Abstract
This is a case report of a patient who presented to the wound care center with LE ulcerations that were subsequently diagnosed with calciphylaxis. She was an insulin dependent diabetic with renal disease, but unaware of her critical kidney status. She was treated with local wound care, a partial parathyroidectomy, and started on dialysis. She is currently healed with no recurrence of ulcerations. Her ulcerations were controlled with conservative wound care and no surgical debridement.
Keywords: Calciphylaxis, Wound care, Lower extremity ulcerations
Introduction
Calciphylaxis is a rare condition defined by small vessel calcification which leads to ischemic injury to the skin then ulceration and infarction.1,2 Calciphylaxis is most commonly reported in patients with chronic kidney disease, specifically ESRD and secondary hyperparathyroidism, but cases have been reported in patients without ESRD.1–6 Margo, Simman, and Jackson stated in their review article that there is an emerging body of literature describing calciphylaxis in diverse clinical settings unassociated with renal dysfunction.5 Brandenburg, Kramann, Specht, and Ketteler state in their article that calciphylaxis is presumably at least in part an iatrogenic man-made problem since it does not belong to the well-known natural sequel of CKD (such as renal bone disease or renal hypertension or renal anemia).6 Bryant and White first recorded the occurrence of calciphylaxis in uremia in 1898 in Guy's Hospital Reports, but Selye coined the term calciphylaxis and a theory for its pathophysiology.7 He used experimental rat models to break down calciphylaxis into three stages: sensitization, latency, and challenging.8 Sensitizers include hyperphosphatemia, hypercalcemia, an elevated calcium × phosphate product, increased intact parathyroid hormone, and vitamin D.1,8–11 Challengers included local trauma, steroids, iron salts, egg albumin, mast cell releasers, and cytotoxic medications.2,8,12 Selye's rat model may not correlate with the vascular calcification syndrome seen in human patients. Calcific uremic arteriolopathy, uremic gangrene syndrome, metastatic calcinosis, and azotemic calcific arteriopathy are all other names used to identify calciphylaxis, but the term remains widely used.13–15 The pathogenesis of calciphylaxis remains uncertain, but recent evidence indicates that vascular calcification is an active process similar to bone formation which is subject to regulation by osteotrophic hormones in addition to key inhibitors of passive mineralization.1,14,16 Also, several cases have documented a hypercoagulabiltiy that may play a role, such as with Protein C and S deficiency.8
The presentation of calciphylaxis in a patient usually begins with painful, violaceous, mottled skin lesions identified as livedo reticularis that are commonly symmetrical.7–9 These lesions then evolve into demarcated nonhealing ulcerations which become necrotic and gangrenous.7 These ulcerations may occur on the abdomen, back, buttocks, thighs, lower extremities, forearms, acral sites, and genitalia.7,9,17 If a patient with this presentation enters a wound center with other risk factors, a skin biopsy may aid in diagnosis. A skin biopsy is not definitive in making the diagnosis of calciphylaxis.14 The pathologic examination of a biopsy reveals arterial medial arteriolar calcification, subintimal fibrosis, and arterial occlusion in the absence of vasculitic change with acute and chronic calcifying septal panniculitis. Subcutaneous and dermal vascular thrombosis may also be seen.14,18,19 In the case presented below, the patient's ulcerations fit this presentation and after admission to the hospital with the confirmation of laboratory data and a skin biopsy the diagnosis of calciphylaxis was determined.
Case Report
A 54 y/o Caucasian female presented to our wound care clinic with bilateral anterior lower extremity ulcerations with irregular borders and a dark solid eschar. (Figure A for initial ulcer presentation) The patient related a past medical history of IDDM (uncontrolled), Hepatitis C, chronic renal insufficiency, HTN, and s/p right nephrectomy approximately 5 years ago. She denied any trauma to the lower extremities and stated that the ulcers started as reddened areas. She also admitted to being hospitalized approximately 1–2 months prior to her first visit to the wound center with cellulitis and was told that she had diabetic ulcers. She was evaluated and had noted local signs of erythema surrounding the eschars and pain around the areas. A plan of care was started that included an enzymatic debriding agent and dry wrap of kerlix and ace from the base of the toes to below the knee. She was placed on an oral antibiotic as well for the local cellulitis. She was to do her dressing changes at home and return to the clinic in 1 week to reevaluate.
Figure.
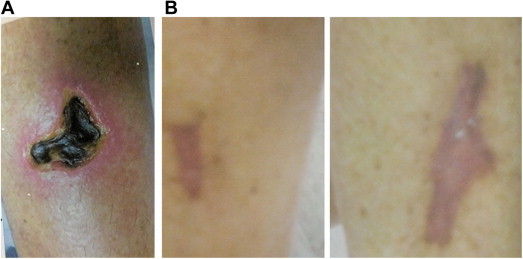
A. Initial wound. B. Healed wound.
The patient returned in 1 week with increased erythema/warmth to the bilateral lower extremities surrounding the ulcers and extending lymphangitis despite oral antibiotics. She was also unable to tolerate the enzymatic debriding agent for dressing changes. At this time, she was admitted to the hospital with ascending cellulitis of the lower extremities. The patient was admitted and a full work up was done with pertinent lab results as follows: BUN – 62 mg/dl (6–23), Creatinine – 4.40 mg/dl (0.60–1.30), ESR – 134 mm/h (0–30), Parathormone Intact, Serum – 127 pg/ml, Phosphorus – 7.3 mg/dl (2.5–4.9), Vitamin D 25-hydroxy – 16 ng/ml, Serum Albumin – 1.7 g/dl (3.4–5.0). She had a biopsy done during this admission with microscopic exam revealing subcutaneous fat calcifications of the large and small blood vessels with extravascular calcification of the septum and fat lobules compatible with calciphylaxis. She had lower extremity vascular testing done during her inpatient stay that revealed noncompressible vessels bilateral lower extremities. The cellulitis was controlled with IV antibiotics and she was discharged to follow up in the wound center as an outpatient. She was seen by nephrology and a decision was made to place her on dialysis. She gained control of her glucose levels with diet and a change in medications. She followed up in the wound center. Due to the increased pain at the ulcer sites and pain with the enzymatic debridement agent, she was placed on a wound care plan of Curasol (Healthpoint) and 2% Lidocaine gel with a dry wrap of kerlix and an Ace wrap from the base of the toes to below the knee. This helped to relieve the discomfort of the ulcer sites and the Curasol, although a hydrogel, allowed for gentle sloughing/autolytic debridement of the wounds and allowed for a moist wound base. She was to change the dressings every 1–2 days and was followed up in the wound center. She noted decreased pain with this treatment therapy. She was showing improvement with this therapy and no manual debridement was required. Approximately 5 months after initial diagnosis with calciphylaxis, she had a subtotal parathyroidectomy. She was discharged from the wound center approximately 10 months after her first visit with healed wounds with no recurrence upon speaking to the patient during a follow up phone interview (Figure B for healed wound).
Discussion
The patient in this case report was fortunate enough to be diagnosed early with calciphylaxis and began a full range of treatment plan. As mentioned above, presentation of ulcerations and histology was discussed. A high suspicion of calciphylaxis would also help dictate diagnosis. The following risk factors would give the treating physician a high suspicion for a possible calciphylaxis diagnosis. These risk factors include female undergoing dialysis, ESRD patients with BMI greater than 30, increasing increments of phosphorous levels in ESRD patients, low serum albumin, and administration of medications with higher chance of calciphylaxis, such as warfarin, calcium based binders, Vitamin D analogue.1,14 There is a patient population that does not have ESRD whom may be placed in the risk factor category and include primary hyperparathyroidism, Crohn's disease, AIDS, polymyositis, Sjogren's syndrome, rheumatoid arthritis, sarcoidosis, and systemic lupus erythematosus, cirrhosis, multiple myeloma, and POEMS.5,8,14 Brandenburg et al6 state in their article that calciphylaxis turns out to be most likely a multifactorial disease. Other differential diagnoses to consider and rule out when suspicious of calciphylaxis include, but are not limited to cholesterol embolization, warfarin necrosis, cryoglobulinemia, cellulitis, and nephrogenic systemic fibrosis.14
The incidence of calciphylaxis is approximately 4% in hemodialysis patients.2,8,10,14 The incidence of calciphylaxis in chronic renal failure patients is approximately 1%.13 The outcome is grim for patients diagnosed with calciphylaxis. The mortality rates vary from a 1 year survival rate of 46%13 to 60–70% overall survival rate.7 The prognosis may vary depending on the location of the ulcerations. Patients with calciphylaxis had proximal lesions including the trunk, knees, and elbows 68% of the time and distal involvement below the knees or distal to the elbows 32% of the time.2,8,20 Roe et al9 stated that proximal lesions have a poorer prognosis than patients with distal lesions. The high mortality rate in calciphylaxis is often secondary to sepsis.2,8,9,14
Treatment of calciphylaxis is in most cases multifactorial and no one treatment stands alone above the rest. Treatments include phosphate binders, decreased calcium in dialysate, antibiotics, low phosphate diet, sodium thiosulfate, cinacalet, bisphosphates, anticoagulation, cyclosporine, stanozolo, parathyroidectomy, local wound care, debridement, renal transplantation, skin grafting, avoidance of challenging agent.2,5,9,13,14 Hyperbaric oxygen treatment and systemic steroids, even though listed as a challenger, have also been suggested with a few studies as treatment.7,8,14 Multiple case studies suggest aggressive wound care and debridement.2,8,14 In the above patient case, we did not aggressively debride the wound sites and she failed the use of enzymatic debridement with reports of pain when using the product. Instead, we opted for Curasol (Healthpoint) and lidocaine gel for gentle autolytic debridement. Milas et al10 also used Curasol without lidocaine gel for their wound care regimen, but performed wound debridements as well. Our patient had a subtotal parathyroidectomy which in literature is not a definitive treatment. Roe et al9 felt that patients with ulcerations more peripheral and who had induration, with or without small eschar, were more likely to experience symptomatic relief, healing, and survival after parathyroidectomy. Early parathyroidectomy is recommended prior to sepsis.7
Conclusion
The patient in this case report, at this point, has had a favorable outcome. She had an early diagnosis of calciphylaxis. She began dialysis for her ESRD after diagnosis and had a subtotal parathyroidectomy. We decided to opt for autolytic debridement rather than aggressive wound debridement or a more aggressive approach to her wound care with enzymatic debridement after she had too much pain with this wound care product. Our patient's favorable outcome may also be linked to the location of her ulcerations which were on her anterior lower extremities below the knee. She was diagnosed and treated in a multifactorial approach with resolution of her ulcerations within 1 year of diagnosis with no recurrence at this time.
Footnotes
Dr. Marshall is the director of the University Hospitals Richmond Medical Center Wound Care/Infectious Disease Fellowship. She also works as an attending with podiatric residents at the University Hospital Richmond Medical Center/Kent State University College of Podiatric Medicine.
Dr. Johnson is currently an Assistant Professor of the Podiatric Medicine Department of Kent State University College of Podiatric Medicine. She graduated from the Wound Care/Infectious Disease Fellowship through the University Hospitals Richmond Medical Center. She is a graduate of the University Hospitals Richmond Medical Center/Ohio College of Podiatric Medicine PM & S 36 residency program.
There were no grant funds with this project. This case report was not adapted from any presentation.
Disclaimers: None.
References
- 1.Couto F.M., Chen H., Blank R.D., Drezner M.K. Calciphylaxis in the absence of end-stage renal disease. Endocr Pract. 2006;4:406–410. doi: 10.4158/EP.12.4.406. [DOI] [PubMed] [Google Scholar]
- 2.Mureebe L., Moy M., Balfour E., Blume P., Gahtan V. Calciphylaxis: a poor prognostic indicator for limb salvage. J Vasc Surg. 2001;33(6):1275–1279. doi: 10.1067/mva.2001.115378. [DOI] [PubMed] [Google Scholar]
- 3.Coates T., Kirkland G.S., Dymock R.B. Cutaneous necrosis form calcific uremic arteriolopathy. Am J Kidney Dis. 1998;32:384–391. doi: 10.1053/ajkd.1998.v32.pm9740153. [DOI] [PubMed] [Google Scholar]
- 4.Duh Q.Y., Lim R.C., Clark O.H. Calciphylaxis in secondary hyperparathyroidism: diagnosis and parathyroidectomy. Arch Surg. 1991;126:1213–1219. doi: 10.1001/archsurg.1991.01410340055008. [DOI] [PubMed] [Google Scholar]
- 5.Margo C.M., Simman R., Jackson S. Calciphylaxis: a review. J Am Coll Cert Wound Spec. 2010;2:66–72. doi: 10.1016/j.jcws.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandenburg V.M., Kramann R., Specht P., Ketteler M. Calciphylaxis in CKD and beyond. Nephrol Dial Transplant. 2012;27:1314–1318. doi: 10.1093/ndt/gfs015. [DOI] [PubMed] [Google Scholar]
- 7.Mathur R.V., Shortland J.R., El Nahas A.M. Calciphylaxis. Postgrad Med J. 2001;77:557–561. doi: 10.1136/pmj.77.911.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean S.M., Werman H. Calciphylaxis: a favorable outcome with hyperbaric oxygen. Vasc Med. 1998;3:115–120. doi: 10.1177/1358836X9800300205. [DOI] [PubMed] [Google Scholar]
- 9.Roe S.M., Graham L.D., Brock W.B., Baker D.E. Calciphylaxis: early recognition and management. Am Surg. 1994;60:81–86. [PubMed] [Google Scholar]
- 10.Milas M., Bush R.L., Lin P. Calciphylaxis and nonhealing wounds: the role of the vascular surgeon in a multidisciplinary treatment. J Vasc Surg. 2003:501–507. doi: 10.1067/mva.2003.70. [DOI] [PubMed] [Google Scholar]
- 11.Seyle H. University of Chicago Press; Chicago, IL: 1962. Calciphylaxis. [Google Scholar]
- 12.Seyle H., Gabbiani G., Strebel R. Sensitization to calciphylaxis by endogenous parathyroid hormone. Endocrinology. 1962;71:554–558. doi: 10.1210/endo-71-4-554. [DOI] [PubMed] [Google Scholar]
- 13.Baranoski S., Ayello E.A. 3rd ed. Lippincott Williams & Wilkins; Philadephia: 2012. Wound Care Essentials; pp. 502–503. [Google Scholar]
- 14.Santos P.W., Hartle J.E., Quarles L.D. Calciphylaxis. In: Sheridan A., editor. Up to Date. 2012. www.uptodate.com Available at: [Google Scholar]
- 15.Rogers N.M., Coates P.T. Calcific uraemic arteriolopathy: an update. Curr Opin Nephrol Hypertens. 2008;17:629–634. doi: 10.1097/MNH.0b013e32830f4566. [DOI] [PubMed] [Google Scholar]
- 16.Vattikuti R., Towler D.A. Osteogenic regulation of vascular calcification: an early perspective. Am J Physiol Endocrinol Metab. 2004;286:E686–E696. doi: 10.1152/ajpendo.00552.2003. [DOI] [PubMed] [Google Scholar]
- 17.Jhaveri F.M., Woosley J.T., Fried F.A. Penile calciphylaxis: rare necrotic lesions in chronic renal failure patients. J Urol. 1998;160:764–767. doi: 10.1016/S0022-5347(01)62781-2. [DOI] [PubMed] [Google Scholar]
- 18.Weenig R.H., Sewell L.D., David M.D. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;(56):569. doi: 10.1016/j.jaad.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 19.Essary L.R., Wick M.R. Cutaneous calciphylaxis: an underrecongnized clinicopathologic entity. Am J Clin Pathol. 2000;(113):280. doi: 10.1309/AGLF-X21H-Y37W-50KL. [DOI] [PubMed] [Google Scholar]
- 20.Budiasavljevic M.N., Cheek D., Ploth D.W. Calciphylaxis in chronic renal failure. J Am Soc Nephrol. 1996;(7):979–984. doi: 10.1681/ASN.V77978. [DOI] [PubMed] [Google Scholar]


