Abstract
Obesity is an energy balance disorder associated with dyslipidemia, insulin resistance and diabetes type 2, also summarized with the term metabolic syndrome or syndrome X. Increasing evidence points to “adipocyte dysfunction”, rather than fat mass accretion per se, as the key pathophysiological factor for metabolic complications in obesity. The dysfunctional fat tissue in obesity characterizes a failure to safely store metabolic substrates into existing hypertrophied adipocytes and/or into new preadipocytes recruited for differentiation. In this review we briefly summarize the potential of redox imbalance in fat tissue as an instigator of adipocyte dysfunction in obesity. We reveal the challenge of the adipose redox changes, insights in the regulation of healthy expansion of adipose tissue and its reduction, leading to glucose and lipids overflow.
Abbreviations: AD, antioxidant defense; AGE, advanced glycation end products; ATMs, adipose tissue macrophages; BMI, body mass index; C/EBP, CCAAT/enchancer binding protein; Cu, ZnSOD, copper zinc superoxide dismutase; CR, calorie restriction; ETC, electron transport chain; FIRKO, fat-specific insulin receptor knockout; FOXO, forkhead-O-box; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GLUT1, glucose transporter 1; GLUT4, glucose transporter 4; GSH, glutathione; GPx, glutathione peroxidase; H2O2, hydrogen peroxide; HIF, 1-hypoxia inducible factor 1; InsR, insulin receptor; IRS-1, insulin receptor substrate 1; MnSOD, manganese superoxide dismutase; mTOR, mammalian target of rapamycin; NADH, nicotinamide-adenine dinucleotide; NADPH, nicotinamide-adenine dinucleotide phosphate; NO, nitric oxide; NOS, nitric oxide synthase; NOX, NADPH oxidase; NOX4, NADPH oxidase, isoform 4; O2•−, superoxide anion radical; OXPHOS, oxidative phosphorylation; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1alpha; PKB, protein kinase B; PKC, protein kinase C; PPP, pentose phosphate pathway; PPARγ, peroxisome proliferator-activated receptor γ; Prx, peroxyredoxin; PTPs, protein tyrosine phosphatases; RAAS, renin–angiotensin–aldosterone-system; RAGE, receptor for advanced glycation end-products; ROS, reactive oxygen species; SIRT, sirtuin; SREBP1c, sterol-regulatory element binding protein 1c; TAG, triacylglycerol; TCA, tricarboxylic acid; TNFα, tumor necrosis factor α; Trx, thioredoxin; TR, thioredoxin reductase; UCPs, uncoupling proteins; VCAM-1, vascular cell adhesion molecule 1; WAT, white adipose tissue
Keywords: Adipose tissue, Obesity, Insulin resistance, Redox
Graphical abstract
Highlights
-
•
Adipose tissue (AT) buffers nutrient excess determining overall metabolic health.
-
•
Redox insight in lipid storage and adipogenesis of AT is reviewed.
-
•
Redox modulation of AT as therapeutic target in obesity/syndrome X is considered.
1. Introduction
Mostly due to the simplicity of the appearance of the adipose tissue and adipocytes themselves, they are regarded as a long-term storage of triacylglycerols (TAG) that is under dominant insulin regulation. Adipose lipid reserves are deposited by lipogenesis in the state of nutrient abundance (high insulin level) and hydrolyzed in the time of increased energy needs (low insulin level) [1,2]. Up to now, the general belief was that normal adipose tissue expands only passively to accommodate nutrient excess, as well as that its excess per se (crudely measured as body mass index, BMI>30, mass (kg)/height2 (m)) is enough for systemic insulin resistance. In-depth investigation of adipose tissue was instigated by the obesity pandemic in the last decades. It revealed that prodigious morpho-functional plasticity and intricately controlled flow of glucose and fatty acids through the adipocytes, under different nutrient conditions orchestrate the nutrient flow through the whole body, defining overall lipid and glucose homeostasis [3–5]. As a matter of fact, regardless of the overall BMI, the disruption of insulin-stimulated glucose uptake in adipose tissue and insulin-mediated lipogenesis and adipogenesis, which is most evident in visceral fat accumulation, appears to be a sufficient trigger of peripheral insulin resistance [4].
There is increasing evidence for the concept of redox-driven alterations of mitochondrial function as well as augmented mitochondrial reactive oxygen species (ROS) formation as a key event for the development of insulin resistance [6–8]. Moreover a hypothesis emerged that excessive nutritional overload triggers redox changes in adipocytes’ mitochondria, which impair local and systemic insulin sensitivity [9–14].
The concept of redox signaling in adipocyte biology, and the difficulties in understanding the roles of fat tissue in the maintenance of metabolic homeostasis, advanced in parallel a thorny path. The offensive/defensive tendencies of oxidants/antioxidants underwent in-depth reconsiderations after key observations that were made over 40 years ago that oxidants can facilitate and mimic insulin action [15], and that hydrogen peroxide (H2O2) is intentionally generated by a specific enzyme in response to insulin stimulation to initiate the insulin signaling cascade [16]. Vigilantly, it had been revealed that both oxidants and antioxidants determine a highly dynamic, spatio-temporal redox metabolism intricately involved in nutrient sensing and handling by the adipocyte [17]. The focus of this review is to summarize the most important redox insights into adipocyte storage function, while greatly emphasizing its changes in nutrient overload leading to insulin resistance at the local and ultimately systemic level.
2. Adipose organ: adipocyte cell types and depot-diversities
Anatomically, adipose tissue in the form of loose connective tissue consisting of adipocytes and stromal-vascular cells is organized in distinct fat depots. Functionally quite heterogenous, fat depots are integrated in a highly dynamic multidepot adipose organ [18]. In regard to energy balance, this adipose organ may appear as predominantly white – energy saving, brown – energy dissipating, or beige (convertible) depending on the relative amount of brown, beige/brite and white adipocyte types (Fig. 1). These adipocytes, in respect to energy metabolism have a completely different function and can be differentiated by their characteristic molecular signature [19]. Human adipose organ consists mostly from white adipocytes, reflecting its primary, energy storage function. Discrete functional characteristics exist even between white adipocytes from different body sites. Compared to subcutaneous adipocytes, adipocytes from the viscera are less insulin sensitive, and the hypertrophic type of growth limits the expanding capacity of the visceral adipose tissue depots [20–23]. Specified differences reflect the greater (theoretically limitless) lipid storing capacity of subcutaneous adipose tissue, in contrast to visceral adipose tissue that may function more as short-term lipid storage. The stated depot-specifics also underlie the differences between the healthy (insulin sensitive) and unhealthy (insulin resistant) obesity in subjects with different adiposity distribution (visceral vs. subcutaneous, respectively) in the long-term nutritional (high calorie) overload.
Fig. 1.
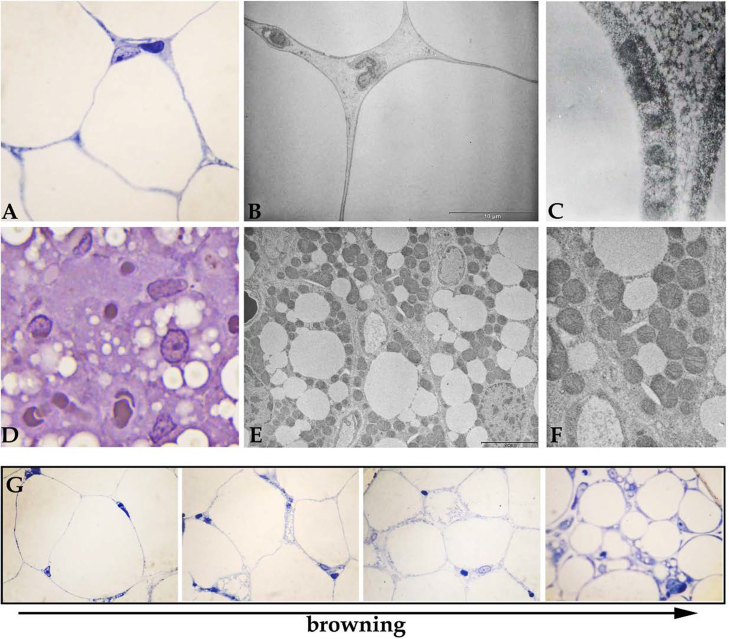
The structure of white adipocyte (A–C) and brown adipocyte (D–F) under light (A, D) and transmission electron microscope (B, C, E, F). The differences between these two adipocytes types comprise of cell size, shape, and lipid droplets number. The most striking contrast is in mitochondrial population (C, F). Under some conditions, the white adipocytes go through “browning”, acquiring properties of brown adipocytes.
3. The role of adipose tissue in maintenance of systemic glucose homeostasis
In the fluctuating nutritional states euglycemia level is tightly upheld by a rate of glucose synthesis in the liver and by a rate of its uptake in many tissues, but especially in liver, muscle and adipose tissue [24]. Postprandial (or after a calorie rich meal) increase in glucose level stimulates insulin secretion and the resulting hyperinsulinemia feedback increases synthesis of glycogen in the liver and facilitates glucose uptake of insulin-sensitive tissues [24]. The significance of adipose tissue insulin signaling in the regulation of overall glucose homeostasis was questioned by data showing that fat-specific insulin receptor knockout (FIRKO) mice are protected from obesity-related glucose intolerance and insulin resistance [25]. On the other side, selective inactivation of the glucose transporter 4 (GLUT4) gene in adipose tissue indeed induced insulin intolerance and hyperinsulinemia, as well as impaired insulin action in muscle and liver [26]. In contrast to rat adipose tissue that metabolizes 3–5% of glucose uptake [27], human adipose tissue is responsible for up to 20% of an orally administered glucose load [28,29]. Thus, the role of insulin signaling and action (glucose uptake and lipid sink) in adipose tissue is of importance in systemic glucose homeostasis, during the feeding/fasting state transitions [26,30,31], especially in obese humans [32].
Chronic nutrient surplus and the resulting hyperinsulinemia increase the adipocytes metabolic glucose flux and consequently lead to an increase in cell size. As adipocytes are loaded with lipids and reach the critical size, precursor cells may be stimulated to differentiate, and an increase in adipocyte number occurs [33,34]. However, adipose tissue eventually becomes unable to store excess lipids, even with enhanced hyperplasia. This results in an increased number of hypertrophic adipocytes. At one time-point, hypertrophic adipocytes develop decreased sensitivity to insulin and insulin mediated effects in adipose tissue: lipogenic and antilipolytic control is impaired. Uncontrolled lipolysis results in free fatty acids and glycerol release, further increasing plasma nonesterified fatty acids level, their redistribution and interference with the glucose uptake and metabolism in the liver and muscle [20]. Impaired function of white adipose tissue (WAT) to buffer excess metabolic substrates from nutritional overload, leaves the non-adipose tissues, in particular liver and muscles to gluco- and lipotoxic insults [35,36]. Overwhelmed glucose and fatty acid buffering capacity of adipose tissue is especially visible postprandially, when the major nutrient overload occurs [37].
4. Adipose tissue function (healthy expansion)
Postprandially, a high level of circulating insulin binds to its receptors and mediates the regulation of the glucose and fatty acids uptake in the adipose tissue, and the activity of enzymes involved in their metabolism and deposition into TAG (lipogenesis). Over the longer term, lipogenesis results in adipose tissue expansion (adipogenesis).
In general, binding of insulin to its receptors, induces receptor autophosphorylation on tyrosine residues, accompanied by the phosphorylation of insulin receptor substrates (IRS) by the activity of insulin receptor tyrosine kinase. The IRSs are associated with the regulatory subunit of phosphatidyl inositol 3-kinase, and after multiple steps, this results in the translocation of GLUT4 containing vesicles toward plasma membrane and glucose uptake [38,39]. Glucose and TAG, ingested beyond the current energy demands, are taken up by adipocytes (as glucose and fatty acids), converted and stored in adipose tissue. Imported, as well as de novo synthesized fatty acids from excess glucose, make up a common fatty acids pool. Fatty acids aimed for storage combine with coenzyme A, form thioester and then they are re-esterified in a stepwise manner to form TAG. The TAG backbone – α-glycerol phosphate, mostly comes from glucose in the fed state (glycolytic intermediates) and lactate or pyruvate in the fasting state (glyceroneogenesis, i.e. shortened version of gluconeogenesis). De novo fatty acid synthesis from glucose is high in insulin sensitive adipocytes, especially after a high carbohydrate diet. Nicotinamide-adenine dinucleotide phosphate (NADPH) needed for this reductive biosynthesis is produced mostly by pentose-phosphate pathway (PPP) (and by malic enzyme). Thus, the glucose uptake and flux through the glycolytic pathway, PPP and glyceroneogenesis are of utmost importance for the lipogenic function of adipocytes (all steps are summarized in [2,4]). Indeed, all metabolic products of glucose increase in the fed state up to 10-fold, and they increase along with adipocyte size. It is estimated that the conventional products of the glucose metabolism, carbon dioxide and TAG account for 40–50% of glucose metabolism in larger, insulin sensitive cells vs. 80–85% of glucose metabolized in small, insulin sensitive cells. Most of the glucose in the fed state in the insulin sensitive, large adipocytes goes into lactate (50% and even 60–70% in human adipocytes) [32]. Pyruvate conversion into lactate reoxidizes nicotinamide-adenine dinucleotide (NADH) into NAD+ needed for glycolitic pathway maintenance. Accumulated lactate may be exported into the circulation and one part may also serve as a precursor for de novo fatty acid synthesis. Thus, it has been shown that increased glucose flux into lactate is paralleled by increased lipogenesis [32]. According to the expanding literature data, NADH/NAD+ ratio has an essential role in the adipocyte function, its healthy expansion after increased energy intake, but also in its dysfunction to expand and to accommodate nutritional overload [40,41].
5. NADH/NAD+ ratio is a new redox modulator of the adipose tissue
In the fed state glucose is taken up by adipocytes, through insulin-mediated glucose transport. Subsequently intensified flux through the glycolysis and PPP supplies energy needed for adipocyte activities and directs excess of metabolic substrates into TAG synthesis (lipogenesis) [32]. The released electrons from PPP are transferred to coenzyme NADP+ generating NADPH needed for de novo lipogenesis, i.e. for the α-glycerol phosphate, fatty acids and TAG synthesis [42]. In parallel, in the fed state, adipocytes shift from oxidative to glycolytic ATP production. High glycolytic flux generates NADH in cytosol originating from the oxidation of glyceraldehyde 3-phosphate reaction, and NAD+ must be regenerated for glycolysis to continue. One part of NAD+ is recycled by lactate dehydrogenase with the raising lactate level. A moderate shift in the NADH/NAD+ ratio due to intensified glycolysis in the fed state decreases the activities of all NAD+-dependent dehydrogenases downstream in regards to glucose oxidative degradation [40]. Moreover, the main sensor of the insulin-mediated glucose flux through the cells, NAD+-dependent histone deacetylase (like Sirtuin 1 – SIRT1) are highly susceptible to decrease in the NAD+/NADH level [43]. Depending on the nutrient availability and NAD+/NADH ratio SIRT1 couples the cellular metabolic status sensed via NAD+ to the peroxisome proliferator-activated receptor γ (PPARγ) expression and thus overall regulation of lipo- and adipogenesis. Inactivation of SIRT1 by decreased NAD+/NADH ratio, and subsequent inactivity of SIRT1-mediated signaling enables transcriptional upregulation of lipogenesis and adipogenesis, by relieving repression ofPPARγ [43].
6. Reductive pressure, oxidative pressure and counterbalance by mitochondrial antioxidant mechanisms
Enzymatic cofactors NAD+ and flavine adenine dinucleotide (FAD) “take over” and transfer the electrons from the oxidation of metabolic substrates (glucose and fatty acids) to the complexes of the mitochondrial electron transport chain (ETC) localized in the inner mitochondrial membrane. As electrons are transferred along the ETC to molecular oxygen, protons (H+) are pumped from the mitochondrial matrix into the intermembrane space, establishing a proton gradient across the inner mitochondrial membrane. The energy of the proton gradient drives the synthesis of ATP by ATP synthase and protons are transported back from the intermembrane space into the mitochondrial matrix [44].
In all aerobic cells most of the oxygen is reduced to water in the mitochondrial ETC, but a significant part of oxygen molecules are incompletely converted to H2O and end-up as superoxide anion radical (O2•−), nonenzymatically, by the electron leak from the complexes I and III [45,46]. The production of O2•− is favored if the ETC carriers are in a more reduced state achieved by (i) an increased inflow of reducing equivalents (increased “push force” for oxidative phosphorylation – OXPHOS) or by (ii) a decreased electron transfer capability of these carriers (decreased flow along the ETC, and/or decreased “pull force” for OXPHOS) [47]. Thus, it has been shown that in muscle high nutritional load increases the NADH level (high “push force”) and augments the pressure on ETC complexes, increasing the probability of electron leak, accelerating O2•− i.e. H2O2 production [47].
To prevent ROS excess, mitochondria are equipped with antioxidant defense (AD) enzymes, like manganese superoxide dismutase (MnSOD) that convert O2•− into H2O2. This notably reactive, non-radical molecule is further reduced to H2O in the mitochondrial matrix by glutathione (GSH) peroxidase (GPx) and/or thioredoxin/peroxyredoxin (Trx/Prx) systems. In contrast to the tissues with high oxidative capacity, like muscle tissue, fat tissue is characterized by a far lesser oxidative capacity and accordingly, a lower level of AD components [48]. Similarly, there are differences even between individual fat depots both in oxidative and antioxidant capacity [49].
Apart from antioxidant systems, electron transfer capability of the ETC regulated by uncoupling, plays an important role in the setting of physiological ROS production by mitochondria during the higher metabolite oxidation and NADH shift [50–52]. Mitochondrial uncoupling decreases the pressure on ETC complexes, increases their electron-transfer capability, thereby restraining the leak of electrons and subsequent production of O2•−. Concurrently, the leak of H+ uncouples the electron transport and ATP synthesis wasting the proton gradient (energy of metabolic substrates) as heat. It may be spontaneous, or mediated by mitochondrial uncoupling proteins (UCPs) [53]. Both, antioxidant and energy dissipating effects of UCPs are mutually connected. This refers even to thermogenin or uncoupling protein 1 (UCP1) in brown adipocytes, essential effectors of thermoregulatory diet- and cold-induced non-shivering thermogenesis [54–56]. The proton leak catalyzed by UCP1 is fatty acid dependent, and two proposed mechanism of fatty acid dependent-UCP1 function seem to operate in vivo. According to the first mechanism, fatty acids act as cofactors/activators [57], while the second “flip-flop” mechanism, proposes that protonated fatty acids freely cross the mitochondrial inner membrane into the matrix, and upon deprotonation, the resulting fatty acid anions are “flipped back” across the inner membrane by UCP1 [58,59]. Thus, UCP1 activity increase is closely associated with increased mitochondrial flux (cycling) of fatty acids.
The UCP1 in non-brown/beige adipocytes or non-adipose tissue (e.g. thymus) seems to have a more general physiological role, beyond energy wasting [60]. Transient increase of UCP1 expression in rat white adipocytes observed under cold exposure may be rather associated with increased lipolysis and fatty acid flux [61]. Actually, different roles of UCP1 in white (with low oxidative capacity) vs. beige/brown adipocytes (highly specialized to oxidize fatty acids) within whole adipose organ seems to be analogous to the different roles that UCP3 has in glycolytic type 2b and oxidative type 1 muscle fibers within muscle, respectively [62]. Thus, it may be speculated that transient upregulation of UCP1 in white adipocytes, like UCP3 in glycolytic muscle fibers, occurs as a defense, when fuel delivery and/or intensified flux of fatty acids exceed the oxidation capacity of adipocytes. The physiological meaning of UCP1 increase in white adipocytes is still vague, but seems to intricately link the oxidative metabolism, reductive pressure, i.e. consequent oxidative pressure in these cells.
In support, Carriere et al. [63] show that an increase in NADH production in mouse and human white adipocytes induces their “browning”, with the morphological characteristics of brown adipocytes. At the metabolic level there is a clear switch towards an oxidative, mitochondrial phenotype. This is followed by a strong expression of functionally active UCP1 in these brown-like adipocytes [63]. The authors elegantly show that L-lactate (and also β-hydroxybutyrate) induces white adipocyte remodeling, directly affecting the intracellular redox state by increasing NADH production in the cytoplasm or mitochondria. L-Lactate, either produced or taken up by white adipocytes via monocarboxylate transporters, can be oxidized to pyruvate in cytoplasm by lactate dehydrogenase. Also, L-lactate can enter mitochondria (most probably via symport with protons), and further be oxidized in the matrix by mitochondrial lactate dehydrogenase, whose existence has been documented in different cell types [64]. Increased NADH in the matrix elevates mitochondrial oxidative phosphorylation and the entire oxidative metabolism (pyruvate or lactate oxidation), which as a result has the higher oxidative pressure in white adipocyte mitochondria. The authors point out that the increase of UCP1 expression is in fact an adaptive response to L-lactate–NADH-induced redox pressure in white adipocytes. In other words, the redox-dependent UCP1 induction in white adipocytes does not have a thermoregulatory uncoupling function, as in brown adipose tissue, but better suits the so called “mild” uncoupling postulated by Skulachev [50], as a special mechanism in mitochondria which prevents a strong increase of electrochemical potential and consequent increment of ROS production. The observation that a lower level of UCP1 usually characterizes obese subjects, in comparison to normal-weight subjects [65], has raised the hypothesis that increasing UCP1 in human adipose tissue in physiological frameworks may be a promising anti-obesity target. However, if the main role of UCP1 induction in WAT (browning) is to alleviate the oxidative pressure because of incoming nutrient (reducing equivalent) excess on the ETC, it would be increased, but only in the extent of limiting the ROS burst. Thus, increasing white adipocyte browning by different agents may in turn cause insulin-sensitization opposed to presenting us with an anti-obesity tool. Furthermore these effects are not mutually exclusive, nor do they preclude the thermogenic role of UCP1, and they may in fact be a part of the same phenomenon.
7. Nutritional signaling in adipose tissue: mitochondria, NADH, and H2O2
Emerging lines of evidence indicate that both genuine AD and antioxidant effect of uncoupling in tissues, including the adipose tissue, are important as nutrient sensors (the sensors of reducing equivalents) in mitochondria and the active effectors mediating ROS-dependent signaling from mitochondria [66].
Initially, O2•− and H2O2, generated at the mitochondrial respiratory complexes, were regarded as inevitable injurious by-products, and it was thought that their passive accumulation leads to different degenerative diseases and aging [67–69]. Thus, the superoxide dismutases (SODs), MnSOD and Cu, ZnSOD, as well as H2O2-metabolizing enzymes and antioxidant molecules have been considered only as essential cellular protectant molecules. However, discovery of the enzymes that intentionally produce ROS, like the NAD(P)H oxidases suggested that fluxes of O2•− and H2O2, in the low, physiological range, play important signaling functions [70–72]. In this context, antioxidant enzymes now appear to be major players in both antioxidant defense and redox signaling.
As a mild oxidant, passing throughout the various cell compartments, H2O2 may act directly, specifically and reversibly with the redox-sensitive sulfhydryl groups in key cysteine residues of various signaling proteins/their binding partners, especially protein phosphatases, different metabolic enzymes and transcription factors [73]. Likewise, H2O2 may shift the ratio of reduced glutathione to oxidized glutathione, the main redox buffer in the cell, and thereby also promote redox-regulation of enzymatic activity by S-glutathionylation. Also important enzymes of the energy metabolism (e.g. glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and isocitrate dehydrogenase) may be redox-controlled by this mechanism [67,74,75]. Reversible oxidation of the cysteine sulfhydryl group acts as an “on-off” switch for changing structural configuration of proteins and thereby affecting their activity, localization and half-life [76,77]. Recently, it has been revealed that mitochondrial O2•−, i.e. H2O2 emission in different cells serves as both, an estimate of energy balance and as a determining factor of cellular redox environment, thus linking cellular metabolic balance to the control of their insulin sensitivity [51,78]. Thus, low, intermittent emission of H2O2 from mitochondria, following nutrient load, seems to be essential in the transient shifts in the intracellular redox environment to a more oxidized state [51]. This transient peroxidative shift may lead to oxidations and thus regulation of activity of numerous enzymes (mainly by oxidizing their Cys residues in catalytic or regulatory sites) in the cytosol, including the highly susceptible protein tyrosine phosphatases (PTPs), involved in insulin signaling [79]. Their transient inactivation thereby may relieve many kinases from the insulin signaling to promote transduction of the insulin action in the adipocyte. Once the level of H2O2 has returned to basal level, the alteration is reversed, and the activity of the protein reverts to its initial level, in the case of PTPs, their activation, and thus insulin signaling cessation [80]. Likewise, activation of the renin–angiotensin–aldosterone-system (RAAS) was reported for hyperglycemic and obese patients [81,82] leading to increased peroxynitrite formation associated with nitration/inactivation of Akt, nitration/activation of MAP kinases ERK1/2 and impaired insulin signaling [83].
In the context of normal nutrient supply, thereby transient rises in metabolic fluxes, and subsequent intermittent increase of ROS (O2•−, and H2O2) from the mitochondria has been recognized in various tissues, primarily muscles [51], brain [84], hypothalamus [85,86] and pancreatic β-cells [32], and recently also in the adipose tissue during the adipogenesis [87]. In adipose tissue, mitochondrial ROS production under increased glucose (or glucose metabolites) fluxes has mostly been studied in hyperglycemic diabetics [88,89], but it can be hypothesized that the similar redox changes, although less evident, may be seen in the prediabetic states, notably after continuous overconsumption of high carbohydrate, high fat diet.
8. Adipose tissue (dys)function
8.1. H2O2 output by NADPH oxidase
Oxidative stress, commonly defined as the consequence of an imbalance between pro-oxidant processes and antioxidant mechanisms leading to a deviation from the steady-state [90], play an instigating (preceding) role in the development of local and peripheral insulin resistance – and not only represent the consequence of the existing hyperglycemia [91]. From the recognition that insulin itself elicits the generation of H2O2 in adipocytes [92], and that excess of H2O2 may inhibit insulin signaling pathway [93], the insulin-instigated H2O2 production and redox signaling were in the center of the adipocytes insulin sensitivity regulation [79,80,94] and dysfunction [91].
NADPH oxidase (NOX) proteins are membrane-associated multimeric enzymes, that transport electrons preferentially from cytosolic NADPH (although nonphagocytic NADPH oxidases may also use NADH as substrate) down an electrochemical gradient through FAD, NOX heme groups, and finally through the membrane to oxygen, generating O2•−, which is rapidly converted to H2O2 [95,96]. Among the (currently seven) NOX isoforms, phagocytic NOX2 (in the macrophages) and NOX4 (in the adipocytes) are highly expressed in adipose tissues [97]. NOX4 can act as a switch between differentiation and proliferation in preadipocytes [98]. Unlike other NOX proteins, NOX4 can directly generate H2O2 even under basal conditions, in the absence of exogenous stimuli [96,99]. However, insulin, as well as some other (anabolic) hormones and cytokines induce a multi-fold increase in intraadipocyte ROS production, which was further increased by expression of NOX4 [80]. In fact, it is noted that H2O2 production by NOX may be activated by various cellular stressors, including ROS itself [100,101].
NOX4 upregulation by insulin is well known, but the underlying mechanisms are not solved finally. It is clear however, that besides the gene expression level, NOX4 activity may be regulated by protein modification [94]. Most importantly, as stated above, NOX4 enzyme activity is redox-regulated by its own product, H2O2 [94,102]. It is hypothesized that a self-limiting (feedback) mechanism may be involved in the low, physiological H2O2 output of non-phagocytic NOX. Li et al. showed also a feed-forward mechanism of NOX regulation by its product [103]. Precisely, it has been shown that exogenous exposure of fibroblasts to H2O2 activates NOX to produce endogenous O2•−, thereby amplifying ROS-mediated injury. This is important since it reveals that redox-dependent regulation of NOX activity in adipocytes is functionally important in transducing extracellular oxidative stress signals in vivo, through interaction with neighboring cells, such as macrophages.
9. NOX-driven redox communication between adipocytes and macrophages
Obesity is accompanied by a phenotypic transformation of adipose tissue macrophages (ATMs), from an anti-inflammatory “alternatively activated” M2 form, to a more proinflammatory “classically activated” M1 form that express different proinflammatory factors, which include tumor necrosis factor α (TNF-α) and inducible nitric oxide synthase iNOS [104,105]. The pro-inflammatory state of adipose tissue imposed by phenotypic transformation of ATMs may be a potential mechanism, whereby obesity leads to insulin resistance [105]. Primarily, a paracrine loop involving saturated fatty acids and TNF-α derived from adipocytes and macrophages, respectively, aggravates obesity-induced adipose tissue inflammation and dysfunction, i.e. systemic insulin resistance [106]. A growing body of evidence indicates that adipocytes and macrophages (and possibly other cells in the stromo-vascular fraction of adipose tissue) communicate and affect each other function/phenotype and overall (dys)function of adipose tissue – through redox mechanisms.
Increased oxidative pressure can lead to immune cell activation [101,107] or vice versa inflammatory cells upon infiltration to tissues can lead to cellular oxidative damage, as was recently demonstrated in the angiotensin II infusion model [108]. In order to introduce the vicious circle initiated by immune cells in adipose tissue, a pro-inflammatory state in obesity and hyperglycemia was reported [109], leading to activation of the potent ROS source NOX2 in infiltrating phagocytic cells. Most importantly, reducing the mitochondrial hydrogen peroxide levels by overexpression of catalase in mitochondria resulted in impaired bacterial killing [110]. Finally, increased superoxide/hydrogen peroxide formation was associated with the activation of the NLRP3 inflammasome, innate immune defenses and subsequent production of pro-inflammatory cytokines [111].
Still, macrophages are recognized to play an important role in adipose tissue healthy remodeling during both, expansion and regression of adipose tissue (e.g. in obesity or after 24 h fasting, respectively) [5]. In fact, increased flux of fatty acids in adipose tissue requires ATMs to create a permissive environment for the remodeling process i.e. homeostatic expansion/regression of adipose tissue [5]. However, a sustained interaction between endogenous ligands (fatty acids) derived from adipocytes and pathogen sensors expressed in macrophages leads to chronic inflammatory responses ranging from the basal homeostatic state to diseased, dysfunctional adipose tissue remodeling, which may be referred to as “homeostatic inflammation” [112]. In parallel with this, it can be hypothesized that a sustained feed-forward regulation of NOXs in adipocytes by NOXs from macrophages and vice versa, has a significant role in the adipose tissue redox state, and remodeling, ranging from homeostatic to dysfunctional. Finally, considering the role of NOX4 in the propagation of insulin signal in adipocytes, insulin sensitivity of adipocytes may be regulated through the complex intercellular NOXs, i.e. H2O2 mediated cross-communication.
10. NOX-mitochondrial cross-talk
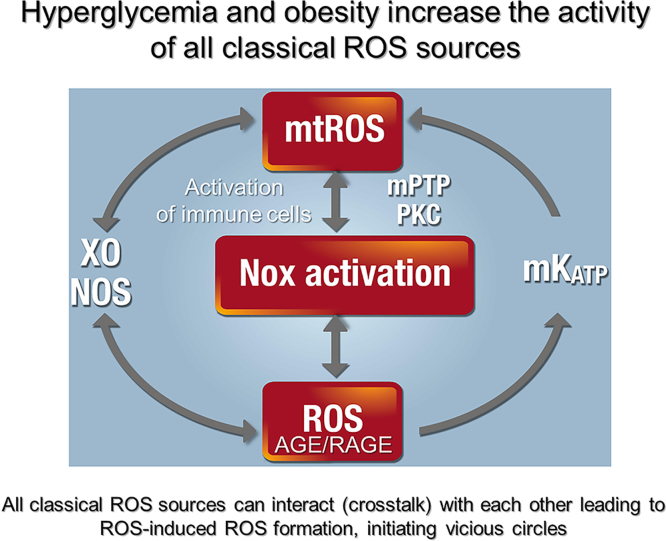
Emerging evidence suggests important NOX4 cross-talk with the mitochondria [113], activation of NOX1/2 by mitochondrial ROS [107,114] and vice versa of mitochondrial ROS by NOX2 [115,116]. In particular, mild mitochondrial dysfunction and mitochondrial ROS formation might abolish the responsiveness of NOX, although the baseline H2O2 production by NOX is in fact enhanced [117]. Dysfunctional mitochondria can also activate ROS formation in neighboring mitochondria in a redox-dependent fashion [118,119]. In general, any biological source of ROS may be activated in a crosstalk fashion by ROS derived from other sources (e.g. NOX-derived ROS may uncouple nitric oxide (NO) synthases (NOSs) and trigger NOS-derived ROS formation or xanthine oxidase is activated by ROS-dependent cysteine oxidation) as shown in Fig. 2 [101]. As a consequence, all classical sources of ROS were found to be activated under hyperglycemic conditions [120]. Overall, the studies on different cells consistently indicate that NOX–H2O2-producing system works both, upstream and downstream of mitochondria [114,121]. This cross-talk may be of utmost significance in defining redox insights into adipose tissue function and insulin insensitivity development (dysfunction).
Fig. 2.
Crosstalk between different sources of reactive oxygen and nitrogen species (mitochondria, NADPH oxidases, xanthine oxidase (XO) and nitric oxide (NO) synthase). Hyperglycemia and obesity lead to activation of primary ROS sources such as mitochondria (mtROS, due to metabolic dysregulation) and NADPH oxidases (Nox-derived ROS, due to low-grade inflammation). Signaling by advanced glycation end products (AGE) and their receptor (RAGE) might also trigger the activation of NADPH oxidases. These primary ROS sources can activate each other in a crosstalk fashion via so-called “kindling radicals” with the potential involvement of redox-sensitive mitochondrial pores (mPTP, mitochondrial permeability transition pore and mKATP, mitochondrial ATP-sensitive potassium channel) and protein kinases (PKC). Primary ROS from mitochondria and Nox enzymes can also activate secondary ROS sources such as xanthine oxidase (by oxidative conversion from the dehydrogenase form) and uncoupled nitric oxide synthases (by uncoupling via several redox switches). The ROS-induced ROS formation can initiate vicious circles that further aggravate the disease progression and stimulate AGE/RAGE signaling as well as low-grade inflammation.
Modified from Biochim Biophys Acta 1797 (2010) 897–906. With permission by Elsevier.
Another major concept of diabetic pathology (also applying to the metabolic syndrome as observed in obesity) is based on direct glucotoxicity, including increased formation of advanced glycation end products (AGE) and their signaling via specific receptors (RAGE) leading to vascular dysfunction and end organ damage [122,123]. Most importantly, oxidative stress and AGE/RAGE components interact with each other in a cross-talk fashion, wherein AGE/RAGE signaling can activate sources of ROS [124,125] and normalization of mitochondrial ROS formation in turn normalizes hyperglycemic damage by decreasing AGE/RAGE signaling [126] (Fig. 2). In 2001, it was demonstrated that macrophages from gp91phox deficient mice responded less to AGE stimulation providing a direct link between AGE/RAGE signaling and NADPH oxidase expression/activity [125]. The same authors also demonstrated a connection between AGE/RAGE signaling and inflammation by increased vascular cell adhesion molecule-1 (VCAM-1) and tissue factor expression in human endothelial cells in response to AGE treatment.
11. Instigating role of mitochondrial ROS in insulin resistant adipocyte response
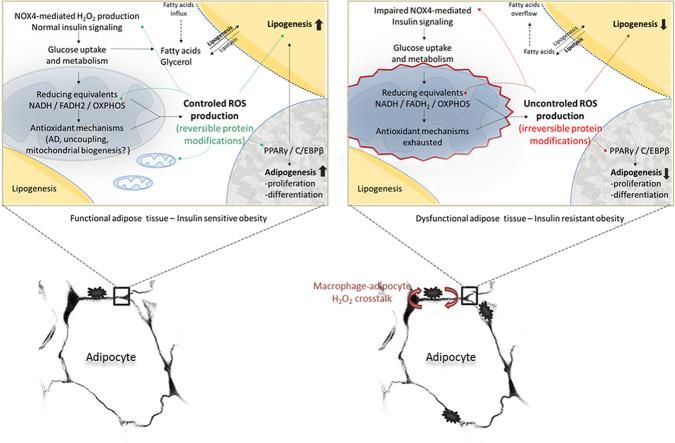
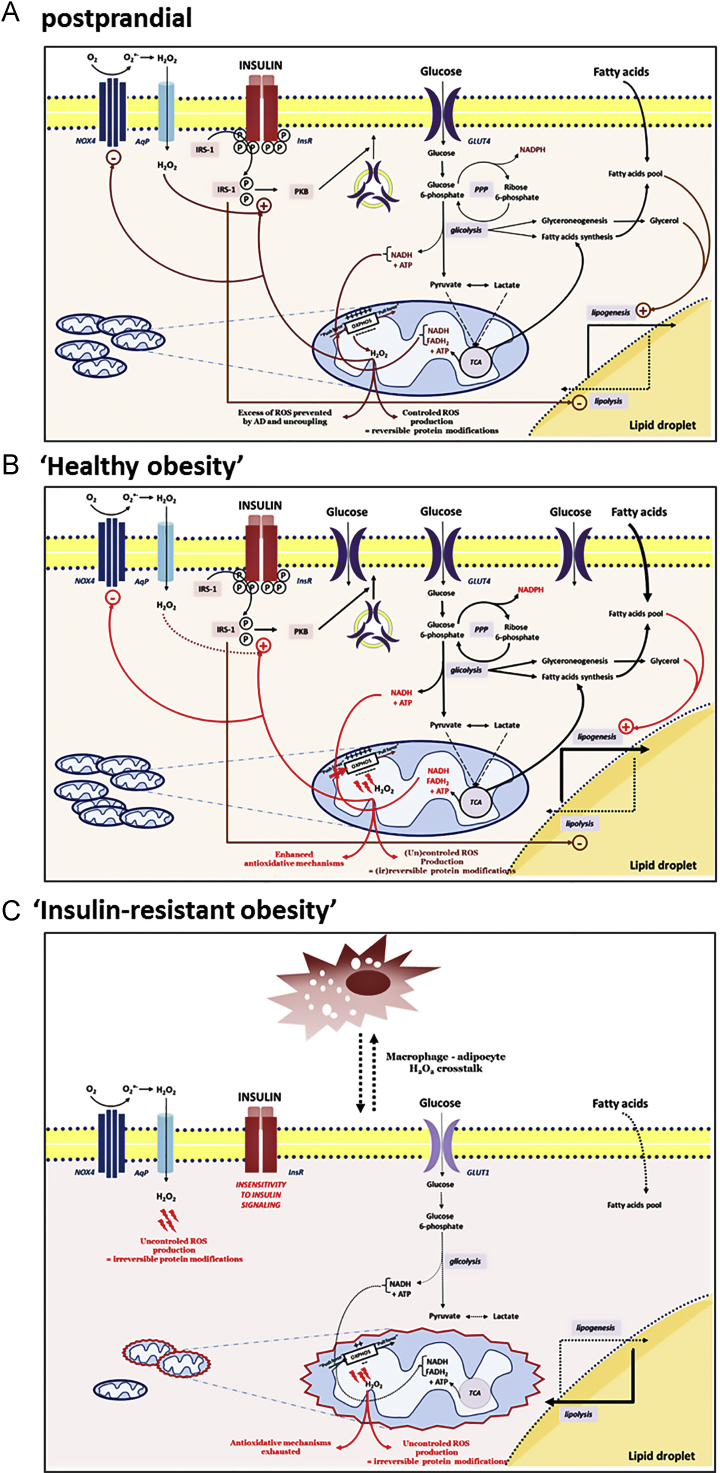
Mitochondrial dysfunction and oxidative stress have been consistently recognized to be implicated in the etiology of insulin resistance [10,12]. However, the evidence supporting a specific role for mitochondrial O2•− in adipocytes insulin resistance was limited in comparison to well recognized responsibility of NOX4–H2O2-producing system [91]. The (patho)physiological role of mitochondrial ROS in insulin sensitivity was mostly recognized in muscles, the tissue with high oxidative capacity, while in adipocytes, as mostly oxidative dormant cells, this source of ROS, as well as the mitochondria as a whole were mostly underestimated. The first evidences that mitochondrial ROS play an important role in adipocyte insulin resistance were provided by Houstis et al. [10], after the recognition of an increase in adipocyte mitochondria during the differentiation of adipocytes [127]. It was recently accentuated that increased mitochondrial phosphorylation due to increased influx of nutrients in the absence of ATP consumption increases ADP availability and increases the occupancy of the electron carriers, increasing the probability of an electron leak and O2•− generation [12]. Namely, by using the mitochondria-targeted dye, highly specific for O2•−, Hoehn et al. [12] have shown that increased mitochondrial O2•− production actually lies upstream of insulin resistance. Using a minimal but chronic exposure to insulin, corticosteroids, cytokines and lipids did not show consistent change in the proximal part of the insulin signaling (PI3K/Akt) pathway [128], indicating indeed that there is a direct causal link and instigating role of the mitochondrial ROS, with insulin resistant response of adipocytes. These data were confirmed by the results showing that both, uncoupling agents and MnSOD mimetics, that alleviate O2•− in the ETC rapidly restore insulin sensitivity of adipocytes [12]. Finally, although the insulin resistance and diabetes type 2 are associated with mitochondrial dysfunction, hyperinsulinemia is in fact, associated with an increase in mitochondrial oxidative phosphorylation [129]. These results support the assumption that mitochondrial oxidative pressure in nutrient oversupply (especially high calorie intake) is the preceding factor that – if not compensated with UCPs or mitochondrial-targeted antioxidants, over time, may lead to mitochondrial dysfunction – evident in insulin resistance (Fig. 3). Intriguingly, insulin resistant response of adipocytes is not viewed as dysfunction, but as an appropriate, functional response of adipocytes to defend themselves from nutrient excess. In fact, by suppressing glucose uptake, adipocytes decrease the metabolic pressure (inflow of electrons to ETC from high NADH), and consequently ROS release. In such context insulin resistant response of adipocytes becomes the last-line antioxidant defense response.
Fig. 3.
Hypothetical model of insulin resistance development in response to nutrition overload. (A) Postprandially, adipocytes serve as sink for excess fatty acids, most of which is thereby stored in form of triacylglycerols. Storage of lipids requires marked uptake, conversion and oxidation of glucose molecules to build up the ATP pool (via glycolysis and OXPHOS), intermediates for fatty acid and glycerol synthesis (via glycolysis and TCA cycle), as well as reducing equivalents for biosynthetic processes (via glycolysis, TCA cycle and PPP). An increased level of serum insulin after a meal stimulates glucose uptake by adipocytes via GLUT4, while simultaneously showing a great anti-lipolytic effect. Glucose uptake further leads to draining of electron-driven energy from the fuel, and subsequently, results in an increase of reductive pressure within the cell. Enhanced ‘push force’ (NADH and FADH2) for the OXPHOS speeds up flux through the respiratory chain, and thus, increases ROS production. Leakage of the electrons from the complexes I and III of OXPHOS onto O2, generates O2•−, which is promptly converted to H2O2. This freely diffusible and notably reactive molecule, when produced in a controlled manner, may oxidize and thus regulate the activities of numerous enzymes (mainly by oxidizing their Cys residues in catalytic or regulatory sites). Due to this, H2O2 has long been known as an insulin-mimicking molecule, which amplifies insulin-triggered signaling through the cell. Besides this casually-based mitochondrial production, H2O2 is also generated in a strictly-controlled, intentional way by NOX4. These pathways of H2O2-productions are mutually interrelated, thus providing an optimal range of H2O2 within the cell. Also, there are some other mechanisms that prevent excessive production of ROS, such as AD and uncoupling that decreases the electrochemical gradient on the two sides of the inner mitochondrial membrane. However, in the case of nutritional overload (B), all of the above-described pathways are markedly up-regulated, leading to a significant increase in reductive pressure, and consequently, an increase in oxidative pressure as well. In these conditions, the cell protects itself from oxidative injures by increasing antioxidant mechanisms: antioxidants, antioxidant enzymes, uncoupling etc. (newly established oxidative-antioxidant balance). Up to here, no or few pathological events occur, and thus it is still legitimate to talk about cell function. However, this is ‘walking on a string’ and it is a question of when this up-regulated metabolism will cause slipping into pathology. (C) Namely, persistent nutritional overload, and consequent oxidative pressure, may easily and at any moment, exceed the antioxidant capacity of the cell. If this happen, oxidative injures begin to accumulate leading to irreversible damage of proteins and other biomolecules, and subsequently, signaling pathways (insulin resistance), organelles (mitochondria) and the cell in whole. Blockage of the insulin-signaling pathway in adipocytes removes the anti-lipolytic effect of insulin, leading to hydrolysis of triacylglycerols and enhanced outflow of fatty acids from WAT, which now have to deposit in the liver and muscle, and may induce insulin resistance on the systemic level. As for the arbitrary correlation between function and dysfunction, it is important to note that insulin resistance may be seen as function, not dysfunction, of adipocytes, because in this way the cells protect themselves from excessive uptake and metabolism of glucose, which drives ROS generation (the last line of defense in the cell).
According to Frizzel et al. [89] when elevated carbohydrate fuel supply exceeds the metabolic needs of the cell, there is an overproduction of reducing equivalents, namely NADH. ATP accumulation in adipocytes may lead to feedback inhibition of OXPHOS and ATP synthase and induce a high membrane potential. Accordingly, electron flow is inhibited and the NADH concentration rises. This increase of the NADH/NAD+ ratio, in diabetes type 2 is well described as “reductive stress”, i.e. pseudohypoxia in the absence of actual hypoxia [130]. A dramatic increase in NADH may cause (i) feedback inhibition of all NAD+-dependent dehydrogenases in the tricarboxylic acid (TCA) cycle and the accumulation of TCA intermediates, like the fumarate and malate, further leading to succination of Cys in wide range of proteins [89]. Moreover, raised NADH levels may exceed the acceptor capacity of its main electron acceptor in the ETC – complex I, perpetuating an electron leak, and O2•− i.e. H2O2 generation. Both, a leak of electrons from ETC, as well as the increased fumarate lead to irreversible modifications of prolyl-4-hydroxylase, enzyme that catalyzes hydroxylation of the Pro residues in the hypoxia-inducible factor 1 alpha (HIF-1α), and thus permanently stabilizes HIF-1α, leading to the state of “pseudohypoxia” [131].
Excessive increase of ROS, i.e. by mitochondria, may have profound effects on multiple levels, including different tissue compartments, from the mitochondrial ETC and the mitochondria itself (TCA cycle enzymes etc.) through the upstream metabolic and regulatory enzymes, like GAPDH. Important enzymes of the energy metabolism (e.g. GAPDH and isocitrate dehydrogenase) may be redox-controlled by oxidation/reduction of cysteine residues [67,74,75]. Thereby, accumulation of intermediate of glycolysis stimulate the other related pathways (polyol, hexosamine, the protein kinase C – PKC activation, advanced glycation end products, and glyceraldehyde autooxidation) [68], further disseminating ROS, particularly O2•−, and consequently, H2O2.
The moderate, transient (oscillatory) mitochondrial ROS may induce opening of mitochondrial permeability transition pore (mPTP) and associated ROS release from mitochondria has been regarded as an adaptive housekeeping function by the timely release from mitochondria of accumulated potentially damaging levels of ROS [119]. However, higher ROS levels and longer mPTP openings may release a ROS burst (ROS-induced ROS release) leading to destruction of mitochondria, and if propagated from mitochondrion to mitochondrion, of the cell itself.
ROS released out of mitochondria may activate local pools of redox-sensitive enzymes [119]. ROS, primarily H2O2, may ascend even the proximal parts of the insulin signaling pathway and NOX4 itself. Even more, H2O2 may diffuse out of the adipocyte affecting neighboring adipocytes, preadipocytes, endothelial cells and tissue macrophages. H2O2 may pass membranes of different cellular compartments also by diffusion that is facilitated by aquaporins, in a more regulated manner [132] and depending on the H2O2 concentration as well as the membrane phospholipid composition, differently affecting cellular compartments.
Thus, H2O2 metabolism and transduction inside and out of adipocytes may be regarded as an auto/paracrine signal integrating the nutrient sensing of adipose tissue with its metabolic and structural remodelling enabling functional fat expansion. Its impairment however, may lead to adipose tissue dysfunction.
12. NOS – source of ROS and NO in adipose tissue
Besides mitochondria, different oxido-reductase systems, like nitric oxide (NO) synthases (NOS) and NOX, can transfer electrons to O2 in NAD(P)H-dependent reactions [133,134]. These enzymes can serve as an important source of superoxide in adipose tissue. In this regard, NOSs, apart from NOX, are of particular importance.
NOS catalyzes the formation of NO from L-arginine via two successive steps involving an electron transfer between the C-terminal flavin-containing reductase domain and N-terminal heme-containing oxygenase domain [135]. Under certain conditions, the enzymatic activity of NOS can be uncoupled to produce O2•− rather than NO, e.g. when the concentration of NOS cofactor tetrahydrobiopterin or substrate L-arginine is too low as well as several other redox-controlled pathways (e.g. accumulation of asymmetric dimethylarginine, adverse phosphorylation by PKC or protein tyrosine kinase-2, oxidative disruption of the zinc–sulfur-complex at the dimer binding interface) [101,109]. The oxidized form of tetrahydrobiopterin is unable to donate an electron to synthesize NO, while peroxynitrite is formed from concomitant generation of superoxide and NO by uncoupled NOS [136]. The NO has important roles in the adipocytes metabolism and particularly mitochondrial bioenergetics, in both brown [137,138] and white adipocytes [139,140]. There are also direct in vivo evidences that insulin stimulation of glucose uptake [141] and the induction of UCP1 (unpublished data), antioxidant defense [142] and oxidative capacity [143] in WAT are all stimulated by L-arginine, i.e. NO. Moreover, the effects of NO depend on the rate of its synthesis by NOSs and its availability that may be determined by the current O2•− level. There is clear evidence that adipocytes contain nitric oxide synthase, most likely endothelial NOS [144–146]. Unfortunately, the role of NO in WAT remains out of the scope of the current review but the close interaction of inflammation in fat tissue, adipocyte (dys)function and regulation of the vascular tone is currently a major field of interest [147].
13. Redox insights into adipogenesis
Some transcription factors may be activated, leading to enhanced target gene expressions in the presence of oxidants. Besides well-known redox-dependent transcription factors involved in the upregulation of AD enzymes (nuclear factor-erythroid 2-related factor 2, nuclear factor-kappa B, activator protein-1, and tumor protein p53), the crucial adipogenic factors – PPARγ and CCAAT/enchancer binding proteins (C/EBPs) are redox sensitive [148]. According to the raising data impaired redox regulation interferes with the adipogenic capacity of adipose tissue through the regulation of pro- and anti-adipogenic factors.
As a response to nutritional overloads and the need for additional fat stores, adipose tissue can expand by means of proliferation and differentiation [149]. Obesity in the adult human is mainly hypertrophic, caused by hypertrophy of adipocytes with increased lipid accumulation. De novo adipocyte formation from stem cells might be responsible for continuous turnover of fat cells in the body [150]. Remarkably, evidence has indicated that the recruitment of new preadipocytes, in subcutaneous adipose tissue, capable of undergoing adipogenic differentiation to mature adipocytes is actually reduced. Based on this, it is estimated that restoring the impaired preadipocyte differentiation in subcutaneous adipose tissue may be a new approach to increase its expanding capacity, prevent the lipid overfill to visceral and nonadipose tissues and resultant insulin resistance [150,151]. It is likely that ROS may produce contrasting effects on adipogenesis depending on the adipogenesis state and intensity of ROS generation [152].
Adipogenesis is a complex two-step process involving a highly orchestrated program of transcriptional regulation of pro- and anti-adipogenic factors, mitochondriogenesis and mitochondrial remodelling paralleled by increasing insulin signalling, triglycerides accumulation and the redox changes [127,152,153]. Adipogenic process occurs via the commitment of mesenchymal stem cells toward preadipocyte fate, and terminal differentiation of preadipocytes into mature adipocytes [152]. A number of different pro- and anti-adipogenic transcription factors control the adipogenic program, and the PPARγ and C/EBPα are considered to be master regulators of the adipogenic process [4,152]. These factors are upregulated by transient upregulation of C/EBPβ and C/EBPδ. Besides, sterol-regulatory element binding protein 1c (SREBP1c) itself regulated by insulin and lipids activates PPARγ and directly upregulates lipogenic enzymes (fatty acid synthase, lipoprotein lipase). Together, these factors contribute to lipogenesis and the final differentiation of adipocytes [154–156].
ROS are involved in both steps of adipogenesis. Increased O2•− and H2O2 production occur in the early and terminal adipocyte differentiation, primary from the increased mitochondriogenesis, mitochondrial function and the NOX4 [98,157]. Carriere et al. [158] demonstrated that an increase in mitochondrial ROS production caused by inhibition of the ETC impedes preadipocyte proliferation, while it was also observed that in the early phase of adipocyte differentiation of human mesenchymal stem cells, there was an increase in mitochondrial metabolism and ROS generation. Moreover, the authors demonstrated that ROS production from mitochondrial complex III was required for activation of the adipogenic transcriptional cascade via upregulation of C/EBPα and PPARγ [157].
In fact, various oxidant compounds, including the inhibition of glutathione synthesis, increase transcriptional activity of PPARγ [159,160]. Recently, Schopfer et al. have recognized redox-sensitive Cys285, located in the ligand-binding domain of PPARγ [161]. Also, it was shown that oxidative conditions induce disulfide bond formation between Cys296 and Cys143 and dimerization of C/EBPβ, additionally potentiating its phosphorylation-, oxidation-, and dimerization-dependent DNA binding activity [162]. Among different oxidants, H2O2 seems to be an important ROS signaling molecule in the transcriptional regulation of adipocytes differentiation and proliferation. Namely, it has been recognized that this molecule increases the expression and/or the binding activity of PPARγ [161] and C/EBPβ [152].
MnSOD, catalase, peroxiredoxins, thioredoxin reductase (TR), and glutathione peroxidase play important roles in the prevention of the excessive level of O2•− and H2O2 during adipocytes differentiation [153,163–165]. Actually, the expression of selenoproteins P and S and GPx (GPx 1, GPx 3 and GPx 4) has been detected in the adipose tissue, while its decreased expression or knock-down impaired adipogenesis, and insulin signaling in the mature adipocytes [156,166]. The role and regulation of thioredoxin-dependent redox system in the human non-diabetic obesity seems to be of the utmost importance, since in the obese non-diabetic humans, a positive correlation between TR1 mRNA [167], TR activity and thioredoxin protein level [49] and fat mass of subcutaneous adipose tissue level was observed. Contrary, different anti-adipogenic factors (TNF-α, rapamycin, an inhibitor of the mammalian target of rapamycin – mTOR) suppressed the induction of TR1 and thioredoxin 2 during the course of adipogenesis [165]. Meanwhile, it has been shown that both, the N-acetyl cysteine a glutathione precursor and its antagonist buthionine sulfoximine, an inhibitor of γ-glutamylcysteine synthetase and de novo GSH synthesis inhibit adipocyte differentiation [168,169]. Unexpectedly, inhibition of GPx, addition of GSH and H2O2 all resulted in impaired insulin signaling in 3T3-L1 adipocytes [166]. According to all these results, it is conceivable that the differentiation of adipocytes is paralleled and dependent on the shift in the redox balance, reflected by both, the progressive increase of ROS (H2O2) and their counterbalancing reductants. Finally, it seems that compared to less differentiated adipocytes, mature insulin-sensitive adipocytes during the differentiation process attain and operate in new, higher redox equilibrium. That being so, increasing/decreasing of both redox sites (oxidant and/or reductant) may disrupt the homeostatic redox balance for the current differentiation state of adipocytes, potentially restricting their adipogenic and lipogenic capacity to expand under nutritional overload. However, increased markers of oxidative stress seen in adipose tissue of insulin resistant rodents and humans possibly result from exhausted capacity of endogenous antioxidants to provide redox balance over the long term [49,91].
The beneficial effect of calorie restriction (CR) on insulin sensitivity and metabolic health is partially mediated by its effects on adipose tissue adipogenesis. Attenuated glucose flux trough metabolic pathways in CR, increases NAD+ and this is sensed trough the NAD+-dependent class III histone deacetylases, i.e. Sirtuins (SIRT1–SIRT7) [170]. Among them, SIRT1 directly block lipid anabolism and adipogenesis by interfering with transcriptional activity of PPARγ in adipose tissue [43]. Importantly, SIRT1 targets also the Forkhead-O-box (FOXO) family of transcription factors [171,172] and PPARγ coactivator 1α (PGC-1α) [173], master regulators of lipid metabolism, stress resistance and apoptosis [174,175], i.e. mitochondrial gene expression and mitochondrial biogenesis [176], respectively. Deacetylation of PGC-1α and FOXOs by SIRT1 enhances lipid catabolism and mitochondrial respiration and reinforces mitochondrial antioxidant enzymes and uncoupling protein 2 [177,178]. In turn, mitochondrially localized member of sirtuin family of proteins – SIRT3, functions as a downstream target gene of PGC-1α, mediating the PGC-1α effects on cellular ROS production and mitochondrial biogenesis [179]. The biological functions of SIRT3 are beginning to emerge; its overexpression seems to increase respiration, while decreasing ROS production [180]. Overall, SIRT1/SIRT3-mediated transcriptional reprograming of adipogenesis/lipogenesis toward adipostasis and improved oxidative capacity – “metabolic fitness” of adipose tissue underlie beneficial effects of CR/CR mimetics on systemic insulin sensitivity and metabolic homeostasis.
14. Conclusion and clinical implication
In summary, the current data indicate that the function of adipose tissue, primarily the response to insulin to accommodate energy surplus safely, is intricately linked with the redox changes. Both the lipogenesis and adipogenesis are controlled by the redox shift, a mostly moderate change in the H2O2 metabolism, possibly instigated by the mitochondrial nutrient sensing. In increased nutrient overload, as well as during the adipogenesis both sites of the redox balance shift to new redox equilibrium, as a part of a new metabolic homeostasis – to accommodate its lipid storage function. Thus, healthy obesity is consistent with the lack of the redox stress signature, at least in the adipose tissue. On the other side, persistent nutritional overload, and consequent oxidative pressure, may effortlessly and abruptly end, exceed the antioxidant capacity of the cell. In contrast to visceral, subcutaneous adipose tissue appears to be well protected against an increased generation of oxygen species in obese subjects [49]. In this regard, low glutathione/thioredoxin levels are of critical significance as the main redox buffers in the cells. If this happens, oxidative injures begin to accumulate leading to irreversible damage of proteins and other biomolecules, signaling pathways (insulin resistance), organelles (mitochondria) and adipocytes/adipose tissue in whole. Consistently data suggest that oxidative damage, especially at the level of expanding visceral adipose tissue may precede adipocyte dysfunction and the development of further metabolic disorders even in obese, still metabolically healthy individuals [4,7,49,181–187].
Of high clinical importance, recent observations indicate that some specific antioxidants by suppression of oxidative stress can prevent accumulation of “bad” adipose tissue and weight gain. Celastrol, triterpenoid compounds with antioxidant and anti-inflammatory properties, was able to effectively suppress weight and alleviate high-fat mediated cardiovascular injury via mitigating oxidative stress and improving lipid metabolism [188]. Also, α-lipoic acid increased mitochondrial content, enhanced oxygen consumption and fatty acid oxidation enzymes in cultured white subcutaneous adipocytes from overweight/obese donors [189]. Mitochondria from α-lipoic acid-treated adipocytes exhibited some morphological characteristics of brown mitochondria, and α-lipoic acid also induced up-regulation of some brown/beige adipocytes markers. Bilobalide, a bioactive from Gingko biloba, significantly protected adipocytes from adverse effects of hypoxia attenuating superoxide generation, inflammation and protecting mitochondria [190].
Actually, different agents that improve mitochondrial redox status by reducing ROS levels and/or by increasing mitochondrial antioxidant activity may be beneficial in both, reducing obesity and improving the metabolic profile. A recent study demonstrated that chronic oral melatonin improves mitochondrial respiration and reduces the oxidative status in inguinal white adipose tissue of Zucker diabetic fatty rats [191]. Besides, growing evidence shows that dietary supplementation with arginine, a precursor of nitric oxide, effectively reduces white adipose tissue in Zucker diabetic fatty rats, diet-induced obese rats, and obese patients with type II diabetes [143,192,193]. L-Arginine upregulates lipolysis and fatty acid oxidation, and inhibits fatty acid synthesis an adipose tissue. The underlying mechanisms involve increases in the expression of PGC-1α, mitochondrial biogenesis, the growth of brown adipose tissue. More importantly, oxidative stress in white adipose tissue of high fat-fed rats was prevented by L-arginine supplementation probably by inducing endogenous antioxidant enzymes [143]. Accordingly, L-arginine holds promise as a safe nutrient to reduce adiposity and improve the metabolic profile in animals and humans.
It appears that the modulation of redox metabolism in adipose tissue by different redox active molecules may provide a promising strategy for the prevention/treatment of insulin resistance and metabolic syndrome. On the other hand, due to the complex ROS function in adipocytes and different redox tone in adipose tissue from different body sites, it is not surprising that conventional AD therapies with vitamins even show a negative effect on systemic insulin sensitivity [194,195]. The explanation would be that any intervention from this direction (by continuous exogenous antioxidant supplementation) interrupts the adipose tissue depot-specific dynamic steady state balance.
Overall, antioxidant supplements cannot compensate for a Western diet and sedentary lifestyle, but antioxidant-rich foods and/or supplements that reinforce endogenous antioxidant defense in parallel with promoting of browning of adipose tissue can improve the organ damage and late complications of obesity and hyperglycemia [140,196].
Acknowledgements
This work was supported by the Ministry of Science and Technological Development of the Republic of Serbia, Grant 173055 and by the European Cooperation in Science and Technology (COST Action BM1203/EU-ROS).
References
- 1.DeCaro L.G. The mobilization of depot fats. Hormonal mechanisms of regulation of the metabolism of adipose tissue. Arch. Sci. Med. 1964;118:10–36. [PubMed] [Google Scholar]
- 2.Large V., Peroni O., Letexier D., Ray H., Beylot M. Metabolism of lipids in human white adipocyte. Diabetes Metab. 2004;30:294–309. doi: 10.1016/s1262-3636(07)70121-0. [DOI] [PubMed] [Google Scholar]
- 3.Yu Y.H., Ginsberg H.N. Adipocyte signaling and lipid homeostasis: sequelae of insulin-resistant adipose tissue. Circ. Res. 2005;96:1042–1052. doi: 10.1161/01.RES.0000165803.47776.38. [DOI] [PubMed] [Google Scholar]
- 4.Sethi J.K., Vidal-Puig A.J. Adipose tissue function and plasticity orchestrate nutritional adaptation. J. Lipid Res. 2007;48:1253–1262. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun K., Kusminski C.M., Scherer P.E. Adipose tissue remodeling and obesity. J. Clin. Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrauwen-Hinderling V.B., Kooi M.E., Schrauwen P. Mitochondrial function and diabetes; consequences for skeletal and cardiac muscle metabolism. Antioxid. Redox Signal. 2015 doi: 10.1089/ars.2015.6291. 10.1089/ars.2015.6291, in press. [DOI] [PubMed] [Google Scholar]
- 7.García-Ruiz C., Baulies A., Mari M., García-Rovés P.M., Fernandez-Checa J.C. Mitochondrial dysfunction in non-alcoholic fatty liver disease and insulin resistance: cause or consequence? Free Radic. Res. 2013;47:854–868. doi: 10.3109/10715762.2013.830717. [DOI] [PubMed] [Google Scholar]
- 8.Szendroedi J., Phielix E., Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2011;8:92–103. doi: 10.1038/nrendo.2011.138. [DOI] [PubMed] [Google Scholar]
- 9.Erol A. Insulin resistance is an evolutionarily conserved physiological mechanism at the cellular level for protection against increased oxidative stress. Bioessays. 2007;29:811–818. doi: 10.1002/bies.20618. [DOI] [PubMed] [Google Scholar]
- 10.Houstis N., Rosen E.D., Lander E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 11.Matsuzawa-Nagata N., Takamura T., Ando H., Nakamura S., Kurita S., Misu H., Ota T., Yokoyama M., Honda M., Miyamoto K., Kaneko S. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism. 2008;57:1071–1077. doi: 10.1016/j.metabol.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Hoehn K.L., Salmon A.B., Hohnen-Behrens C., Turner N., Hoy A.J., Maghzal G.J., Stocker R., Van Remmen H., Kraegen E.W., Cooney G.J., Richardson A.R., James D.E. Insulin resistance is a cellular antioxidant defense mechanism. Proc. Natl. Acad. Sci. U.S.A. 2009;106:17787–17792. doi: 10.1073/pnas.0902380106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Pauw A., Tejerina S., Raes M., Keijer J., Arnould T. Mitochondrial (dys)function in adipocyte (de)differentiation and systemic metabolic alterations. Am. J. Pathol. 2009;175:927–939. doi: 10.2353/ajpath.2009.081155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao C.L., Zhu C., Zhao Y.P., Chen X.H., Ji C.B., Zhang C.M., Zhu J.G., Xia Z.K., Tong M.L., Guo X.R. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol. Cell. Endocrinol. 2010;320:25–33. doi: 10.1016/j.mce.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 15.Postner B.L., Shaver A., Fantus I.G. In: New Antidiabetic Drugs. Bailey C.J., Flatt P.R., editors. Smith-Gordon; London: 1990. pp. 107–117. [Google Scholar]
- 16.Mukherjee S.P., Lynn W.S. Reduced nicotinamide adenine dinucleotide phosphate oxidase in adipocyte plasma membrane and its activation by insulin. Possible role in the hormone’s effects on adenylate cyclase and the hexose monophosphate shunt. Arch. Biochem. Biophys. 1977;184:69–76. doi: 10.1016/0003-9861(77)90327-7. [DOI] [PubMed] [Google Scholar]
- 17.Bashan N., Kovsan J., Kachko I., Ovadia H., Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol. Rev. 2009;89:27–71. doi: 10.1152/physrev.00014.2008. [DOI] [PubMed] [Google Scholar]
- 18.Cinti S. The adipose organ. Prostaglandins Leukot. Essent. Fatty Acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Waldén T.B., Hansen I.R., Timmons J.A., Cannon B., Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am. J. Physiol. Endocrinol. Metab. 2012;302:19–31. doi: 10.1152/ajpendo.00249.2011. [DOI] [PubMed] [Google Scholar]
- 20.Engfeldt P., Arner P. Lipolysis in human adipocytes, effects of cell size, age and of regional differences. Horm. Metab. Res. Suppl. 1988;19:26–29. [PubMed] [Google Scholar]
- 21.Frayn K. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45:1201–1210. doi: 10.1007/s00125-002-0873-y. [DOI] [PubMed] [Google Scholar]
- 22.Wajchenberg B.L., Giannella-Neto D., da Silva M.E., Santos R.F. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm. Metab. Res. 2002;34:616–621. doi: 10.1055/s-2002-38256. [DOI] [PubMed] [Google Scholar]
- 23.Bjørndal B., Burri L., Staalesen V., Skorve J., Berge R.K. Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J. Obes. 2011;2011:490650. doi: 10.1155/2011/490650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeFronzo R.A., Ferrannini E. Regulation of hepatic glucose metabolism in humans. Diabetes Metab. Rev. 1987;3:415–459. doi: 10.1002/dmr.5610030204. [DOI] [PubMed] [Google Scholar]
- 25.Blüher M., Michael M.D., Peroni O.D., Ueki K., Carter N., Kahn B.B., Kahn C.R. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev. Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 26.Abel E.D., Peroni O., Kim J.K., Kim Y.B., Boss O., Hadro E., Minnemann T., Shulman G.I., Kahn B.B. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 27.James D.E., Burleigh K.M., Kraegen E.W. Time dependence of insulin action in muscle and adipose tissue in the rat in vivo. An increasing response in adipose tissue with time. Diabetes. 1985;34:1049–1054. doi: 10.2337/diab.34.10.1049. [DOI] [PubMed] [Google Scholar]
- 28.Jansson P.-A., Larsson A., Smith U., Lonnroth P. Lactate release from the subcutaneous tissue in lean and obese men. J. Clin. Invest. 1994;93:240–246. doi: 10.1172/JCI116951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kashiwagi A., Verso M.A., Andrews J., Vasques G., Reaven G., Foley J.E. In vitro insulin resistance of human adipocytes isolated from subjects with non-insulin-dependent diabetes mellitus. J. Clin. Invest. 1983;72:1246–1254. doi: 10.1172/JCI111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahn B.B., Cushman S.W., Flier J.S. Regulation of glucose transporter-specific mRNA levels in rat adipose cells with fasting and refeeding. Implications for in vivo control of glucose transporter number. J. Clin. Invest. 1989;83:199–204. doi: 10.1172/JCI113859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herman M.A., Kahn B.B. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J. Clin. Invest. 2006;116:1767–1775. doi: 10.1172/JCI29027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiGirolamo M., Newby F.D., Lovejoy J. Lactate production in adipose tissue: a regulated function with extra-adipose implications. FASEB J. 1992;6:2405–2412. doi: 10.1096/fasebj.6.7.1563593. [DOI] [PubMed] [Google Scholar]
- 33.Bjorntorp P. Size, number and function of adipose tissue cells in human obesity. Horm. Metab. Res. 1974;4:77–83. [PubMed] [Google Scholar]
- 34.Hirsch J. B. Bachelor, Adipose tissue cellularity in human obesity. Clin. Endocrinol. Metab. 1976;5:299–311. doi: 10.1016/s0300-595x(76)80023-0. [DOI] [PubMed] [Google Scholar]
- 35.Heilbronn L., Smith S.R., Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int. J. Obes. Relat. Metab. Disord. 2004;4:S12–S21. doi: 10.1038/sj.ijo.0802853. [DOI] [PubMed] [Google Scholar]
- 36.Unger R.H., Scherer P.E. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol. Metab. 2010;21:345–352. doi: 10.1016/j.tem.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McQuaid S.E., Hodson L., Neville M.J., Dennis A.L., Cheeseman J., Humphreys S.M., Ruge T., Gilbert M., Fielding B.A., Frayn K.N., Karpe F. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes. 2011;60:47–55. doi: 10.2337/db10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kahn B.B., Cushman S.W. Subcellular translocation of glucose transporters: role in insulin action and its perturbation in altered metabolic states. Diabetes Metab. Rev. 1985;1:203–227. doi: 10.1002/dmr.5610010301. [DOI] [PubMed] [Google Scholar]
- 39.Watson R.T., Kanzaki M., Pessin J.E. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr. Rev. 2004;25:177–204. doi: 10.1210/er.2003-0011. [DOI] [PubMed] [Google Scholar]
- 40.Ido Y. Pyridine nucleotide redox abnormalities in diabetes. Antioxid. Redox Signal. 2007;9:931–942. doi: 10.1089/ars.2007.1630. [DOI] [PubMed] [Google Scholar]
- 41.Hwang J.H., Kim D.W., Jo E.J., Kim Y.K., Jo Y.S., Park J.H., Yoo S.K., Park M.K., Kwak T.H., Kho Y.L., Han J., Choi H.S., Lee S.H., Kim J.M., Lee I., Kyung T., Jang C., Chung J., Kweon G.R., Shong M. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes. 2009;58:965–974. doi: 10.2337/db08-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins Y., Chouchani E.T., James A.M., Menger K.E., Cochemé H.M., Murphy M.P. Mitochondrial redox signalling at a glance. J. Cell Sci. 2012;125:801–806. doi: 10.1242/jcs.098475. [DOI] [PubMed] [Google Scholar]
- 43.Picard F., Kurtev M., Chung N., Topark-Ngarm A., Senawong T., Machado De Oliveira R., Leid M., McBurney M.W., Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 45.Turrens J.F., Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem. J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson B.H. The role of manganese superoxide dismutase in health and disease. J. Inherit. Metab. Dis. 1998;21:598–603. doi: 10.1023/a:1005427323835. [DOI] [PubMed] [Google Scholar]
- 47.Fridlyand L.E., Philipson L.H. Reactive species and early manifestation of insulin resistance in type 2 diabetes. Diabetes Obes. Metab. 2006;8:136–145. doi: 10.1111/j.1463-1326.2005.00496.x. [DOI] [PubMed] [Google Scholar]
- 48.Galinier A., Carrière Y., Fernandez C., Carpéné M., André S., Caspar-Bauguil J.P., Thouvenot B., Périquet L., Pénicaud L. Casteilla, Adipose tissue proadipogenic redox changes in obesity. J. Biol. Chem. 2006;281:12682–12687. doi: 10.1074/jbc.M506949200. [DOI] [PubMed] [Google Scholar]
- 49.Jankovic A., Korac A., Srdic-Galic B., Buzadzic B., Otasevic V., Stancic A., Vucetic M., Markelic M., Velickovic K., Golic I., Korac B. Differences in the redox status of human visceral and subcutaneous adipose tissues—relationships to obesity and metabolic risk. Metabolism. 2014;63:661–671. doi: 10.1016/j.metabol.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 50.Skulachev V.P. Uncoupling: new approaches to an old problem of bioenergetics. Biochim. Biophys. Acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 51.Anderson E.J., Lustig M.E., Boyle K.E., Woodlief T.L., Kane D.A., Lin C.T., Price J.W., 3rd, Kang L., Rabinovitch, Szeto P.S., Houmard J.A., Cortright R.N., Wasserman D.H., Neufer P.D. Mitochondrial HH H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sivitz W.I., Yorek M.A. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signal. 2010;12:537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krauss S., Zhang C.Y., Lowell B.B. The mitochondrial uncoupling-protein homologues. Nat. Rev. Mol. Cell Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- 54.Nicholls D.G., Locke R.M. Thermogenic mechanisms in brown fat. Physiol. Rev. 1984;64:1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- 55.Nedergaard J., Golozoubova V., Matthias A., Asadi A., Jacobsson A., Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim. Biophys. Acta. 2001;1504:82–106. doi: 10.1016/s0005-2728(00)00247-4. [DOI] [PubMed] [Google Scholar]
- 56.Petrović V., Buzadzić B., Korać A., Vasilijević A., Janković A., Korać B. Free radical equilibrium in interscapular brown adipose tissue: relationship between metabolic profile and antioxidative defense. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006;142:60–65. doi: 10.1016/j.cbpc.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Klingenberg M., Huang S.-G. Structure and function of the uncoupling protein from brown adipose tissue. Biochim. Biophys. Acta. 1999;1415:271–296. doi: 10.1016/s0005-2736(98)00232-6. [DOI] [PubMed] [Google Scholar]
- 58.Skulachev V.P. Fatty acid circuit as a physiological mechanism of uncoupling of oxidative phosphorylation. FEBS Lett. 1991;294:158–162. doi: 10.1016/0014-5793(91)80658-p. [DOI] [PubMed] [Google Scholar]
- 59.Garlid K.D., Jabůrek M., Ježek P., Vařecha M. How do uncoupling proteins uncouple? Biochim. Biophys. Acta. 2000;1459:383–389. doi: 10.1016/s0005-2728(00)00175-4. [DOI] [PubMed] [Google Scholar]
- 60.Porter R.K. A new look at UCP 1. Biochim. Biophys. Acta. 1757;2006:446–448. doi: 10.1016/j.bbabio.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 61.Jankovic A., Golic I., Markelic M., Stancic A., Otasevic V., Buzadzic B., Korac A., Korac B. Two key temporally distinguishable molecular and cellular components of white adipose tissue browning during cold acclimation. J. Physiol. 2015 doi: 10.1113/JP270805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hesselink M.K.C., Keizer H.A., Borghouts L.B., Schaart G., Kornips C.F.P., Slieker L.J., Sloop K.W., Saris W.H.M., Schrauwen P. Protein expression of UCP3 differs between human type 1, type 2a and type 2b fibers. FASEB J. 2001;15:1071–1073. [PubMed] [Google Scholar]
- 63.Carriere A., Jeanson Y., Berger-Muller S., Andre M., Chenouard V., Arnaud E., Barreau C., Walther R., Galinier A., Wdziekonski B., Villageois P., Louche K., Collas P., Moro C., Dani C., Villarroya F., Casteilla L. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes. 2014:3253–3265. doi: 10.2337/db13-1885. [DOI] [PubMed] [Google Scholar]
- 64.Passarellaa S., de Barib L., Valentib D., Pizzutoa R., Paventia G., Atlanteb A. Mitochondria and l-lactate metabolism. FEBS Lett. 2008;582:3569–3576. doi: 10.1016/j.febslet.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 65.Esterbauer H., Oberkofler H., Liu Y.M., Breban D., Hell E., Krempler F., Patsch W. Uncoupling protein-1 mRNA expression in obese human subjects: the role of sequence variations at the uncoupling protein-1 gene locus. J. Lipid Res. 1998;39:834–844. [PubMed] [Google Scholar]
- 66.Casteilla L., Rigoulet M., Pénicaud L., Mitochondrial R.O.S. metabolism: modulation by uncoupling proteins. IUBMB Life. 2001;52:181–188. doi: 10.1080/15216540152845984. [DOI] [PubMed] [Google Scholar]
- 67.Kil I.S., Park J.W. Regulation of mitochondrial NADP+-dependent isocitrate dehydrogenase activity by glutathionylation. J. Biol. Chem. 2005;280:10846–10854. doi: 10.1074/jbc.M411306200. [DOI] [PubMed] [Google Scholar]
- 68.Yan M.H., Wang X., Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic. Biol. Med. 2013;62:90–101. doi: 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flynn J.M., Melov S. SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic. Biol. Med. 2013;62:4–12. doi: 10.1016/j.freeradbiomed.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Halliwell B., Gutteridge J.M. The antioxidants of human extracellular fluids. Arch. Biochem. Biophys. 1990;280:1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- 71.Kamata H., Hirata H. Redox regulation of cellular signaling. Cell. Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 72.Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 73.Rhee S.G., Bae Y.S., Lee S.R., Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci. STKE. 2000;53:pe1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 74.Oudot C., Jaquinod M., Cortay J.C., Cozzone A.J., Jault J.M. The isocitrate dehydrogenase kinase/phosphatase from Escherichia coli is highly sensitive to in-vitro oxidative conditions role of cysteine67 and cysteine108 in the formation of a disulfide-bonded homodimer. Eur. J. Biochem. 1999;262:224–229. doi: 10.1046/j.1432-1327.1999.00395.x. [DOI] [PubMed] [Google Scholar]
- 75.Hildebrandt T., Knuesting J., Berndt C., Morgan B., Scheibe R. Cytosolic thiol switches regulating basic cellular functions: GAPDH as an information hub? Biol. Chem. 2015;396:523–537. doi: 10.1515/hsz-2014-0295. [DOI] [PubMed] [Google Scholar]
- 76.Dalle-Donne I., Rossi R., Giustarini D., Colombo R., Milzani A. S-glutathionylation in protein redox regulation. Free Radic. Biol. Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 77.Jones D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fisher-Wellman K.H., Neufer P.D. Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol. Metab. 2012;23:142–153. doi: 10.1016/j.tem.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mahadev K., Wu X., Zilbering A., Zhu L., Lawrence J.T., Goldstein B.J. Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J. Biol. Chem. 2001;276:48662–48669. doi: 10.1074/jbc.M105061200. [DOI] [PubMed] [Google Scholar]
- 80.Goldstein B.J., Mahadev K., Wu X., Zhu L., Motoshima H. Role of insulin-induced reactive oxygen species in the insulin signaling pathway. Antioxid. Redox Signal. 2005;7:1021–1031. doi: 10.1089/ars.2005.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oelze M., Schuhmacher S., Daiber A. Organic nitrates and nitrate resistance in diabetes: the role of vascular dysfunction and oxidative stress with emphasis on antioxidant properties of pentaerithrityl tetranitrate. Exp. Diabetes Res. 2010;2010:213176. doi: 10.1155/2010/213176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mollnau H., Oelze M., Zinßius E., Hausding M., Wu Z., Knorr M., Ghaemi Kerahrodi J., Kröller-Schön S., Jansen T., Teutsch C., Foster C., Li H., Wenzel P., Schulz E., Münzel T., Daiber A. Effects of telmisartan or amlodipine monotherapy versus telmisartan/amlodipine combination therapy on vascular dysfunction and oxidative stress in diabetic rats. Naunyn Schmiedebergs Arch. Pharmacol. 2013;386:405–419. doi: 10.1007/s00210-013-0842-7. [DOI] [PubMed] [Google Scholar]
- 83.Csibi A., Communi D., Müller N., Bottari S.P. Angiotensin II inhibits insulin-stimulated GLUT4 translocation and Akt activation through tyrosine nitration-dependent mechanisms. PLoS One. 2010;5:e10070. doi: 10.1371/journal.pone.0010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benani A., Troy S., Carmona M.C., Fioramonti X., Lorsignol A., Leloup C., Casteilla L., Pénicaud L. Role for mitochondrial reactive oxygen species in brain lipid sensing: redox regulation of food intake. Diabetes. 2007;56:152–160. doi: 10.2337/db06-0440. [DOI] [PubMed] [Google Scholar]
- 85.Colombani A.L., Carneiro L., Benani A., Galinier A., Jaillard T., Duparc T., Offer G., Lorsignol A., Magnan C., Casteilla L., Pénicaud L., Leloup C. Enhanced hypothalamic glucose sensing in obesity: alteration of redox signaling. Diabetes. 2009;58:2189–2197. doi: 10.2337/db09-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leloup C., Tourrel-Cuzin C., Magnan C., Karaca M., Castel J., Carneiro L., Colombani A.L., Ktorza A., Casteilla L., Pénicaud L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes. 2009;58:673–681. doi: 10.2337/db07-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leloup C., Casteilla L., Carrière A., Galinier A., Benani A., Carneiro L., Pénicaud L. Balancing mitochondrial redox signaling: a key point in metabolic regulation. Antioxid. Redox Signal. 2011;14:519–530. doi: 10.1089/ars.2010.3424. [DOI] [PubMed] [Google Scholar]
- 88.Yan L.J. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J. Diabetes Res. 2014;2014:137919. doi: 10.1155/2014/137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frizzell N., Thomas S.A., Carson J.A., Baynes J.W. Mitochondrial stress causes increased succination of proteins in adipocytes in response to glucotoxicity. Biochem. J. 2012;445:247–254. doi: 10.1042/BJ20112142. [DOI] [PubMed] [Google Scholar]
- 90.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4C:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., Nakayama O., Makishima M., Matsuda M., Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.May J.M., de Haën C. Insulin-stimulated intracellular hydrogen peroxide production in rat epididymal fat cells. J. Biol. Chem. 1979;254:2214–2220. [PubMed] [Google Scholar]
- 93.Rudich A., Tirosh R., Potashnik R., Hemi H., Bashan N.K. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes. 1998;47:1562–1569. doi: 10.2337/diabetes.47.10.1562. [DOI] [PubMed] [Google Scholar]
- 94.Li J.M., Shah A.M. ROS generation by nonphagocytic NADPH oxidase: potential relevance in diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14:S221–S226. doi: 10.1097/01.asn.0000077406.67663.e7. [DOI] [PubMed] [Google Scholar]
- 95.Griendling K.K., Sorescu D., Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 96.Martyn K.D., Frederick L.M., von Loehneysen K., Dinauer M.C., Knaus U.G. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell. Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 97.Mouche S., Mkaddem S.B., Wang W., Katic M., Tseng Y.H., Carnesecchi S., Steger K., Foti M., Meier C.A., Muzzin P., Kahn C.R., Ogier-Denis E., Szanto I. Reduced expression of the NADPH oxidase NOX4 is a hallmark of adipocyte differentiation. Biochim. Biophys. Acta. 1773;2007:1015–1027. doi: 10.1016/j.bbamcr.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 98.Schröder K., Wandzioch K., Helmcke I., Brandes R.P. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler. Thromb. Vasc. Biol. 2009;29:239–245. doi: 10.1161/ATVBAHA.108.174219. [DOI] [PubMed] [Google Scholar]
- 99.Takac I., Schröder K., Zhang L., Lardy B., Anilkumar N., Lambeth J.D., Shah A.M., Morel F., Brandes R.P. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jiang F., Zhang Y., Dusting G.J. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol. Rev. 2011;63:218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 101.Schulz E., Wenzel P., Münzel T., Daiber A. Mitochondrial redox signaling: interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid. Redox Signal. 2014;20:308–324. doi: 10.1089/ars.2012.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kovacic H.N., Irani K., Goldschmidt-Clermont P.J. Redox regulation of human Rac1 stability by the proteasome in human aortic endothelial cells. J. Biol. Chem. 2001;276:45856–45861. doi: 10.1074/jbc.M107925200. [DOI] [PubMed] [Google Scholar]
- 103.Li W.G., Miller F.J., Jr., Zhang H.J., Spitz D.R., Oberley L.W., Weintraub N.L. H(2)O(2)-induced O(2) production by a non-phagocytic NAD(P)H oxidase causes oxidant injury. J. Biol. Chem. 2001;276:29251–29256. doi: 10.1074/jbc.M102124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lumeng C.N., Deyoung S.M., Bodzin J.L., Saltiel A.R. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 106.Suganami T., Ogawa Y. Adipose tissue macrophages: their role in adipose tissue remodeling. J Leukoc. Biol. 2010;88:33–39. doi: 10.1189/jlb.0210072. [DOI] [PubMed] [Google Scholar]
- 107.Kröller-Schön S., Steven S., Kossmann S., Scholz A., Daub S., Oelze M., Xia N., Hausding M., Mikhed Y., Zinssius E., Mader M., Stamm P., Treiber N., Scharffetter-Kochanek K., Li H., Schulz E., Wenzel P., Münzel T., Daiber A. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid. Redox Signal. 2014;20:247–266. doi: 10.1089/ars.2012.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wenzel P., Knorr M., Kossmann S., Stratmann J., Hausding M., Schuhmacher S., Karbach S.H., Schwenk M., Yogev N., Schulz E., Oelze M., Grabbe S., Jonuleit H., Becker C., Daiber A., Waisman A., Münzel T. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 109.Karbach S., Wenzel P., Waisman A., Munzel T., Daiber A. eNOS uncoupling in cardiovascular diseases—the role of oxidative stress and inflammation. Curr. Pharm. Des. 2014;20:3579–3594. doi: 10.2174/13816128113196660748. [DOI] [PubMed] [Google Scholar]
- 110.West A.P., Brodsky Rahner I.E., Woo D.K., Erdjument-Bromage H., Tempst P., Walsh M.C., Choi Y., Shadel G.S., Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 112.Itoh M., Suganami T., Hachiya R., Ogawa Y. Adipose tissue remodeling as homeostatic inflammation. Int. J. Inflam. 2011;2011:720926. doi: 10.4061/2011/720926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta. 1797;2010:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 114.Wenzel P., Mollnau H., Oelze M., Schulz E., Wickramanayake J.M., Müller J., Schuhmacher S., Hortmann M., Baldus S., Gori T., Brandes R.P., Münzel T., Daiber A. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid. Redox Signal. 2008;10:1435–1447. doi: 10.1089/ars.2007.1969. [DOI] [PubMed] [Google Scholar]
- 115.Doughan A.K., Harrison D.G., Dikalov S.I. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 116.Dikalova A.E., Bikineyeva A.T., Budzyn K., Nazarewicz R.R., McCann L., Lewis W., Harrison D.G., Dikalov S.I. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wosniak J., Jr., Santos C.X., Kowaltowski A.J., Laurindo F.R. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid. Redox Signal. 2009;11:1265–1278. doi: 10.1089/ars.2009.2392. [DOI] [PubMed] [Google Scholar]
- 118.Zorov D.B., Filburn C.R., Klotz L.O., Zweier J.L., Sollott S.J. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wendt M.C., Daiber A., Kleschyov A.L., Mülsch A., Sydow K., Schulz E., Chen K., Keaney J.F., Jr, Lassègue B., Walter U., Griendling K.K., Münzel T. Differential effects of diabetes on the expression of the gp91phox homologues nox1 and nox4. Free Radic. Biol. Med. 2005;39:381–391. doi: 10.1016/j.freeradbiomed.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 121.Rathore R., Zheng Y.M., Niu C.F., Liu Q.H., Korde A., Ho Y.S., Wang Y.X. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic. Biol. Med. 2008;45:1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Forbes J.M., Cooper M.E., Thallas V., Burns W.C., Thomas M.C., Brammar G.C., Lee F., Grant S.L., Burrell L.M., Jerums G., Osicka T.M. Reduction of the accumulation of advanced glycation end products by ACE inhibition in experimental diabetic nephropathy. Diabetes. 2002;51:3274–3282. doi: 10.2337/diabetes.51.11.3274. [DOI] [PubMed] [Google Scholar]
- 123.Yamamoto Y., Yamagishi S., Yonekura H., Doi T., Tsuji H., Kato I., Takasawa S., Okamoto H., Abedin J., Tanaka N., Sakurai S., Migita H., Unoki H., Wang H., Zenda T., Wu P.S., Segawa Y., Higashide T., Kawasaki K., Yamamoto H. Roles of the AGE-RAGE system in vascular injury in diabetes. Ann. N.Y. Acad. Sci. 2000;902:163–170. doi: 10.1111/j.1749-6632.2000.tb06311.x. [DOI] [PubMed] [Google Scholar]
- 124.Coughlan M.T., Thorburn D.R., Penfold S.A., Laskowski A., Harcourt B.E., Sourris K.C., Tan A.L., Fukami K., Thallas-Bonke V., Nawroth P.P., Brownlee M., Bierhaus A., Cooper M.E., Forbes J.M. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J. Am. Soc. Nephrol. 2009;20:742–752. doi: 10.1681/ASN.2008050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wautier M.P., Chappey O., Corda S., Stern D.M., Schmidt A.M., Wautier J.L. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am. J. Physiol. Endocrinol. Metab. 2001;280:E685–E694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 126.Nishikawa T., Edelstein D., Du X.L., Yamagishi S., Matsumura T., Kaneda Y., Yorek M.A., Beebe D., Oates P.J., Hammes H.P., Giardino I., Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 127.Wilson-Fritch L., Burkart A., Bell G., Mendelson K., Leszyk J., Nicoloro S., Czech M., Corvera S. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol. Cell Biol. 2003;23:1085–1094. doi: 10.1128/MCB.23.3.1085-1094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hoehn K.L., Hohnen-Behrens C., Cederberg A., Wu L.E., Turner N., Yuasa T., Ebina Y., James D.E. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 2008;7:421–433. doi: 10.1016/j.cmet.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hall J.C., Sordahl L.A., Stefko P.L. The effect of insulin on oxidative phosphorylation in normal and diabetic mitochondria. J. Biol. Chem. 1960;235:1536–1539. [PubMed] [Google Scholar]
- 130.Williamson J.R., Chang K., Frangos M., Hasan K.S., Ido Y., Kawamura T., Nyengaard J.R., van den Enden M., Kilo C., Tilton R.G. Hyperglycemic pseudohypoxia and diabetic complications. Diabetes. 1993;42:801–813. doi: 10.2337/diab.42.6.801. [DOI] [PubMed] [Google Scholar]
- 131.Nagai R., Brock J.W., Blatnik M., Baatz J.E., Bethard J., Walla M.D., Thorpe S.R., Baynes J.W., Frizzell N. Succination of protein thiols during adipocyte maturation: a biomarker of mitochondrial stress. J. Biol. Chem. 2007;282:34219–34228. doi: 10.1074/jbc.M703551200. [DOI] [PubMed] [Google Scholar]
- 132.Almasalmeh A., Krenc D., Wu B., Beitz E. Structural determinants of the hydrogen peroxide permeability of aquaporins. FEBS J. 2014;281:647–656. doi: 10.1111/febs.12653. [DOI] [PubMed] [Google Scholar]
- 133.Münzel T., Daiber A., Ullrich V., Mülsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler. Thromb. Vasc. Biol. 2005;25:1551–1557. doi: 10.1161/01.ATV.0000168896.64927.bb. [DOI] [PubMed] [Google Scholar]
- 134.Förstermann U., Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 135.Vásquez-Vivar J., Kalyanaraman B., Martásek P., Hogg N., Masters B.S., Karoui H., Tordo P., Pritchard K.A., Jr. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc. Natl. Acad. Sci. U.S.A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schulz E., Jansen T., Wenzel P., Daiber A., Münzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid. Redox Signal. 2008;10:1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 137.Otasevic V., Korac A., Buzadzic B., Stancic A., Jankovic A., Korac B. Nitric oxide and thermogenesis-challenge in molecular cell physiology. Front. Biosci. 2011;3:1180–1195. doi: 10.2741/219. [DOI] [PubMed] [Google Scholar]
- 138.Vucetic M., Otasevic V., Korac A., Stancic A., Jankovic A., Markelic M., Golic I., Velickovic K., Buzadzic B., Korac B. Interscapular brown adipose tissue metabolic reprogramming during cold acclimation: Interplay of HIF-1α and AMPKα. Biochim. Biophys. Acta. 1810;2011:1252–1261. doi: 10.1016/j.bbagen.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 139.Fu W.J., Haynes T.E., Kohli R., Hu J., Shi W., Spencer T.E., Carroll R.J., Meininger C.J., Wu G. Dietary l-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J. Nutr. 2005;135:714–721. doi: 10.1093/jn/135.4.714. [DOI] [PubMed] [Google Scholar]
- 140.Stančić A., Korać A., Buzadžić B., Otašević V., Janković A., Vučetić M., Korać B. l-Arginine in nutrition: multiple beneficial effects in the etiopathology of diabetes. J. Nutr Ther. 2012;1:114–131. [Google Scholar]
- 141.Roy D., Perreault M., Marette A. Insulin stimulation of glucose uptake in skeletal muscles and adipose tissues in vivo is NO dependent. Am. J. Physiol. 1998;274:E692–E699. doi: 10.1152/ajpendo.1998.274.4.E692. [DOI] [PubMed] [Google Scholar]
- 142.Janković A., Buzadžić B., Korać A., Petrović V., Vasilijević A., Korać B. Antioxidative defense organization in retroperitoneal white adipose tissue during acclimation to cold—the involvement of l-arginine/NO pathway. J. Therm. Biol. 2009;34:358–365. [Google Scholar]
- 143.Jobgen W.S., Fried S.K., Fu W.J., Meininger C.J., Wu G. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J. Nutr. Biochem. 2006;17:571–588. doi: 10.1016/j.jnutbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 144.Ribiere C., Jaubert A.M., Gaudiot N., Sabourault D., Marcus M.L., Boucher J.L., Denis-Henriot D., Giudicelli Y. White adipose tissue nitric oxide synthase: a potential source for NO production. Biochem. Biophys. Res. Commun. 1996;222:706–712. doi: 10.1006/bbrc.1996.0824. [DOI] [PubMed] [Google Scholar]
- 145.Tedesco L., Valerio A., Cervino C., Cardile A., Pagano C., Vettor R., Pasquali R., Carruba M.O., Marsicano G., Lutz B., Pagotto U., Nisoli E. Cannabinoid type 1 receptor blockade promotes mitochondrial biogenesis through endothelial nitric oxide synthase expression in white adipocytes. Diabetes. 2008;57:2028–2036. doi: 10.2337/db07-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Koh E.H., Kim M., Ranjan K.C., Kim H.S., Park H.S., Oh K.S., Park I.S., Lee W.J., Kim M.S., Park J.Y., Youn J.H., Lee K.U. eNOS plays a major role in adiponectin synthesis in adipocytes. Am. J. Physiol. Endocrinol. Metab. 2010;298:E846–E853. doi: 10.1152/ajpendo.00008.2010. [DOI] [PubMed] [Google Scholar]
- 147.Filip M., Maciag J., Nosalski R., Korbut R., Guzik T. Endothelial dysfunction related to oxidative stress and inflammation in perivascular adipose tissue. Postepy Biochem. 2012;58:186–194. [PubMed] [Google Scholar]
- 148.Liu G.S., Chan E.C., Higuchi M., Dusting G.J., Jiang F. Redox mechanisms in regulation of adipocyte differentiation: beyond a general stress response. Cells. 2012;1:976–993. doi: 10.3390/cells1040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bjorntorp P. Adipose-tissue distribution and function. Int. J. Obes. 1991;15:67–81. [PubMed] [Google Scholar]
- 150.Gustafson B., Gogg S., Hedjazifar S., Jenndahl L., Hammarstedt A., Smith U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am. J. Physiol. Endocrinol. Metab. 2009;297:E999–E1003. doi: 10.1152/ajpendo.00377.2009. [DOI] [PubMed] [Google Scholar]
- 151.Després J.P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 152.Cristancho A.G., Lazar M.A. Forming functional fat: a growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ducluzeau P.H., Priou M., Weitheimer M., Flamment M., Duluc L., Iacobazi F., Soleti R., Simard G., Durand A., Rieusset J., Andriantsitohaina R., Malthièry Y. Dynamic regulation of mitochondrial network and oxidative functions during 3T3-L1 fat cell differentiation. J. Physiol. Biochem. 2011;67:285–296. doi: 10.1007/s13105-011-0074-6. [DOI] [PubMed] [Google Scholar]
- 154.Lefterova M.I., Zhang Y., Steger D.J., Schupp M., Schug J., Cristancho A., Feng D., Zhuo D., Stoeckert C.J., Jr., Liu X.S., Lazar M.A. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22:2941–2952. doi: 10.1101/gad.1709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nielsen R., Pedersen T.A., Hagenbeek D., Moulos P., Siersbaek R., Megens E., Denissov S., Børgesen M., Francoijs K.J., Mandrup S., Stunnenberg H.G. Genome-wide profiling of PPARgamma:RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev. 2008;22:2953–2967. doi: 10.1101/gad.501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tontonoz P., Hu E., Spiegelman B.M. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr. Opin. Genet. Dev. 1995;5:571–576. doi: 10.1016/0959-437x(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 157.Tormos K.V., Anso E., Hamanaka R.B., Eisenbart J., Joseph J., Kalyanaraman B., Chandel N.S. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011;14:537–544. doi: 10.1016/j.cmet.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Carrière A., Fernandez Y., Rigoulet M., Pénicaud L., Casteilla L. Inhibition of preadipocyte proliferation by mitochondrial reactive oxygen species. FEBS Lett. 2003;550:163–167. doi: 10.1016/s0014-5793(03)00862-7. [DOI] [PubMed] [Google Scholar]
- 159.Nagy L., Tontonoz P., Alvarez J.G., Chen H., Evans R.M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 160.Zhang Q., Seltmann H., Zouboulis C.C., Konger R.L. Involvement of PPARgamma in oxidative stress-mediated prostaglandin E(2) production in SZ95 human sebaceous gland cells. J. Invest. Dermatol. 2006;126:42–48. doi: 10.1038/sj.jid.5700028. [DOI] [PubMed] [Google Scholar]
- 161.Schopfer F.J., Cole M.P., Groeger A.L., Chen C.S., Khoo N.K., Woodcock S.R., Golin-Bisello F., Motanya U.N., Li Y., Zhang J., Garcia-Barrio M.T., Rudolph T.K., Rudolph V., Bonacci G., Baker P.R., Xu H.E., Batthyany C.I., Chen Y.E., Hallis T.M., Freeman B.A. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: selective ligand activity and anti-diabetic signaling actions. J. Biol. Chem. 2010;285:12321–12333. doi: 10.1074/jbc.M109.091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim J.W., Tang Q.Q., Li X., Lane M.D. Effect of phosphorylation and S–S bond-induced dimerization on DNA binding and transcriptional activation by C/EBPbeta. Proc. Natl. Acad. Sci. U.S.A. 2007;104:1800–1804. doi: 10.1073/pnas.0611137104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Huh J.Y., Kim Y., Jeong J., Park J., Kim I., Huh K.H., Kim Y.S., Woo H.A., Rhee S.G., Lee K.J., Ha H. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Antioxid. Redox Signal. 2012;16:229–243. doi: 10.1089/ars.2010.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Higuchi M., Dusting G.J., Peshavariya H., Jiang F., Hsiao S.T., Chan E.C., Liu G.S. Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and Forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev. 2013;22:878–888. doi: 10.1089/scd.2012.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rajalin A.M., Micoogullari M., Sies H., Steinbrenner H. Upregulation of the thioredoxin-dependent redox system during differentiation of 3T3-L1 cells to adipocytes. Biol. Chem. 2014;395:667–677. doi: 10.1515/hsz-2014-0102. [DOI] [PubMed] [Google Scholar]
- 166.Kobayashi H., Matsuda M., Fukuhara A., Komuro R., Shimomura I. Dysregulated glutathione metabolism links to impaired insulin action in adipocytes. Am. J. Physiol. Endocrinol. Metab. 2009;296:E1326–E1334. doi: 10.1152/ajpendo.90921.2008. [DOI] [PubMed] [Google Scholar]
- 167.Das S.K., Sharma N.K., Hasstedt S.J., Mondal A.K., Ma L., Langberg K.A., Elbein. S.C. An integrative genomics approach identifies activation of thioredoxin/thioredoxin reductase-1-mediated oxidative stress defense pathway and inhibition of angiogenesis in obese nondiabetic human subjects. J. Clin. Endocrinol. Metab. 2011;96:E1308–E1313. doi: 10.1210/jc.2011-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Khamaisi M., Kavel O., Rosenstock M., Porat M., Yuli M., Kaiser N., Rudich A. Effect of inhibition of glutathione synthesis on insulin action: in vivo and in vitro studies using buthionine sulfoximine. Biochem. J. 2000;349:579–586. doi: 10.1042/0264-6021:3490579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Calzadilla P., Sapochnik D., Cosentino S., Diz V., Dicelio L., Calvo J.C., Guerra L.N. N-acetylcysteine reduces markers of differentiation in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2011;12:6936–6951. doi: 10.3390/ijms12106936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Guarente L., Picard F. Calorie restriction—the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 171.Brunet A., Sweeney L.B., Sturgill J.F., Chua K.F., Greer P.L., Lin Y., Tran H., Ross S.E., Mostoslavsky R., Cohen H.Y. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 172.Motta M.C., Divecha N., Lemieux M., Kamel C., Chen D., Gu W., Bultsma Y., McBurney M., Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 173.Dowell P., Otto T.C., Adi S., Lane M.D. Convergence of peroxisome proliferator-activated receptor gamma and Foxo1 signaling pathways. J. Biol. Chem. 2003;278:45485–45491. doi: 10.1074/jbc.M309069200. [DOI] [PubMed] [Google Scholar]
- 174.Armoni M., Harel C., Karni S., Chen H., Bar-Yoseph F., Ver M.R., Quon M.J., Karnieli E. FOXO1 represses peroxisome proliferator-activated receptor-gamma1 and -gamma2 gene promoters in primary adipocytes. A novel paradigm to increase insulin sensitivity. J. Biol. Chem. 2006;281:19881–19891. doi: 10.1074/jbc.M600320200. [DOI] [PubMed] [Google Scholar]
- 175.Rodgers J.T., Lerin C., Haas W., Gygi S.P., Spiegelman B.M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2007;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 176.Puigserver P., Wu Z., Park C.W., Graves R., Wright M., Spiegelman B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 177.Kops G.J., Dansen T.B., Polderman P.E., Saarloos I., Wirtz K.W., Coffer P.J., Huang T.T., Bos J.L., Medema R.H., Burgering B.M. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 178.Olmos Y., Sánchez-Gómez F.J., Wild B., García-Quintans N., Cabezudo S., Lamas S., Monsalve M. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1α complex. Antioxid. Redox Signal. 2013;19:1507–1521. doi: 10.1089/ars.2012.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kong X., Wang R., Xue Y., Liu X., Zhang H., Chen Y., Fang F., Chang Y. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5:e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shi T., Wang F., Stieren E., Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J. Biol. Chem. 2005;280:13560–13567. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- 181.Kim M., Paik J.K., Kang R., Kim S.Y., Lee S.H., Lee J.H. Increased oxidative stress in normal-weight postmenopausal women with metabolic syndrome compared with metabolically healthy overweight/obese individuals. Metabolism. 2013;62:554–560. doi: 10.1016/j.metabol.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 182.Laight D.W., Desai K.M., Gopaul N.K., Anggard E.E., Carrier M.J. Pro-oxidant challenge in vivo provokes the onset of NIDDM in the insulin resistant obese Zucker rat. Br. J. Pharmacol. 1999;128:269–271. doi: 10.1038/sj.bjp.0702801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gopaul N.K., Manraj M.D., Hébé A., Yan S. Lee Kwai, Johnston A., Carrier M.J., Anggard E.E. Oxidative stress could precede endothelial dysfunction and insulin resistance in Indian Mauritians with impaired glucose metabolism. Diabetologia. 2001;44:706–712. doi: 10.1007/s001250051679. [DOI] [PubMed] [Google Scholar]
- 184.Roberts C.K., Barnard R.J., Sindhu R.K., Jurczak M., Ehdaie A., Vaziri N.D. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet induced metabolic syndrome. Metabolism. 2006;55:928–934. doi: 10.1016/j.metabol.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 185.Roberts C.K., Sindhu K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009;84:705–712. doi: 10.1016/j.lfs.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 186.Korkmaz G.G., Altınoglu E., Civelek S., Sozer V., Erdenen F., Tabak O., Uzun H. The association of oxidative stress markers with conventional risk factors in the metabolic syndrome. Metabolism. 2013;62:828–835. doi: 10.1016/j.metabol.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 187.Rains J.L., Jain S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang C., Shi C., Yang X., Yang M., Sun H., Wang C. Celastrol suppresses obesity process via increasing antioxidant capacity and improving lipid metabolism. Eur. J. Pharmacol. 2014;744:52–58. doi: 10.1016/j.ejphar.2014.09.043. [DOI] [PubMed] [Google Scholar]
- 189.Fernández-Galilea M., Pérez-Matute P., Prieto-Hontoria P.L., Houssier M., Burrell M.A., Langin D., Martínez J.A., Moreno-Aliaga M.J. α-Lipoic acid treatment increases mitochondrial biogenesis and promotes beige adipose features in subcutaneous adipocytes from overweight/obese subjects. Biochim. Biophys. Acta. 2015;1851:273–281. doi: 10.1016/j.bbalip.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 190.Priyanka A., Nisha V.M., Anusree S.S., Raghu K.G. Bilobalide attenuates hypoxia induced oxidative stress, inflammation, and mitochondrial dysfunctions in 3T3-L1 adipocytes via its antioxidant potential. Free Radic. Res. 2014;48:1206–1217. doi: 10.3109/10715762.2014.945442. [DOI] [PubMed] [Google Scholar]
- 191.Jimenéz-Aranda A., Fernández-Vázquez G., Mohammad A-Serrano M., Reiter R.J., Agil A. Melatonin improves mitochondrial function in inguinal white adipose tissue of Zücker diabetic fatty rats. J. Pineal Res. 2014;57:103–109. doi: 10.1111/jpi.12147. [DOI] [PubMed] [Google Scholar]
- 192.McKnight J.R., Satterfield M.C., Jobgen W.S., Smith S.B., Spencer T.E., Meininger C.J., McNeal C.J., Wu G. Beneficial effects of l-arginine on reducing obesity: potential mechanisms and important implications for human health. Amino Acids. 2010;39:349–357. doi: 10.1007/s00726-010-0598-z. [DOI] [PubMed] [Google Scholar]
- 193.Tan B., Li X., Yin Y., Wu Z., Liu C., Tekwe C.D., Wu G. Regulatory roles for l-arginine in reducing white adipose tissue. Front. Biosci. 2012;17:2237–2246. doi: 10.2741/4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ristow M., Zarse K., Oberbach A., Klöting N., Birringer M., Kiehntopf M., Stumvoll M., Kahn C.R., Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. U.S.A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gomez-Cabrera M.C., Ristow M., Viña J. Antioxidant supplements in exercise: worse than useless? Am. J. Physiol. Endocrinol. Metab. 2012;302:E476–E477. doi: 10.1152/ajpendo.00567.2011. [DOI] [PubMed] [Google Scholar]
- 196.Abdali D., Samson S.E., Grover A.K. How effective are antioxidant supplements in obesity and diabetes? Med. Princ Pract. 2015;24:201–215. doi: 10.1159/000375305. [DOI] [PMC free article] [PubMed] [Google Scholar]