Identity
Other names: S100; S100-alpha; S100A
HGNC (Hugo): S100A1
Location: 1q21.3
Local order: The S100A1 gene is located at the telomeric end of the 16 member S100A gene cluster on 1q21.3. The S100A1 gene is flanked by the S100A13 (centromeric) and C1orf77 (telomeric) genes.
DNA/RNA
Description
The S100A1 gene structure is highly conserved in mammals and contains three exons separated by two introns (Song and Zimmer, 1996; Zimmer et al., 1996; Kiewitz et al., 2000). The first exon encodes the 5' UTR. The second exon encodes the 5' UTR and amino terminal EF hand Ca2+-binding domain. The third exon encodes the carboxy-terminal canonical EF-hand and the 3' UTR. Cis-acting DNA elements that regulate expression in skeletal muscle, neurons, glia and heart have been identified (Song and Zimmer, 1996; Kiewitz et al., 2000). S100A1 transcription in chondrocytes is regulated by the SOX trio of transcription factors, SOX5, SOX6 and SOX9 (Saito et al., 2007).
Transcription
The S100A1 protein is encoded in a 594 bp mRNA transcript. Protein products have not been verified for the five splice variants reconstructed from low abundance cDNAs (Ensembl and Aceview).
Pseudogene
None.
Protein
Description
S100A1 is a member of the S100 family of Ca2+-binding proteins. S100A1 exists as a dimer of two identical monomers. Each monomer contains 94 amino acids (figure 1), has a molecular weight of 10546 Da, and contains two EF-hand ca2+-binding domains (Wright et al., 2005). The non-canonical amino terminal EF-hand contains 14 amino acids and is characteristic of S100 proteins. The carboxy terminal canonical EF-hand contains 12 amino acids and is characteristic of all S100/calmodulin/troponin superfamily members. Like other members of this superfamily, S100A1 possesses no inherent enzymatic activity and exerts its biological effects by interacting with and modulating the activity of target proteins (Zimmer et al., 2003). S100A1 undergoes an ~90 degree rotation of helix 3 upon binding calcium (figure 2), exposing a hydrophobic pocket that serves as the binding site for intracellular and extracellular proteins targets (Landar et al., 1998; Wright et al., 2009b; Zimmer and Weber, 2010). The amino acid sequence linking the two EF-hands confers family member-specific binding for some target proteins (Zimmer and Weber, 2010).
Figure 1. Amino acid sequence of the human s100A1 monomer.
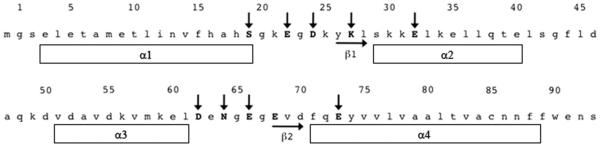
Residues involved in binding Ca2+ are shown with downward arrows. Elements of secondary structure are indicated.
Figure 2. The conformational change of the S100A1 dimer.
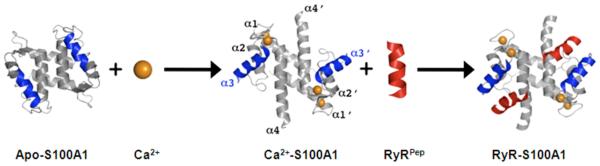
Upon binding Ca2+, alpha-helix 3 (blue) undergoes an ~90 degree rotation, exposing a hydrophobic binding site that can interact with a variety of biological targets. Here, a peptide derived from ryanodine receptor (RyRPep; red) is shown binding to S100A1 (Wright et al., 2008).
Expression
S100 proteins are expressed exclusively in higher chordates in a cell-type and tissue-specific manner (Zimmer et al., 1995; Zimmer et al., 2005). It is the unique complement of S100 family members expressed in individual cells that allows cells to transduce changes in calcium levels into unique biological responses. While there are species-specific differences in S100A1 levels, all species exhibit intermediate to high levels of S100A1 in heart, skeletal muscle, smooth muscle, skin, brain, adipose and kidney (Kato et al., 1986; Zimmer and Van Eldik, 1987; Zimmer et al., 1991; Zimmer et al., 1995; Zimmer et al., 2005; Song and Zimmer, 1996; Kiewitz et al., 2000). Lung, liver, spleen, intestine, testis, thyroid gland, salivary gland, pancreas, ovary, placenta and prostate also contain detectable levels of S100A1 protein/mRNA.
Localisation
In non-muscle cells, S100A1 is located in the perinuclear cytoplasm. In muscle cells, S100A1 colocalizes with the sarcolemma, sarcoplasmic reticulum and mitochondria. Extracellular forms of S100A1 have also been detected (Most et al., 2003; Hernández-Ochoa et al., 2009).
Function
Like other S100 proteins, S100A1 has been implicated in a variety of biological and cellular processes. S100A1 mediates the suppression of chondrocyte differentiation (Saito et al., 2007). In the nervous system, S100A1 modulates innate fear and exploration of novel stimuli (Ackermann et al., 2006). In neuronal cells, S100A1 regulates cell proliferation, dendrite formation, intracellular calcium levels, microtubule stability, and amyloid precursor protein levels (Zimmer et al., 1995; Zimmer et al., 2005). In ganglion neurons, exogenous S100A1 increases sympathetic output by enhancing L-type calcium channel currents in a PKA-dependent manner (Hernández-Ochoa et al., 2009). In the heart, S100A1 regulation of the ryanodine receptor, SERCA pump and phospholamban alters Ca2+ handling; regulation of titin alters contractile performance; and regulation F1-ATPase alters mitochondrial energy metabolism (Rohde et al., 2010). In skeletal muscle, S100A1 interaction with the ryanodine receptor modulates excitation contraction coupling (Prosser et al., 2008; Wright et al, 2008). In endothelial cells, S100A1 moldulates calcium levels and nitric oxide production (Pleger et al., 2008). Additional S100A1 target proteins include the RAGE receptor, aldolase, annexin A6, the actin-capping protein CAPZA1, desmin, glial fibrillary acidic protein, the 43 kD gap junction protein (GJA1), heat shock proteins, immunophilins (CyP40 and FKBP52) phosphoglucomutase, glycogen phosphorylase kinase, tubulin, the calcyclin binding protein, and other S100s (S100A3, S100A4, S100B, S100P) (Wright et al, 2009b). Two structures of S100A1-target complexes have been characterized using NMR (Wright et al., 2008; Wright et al., 2009a).
Homology
Species comparisons
Human - Chimpanzee: 100%
Human - Cow: 99%
Human - Canine: 98%
Human - Rat: 94%
Human - Mouse: 94%
Other S100 family members
Human S100A1 - Human S100A4: 51%
Human S100A1 - Human S100A5: 46%
Human S100A1 - Human S100A7: 24%
Human S100A1 - Human S100A10: 48%
Human S100A1 - Human S100A13: 41%
Human S100A1 - Human S100B: 60%
Human S100A1 - Human S100P: 56%
Mutations
Note
No disease-associated mutations in the S100A1 gene have been reported.
Implicated in
Melanoma
Note
Like other S100 proteins, S100A1 displays altered expression patterns in melanomas. Expression is increased in malignant, relative to benign, melanocytic tumors (Sviatoha et al., 2010) and has been proposed as a possible melanoma biomarker (Nonaka et al., 2008).
Renal cell carcinoma
Note
It has also been observed that renal cell carcinoma is accompanied by increased expression of S100A1 (Teratani et al., 2002). As with melanoma, S100A1 has been proposed as a marker to differentiate between renal cell carcinoma subtypes (Keijser et al., 2006; Li et al., 2007; Yusenko et al., 2009).
Lung cancer
Note
Genomic changes in the S100A1 gene have been reported in lung cancer (Jiang et al., 2010).
Alzheimer's disease
Note
There is evidence suggesting that S100A1 could be involved in Alzheimer's disease (Zimmer et al., 2005). PC12 cells not expressing S100A1 are more resistant to Abeta25-32-induced cell death. Such cells also display a stabilization of intracellular calcium levels similar to that observed in other systems displaying increased resistance to Abeta-induced cell death. These observations suggest that S100A1 antagonists could hinder the progression of Alzheimer's disease.
Heart disease
Note
The importance of S100A1 in cardiac function was hypothesized in light of two early observations - that S100A1 levels are diminished in cardiac tissues from patients with end stage heart failure (Remppis et al., 1996), but increase during pulmonary hypertension leading to myocardial hypertrophy (Ehlermann et al., 2000). S100A1 is now known to modulate excitation-contraction coupling through interactions with the ryanodine receptor, competing for the same binding site occupied by calmodulin (Prosser et al., 2008; Wright et al., 2008). Furthermore, over-expression of S100A1 delays myocardial remodeling, improves contractile performance, and increases survival in response to myocardial infarction (Kraus et al., 2009; Rohde et al., 2010). This protein is now the subject of gene-therapy studies for treating chronic heart failure (Pleger et al., 2007).
References
- Kato K, Kimura S, Haimoto H, Suzuki F. S100a0 (alpha alpha) protein: distrbution in muscle tissues of various animals and purification from human pectoral muscle. J Neurochem. 1986 May;46(5):1555–60. doi: 10.1111/j.1471-4159.1986.tb01776.x. [DOI] [PubMed] [Google Scholar]
- Zimmer DB, Van Eldik LJ. Tissue distribution of rat S100 alpha and S100 beta and S100-binding proteins. Am J Physiol. 1987 Mar;252:C285–9. doi: 10.1152/ajpcell.1987.252.3.C285. 3 Pt 1. [DOI] [PubMed] [Google Scholar]
- Zimmer DB, Song W, Zimmer WE. Isolation of a rat S100 alpha cDNA and distrbution of its mRNA in rat tissues. Brain Res Bull. 1991 Aug;27(2):157–62. doi: 10.1016/0361-9230(91)90061-n. [DOI] [PubMed] [Google Scholar]
- Zimmer DB, Cornwall EH, Landar A, Song W. The S100 protein family: history, function, and expression. Brain Res Bull. 1995;37(4):417–29. doi: 10.1016/0361-9230(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Remppis A, Greten T, Schäfer BW, Hunziker P, Erne P, Katus HA, Heizmann CW. Altered expression of the Ca(2+)-binding protein S100A1 in human cardiomyopathy. Biochim Biophys Acta. 1996 Oct 11;1313(3):253–7. doi: 10.1016/0167-4889(96)00097-3. [DOI] [PubMed] [Google Scholar]
- Song W, Zimmer DB, Brain Res. Expression of the rat S100A1 gene in neurons, glia, and skeletal muscle. 1996 May 20;721(1-2):204–16. doi: 10.1016/0006-8993(96)00172-2. [DOI] [PubMed] [Google Scholar]
- Zimmer DB, Chessher J, Song W. Nucleotide homologies in genes encoding members of the S100 protein family. Biochim Biophys Acta. 1996 Oct 11;1313(3):229–38. doi: 10.1016/0167-4889(96)00094-8. [DOI] [PubMed] [Google Scholar]
- Landar A, Rustandi RR, Weber DJ, Zimmer DB. S100A1 utilizes different mechanisms for interacting with calcium-dependent and calcium-independent target proteins. Biochemistry. 1998 Dec 15;37(50):17429–38. doi: 10.1021/bi9817921. [DOI] [PubMed] [Google Scholar]
- Ehlermann P, Remppis A, Guddat O, Weimann J, Schnabel PA, Motsch J, Heizmann CW, Katus HA. Right ventricular upregulation of the Ca(2+) binding protein S100A1 in chronic pulmonary hypertension. Biochim Biophys Acta. 2000 Feb 21;1500(2):249–55. doi: 10.1016/s0925-4439(99)00106-4. [DOI] [PubMed] [Google Scholar]
- Kiewitz R, Lyons GE, Schäfer BW, Heizmann CW. Transcriptional regulation of S100A1 and expression during mouse heart development. Biochim Biophys Acta. 2000 Dec 20;1498(2-3):207–19. doi: 10.1016/s0167-4889(00)00097-5. [DOI] [PubMed] [Google Scholar]
- Teratani T, Watanabe T, Kuwahara F, Kumagai H, Kobayashi S, Aoki U, Ishikawa A, Arai K, Nozawa R. Induced transcriptional expression of calcium-binding protein S100A1 and S100A10 genes in human renal cell carcinoma. Cancer Lett. 2002 Jan 10;175(1):71–7. doi: 10.1016/s0304-3835(01)00724-8. [DOI] [PubMed] [Google Scholar]
- Most P, Boerries M, Eicher C, Schweda C, Ehlermann P, Pleger ST, Loeffler E, Koch WJ, Katus HA, Schoenenberger CA, Remppis A. Extracellular S100A1 protein inhbits apoptosis in ventricular cardiomyocytes via activation of the extracellular signal-regulated protein kinase 1/2 (ERK1/2) J Biol Chem. 2003 Nov 28;278(48):48404–12. doi: 10.1074/jbc.M308587200. [DOI] [PubMed] [Google Scholar]
- Zimmer DB, Wright Sadosky P, Weber DJ. Molecular mechanisms of S100-target protein interactions. Microsc Res Tech. 2003 Apr 15;60(6):552–9. doi: 10.1002/jemt.10297. [DOI] [PubMed] [Google Scholar]
- Wright NT, Varney KM, Ellis KC, Markowitz J, Gitti RK, Zimmer DB, Weber DJ. The three-dimensional solution structure of Ca(2+)-bound S100A1 as determined by NMR spectroscopy. J Mol Biol. 2005 Oct 21;353(2):410–26. doi: 10.1016/j.jmb.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Zimmer DB, Chaplin J, Baldwin A, Rast M. S100-mediated signal transduction in the nervous system and neurological diseases. Cell Mol Biol (Noisy-le-grand) 2005 Sep 5;51(2):201–14. [PubMed] [Google Scholar]
- Ackermann GE, Marenholz I, Wolfer DP, Chan WY, Schäfer B, Erne P, Heizmann CW. S100A1-deficient male mice exhibit increased exploratory activity and reduced anxiety-related responses. Biochim Biophys Acta. 2006 Nov;1763(11):1307–19. doi: 10.1016/j.bbamcr.2006.08.048. [DOI] [PubMed] [Google Scholar]
- Keijser S, Missotten GS, Bonfrer JM, de Wolff-Rouendaal D, Jager MJ, de Keizer RJ. Immunophenotypic markers to differentiate between benign and malignant melanocytic lesions. Br J Ophthalmol. 2006 Feb;90(2):213–7. doi: 10.1136/bjo.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Barthelemy A, Feng G, Gentil-Perret A, Peoc'h M, Genin C, Tostain J. S100A1: a powerful marker to differentiate chromophobe renal cell carcinoma from renal oncocytoma. Histopathology. 2007 Apr;50(5):642–7. doi: 10.1111/j.1365-2559.2007.02655.x. [DOI] [PubMed] [Google Scholar]
- Pleger ST, Most P, Boucher M, Soltys S, Chuprun JK, Pleger W, Gao E, Dasgupta A, Rengo G, Remppis A, Katus HA, Eckhart AD, Rabinowitz JE, Koch WJ. Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation. 2007 May 15;115(19):2506–15. doi: 10.1161/CIRCULATIONAHA.106.671701. [DOI] [PubMed] [Google Scholar]
- Saito T, Ikeda T, Nakamura K, Chung UI, Kawaguchi H. S100A1 and S100B, transcriptional targets of SOX trio, inhibit terminal differentiation of chondrocytes. EMBO Rep. 2007 May;8(5):504–9. doi: 10.1038/sj.embor.7400934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka D, Chiriboga L, Rubin BP. Differential expression of S100 protein subtypes in malignant melanoma, and benign and malignant peripheral nerve sheath tumors. J Cutan Pathol. 2008 Nov;35(11):1014–9. doi: 10.1111/j.1600-0560.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- Pleger ST, Harris DM, Shan C, Vinge LE, Chuprun JK, Berzins B, Pleger W, Druckman C, Vökers M, Heierhorst J, Øie E, Remppis A, Katus HA, Scalia R, Eckhart AD, Koch WJ, Most P. Endothelial S100A1 modulates vascular function via nitric oxide. Circ Res. 2008 Apr 11;102(7):786–94. doi: 10.1161/CIRCRESAHA.108.172031. [DOI] [PubMed] [Google Scholar]
- Prosser BL, Wright NT, Hernñndez-Ochoa EO, Varney KM, Liu Y, Olojo RO, Zimmer DB, Weber DJ, Schneider MF. S100A1 binds to the calmodulin-binding site of ryanodine receptor and modulates skeletal muscle excitation-contraction coupling. J Biol Chem. 2008 Feb 22;283(8):5046–57. doi: 10.1074/jbc.M709231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NT, Prosser BL, Varney KM, Zimmer DB, Schneider MF, Weber DJ. S100A1 and calmodulin compete for the same binding site on ryanodine receptor. J Biol Chem. 2008 Sep 26;283(39):26676–83. doi: 10.1074/jbc.M804432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Ochoa EO, Prosser BL, Wright NT, Contreras M, Weber DJ, Schneider MF. Augmentation of Cav1 channel current and action potential duration after uptake of S100A1 in sympathetic ganglion neurons. Am J Physiol Cell Physiol. 2009 Oct;297(4):C955–70. doi: 10.1152/ajpcell.00140.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus C, Rohde D, Weidenhammer C, Qiu G, Pleger ST, Voelkers M, Boerries M, Remppis A, Katus HA, Most P. S100A1 in cardiovascular health and disease: closing the gap between basic science and clinical therapy. J Mol Cell Cardiol. 2009 Oct;47(4):445–55. doi: 10.1016/j.yjmcc.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NT, Cannon BR, Wilder PT, Morgan MT, Varney KM, Zimmer DB, Weber DJ. Solution structure of S100A1 bound to the CapZ peptide (TRTK12) J Mol Biol. 2009a Mar 13;386(5):1265–77. doi: 10.1016/j.jmb.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NT, Cannon BR, Zimmer DB, Weber DJ. S100A1: Structure, Function, and Therapeutic Potential. Curr Chem Biol. 2009b May 1;3(2):138–145. doi: 10.2174/187231309788166460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusenko MV, Zubakov D, Kovacs G. Gene expression profiling of chromophobe renal cell carcinomas and renal oncocytomas by Affymetrix GeneChip using pooled and individual tumours. Int J Biol Sci. 2009 Jul 29;5(6):517–27. doi: 10.7150/ijbs.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Todd NW, Li R, Zhang H, Fang H, Stass SA. A panel of sputum-based genomic marker for early detection of lung cancer. Cancer Prev Res (Phila) 2010 Dec;3(12):1571–8. doi: 10.1158/1940-6207.CAPR-10-0128. [DOI] [PubMed] [Google Scholar]
- Rohde D, Ritterhoff J, Voelkers M, Katus HA, Parker TG, Most P. S100A1: a multifaceted therapeutic target in cardiovascular disease. J Cardiovasc Transl Res. 2010 Oct;3(5):525–37. doi: 10.1007/s12265-010-9211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sviatoha V, Tani E, Kleina R, Sperga M, Skoog L. Immunohistochemical analysis of the S100A1, S100B, CD44 and Bcl-2 antigens and the rate of cell proliferation assessed by Ki-67 antibody in benign and malignant melanocytic tumours. Melanoma Res. 2010 Apr;20(2):118–25. doi: 10.1097/CMR.0b013e3283350554. [DOI] [PubMed] [Google Scholar]
- Zimmer DB, Weber DJ. The Calcium-Dependent Interaction of S100B with Its Protein Targets. Cardiovasc Psychiatry Neurol. 2010 doi: 10.1155/2010/728052. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon BR, Zimmer DB, Weber DJ. S100A1 (S100 calcium binding protein A1) Atlas Genet Cytogenet Oncol Haematol. 2011;15(10):873–876. doi: 10.4267/2042/46035. [DOI] [PMC free article] [PubMed] [Google Scholar]


