandall CG, Shepherd AM. The effect of iontophoresis on the cutaneous vasculature: evidence for current-induced hyperemia. Microvasc Res. 1995;50:444–452. doi: 10.1006/mvre.1995.1070. [DOI] [PubMed] [Google Scholar]