Abstract
Oxidative stress is considered a causative factor in carcinogenesis, but also in the development of resistance to current chemotherapies. The appropriate usage of redox-modulating compounds is limited by the lack of knowledge of their impact on specific molecular pathways. Increased levels of the IKKε kinase, as a result of gene amplification or aberrant expression, are observed in a substantial number of breast carcinomas. IKKε not only plays a key role in cell transformation and invasiveness, but also in the development of resistance to tamoxifen. Here, we studied the effect of in vitro treatment with the redox-modulating triphenylmethane dyes, Gentian Violet and Brilliant Green, and nitroxide Tempol on IKKε expression and cell proliferation in the human breast cancer epithelial cell lines exhibiting amplification of IKKε, MCF-7 and ZR75.1. We show that Gentian Violet, Brilliant Green and Tempol significantly decrease intracellular superoxide anion levels and inhibit IKKε expression and cell viability. Treatment with Gentian Violet and Brilliant Green was associated with a reduced cyclin D1 expression and activation of caspase 3 and/or 7. Tempol decreased cyclin D1 expression in both cell lines, while activation of caspase 7 was only observed in MCF-7 cells. Silencing of the superoxide-generating NOX2 NADPH oxidase expressed in breast cancer cells resulted in the significant reduction of IKKε expression. Taken together, our results suggest that redox-modulating compounds targeting NOX2 could present a particular therapeutic interest in combination therapy against breast carcinomas exhibiting IKKε amplification.
Keywords: Breast cancer, IKKε, NADPH oxidase, Cell growth, Tempol, Triphenylmethane dyes
Graphical abstract
Highlights
-
•
IKKε kinase is amplified in MCF7 and ZR75.1 breast cancer cells.
-
•
Brilliant Green, Gentian Violet and Tempol reduce superoxide levels in MCF7 and ZR75.1 cells.
-
•
Brilliant Green, Gentian Violet and Tempol inhibit IKKε expression in MCF7 and ZR75.1 cells.
-
•
IKKε overexpression in breast cancer cells is dependent on NOX2.
-
•
Brilliant Green, Gentian Violet and Tempol reduce MCF7 and ZR75.1 cell viability.
1. Introduction
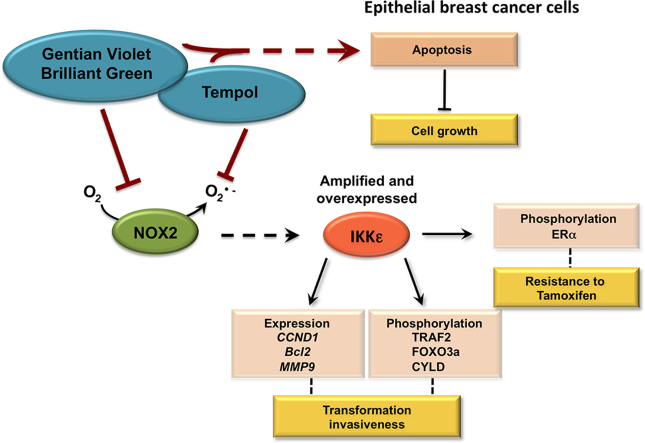
Breast cancer is a leading cause of cancer-related mortality in women, second only to lung cancer. Breast carcinomas are characterized by their biological complexity and heterogeneity. Initiation and progression of breast cancer is a multi-step process that involves the dysregulation of multiple genes controlling cell survival and proliferation [1,2]. IKKε (also known as IKBKE or IKKi) is part of the IKK family of kinases, of which IKKα and IKKβ are the classical members, well known for their role in the activation of the NF-κB transcription factor [3,4]. Increased IKKε activity, as a result of amplification of the 1q32 region comprising the IKBKE locus and/or aberrant expression, was identified in 30% primary breast tumors, in epithelial breast cancer cell lines and in murine mammary breast tumors induced by 7,12-dimethylbenzene(a)anthracene (DMBA) [5,6]. Functional studies have shown that IKKε plays a key role in cell transformation and invasiveness [6–8]. IKKε-mediated mammary epithelial cell transformation is dependent on the phosphorylation of the cylindromatosis tumor suppressor (CYLD), the estrogen receptor α (ERα), the tumor necrosis factor receptor-associated factor 2 (TRAF2) E3 ligase and of the Forkhead box O 3a (FOXO3a) transcription factor [8–11]. Expression of the CCND1 (Cyclin D1), MMP-9 (metalloproteinase-9) and Bcl-2 genes was found to be dependent on IKKε activity [5,6,9]. Importantly, IKKε was also shown to contribute to the development of resistance of hormone-dependent breast cancers to the selective estrogen receptor modulator tamoxifen, likely through its role in ERα phosphorylation [9]. Primary or acquired resistance to tamoxifen severely reduces its clinical effectiveness and constitutes a serious threat to the eradication of breast cancer [12].
Cellular redox homeostasis, fundamental for a proper function of the cell, results from a critical balance between production of reactive oxygen species (ROS) and detoxification ensured by antioxidant enzymes. ROS control various cell responses, ranging from proliferation, motility, senescence, severe cellular damage and cell death, in a cell-type and dose-dependent manner [13–17]. In cancer cells high ROS production/antioxidant capacity results in high ROS levels that are yet compatible with cell survival [18]. Oxidative stress has emerged as an important pathogenic factor in the development of a large number of tumors and malignant cells, including breast carcinomas [17,19,20]. Traditionally, the oxidative stress theory of cancer is associated with the capacity of ROS to induce DNA damage and promote genetic instability. However, ROS are now well appreciated to act as cellular switches for signaling cascades [21,22]. Intriguingly, ROS are double-edged swords that can have dual roles in cancer by either promoting prooncogenic or antitumorigenic signaling pathways, making the use of redox-modulating agents in anticancer therapeutic strategies a complex task [18,23]. Conversion of breast tumors to a Tam-resistant phenotype was also reported to be associated with oxidative stress [24–26].
A major hurdle in the use of most compounds with known redox-modulating activities is the lack of knowledge of their impact on specific molecular pathways. Thus, a better understanding of the pathways that are altered by redox-modulating compounds in breast cancer cells will help define an appropriate therapeutic usage. Here, we studied the impact of the cationic triphenylmethane dyes, Brilliant Green and Gentian Violet, on ERα+ breast cancer epithelial cell lines, MCF-7 and ZR75.1, which exhibit cell growth dependence on amplified IKKε [27]. Brilliant Green and the Federal Drug Administration (FDA)-approved Gentian Violet are of particular interest as they have a long history of human and veterinary use in many conditions including bacterial, fungal and parasitic infections [28,29]. Gentian Violet and Brilliant Green were recently shown to have an impact on host cells with an effect on cellular redox mechanisms by inhibition of NADPH oxidases [30] and modulation of the thioredoxin (Trx) system [31]. The observation that Gentian Violet and Brilliant Green inhibit NADPH oxidases has expanded their use to treatment of cutaneous melanoma metastasis and hemangiomas [30,32]. We also studied the impact of a different class of redox-modulating agent using the nitroxide Tempol, 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl, a cell-permeant superoxide dismutase mimetic that is the lead compounds of a series of nitroxide that have antioxidant activities, mainly through free radical scavenging [33,34].
Here, we show that Gentian Violet, Brilliant Green and Tempol, which all significantly decreased intracellular superoxide levels, impaired the expression of the oncogenic IKKε kinase in MCF-7 and ZR75.1 cells. We also report that either treatment significantly reduced cell viability. Treatment with Gentian Violet and Brilliant Green was associated with a reduced cyclin D1 expression and activation of caspase 3 and/or 7. Tempol decreased cyclin D1 expression in both cell lines, but activation of caspase 7 was only observed in MCF-7 cells, but not in ZR75.1 cells. Distinct isoforms of the NADPH oxidases family (NOX1-5/DUOX1-2) were found expressed in MCF-7 and ZR75.1 cells and in primary breast tumors. Knockdown of the superoxide-generating NOX2 isoform resulted in significant reduction of IKKε expression. Taken together, our data show that the molecular consequences of the treatment of breast cancer cells exhibiting amplification of the IKKε kinase with Gentian Violet and Brilliant Green, or Tempol involve the inhibition of NOX2-dependent IKKε expression and an antiproliferative response. These findings provide valuable insights into the use of redox-modulating agents that target at least NOX2 in the treatment of breast cancers highly expressing IKKε kinase.
2. Methods
2.1. Reagents
Gentian Violet and Brilliant Green were purchased from Sigma-Aldrich. Tempol was from Enzo Life Sciences. Previously described RNAi oligonucleotides (RNAi, [35]) and Dharmafect 1 transfection reagent were from Dharmacon.
2.2. Cell culture
ZR75.1 and MCF-7 breast cancer cell lines were obtained from Dr. S. Mader, Université de Montréal, Canada. ZR75.1 cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FBS (HI-FBS) and 1% l-glutamine. MCF-7 cells were maintained in MEM medium supplemented with 10% HI-FBS, 1% l-glutamine, 1% non-essential amino acids and 1% sodium pyruvate. All media and supplements were obtained from Life Technologies. All cultures were performed without antibiotics and controlled for the absence of mycoplasma contamination on a regular basis using the MycoAlert Mycoplasma Detection Kit (Lonza).
2.3. Primary breast tumor samples
Primary breast tumors samples were provided by the “Banque de tissues et de données” of the “Réseau de Recherche sur le cancer” of the Fonds de Recherche Du Quebec – Santé (FRQS), which is affiliated with the Canadian tumor repository network. Written and informed consent was obtained from each patient. The present research was conducted with the approval of the Ethics Committee of the CRCHUM where the experiments were performed.
2.4. Transfection of RNAi oligonucleotides
Transfection of RNAi oligonucleotides was performed using the Dharmafect1 transfection reagent according to the manufacturer’s instructions. Cells were plated in 35 mm plates to reach 30–40% confluency at the time of transfection and were transfected using 8 µl of transfection reagent and 100 pmol of oligonucleotide. Transfection was pursued for 72 h before harvesting.
2.5. Intracellular superoxide detection
Intracellular superoxide levels were assessed using the fluorescent dye dihydroethidium (DHE, Life Technologies) by flow cytometry. MCF-7 and ZR75.1 were cultured and treated with the indicated compounds in phenol-red free medium for 2–4 h. Cells were loaded with 1 µM DHE for 1 h at 37 °C before being collected by brief trypsinization. Cells were washed twice in PBS, and resuspended in HBSS for analysis using an LSR-II (BD Biosciences). Cells were excited at 488 nm and emission was collected at 575 nm, which are specific for the detection of the superoxide-specific DHE metabolite oxyethidium [36]. No dye cells (data not shown) and vehicle-treated cells were used as controls. All data were acquired with the FACSDiva (BD Biosciences) and analyzed using the FlowJo software (BD Biosciences).
2.6. RT-PCR analyses
Total RNA from MCF-7 and ZR75.1 cells was prepared using the RNAqueous-96 Isolation Kit (Ambion). Total RNA from primary breast tumors was prepared as described in [37]. Total RNA was subjected to reverse transcription using the QuantiTect Reverse Transcription Kit (Qiagen). Quantitative PCR amplifications of IKKε, NOX2, NOX5, S9 and β-actin genes were performed using the Fast start SYBR Green Kit (Roche) in a Rotor-Gene 3000 Real-Time Thermal Cycler (Corbett Research). Absence of genomic DNA contamination was analyzed using a reaction without reverse transcriptase. Gene expression normalized over actin or S9 was analyzed using the ΔΔ Cycle threshold (Ct) method [38] or as absolute mRNA copy numbers using plasmid-based standard curves as described in [39]. RT-PCR analysis of NOX/DUOX isoforms expression was performed as previously described [35]. Positive controls were used for each gene, as follows: total RNA from colon was used for NOX1; total RNA from DMSO-differentiated HL60 was used for NOX2; total RNA from human fetal kidney was used for NOX3; total RNA from MRC-5 were used for NOX4; total RNA from human spleen was used for NOX5; total RNA from human thyroid was used for DUOX1 and DUOX2. Human thyroid total RNA, human fetal kidney total RNA, human colon total RNA, and human spleen total RNA were purchased from BD Clontech.
2.7. Quantification of cell viability
Cell viability was monitored after the indicated time of treatment with the pharmacological inhibitors or the corresponding vehicle using a crystal violet colorimetric assay [40]. The optical density (570 nm) was determined and expressed as a relative value in percent of control cells treated with the vehicle.
2.8. Immunoblot analysis
Whole cell extracts (WCE) were prepared in Nonidet P-40 lysis buffer (50 mM Hepes pH 7.4, 150 mM NaCl, 5 mM EDTA, 10% Glycerol, 1% IGEPAL® CA-630 supplemented with 1 µg/ml leupeptin, 2 µg/ml aprotinin, 5 mM NaF, 1 mM orthovanadate, 2 mM PNPP, 10 mM β-glycerophosphate pH 7.5) for 20 min on ice followed by 3 rounds of freeze and thaw. WCE were collected after centrifugation at 17,000g for 20 min at 4 °C. WCE were subjected to SDS-PAGE electrophoresis and transferred onto nitrocellulose membrane (Biorad). The membrane was blocked in PBS containing 0.5% Tween (PBS-T) and 5% nonfat dry milk for 1 h at room temperature before incubation with the following primary antibodies: anti-cleaved Caspase 3, anti-cleaved Caspase 7, anti-PARP, anti-IKKε and anti-cyclin D1 (all from Cell Signaling), anti-TBK1 (Imgenex) and anti-actin (Chemicon International). Antibodies were diluted in PBS-T containing either 5% nonfat dry milk or BSA. After 5 washes in PBS-T, membranes were incubated for 1 h with HRP-conjugated goat anti-rabbit (Kirkegaard & Perry Laboratories or Jackson Immunoresearch Labs) or goat anti-mouse secondary antibodies (Kirkegaard & Perry Laboratories) in blocking solution. Immunoreactive bands were visualized by enhanced chemiluminescence using the Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Sciences) and detected using a LAS4000mini CCD camera apparatus (GE Healthcare). Quantification was performed using the ImageJ v1.42q software.
2.9. Statistical analyses
Results are presented as the mean of a minimum of 3 independent replicates±standard deviation (SD) or standard deviation to the mean (SEM) as indicated. Statistical analyses were performed with the GraphPad Prism software version 5.0 using the indicated tests. Statistical relevance was evaluated using the following p values: p<0.05 (*), p<0.01 (**) and p<0.001 (***).
3. Results
Tempol, Gentian Violet and Brilliant Green reduce intracellular superoxide levels in MCF-7 and ZR75.1 breast cancer cell lines exhibiting amplification of IKKε.
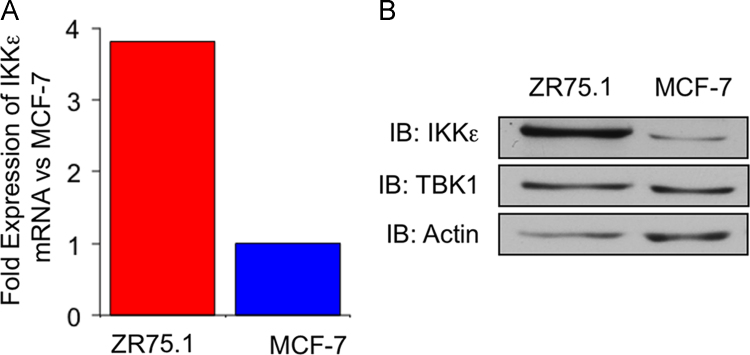
A previous report showed that MCF-7 and ZR75.1 exhibit amplification of the IKBKE gene encoding the IKKε kinase, reflecting a similar observation made in a substantial fraction of primary breast tumors [6]. Ten copies of IKBKE were detected in ZR75.1 cells and five copies in MCF-7 cells [6]. Here, we confirmed that ZR75.1 cells express higher IKKε mRNA (Fig. 1A) and protein levels (Fig. 1B) than MCF-7 cells. In contrary, protein expression levels of the closely related IKKε homolog Tank Binding Kinase 1 (TBK1) was similar in the two cell lines (Fig. 1B).
Fig. 1.
IKKε expression levels in MCF-7 compared to ZR75.1 cells. (A) Total RNA were extracted from MCF-7 and ZR75.1 cells, subjected to reverse transcription and IKKε mRNA expression was quantified by RT-qPCR and expressed as fold over MCF-7 level after normalization to actin expression. (B) WCE extracts were prepared and resolved by SDS-PAGE. Immunoblot analyses were performed using anti-IKKε and anti-TBK1 antibodies. Anti-actin antibodies were used to control equal loading. Data are representative of two experiments.
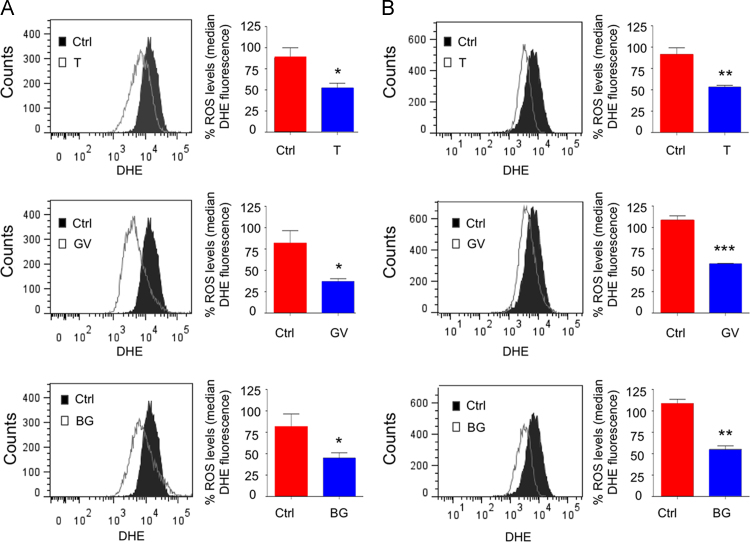
MCF-7 and ZR75.1 cells were left untreated (control) or individually treated with the triphenylmethane dyes, Gentian Violet and Brilliant Green, and the nitroxide Tempol before staining with the dihydroethidium (DHE) fluorescent probe and analysis by flow cytometry (ex: 488 nm/em: 575 nm). Consistent with a reduction in superoxide levels, a significant reduction in the median DHE fluorescence was observed in MCF-7 (Fig. 2A) and in ZR75.1 cells (Fig. 2B) treated with 5 µM Gentian Violet or 5 µM Brilliant Green compared to control cells. Treatment of MCF-7 cells with 5 mM Tempol (Fig. 2A) and ZR75.1 cells with 3 mM Tempol (Fig. 2B) also significantly reduced the median DHE fluorescence. These observations demonstrate that triphenylmethane dyes and nitroxide Tempol significantly reduce intracellular superoxide levels in IKKε-expressing MCF-7 and ZR75.1 breast cancer cell lines.
Fig. 2.
Reduction of intracellular superoxide levels in cells treated with tempol, Gentian Violet or Brilliant Green. In (A), MCF-7 cells were left untreated or treated with Tempol (T, 5 mM), Gentian Violet (GV, 5 µM) or Brilliant Green (BG, 5 µM). In (B), ZR75.1 cells were left untreated or treated with Tempol (3 mM), Gentian Violet (5 µM) or Brilliant Green (5 µM). Intracellular superoxide levels were monitored using staining with the fluorescent dye DHE. Fluorescence (ex: 488 nm/em: 575 nm) was acquired by flow cytometry and the median of fluorescence was quantified. Values are means±SEM from n≥3. Statistical comparison (treated cells vs untreated cells) was performed using a t-test.
Tempol and triphenylmethane dyes inhibit IKKε expression in MCF-7 and ZR75.1 breast cancer cell lines.
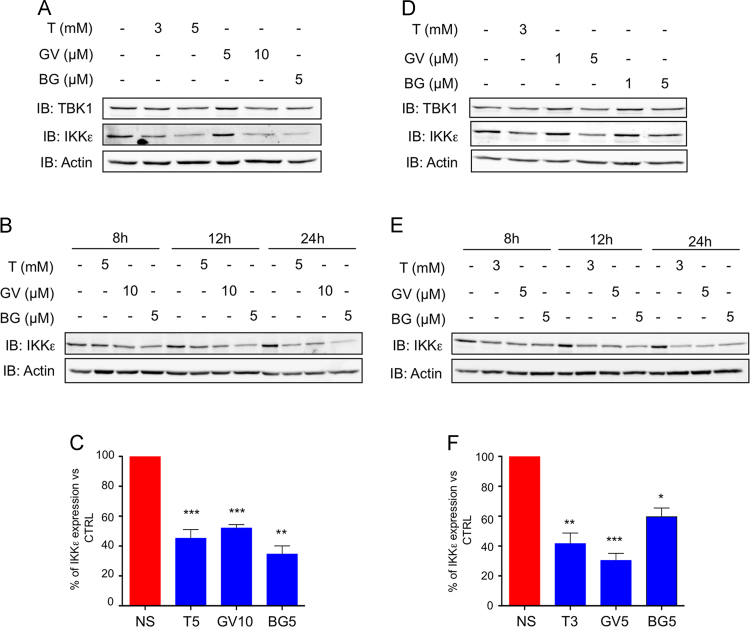
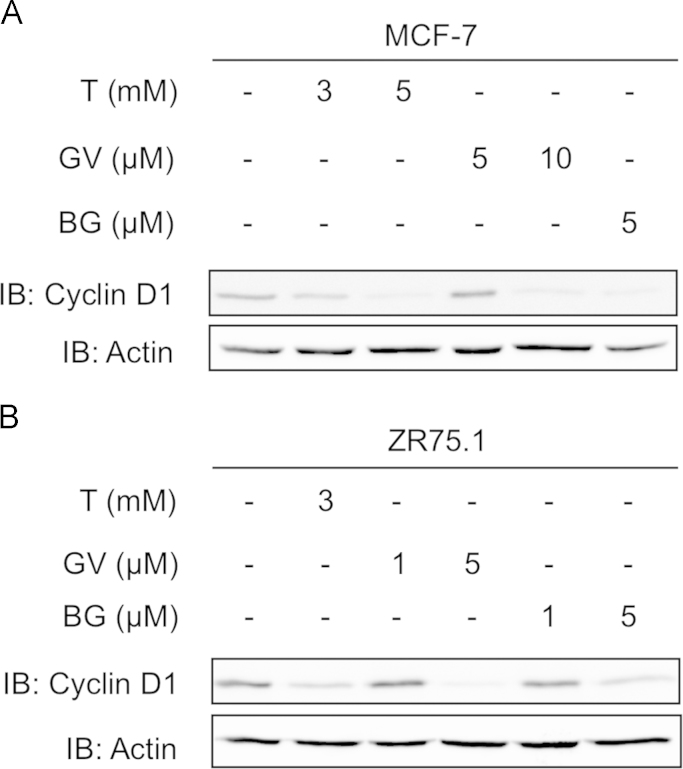
To determine if treatment of breast cancer cells with the redox-modulating triphenylmethane dyes or Tempol modulates IKKε expression levels, MCF-7 and ZR75.1 cells were subjected to treatment with each of the compounds for 8–24 h and further analyzed by immunoblot using anti-IKKε antibodies. As shown in Fig. 3, Tempol, Gentian Violet and Brilliant Green decreased IKKε expression with significant inhibition at 5 mM, 10 and 5 µM, respectively in MCF-7 cells (Fig. 3A–C) and at 3 mM, 5 and 5 µM, respectively in ZR75.1 cells (Fig. 3D and E). Of note, in these same conditions the expression levels of TBK1 remained steady (Fig. 3A and D). The CCND1 gene, encoding cyclin D1, was previously shown to be regulated by IKKε in epithelial breast cancer cells [5,6,9]. Immunoblot analyses showed that cyclin D1 expression is impaired by Gentian Violet, Brilliant Green or Tempol treatment in MCF-7 and ZR75.1 cells with a profile similar to that of IKKε expression (Fig. 4). Taken together, these results demonstrate that Tempol, Gentian Violet and Brilliant Green significantly impair expression of the IKKε oncogenic kinase in breast cancer cells exhibiting amplification of the IKBKE gene.
Fig. 3.
Treatment with Gentian Violet, Brilliant Green or Tempol decreases oncogenic IKKε kinase expression levels. MCF-7 (A–C) and ZR75.1 (B–F) were left untreated or treated with Tempol (T), Gentian Violet (GV) or Brilliant Green (BG) at the indicated concentrations for 24 h (A, C, D and F) or the indicated times (B and E). WCE were resolved by SDS-PAGE and analyzed by immunoblot using anti-IKKε and anti-TBK1 antibodies. Equal loading was monitored using anti-actin antibodies. Representative immunoblots of at least three experiments are shown. In (C) and (F), IKKε levels were quantified by densitometric analysis using the ImageJ software. Quantification data are expressed as mean±SEM from n≥3. Statistical comparison was performed using a t-test.
Fig. 4.
Tempol, Gentian Violet and Brillant Green treatment impair cyclin D1 expression. MCF-7 (A) and ZR75.1 (B) were left untreated or treated for 24 h with Tempol (T), Gentian Violet (GV) or Brilliant Green (BG) at the indicated concentrations. WCE were resolved by SDS-PAGE and analyzed by immunoblot using anti-cyclin D1 and anti-actin antibodies. Experiments were performed three times. Representative immunoblots are shown.
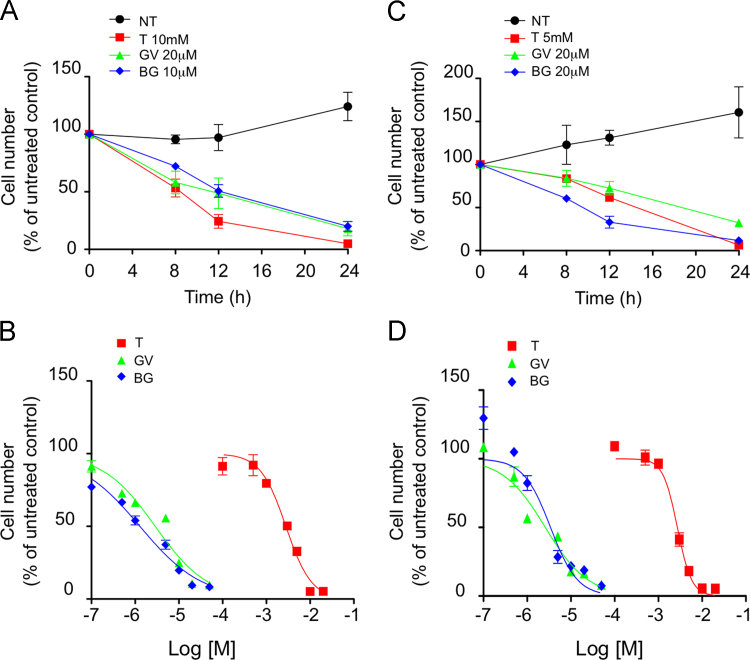
Tempol and triphenylmethane dyes exhibit potent anti-proliferative activity in MCF-7 and ZR75.1 cells.
We next evaluated the impact of Gentian Violet, Brilliant Green and Tempol on breast cancer cells growth. MCF-7 and ZR75.1 were treated with increasing concentrations of either compound for 8–24 h (Fig. 5). Both cell lines exhibited a concentration-dependent susceptibility to Tempol, Gentian Violet or Brilliant Green, albeit with different sensitivities as evidenced by half-maximal inhibitory concentration (IC50) values measured at 24 h (Fig. 5B and D). The IC50 values for Gentian Violet were 2.87 and 2.6 µM in MCF-7 and ZR75.1 cells, respectively. The IC50 value for Brilliant Green was 1.28 µM in MCF-7 cells, while it was of 3.42 µM in ZR75.1 cells. This revealed that Gentian Violet and Brilliant Green were more potent inhibitors than Tempol, which exhibited significantly higher IC50 values of 2.8 mM in MCF-7 cells and 2.7 mM in ZR75.1 cells. Collectively, these results highlight the potent antiproliferative effect of Gentian Violet, Brilliant Green and Tempol in breast cancer cells.
Fig. 5.
Tempol, Gentian Violet and Brilliant Green significantly decrease viability of MCF-7 and ZR75.1 cells. MCF-7 (A and B) and ZR75.1 (C and D) cells were left untreated or treated with Tempol (T), Gentian Violet (GV) or Brilliant Green (BG). In A and C, inhibitors were used at the indicated concentrations and cell viability was determined at various times by the crystal violet assay. In (B) and (D), cell viability was monitored after treatment with increasing concentrations of either inhibitor. Non-linear regression curve fit (log[inhibitor] vs normalized response) were obtained using GraphPad Prism software. Plotted data correspond to the mean±SD (n≥3).
Distinct effect of Tempol and Triphenylmethane dyes on caspase 3 and/or 7 activation in MCF-7 and ZR75.1.
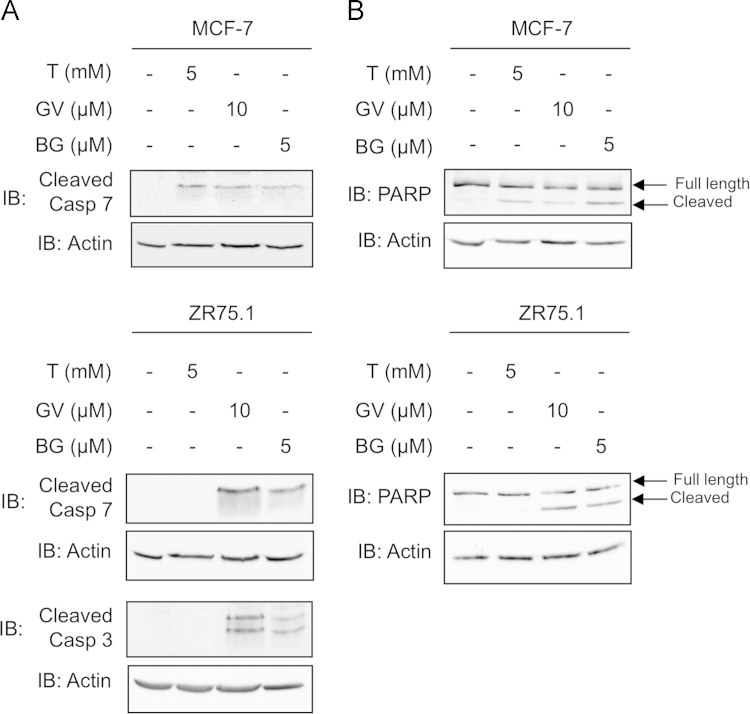
To further document the molecular consequences of treatment with Gentian Violet, Brilliant Green or Tempol, we next sought to analyze caspase 3/7 activation through immunoblot detection of the proapoptotic cleaved caspase 3 and 7, as well as the cleaved caspase substrate PARP, in cells treated with Tempol, Gentian Violet or Brilliant Green (Fig. 6). Caspase 3 cleavage was only assessed in ZR75.1 cells as, as previously described, MCF-7 cells are deficient in caspase 3 [41]. Treatment of MCF-7 and ZR75.1 cells with Gentian Violet or Brilliant Green led to a significant induction of cleaved caspase 7 and PARP (Fig. 6). Additionally, caspase 3 cleavage was detected in Gentian Violet- and Brilliant Green-treated ZR75.1 cells (Fig. 6A). Treatment with Tempol resulted in the cleavage of caspase 7 and PARP in MCF-7 cells, but neither caspase 3, caspase 7 nor PARP cleavage was observed in ZR75.1 cells (Fig. 6). These results show that treatment of breast cancer cells with the triphenylmethane dyes, Gentian Violet and Brilliant Green, induces caspase 3/7 activation. Although Tempol induces inhibition of IKKε expression and cell growth in MCF-7 and ZR75.1 cells, it induces caspase activation in MCF-7 cells, but not in ZR75.1 cells.
Fig. 6.
Cell-type specific activation of caspases by Tempol, Gentian Violet and Brillant Green. MCF-7 (A) and ZR75.1 (B) were treated with the indicated concentration of Tempol (T), Gentian Violet (GV) or Brilliant Green (BG) for 24 h. WCE were analyzed by immunoblot for cleaved-caspase 3 (19 and 17 kDa), cleaved-caspase 7 (20 kDa) and PARP (Full-length 116 kDa and cleaved 89 kDa). Equal loading was monitored using anti-actin antibodies. Representative immunoblots of three different experiments are shown.
IKKε expression in breast cancers cells is dependent on NOX2.
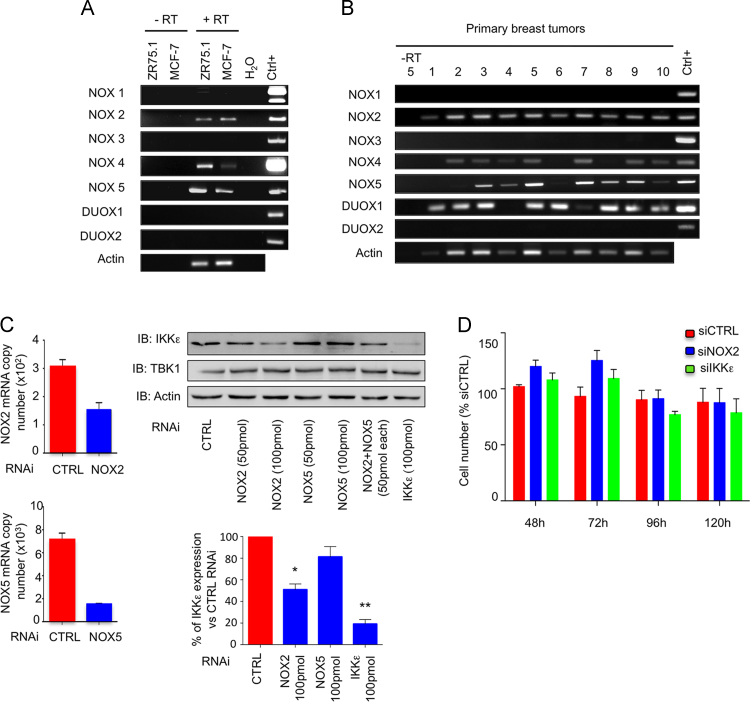
The triphenylmethane dyes, Gentian Violet and Brilliant Green, were recently shown to alter the redox cellular status through inhibition of ROS-generating NADPH oxidase activities [42]. Analysis of NADPH oxidase isoforms, NOX1-5 and DUOX1-2, mRNA expression in MCF-7 and ZR75.1 cells (Fig. 7A) and in primary breast tumors (Fig. 7B) revealed the expression of distinct isoforms of superoxide-generating NOX2 and NOX5 and hydrogen peroxide-generating NOX4 and DUOX1 [43]. According to the observed modulation of intracellular superoxide levels following treatment with Gentian Violet and Brilliant Green (Fig. 1), the role of NOX2 and NOX5 was analyzed. NOX2 and/or NOX5 were downregulated using RNAi in MCF-7 cells and expression of IKKε and TBK1 were monitored by immunoblot. IKKε specific-RNAi was used as a positive control. Silencing of NOX2, but not of NOX5, resulted in the significant inhibition of IKKε expression, while TBK1 levels remained steady (Fig. 7C). Neither silencing of NOX2 nor silencing of IKKε was sufficient to decrease cell viability (Fig. 7D).
Fig. 7.
NOX2-dependent regulation of IKKε expression in MCF-7 cells. Total RNA from MCF-7 or ZR75.1 cells (A) or from 10 distinct primary breast tumors (B) was extracted, subjected to reverse transcription and specific expression of NOX1-5, DUOX1-2 and actin was evaluated by RT-PCR. Specific positive controls (Ctrl+) were used for each gene as detailed in the methods section. In (C), control (CTRL), NOX2-, NOX5- or IKKε-specific RNAi were transfected into MCF-7 cells. Efficiency of NOX2 and NOX5 silencing was controlled by RT-qPCR (left panels). IKKε and TBK1 protein expression were analyzed by immunoblot (IB) using specific antibodies. Actin was used as loading control. IKKε levels were quantified by densitometric analysis using the ImageJ software. Quantification data are expressed as mean±SEM from n≥3. A t-test was performed to compare specific RNAi-transfected to CTRL RNAi-transfected cells. (D) Cell viability was monitored at the indicated times post-RNAi transfection and expressed as % of the corresponding siCTRL-transfected condition at each time point.
Altogether, our results show that treatment of breast cancer cells exhibiting amplification of the IKKε kinase with the redox modulating triphenylmethane dyes, Gentian Violet and Brilliant Green, and nitroxide Tempol leads to a significant decrease in NOX2-dependent IKKε expression.
4. Discussion
Because oxidative stress is considered a causative factor in carcinogenesis, including breast cancer, compounds with redox-modulating activities have attracted much attention as potential therapeutic agents [18,23]. Importantly, ROS have also been associated with the development of resistance to tamoxifen treatment [24–26]. Tamoxifen is the most widely selective estrogen receptor modulator adjuvant used for the treatment of hormone-dependent breast cancers, which allowed 40–50% decrease in the odds of recurrence and reduced mortality by nearly a third [44]. However, primary or acquired resistance to tamoxifen severely reduced its clinical effectiveness, constituting a serious threat to the eradication of breast cancer [12]. Because persistent oxidative stress was also shown to mediate anti-tumorigenic responses [18,23], a better understanding of the pathways that are altered by compounds used for their capacity to modulate the redox state of cancer cells is required to move them toward appropriate clinical applications. In the present study, we report that the triphenylmethane dyes, Gentian Violet and Brilliant Green, and nitroxide Tempol, reduce intracellular superoxide levels and exhibit potent growth inhibiting activities of the breast cancer epithelial cell lines MCF-7 and ZR75.1 that present different levels of IKBKE amplification. Molecular analyses revealed that the three compounds significantly diminished the expression of IKKε, an oncogenic kinase that is not only involved in epithelial breast cancer cell transformation and invasiveness [5–7,45,46], but also contributes to the development of breast cancer cells resistance to tamoxifen treatment likely through the phosphorylation of ERα [9]. Thus, our observation that Gentian Violet, Brilliant Green and Tempol diminish IKKε expression in breast cancer cells encourages the investigation of combination anticancer therapies to limit the development of resistance to tamoxifen. Aberrant expression of IKKε expression has also been observed in prostate tumors [47] and is associated with poor prognosis in ovarian cancer [48]. Therefore, impairment of IKKε expression by redox-modulating agents might also be of interest for the treatment of ovarian and prostate cancers.
Gentian Violet and Brilliant Green were shown to be potent and efficacious inhibitors of ROS-generating NOX2 and NOX4 activities [30]. The role of NADPH oxidases in breast tumor initiation and progression appears to be complex, as different isoforms of NADPH oxidase, NOX1, NOX2 and NOX4, have previously been shown to be involved in ROS production in epithelial breast cancer cells and contribute to cell proliferation, through induction of pro-survival pathways, regulation of cellular senescence, resistance to apoptosis, and tumorigenic transformation [49–53]. Brilliant Green at nanomolar concentrations was also shown to induce mitochondrial Trx2 oxidation and degradation in HeLa cells and fibroblasts [31]. Assessment of NADPH oxidases (NOX1-5/DUOX1-2) expression in MCF-7 and ZR75.1 cells and in 10 primary breast tumor samples confirmed the expression of distinct isoforms of NADPH oxidases. Of note, NOX2, NOX4 and NOX5 were detected in cell lines and primary tumors, while DUOX1 expression was not detected in MCF-7 and ZR75.1 cells. The observation that NOX2 silencing significantly impaired IKKε expression in MCF-7 cells, strongly supports that Gentian Violet and Brilliant Green regulate IKKε expression, at least in part, through their ability to inhibit NOX2. The Trx system is one of the major components of the thiol reducing system that plays an important role in the redox imbalance in cancer cells [54] and is overexpressed in several human cancers, including breast carcinomas [55]. Further studies will be required to determine if Trx play a role in the inhibition of IKKε expression and/or cell growth induced by Gentian Violet and Brilliant Green.
The cell growth inhibition observed following treatment of breast cancer cells with Gentian Violet, Brilliant Green and Tempol (Fig. 5), was not recapitulated by NOX2 silencing (Fig. 7). This suggests that inhibition of NOX2-dependent IKKε expression is not sufficient to mediate the growth inhibiting activities of the compounds. The anti-proliferative action of Gentian Violet and Brilliant Green was associated with cleavage of caspase 7 and of the PARP substrate both in caspase 3-positive ZR75.1 cells and in caspase 3-deficient MCF-7 cells, suggesting that these compounds regulate apoptosis of breast cancer cells independently of the caspase 3 status. However, Tempol displayed distinct responses depending on the cell line. While in MCF-7 Tempol treatment induced activation of caspase 7, Tempol-induced ZR75.1 cell growth inhibition was not correlated with caspase activation. The observation that Tempol induces caspase 7 and downstream PARP cleavage is in agreement with a previous study reporting that Tempol induces apoptosis in MCF-7 cells [56]. The absence of caspase activation and PARP cleavage by Tempol in ZR75.1 cells implies the activation of a caspase-independent cell growth inhibition pathway that remains to be characterized [57]. Further studies will be required to identify the pathway(s) that is responsible for the inhibition of cell viability triggered by Gentian Violet, Brilliant Green and Tempol.
Gentian Violet was recently identified in a high-throughput screen aimed at identifying small molecules inhibiting the degradation of the cyclin-dependent kinase inhibitor p27Kip1, which is associated with many aggressive phenotypes and a poor prognosis in various cancers, including breast cancer [58]. Interestingly, transcriptional regulation of p27Kip1 is under the control of the FOXO3a transcription factors [59], which is a substrate for IKKε [11]. FOXO3a is inhibited by IKKε-mediated phosphorylation [11]. Therefore, it is tempting to speculate that Gentian Violet, and potentially the closely related Brilliant Green, not only inhibit p27Kip1 degradation, but also increase p27Kip1 transcription as a result from inhibition of IKKε expression.
Oral use of Tempol has been widely evaluated in a wide range of animal models [33]. In contrary, the triphenylmethane dyes, Gentian Violet and Brilliant Green, have been mainly used for topical therapeutic indications [42]. During the course of our work, Imipramine-blue, a triphenylmethane analog of Gentian Violet, was created for systemic anti-glioblastoma therapy [60] opening the path to systemic use of this family of compounds. The observation that IKKε expression is dependent on NOX2 also points to the use of a NOX2-specific inhibitor. The quest for specific NOX inhibitors has been underway for more than two decades. The use of NOX2ds-tat, a peptidic NOX2-specific inhibitor, yielded very mild results in our model (data not shown), which might be attributed to a tightly assembled, constitutive NOX2 oxidase [61,62]. We are awaiting availability of small molecule inhibitors with greater potency to test in this system [63].
Our in vitro data demonstrates that inhibition of NOX2-dependent IKKε expression ranks amongst the pleiotropic activities of the redox-modulating triphenylmethane dyes, Gentian Violet and Brilliant Green, and nitroxide Tempol on breast cancer cells. These findings provide valuable insights for the development of novel combined therapies to treat breast tumors highly expressing IKKε kinase.
Acknowledgments
The authors thank members of the laboratory for fruitful discussions. The authors also thank Dr. Sylvie Mader, University of Montreal, for cell lines used in this study. The authors are also grateful to Dr. Amal Nadiri, Dr. Dominique Gauchat, and Dr. Malek Jundi for help with the flow cytometry experiments design and analysis. Tumor banking was supported by the Banque de tissus et de données of the Réseau de recherche sur le cancer of the Fonds de la recherche du Québec-Santé (FRQS), affiliated to the Canadian Tumor Repository Network (CTRNet). This work was funded by grants to NG from the Canadian Breast Cancer Research Alliance and the Canadian Institutes of Health Research (CBCRA #019797 and CIHR/CBCRA MOP-102622). NG is recipient of a Tier II Canada Research Chair. RL holds a senior fellowship from the Fonds de la recherche du Québec-Santé (FRQS).
Contributor Information
Espérance Mukawera, Email: esperance.mukawera.chum@ssss.gouv.qc.ca.
Stefany Chartier, Email: chartiers@live.fr.
Virginie Williams, Email: virginie@gmail.com.
Patrick J. Pagano, Email: pagano@pitt.edu.
Réjean Lapointe, Email: rejean.lapointe@umontreal.ca.
Nathalie Grandvaux, Email: nathalie.grandvaux@umontreal.ca.
References
- 1.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. 10647931 [DOI] [PubMed] [Google Scholar]
- 2.Thiery J.P., Sastre-Garau X., Vincent-Salomon B., Sigal-Zafrani X., Pierga J.Y., Decraene C., Meyniel J.P., Gravier E., Asselain B., De Rycke Y., Hupe P., Barillot E., Ajaz S., Faraldo M., Deugnier M.A., Glukhova M., Medina D., Breast Cancer Group Challenges in the stratification of breast tumors for tailored therapies. Bull. Cancer. 2006;93(8):E81–E89. 16935776 [PubMed] [Google Scholar]
- 3.Clément J.F., Meloche S., Servant M.J. The IKK-related kinases: from innate immunity to oncogenesis. Cell Res. 2008;18(9):889–899. doi: 10.1038/cr.2008.273. 19160540 [DOI] [PubMed] [Google Scholar]
- 4.Hayden M.S., Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132(3):344–362. doi: 10.1016/j.cell.2008.01.020. 18267068 [DOI] [PubMed] [Google Scholar]
- 5.Eddy S.F., Guo S., Demicco E.G., Romieu-Mourez R., Landesman-Bollag E., Seldin D.C., Sonenshein G.E. Inducible IkappaB kinase/IkappaB kinase epsilon expression is induced by CK2 and promotes aberrant nuclear factor-kappaB activation in breast cancer cells. Cancer Res. 2005;65(24):11375–11383. doi: 10.1158/0008-5472.CAN-05-1602. 16357145 [DOI] [PubMed] [Google Scholar]
- 6.Boehm J.S., Zhao J.J., Yao J., Kim S.Y., Firestein R., Dunn I.F., Sjostrom S.K., Garraway L.A., Weremowicz S., Richardson A.L., Greulich H., Stewart C.J., Mulvey L.A., Shen R.R., Ambrogio L., Hirozane-Kishikawa T., Hill D.E., Vidal M., Meyerson M., Grenier J.K., Hinkle G., Root D.E., Roberts T.M., Lander E.S., Polyak K., Hahn W.C. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129(6):1065–1079. doi: 10.1016/j.cell.2007.03.052. 17574021 [DOI] [PubMed] [Google Scholar]
- 7.Qin B., Cheng K. Silencing of the IKKepsilon gene by siRNA inhibits invasiveness and growth of breast cancer cells. Breast Cancer Res. 2010;12(5):R74. doi: 10.1186/bcr2644. 20863366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutti J.E., Shen R.R., Abbott D.W., Zhou A.Y., Sprott K.M., Asara J.M., Hahn W.C., Cantley L.C. Phosphorylation of the tumor suppressor CYLD by the breast cancer oncogene IKKepsilon promotes cell transformation. Mol. Cells. 2009;34(4):461–472. doi: 10.1016/j.molcel.2009.04.031. 19481526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo J.P., Shu S.K., Esposito N.N., Coppola D., Koomen J.M., Cheng J.Q. IKKepsilon phosphorylation of estrogen receptor alpha ser-167 and contribution to tamoxifen resistance in breast cancer. J. Biol. Chem. 2010;285(6):3676–3684. doi: 10.1074/jbc.M109.078212. 19940156 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Shen R.R., Zhou A.Y., Kim E., Lim E., Habelhah H., Hahn W.C. IκB kinase ε phosphorylates TRAF2 to promote mammary epithelial cell transformation. Mol. Cell. Biol. 2012;32(23):4756–4768. doi: 10.1128/MCB.00468-12. 23007157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo J.P., Tian W., Shu S., Xin Y., Shou C., Cheng J.Q. IKBKE phosphorylation and inhibition of FOXO3a: a mechanism of IKBKE oncogenic function. PLOS One. 2013;8(5):e63636. doi: 10.1371/journal.pone.0063636. 23691078 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Hughes-Davies L., Caldas C., Wishart G.C. Tamoxifen: the drug that came in from the cold. Br. J. Cancer. 2009;101(6):875–878. doi: 10.1038/sj.bjc.6605231. 19672259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halliwell B., Gutteridge J. Free Radicals in Biology and Medicine. fourth edition. Oxford University Books; Oxford, UK: 2007. p. 888. [Google Scholar]
- 14.Brown N.S., Bicknell R. Hypoxia and oxidative stress in breast cancer. oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 2001;3(5):323–327. doi: 10.1186/bcr315. 11597322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. 11773609 [DOI] [PubMed] [Google Scholar]
- 16.Giles G.I. The redox regulation of thiol dependent signaling pathways in cancer. Curr. Pharm. Des. 2006;12(34):4427–4443. doi: 10.2174/138161206779010549. 17168752 [DOI] [PubMed] [Google Scholar]
- 17.Jorgenson T.C., Zhong W., Oberley T.D. Redox imbalance and biochemical changes in cancer. Cancer Res. 2013;73(20):6118–6123. doi: 10.1158/0008-5472.CAN-13-1117. 23878188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12(12):931–947. doi: 10.1038/nrd4002. 24287781 [DOI] [PubMed] [Google Scholar]
- 19.Curtis C.D., Thorngren D.L., Nardulli A.M. Immunohistochemical analysis of oxidative stress and DNA repair proteins in normal mammary and breast cancer tissues. BMC Cancer. 2010;10:9. doi: 10.1186/1471-2407-10-9. 20064251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Outschoorn U.E., Balliet R., Lin Z., Whitaker-Menezes D., Birbe R.C., Bombonati A., Pavlides S., Lamb R., Sneddon S., Howell A., Sotgia F., Lisanti M.P. BRCA1 mutations drive oxidative stress and glycolysis in the tumor microenvironment: implications for breast cancer prevention with antioxidant therapies. Cell Cycle. 2012;11(23):4402–4413. doi: 10.4161/cc.22776. 23172369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011;194(1):7–15. doi: 10.1083/jcb.201102095. 21746850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen-Heininger Y.M.W., Mossman B.T., Heintz N.H., Forman H.J., Kalyanaraman B., Finkel T., Stamler J.S., Rhee S.G., van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic. Biol. Med. 2008;45(1):1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. 18423411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saeidnia S., Abdollahi M. Antioxidants: friends or foe in prevention or treatment of cancer: the debate of the century. Toxicol. Appl. Pharmacol. 2013;271(1):49–63. doi: 10.1016/j.taap.2013.05.004. 23680455 [DOI] [PubMed] [Google Scholar]
- 24.Schiff R., Reddy P., Ahotupa M., Coronado-Heinsohn E., Grim M., Hilsenbeck S.G., Lawrence R., Deneke S., Herrera R., Chamness G.C., Fuqua S.A., Brown P.H., Osborne C.K. Oxidative stress and AP-1 activity in tamoxifen-resistant breast tumors in vivo. J. Natl. Cancer Inst. 2000;92(23):1926–1934. doi: 10.1093/jnci/92.23.1926. 11106684 [DOI] [PubMed] [Google Scholar]
- 25.Singh B., Bhat N.K., Bhat H.K. Partial inhibition of estrogen-induced mammary carcinogenesis in rats by tamoxifen: balance between oxidant stress and estrogen responsiveness. PLOS One. 2011;6(9):e25125. doi: 10.1371/journal.pone.0025125. 21966433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weitsman G.E., Weebadda W., Ung K., Murphy L.C. Reactive oxygen species induce phosphorylation of serine 118 and 167 on estrogen receptor alpha. Breast Cancer Res. Treat. 2009;118(2):269–279. doi: 10.1007/s10549-008-0221-0. 18941890 [DOI] [PubMed] [Google Scholar]
- 27.Barbie T.U., Alexe G., Aref A.R., Li S., Zhu Z., Zhang X., Imamura Y., Thai T.C., Huang Y., Bowden M., Herndon J., Cohoon T.J., Fleming T., Tamayo P., Mesirov J.P., Ogino S., Wong K.K., Ellis M.J., Hahn W.C., Barbie D.A., Gillanders W.E. Targeting an IKBKE cytokine network impairs triple-negative breast cancer growth. J. Clin. Invest. 2014;124(12):5411–5423. doi: 10.1172/JCI75661. 25365225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Docampo R., Moreno S.N. The metabolism and mode of action of gentian violet. Drug Metab. Rev. 1990;22(2–3):161–178. doi: 10.3109/03602539009041083. 2272286 [DOI] [PubMed] [Google Scholar]
- 29.Balabanova M., Popova L., Tchipeva R. Dyes in dermatology. Clin. Dermatol. 2003;21(1):2–6. doi: 10.1016/s0738-081x(02)00330-9. 12609582 [DOI] [PubMed] [Google Scholar]
- 30.Perry B.N., Govindarajan B., Bhandarkar S.S., Knaus U.G., Valo M., Sturk C., Carrillo C.O., Sohn A., Cerimele F., Dumont D., Losken A., Williams J., Brown L.F., Tan X., Ioffe E., Yancopoulos G.D., Arbiser J.L. Pharmacologic blockade of angiopoietin-2 is efficacious against model hemangiomas in mice. J. Invest. Dermatol. 2006;126(10):2316–2322. doi: 10.1038/sj.jid.5700413. 16741507 [DOI] [PubMed] [Google Scholar]
- 31.Zhang X., Zheng Y., Fried L.E., Du Y., Montano S.J., Sohn A., Lefkove B., Holmgren L., Arbiser J.L., Holmgren A., Lu J. Disruption of the mitochondrial thioredoxin system as a cell death mechanism of cationic triphenylmethanes. Free Radic. Biol. Med. 2011;50(7):811–820. doi: 10.1016/j.freeradbiomed.2010.12.036. 21215310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arbiser J.L., Bips M., Seidler A., Bonner M.Y., Kovach C. Combination therapy of imiquimod and gentian violet for cutaneous melanoma metastases. J. Am. Acad. Dermatol. 2012;67(2):e81–e83. doi: 10.1016/j.jaad.2011.10.028. 22794825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilcox C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010;126(2):119–145. doi: 10.1016/j.pharmthera.2010.01.003. 20153367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batinić-Haberle I., Rebouças J.S., Spasojević I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Signal. 2010;13(6):877–918. doi: 10.1089/ars.2009.2876. 20095865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fink K., Duval A., Martel A., Soucy-Faulkner A., Grandvaux N. Dual role of NOX2 in respiratory syncytial virus- and Sendai virus-induced activation of NF-kappaB in airway epithelial cells. J. Immunol. 2008;180(10):6911–6922. doi: 10.4049/jimmunol.180.10.6911. 18453612 [DOI] [PubMed] [Google Scholar]
- 36.Zhao H., Kalivendi S., Zhang H., Joseph J., Nithipatikom K., Vásquez-Vivar J., Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic. Biol. Med. 2003;34(11):1359–1368. doi: 10.1016/s0891-5849(03)00142-4. 12757846 [DOI] [PubMed] [Google Scholar]
- 37.Forget M.A., Turcotte S., Beauseigle D., Godin-Ethier J., Pelletier S., Martin J., Tanguay S., Lapointe R. The Wnt pathway regulator DKK1 is preferentially expressed in hormone-resistant breast tumours and in some common cancer types. Br. J. Cancer. 2007;96(4):646–653. doi: 10.1038/sj.bjc.6603579. 17245340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dussault A.A., Pouliot M. Rapid and simple comparison of messenger RNA levels using real-time PCR. Biol. Proced. Online. 2006;8:1–10. doi: 10.1251/bpo114. 16446781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fink K., Martin L., Mukawera E., Chartier S., De Deken X., Brochiero E., Miot F., Grandvaux N. IFNβ/TNFα synergism induces a non-canonical STAT2/IRF9-dependent pathway triggering a novel DUOX2 NADPH oxidase-mediated airway antiviral response. Cell Res. 2013;23(5):673–690. doi: 10.1038/cr.2013.47. 23545780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lundholt B.K., Briand P., Lykkesfeldt A.E. Growth inhibition and growth stimulation by estradiol of estrogen receptor transfected human breast epithelial cell lines involve different pathways. Breast Cancer Res. Treat. 2001;67(3):199–214. doi: 10.1023/a:1017977406429. 11561766 [DOI] [PubMed] [Google Scholar]
- 41.Jänicke R.U., Sprengart M.L., Wati M.R., Porter A.G. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 1998;273(16):9357–9360. doi: 10.1074/jbc.273.16.9357. 9545256 [DOI] [PubMed] [Google Scholar]
- 42.Maley A.M., Arbiser J.L. Gentian violet: a 19th century drug re-emerges in the 21st century. Exp. Dermatol. 2013;22(12):775–780. doi: 10.1111/exd.12257. 24118276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nauseef W.M. Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases. Biochim. Biophys. Acta. 2014;1840(2):757–767. doi: 10.1016/j.bbagen.2013.04.040. 23660153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osborne C.K. Tamoxifen in the treatment of breast cancer. N. Engl. J. Med. 1998;339(22):1609–1618. doi: 10.1056/NEJM199811263392207. 9828250 [DOI] [PubMed] [Google Scholar]
- 45.Romieu-Mourez R., Landesman-Bollag E., Seldin D.C., Traish A.M., Mercurio F., Sonenshein G.E. Roles of IKK kinases and protein kinase CK2 in activation of nuclear factor-kappaB in breast cancer. Cancer Res. 2001;61(9):3810–3818. 11325857 [PubMed] [Google Scholar]
- 46.Romieu-Mourez R., Landesman-Bollag E., Seldin D.C., Sonenshein G.E. Protein kinase CK2 promotes aberrant activation of nuclear factor-kappaB, transformed phenotype, and survival of breast cancer cells. Cancer Res. 2002;62(22):6770–6778. 12438279 [PubMed] [Google Scholar]
- 47.Péant B., Diallo J.S., Lessard L., Delvoye N., Le Page C., Saad F., Mes-Masson A.M. Regulation of IkappaB kinase epsilon expression by the androgen receptor and the nuclear factor-kappaB transcription factor in prostate cancer. Mol. Cancer Res. 2007;5(1):87–94. doi: 10.1158/1541-7786.MCR-06-0144. 17259348 [DOI] [PubMed] [Google Scholar]
- 48.Guo J.P., Shu S.K., He L., Lee Y.C., Kruk P.A., Grenman S., Nicosia S.V., Mor G., Schell M.J., Coppola D., Cheng J.Q. Deregulation of IKBKE is associated with tumor progression, poor prognosis, and cisplatin resistance in ovarian cancer. Am. J. Pathol. 2009;175(1):324–333. doi: 10.2353/ajpath.2009.080767. 19497997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graham K.A., Kulawiec M., Owens K.M., Li X., Desouki M.M., Chandra D., Singh K.K. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol. Ther. 2010;10(3):223–231. doi: 10.4161/cbt.10.3.12207. 20523116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rao Malla R., Raghu H., Rao J.S. Regulation of NADPH oxidase (Nox2) by lipid rafts in breast carcinoma cells. Int. J. Oncol. 2010;37(6):1483–1493. doi: 10.3892/ijo_00000801. 21042717 [DOI] [PubMed] [Google Scholar]
- 51.Choi J.A., Lee J.W., Kim H., Kim E.Y., Seo J.M., Ko J., Kim J.H. Pro-survival of estrogen receptor-negative breast cancer cells is regulated by a BLT2-reactive oxygen species-linked signaling pathway. Carcinogenesis. 2010;31(4):543–551. doi: 10.1093/carcin/bgp203. 19748928 [DOI] [PubMed] [Google Scholar]
- 52.Desouki M.M., Kulawiec M., Bansal S., Das G.M., Singh K.K. Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol. Ther. 2005;4(12):1367–1373. doi: 10.4161/cbt.4.12.2233. 16294028 [DOI] [PubMed] [Google Scholar]
- 53.Ostrakhovitch E.A., Li S.S. NIP1/DUOXA1 expression in epithelial breast cancer cells: regulation of cell adhesion and actin dynamics. Breast Cancer Res. Treat. 2010;119(3):773–786. doi: 10.1007/s10549-009-0372-7. 19322654 [DOI] [PubMed] [Google Scholar]
- 54.Karlenius T.C., Tonissen K.F. Thioredoxin and Cancer: a role for thioredoxin in all states of tumor oxygenation. Cancers. 2010;2(2):209–232. doi: 10.3390/cancers2020209. 24281068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cha M.K., Suh K.H., Kim I.H. Overexpression of peroxiredoxin I and thioredoxin1 in human breast carcinoma. J. Exp. Clin. Cancer Res. 2009;28:93. doi: 10.1186/1756-9966-28-93. 19566940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gariboldi M.B., Lucchi S., Caserini C., Supino R., Oliva C., Monti E. Antiproliferative effect of the piperidine nitroxide tempol on neoplastic and nonneoplastic mammalian cell lines. Free Radic. Biol. Med. 1998;24(6):913–923. doi: 10.1016/s0891-5849(97)00372-9. 9607601 [DOI] [PubMed] [Google Scholar]
- 57.Constantinou C., Papas K.A., Constantinou A.I. Caspase-independent pathways of programmed cell death: the unraveling of new targets of cancer therapy? Curr. Cancer Drug Targets. 2009;9(6):717–728. doi: 10.2174/156800909789271512. 19754356 [DOI] [PubMed] [Google Scholar]
- 58.Chu I.M., Hengst L., Slingerland J.M. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat. Rev. Cancer. 2008;8(4):253–267. doi: 10.1038/nrc2347. 18354415 [DOI] [PubMed] [Google Scholar]
- 59.Burhans W.C., Heintz N.H. The cell cycle is a redox cycle: linking phase-specific targets to cell fate. Free Radic. Biol. Med. 2009;47(9):1282–1293. doi: 10.1016/j.freeradbiomed.2009.05.026. 19486941 [DOI] [PubMed] [Google Scholar]
- 60.Munson JM, Fried L, Rowson SA, Bonner MY, Karumbaiah L, Diaz B, Courtneidge SA, Knaus UG, Brat DJ, Arbiser JL, Bellamkonda RV. Anti-invasive adjuvant therapy with imipramine blue enhances chemotherapeutic efficacy against glioma. Sci. Transl. Med. 2012;4(127):127ra36. doi: 10.1126/scitranslmed.3003016. [DOI] [PubMed] [Google Scholar]
- 61.Rey F.E., Cifuentes M.E., Kiarash A., Quinn M.T., Pagano P.J. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(−) and systolic blood pressure in mice. Circ. Res. 2001;89(5):408–414. doi: 10.1161/hh1701.096037. 11532901 [DOI] [PubMed] [Google Scholar]
- 62.Csányi G., Cifuentes-Pagano E., Al Ghouleh I., Ranayhossaini D.J., Egaña L., Lopes L.R., Jackson H.M., Kelley E.E., Pagano P.J. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic. Biol. Med. 2011;51(6):1116–1125. doi: 10.1016/j.freeradbiomed.2011.04.025. 21586323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cifuentes-Pagano E., Saha J., Csányi G., Ghouleh I.A., Sahoo S., Rodríguez A., Wipf P., Pagano P.J., Skoda E.M. Bridged tetrahydroisoquinolines as selective NADPH oxidase 2 (Nox2) inhibitors. Medchemcomm. 2013;4(7):1085–1092. doi: 10.1039/C3MD00061C. 24466406 [DOI] [PMC free article] [PubMed] [Google Scholar]