Article first published online 2 July 2015
Key Words: extraintestinal manifestations, inflammatory bowel disease, arthritis, uveitis
Abstract:
Extraintestinal manifestations (EIM) in inflammatory bowel disease (IBD) are frequent and may occur before or after IBD diagnosis. EIM may impact the quality of life for patients with IBD significantly requiring specific treatment depending on the affected organ(s). They most frequently affect joints, skin, or eyes, but can also less frequently involve other organs such as liver, lungs, or pancreas. Certain EIM, such as peripheral arthritis, oral aphthous ulcers, episcleritis, or erythema nodosum, are frequently associated with active intestinal inflammation and usually improve by treatment of the intestinal activity. Other EIM, such as uveitis or ankylosing spondylitis, usually occur independent of intestinal inflammatory activity. For other not so rare EIM, such as pyoderma gangrenosum and primary sclerosing cholangitis, the association with the activity of the underlying IBD is unclear. Successful therapy of EIM is essential for improving quality of life of patients with IBD. Besides other options, tumor necrosis factor antibody therapy is an important therapy for EIM in patients with IBD.
Inflammatory bowel disease (IBD), which includes Crohn's disease (CD) and ulcerative colitis (UC), should be regarded as a systemic disorder not limited to the gastrointestinal tract because many patients will develop extraintestinal symptoms. Extraintestinal symptoms may involve virtually any organ system with a potentially detrimental impact on the patient's functional status and quality of life.
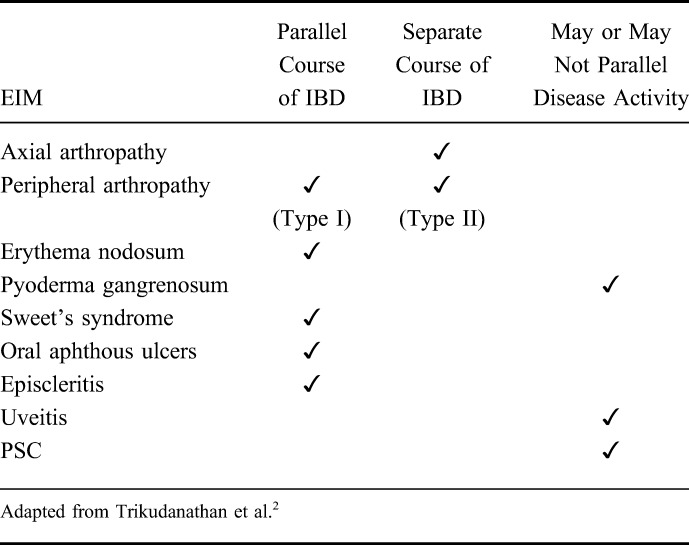
Extraintestinal symptoms can be divided in 2 groups: extraintestinal manifestations (EIM) and extraintestinal complications. EIM most frequently affect joints (peripheral and axial arthropathies), the skin (erythema nodosum, pyoderma gangrenosum, Sweet's syndrome, aphthous stomatitis), the hepatobiliary tract (primary sclerosing cholangitis [PSC]), and the eye (episcleritis, uveitis) (Fig. 1). Less frequently, EIM also affect the lungs, the heart, the pancreas, or the vascular system. Extraintestinal complications are mainly caused by the disease itself and include conditions such as malabsorption with consequent micronutrient deficiencies, osteoporosis, peripheral neuropathies, kidney stones, gallstones, and IBD drug-related side effects.
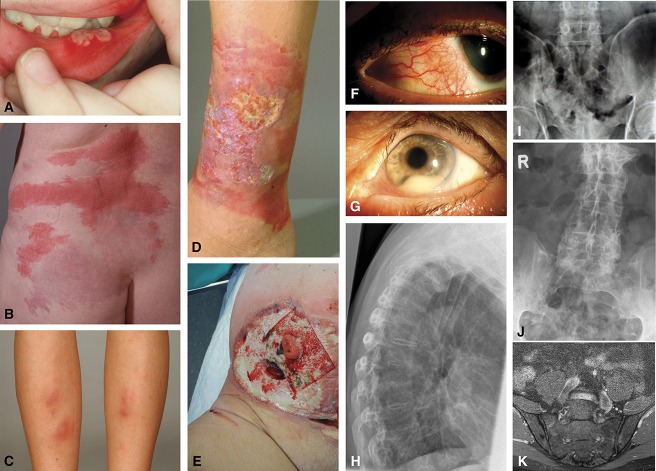
FIGURE 1.
A, Oral aphthous ulcers, (B) Sweet's syndrome, (C) erythema nodosum, (D) pyoderma gangrenosum, (E) peristomal pyoderma gangrenosum, (F) episcleritis, (G) uveitis with hypopyon and dilated iris vessels, (H) conventional x-ray of the lateral spine demonstrating syndesmophytes (bamboo spine), (I) plane radiograph of the ileosacral joints with bilateral sacroiliitis, (J) plane radiography of the sacrum with bilateral ankylosis, (K) coronal magnetic resonance image of the sacroiliac joints with active inflammation mainly on the left side and chronic inflammatory changes on both sides.
This article focuses on the clinical features of EIM. Certain EIM such as pauciarticular arthritis, oral aphthous ulcers, erythema nodosum, or episcleritis usually occur with increased intestinal disease activity.1,2 Other EIM such as ankylosing spondylitis and uveitis usually follow an independent course from IBD disease activity.1,2 And finally, some EIM such as PSC and pyoderma gangrenosum may or may not be related to IBD disease activity (Table 1).2–4
TABLE 1.
Relationship Between EIM Activity and Intestinal Activity
EIM in IBD are reported with frequencies ranging from 6% to 47%.5–13 Multiple EIM may occur concomitantly, and the presence of 1 EIM confers a higher likelihood to develop other EIM.14 Recently, we reported based on data from the Swiss IBD Cohort study that up to 1 quarter of EIM-affected patients with IBD tend to suffer from a combination of several EIMs (up to 5).14 Furthermore, our group recently published data regarding the chronological order of EIM appearance relative to IBD diagnosis.15 A summary of the chronologic appearance of EIMs relative to IBD diagnosis is presented in Figure 2. In 25.8% of cases, a first EIM occurred before IBD was diagnosed (median time 5 mo before IBD diagnosis; range, 0–25 mo). In 74.2% of cases, the first EIM manifested after IBD diagnosis was made (median, 92 mo; range, 29–183 mo) (Fig. 2). We found that up to 4 different EIM occurred before IBD was diagnosed, and that at 30 years after IBD diagnosis, 50% of patients had suffered from at least 1 EIM. Perianal CD, colonic involvement, and cigarette smoking increased the likelihood to suffer from EIMs.16
FIGURE 2.
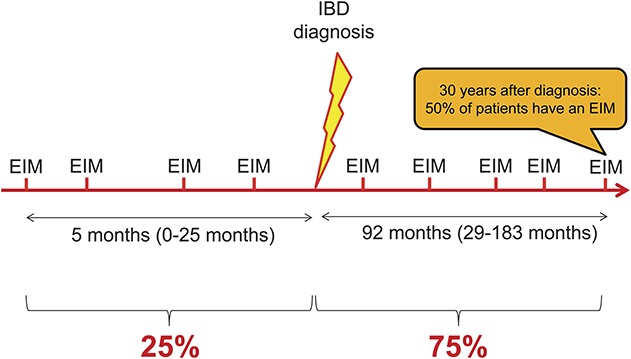
Chronology of EIM in patients with IBD. In one quarter of patients with IBD, up to 4 EIM appeared before the time of IBD diagnosis. The median time before IBD diagnosis is 5 mo (range, 0–25 mo). In 75% of cases, the first EIM manifested after IBD diagnosis (median, 92 mo; range, 29–183 mo). Thirty years after diagnosis up to 50% of patients with IBD have suffered from at least 1 EIM.15
PATHOGENESIS OF EIM
The pathogenesis of EIM in IBD is not well understood. It is believed that the diseased gastrointestinal mucosa may trigger immune responses at the extraintestinal site due to shared epitopes, e.g., of intestinal bacteria and the synovia.17–21 This would mean that bacteria that are translocated across the leaky intestinal barrier trigger an adaptive immune response that finally is unable to discriminate between bacterial epitopes and epitopes of joints or the skin. Triggers of the autoimmune responses in certain organs seem to be influenced by genetic factors. Concordance in EIM was present in 70% of parent–child pairs and 84% in sibling pairs.10,22 Associations of EIM in IBD with major histocompatibility complex loci have been demonstrated. EIM in patients with CD are more frequently observed in patients with HLA-A2, HLA-DR1, and HLA-DQw5, whereas EIM in patients with UC are more likely to appear when the HLA-DR103 genotype is present.23 Particular HLA complexes have also been linked to specific EIM. HLA-B8/DR3 is associated with an increased risk of PSC in UC, whereas HLA-DRB1*0103, HLA-B*27, and HLA-B*58 are associated with EIM of joints, the skin, and eyes, respectively, in patients with IBD.4,24,25 HLA-B*27 itself does not seem to be associated with IBD5 but HLA-B*27 shows a strong association with the development of ankylosing spondylitis, as 50% to 90% of patients with IBD are positive for this marker.26 As HLA-B*27 per se shows an association with ankylosing spondilitis or rheumatoid arthritis it remains unclear whether there is a specific role for EIM in IBD.
MUSCULOSCELETAL EIM
Musculoskeletal EIM including joint complaints represent the most common EIMs in IBD. Joint symptoms affecting peripheral large and small joints or the axial joints occur in up to 40% of patients with IBD.14,27,28
Peripheral Arthralgia/Arthritis
Peripheral arthralgia/arthritis in patients with IBD, in contrast to other specific forms of arthritis such as rheumatoid arthritis or psoriatic arthritis, shows little or no joint destruction. Classically, it presents as a seronegative arthralgia/arthritis,1 which affects 5% to 10% of patients with UC and 10% to 20% of patients with CD.29 A higher risk for peripheral arthralgia/arthritis is seen in patients with IBD with colonic involvement and in patients suffering from perianal disease, erythema nodosum, stomatitis, uveitis, and pyoderma gangrenosum.1,20
Peripheral arthralgia/arthritis has been classified into 2 entities (Table 2): type I (pauciarticular) arthralgie/arthritis usually affects less than 5 large joints, such as ankles, knees, hips, wrists, elbows, and shoulders and is often acute, asymmetrical, and migratory. The knee is commonly involved. Approximately, 20% to 40% of all patients have more than 1 episode of arthralgia/arthritis. Pauciarticular arthralgia/arthritis is usually related to IBD activity and self-limiting with a maximum duration of up to 10 weeks.29 Consequently, medical or surgical treatment of the underlying intestinal inflammation (i.e., colitis) is usually associated with improvement of type I arthritis. Type II (polyarticular) arthralgia/arthritis frequently is a symmetrical arthritis involving 5 or more small joints. It is not related with intestinal disease activity and may precede IBD diagnosis. Type II arthropathy can persist for years (median of 3 yr).29 The metacarpophalangeal joint is most commonly involved. Type II arthritis is associated with an increased risk of uveitis but not erythema nodosum.29
TABLE 2.
Classification of Peripheral Arthropathy Associated with IBD
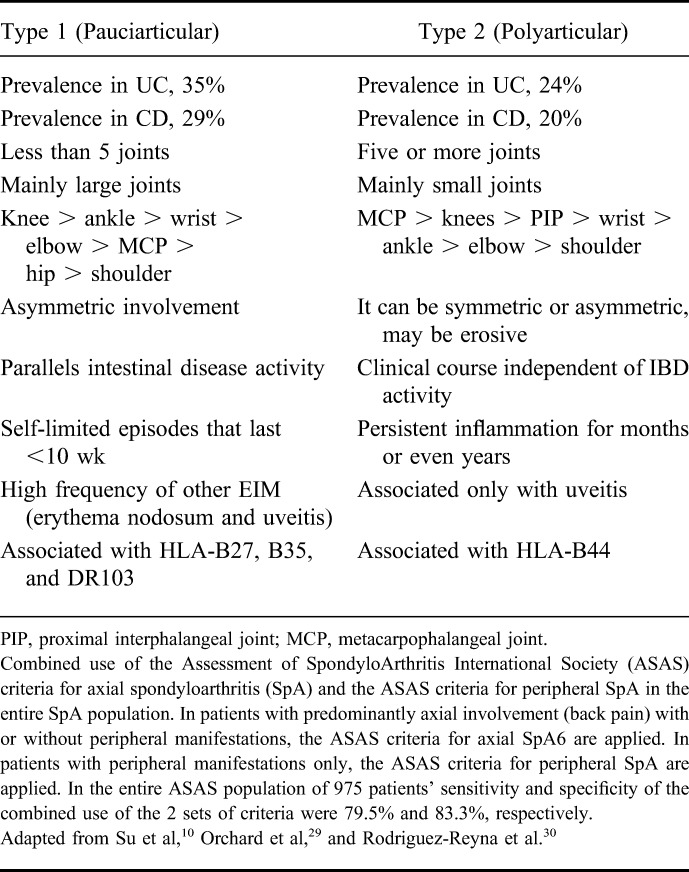
The diagnosis/classification of type 1 and type 2 arthropathies is purely clinical, as imaging is most often normal with no evidence of significant inflammation or joint destruction.31 Both types are seronegative (i.e., rheumatoid factor-negative), but may represent immunogenetically distinct entities. Type 1 peripheral arthropathy is associated with HLA-B27, HLA-B35, and HLA-DR103, whereas type 2 is associated with HLA-B44.25
As type II peripheral arthropathy usually occurs independently from intestinal activity and anti-inflammatory treatment may not be successful, physiotherapy and treatment of associated pain is the main treatment option in those cases.
Table 3 summarizes treatment options of EIM in IBD. Other treatment modalities include rest, and intra-articular steroid injections. Use of sulphasalazine has been reported to improve peripheral arthropathies.45 Therapy with nonsteroidal anti-inflammatory drugs (NSAIDs) in the management of IBD-associated peripheral arthropathies requires caution because of the reported association of exacerbation of IBD with NSAID use.46–50
TABLE 3.
Treatment Options of EIM in IBD
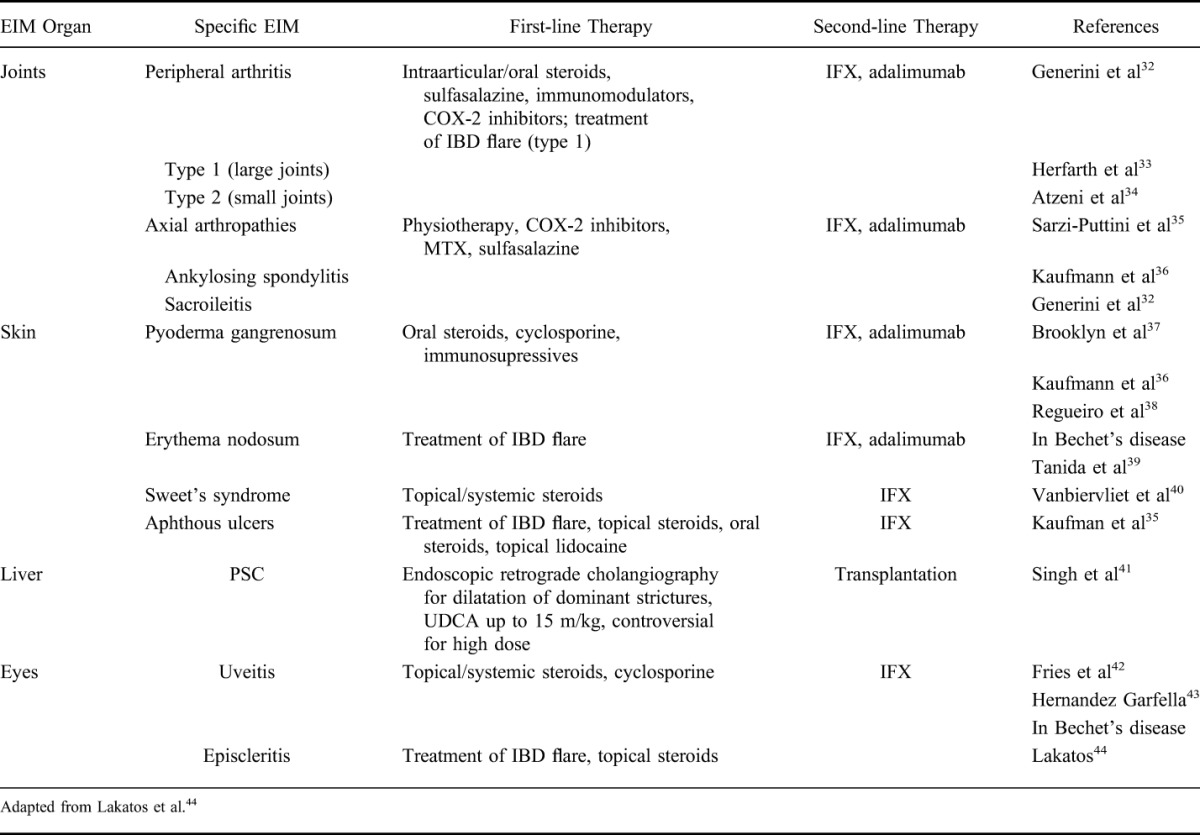
In a study by Takeuchi et al, up to 25% of patients in remission experienced a disease flare when provided certain NSAIDs. It seems that COX-2 inhibitors may show a better safety profile and might be used with caution in patients with IBD suffering from peripheral arthropathies.49,51–54
Whether a discrimination of type I and type II arthropathy is clinically useful and meaningful has never been studied in detail. In most large IBD centers, this discrimination is not used. In fact, affection of small joints may disappear as well with the treatment of the underlying disease, whereas inflammation of the large joints may also occur as side effect of anti-TNF therapy. A careful documentation of joint affections, which is standard in rheumatology, certainly would be helpful and could improve the outcome of patients with IBD. This should be requested as a standard in IBD centers.
Axial Arthropathies
Axial arthropathies are less frequent than peripheral arthralgia/arthritis in patients with IBD, occurring in 3% to 5% of patients although frequencies of up to 25% have been reported.9–13,55 Males are more frequently affected than females. In contrast to peripheral arthralgia/arthritis (at least in contrast to type I arthropathy), axial arthropathies are usually independent of the intestinal IBD activity. Axial arthropathies can be categorized into ankylosing spondylitis and sacroiliitis. Ankylosing spondylitis in patients with IBD occurs in 5% to 10% of patients and is mainly HLA-B27–positive.10,56,57 Patients with ankylosing spondylitis often experience severe onset of back pain at young age, usually associated with morning stiffness or pain exacerbation by periods of rest. Physical examination reveals limited spinal flexion (Schober's test) and reduced chest expansion. Radiographs in early stages may be normal or show only minimal sclerosis. The disease course is usually progressive, resulting in permanent skeletal damage. Patients with IBD with advanced ankylosing spondylitis may show squaring of vertebral bodies, marginal syndesmophytes, bone proliferation, and ankylosis, features classically described as bamboo spine.
Sacroiliitis is observed radiographically in up to 25% of patients.6,48 Most patients with sacroiliitis are HLA-B27–negative and do not progress to ankylosing spondylitis. Patients with the radiographic finding of bilateral sacroiliitis are more likely to progress to ankylosing spondylitis.58
Treatment options of peripheral arthralgia/arthritis and axial arthropathies in IBD are summarized in Table 3. Therapeutic agents for axial arthropathies that have been reported include sulfasalazine, mesalamine, methotrexate (MTX), azathioprine, thalidomide, and anti-tumor necrosis factor therapy.32,59–61 TNF-antibodies such as infliximab and adalimumab have shown an improvement of axial arthropathies in several studies in patients with IBD and should be used especially in refractory cases.31,32,44,61–64
Axial arthropathies can impact work ability and cause an additional burden for patients with IBD. The diagnosis of axial arthropathies is followed by an access to medications that frequently are not approved for the treatment of patients with IBD. The treatment of axial arthropathies frequently is initiated by rheumatologists. However, it needs to be highlighted that this has to happen in close collaboration with the gastroenterologist. Enbrel, which has been found not to be effective in IBD, is not a good treatment option for ankylosing spondylitis in patients with IBD.
EIM OF THE SKIN
The diagnosis of cutaneous EIM in IBD is based on the clinical picture and on their characteristic features and the exclusion of other specific skin disorders. Cutaneous disorders associated with IBD occur in up to 15% of patients.11,14
Erythema Nodosum
Erythema nodosum occurs in up to 15% of patients with CD and 10% of patients with UC.11 Other publications report a considerably lower frequency.4,14,65 Preponderance in female patients has been suggested.65,66 Furthermore, erythema nodosum is frequently associated with eye and joint involvement, isolated colonic involvement, and pyoderma gangrenosum.65
Erythema nodosum is usually easily recognized as raised, tender, red, or violet inflammatory subcutaneous nodules of 1 to 5 cm in diameter, typically on the anterior extensor surface of the lower extremities but rarely on the face and trunk.11 It shows preponderance in females and patients with CD.14,66 Unpublished data from the Swiss IBD Cohort Study indicate that location of erythema nodosum does not differ significantly between patients with CD and UC. Figure 3 illustrates the distribution of erythema nodosum lesions in male and female patients with IBD. The diagnosis is established based on clinical judgment, and skin biopsies are rarely required. Erythema nodosum usually heals without scars. Its onset coincides with acute flares of IBD and is frequently self-limiting or improves with treatment of the underlying IBD.13 Mild cases may be treated with leg elevation, use of analgesics, potassium iodine, systemic corticosteroids, and compression stockings.67
FIGURE 3.
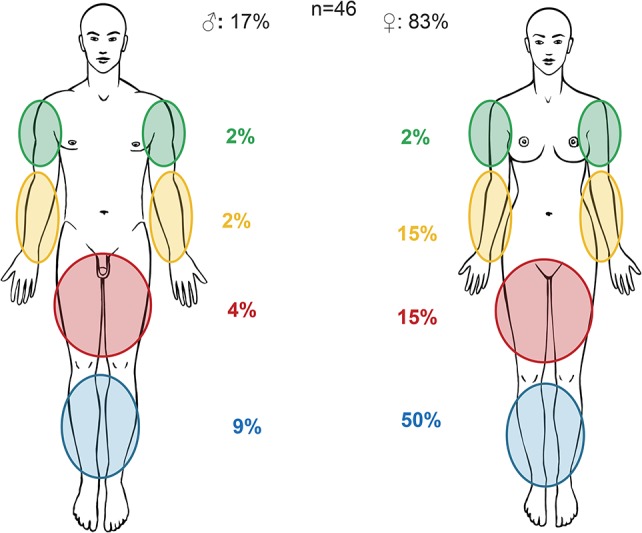
Unpublished data from the Swiss IBD cohort study.15 Location of erythema nodosum in male and female patients suffering from IBD.
In severe or refractory cases, alternative causes of erythema nodosum should be investigated such as infections with Streptococcus, Yersinia pseudotuberculosis, Yersinia enterocolitica, syphilis, sarcoidosis, Behcet's disease, and use of oral contraceptives or other medication. After exclusion of other causes, severe cases may require systemic corticosteroids or immunosuppressive therapy or TNF antibodies. Only a few case reports highlight the benefit of infliximab and adalimumab for erythema nodosum.68–71 Please refer to Table 3 for an overview of treatment options of EIM in IBD.
Pyoderma Gangrenosum
Pyoderma gangrenosum is a much rarer, more severe, debilitating EIM, more common in UC than in CD. It affects women more frequently than men72,73 and is associated with black African origin, familial history of UC, and pancolitis as the initial location of IBD, permanent stoma, eye involvement, and erythema nodosum.65 The prevalence of pyoderma gangrenosum in IBD is 0.4% to 2%.4,6,14,66,74 Vice versa, up to 50% of patients with pyoderma gangrenosum have underlying IBD.1 The lesions are usually preceded by a trauma (even many years earlier) through a phenomenon known as pathergy. This trauma can even be minimal such as venous puncture or biopsy. Figure 4 shows the distribution of location of pyoderma gangrenosum lesions in male and female patients suffering from IBD in the SIBDCS.
FIGURE 4.
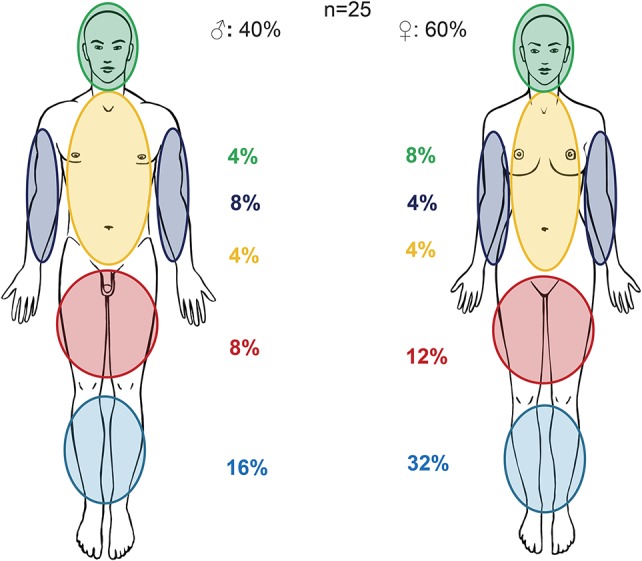
Unpublished data from the Swiss IBD cohort study.15 Location of pyoderma gangrenosum in male and female patients suffering from IBD.
Patients with severe disease and colonic involvement are most likely to develop this complication.75 The disease course is unpredictable. Pyoderma gangrenosum usually begins as an erythematous pustule or nodule that spreads rapidly to the adjacent skin and develops into a burrowing ulcer with irregular violaceous edges.76 The deep ulcerations often contain purulent material, which is sterile on culture. These ulcers can be solitary or multiple, unilateral, or bilateral, and can range in size from several centimeters to an entire limb. The most common sites includes extensor surfaces of the legs (shins) and adjacent to a postsurgical stoma but can occur anywhere on the body, including the genitalia.75 Peristomal pyoderma gangrenosum is seen occasionally as a complication in patients with IBD. One study followed 20 consecutive peristomal pyoderma gangrenosum patients and reported only limited success with local enterostomal care, debridement, and/or stomal revision but responded to a variety of medical therapies.77 All these peristomal ulcers healed completely within a median of 11.4 months (range, 1–42 mo). Another smaller study reported on 7 patients with peristomal pyoderma gangrenosum,78 4 of which occurred in patients with IBD. Intravenous cyclosporine and infliximab were tried in some of those patients with success.
Cases of pyoderma gangrenosum evolving from preceding erythema nodosum also have been reported.79 The diagnosis is made clinically, although wound swabs and a skin biopsy may be needed to exclude other conditions. There are no pathognomonic histological features, generally revealing only diffuse neutrophil infiltration and dermolysis. Pyoderma gangrenosum has typically no association to the clinical activity of the underlying intestinal disease; however, pyoderma gangrenosum may resolve with treatment of the IBD. Up to 36% to 50% of patients with pyoderma gangrenosum have IBD.80 Pyoderma gangrenosum may resolve with treatment of the underlying IBD (Table 3). Mild cases usually respond to local and topical therapy, including intralesional corticosteroid injections, moist treatment with hydroactive dressings, and topical sodium cromoglycate.76,81,82 Effective systemic agents include oral sulfasalazine, dapsone, corticosteroids, and immunomodulators such as azathioprine, cyclophosphamide, cyclosporine, methotrexate, tacrolimus, and mycophenolate mofetil.67,76,81,83,84
Rapid healing of these lesions should be the therapeutic aim because pyoderma gangrenosum can be a debilitating skin disorder. However, response to therapy varies, and many patients with pyoderma gangrenosum have a disease course that is refractory to these agents. Adalimumab and infliximab are efficient treatment options in severe pyoderma gangrenosum cases and have been reported in several case reports and case series.35–37,64,77,78,85–105 For an overview on TNF-antibody therapies in EIM, please see Vavricka et al.64
PG is initially sometimes treated by surgical debridement. A surgical intervention typically worsens PG. If there is any doubt about the nature of an ulcer in patients with IBD, surgical debridement should be avoided until a PG is excluded. It has been discussed whether a maintenance treatment also is necessary for PG.
Sweet's Syndrome
Sweet's syndrome, or acute febrile neutrophilic dermatosis, is a rare dermatologic manifestation associated with CD and UC.106,107 Besides IBD, Sweet's syndrome may also be associated with other systemic diseases such as malignancy. The cutaneous lesion of Sweet's syndrome manifests as tender or papulosquamous exanthema or nodules involving the arm, legs, trunk, hands, or face. Other characteristic features of Sweet's syndrome are leukocytosis and histologic findings of a neutrophilic infiltrate. Associated systemic manifestations include arthritis, fever, and ocular symptoms, such as conjunctivitis. Its association with IBD usually parallels the gastrointestinal disease activity but may precede the diagnosis of IBD.108 The use of azathioprine has been implicated in the development of Sweet's syndrome in a patient with IBD.109,110 Table 3 reports on the treatment options of EIMs in IBD. Most cases of Sweet's syndrome respond to topical or systemic corticosteroid therapy111 and heal without scarring. Metronidazole has been reported to be effective in 1 case report.108
Oral Lesions
The oral cavity is frequently affected in patients with IBD, especially the ones suffering from CD. Periodontitis and other lesions such as aphthous stomatitis and, in more severe cases, pyostomatitis vegetans are found in up to 10% of patients with IBD.10,14,112 Both diseases follow the course of the underlying IBD. Aphthous lesions are typically located on the labial and buccal mucosa but may also affect the tongue and oropharynx. Pyostomatitis vegetans manifests as multiple pustular sometimes hemorrhagic eruptions anywhere on the oral mucosa with a cobblestone pattern. Therapy includes antiseptic mouthwashes and topical steroids (Table 3).67,105
OCULAR MANIFESTATIONS
Beside joints and skin, the eye is the third major tissue type predisposed to immune-mediated EIMs. Nearly, 2% to 5% of patients with IBD experience ocular manifestations,10,13,113 particularly associated with concomitant musculoskeletal manifestations.4 Ocular manifestations are reported more frequently in patients with CD (3.5%–6.3%) than patients with UC (1.6%–4.6%) and include episcleritis and uveitis.9,11,13,14,55 Patients older than 40 years have more likely iritis/uveitis than those younger than 40 years.6
Episcleritis is defined as painless hyperemia of the conjunctiva and sclera without changes of virus and often parallels the activity of the underlying IBD. Besides episcleritis, anterior uveitis is the most common ocular manifestations of IBD. The different types of uveitis are divided as follows: (1) anterior uveitis has its primary site of inflammation in the anterior chamber, (2) intermediate uveitis with its primary site of inflammation being the vitreous, (3) posterior uveitis with its primary site of inflammation being the retina and the choroid, and (4) panuveitis with its primary site of inflammation including anterior chamber, vitreous, retina, and choroid. Uveitis occurs independently of disease activity and is defined as inflammation of the middle chamber of the eye. Uveitis occurs acutely or subacutely and is usually very painful. Anterior uveitis is also referred to as iritis, which typically presents as pain, photophobia, and red eye and can be associated with blurry vision or floaters. Diagnosis is confirmed by slit-lamp examination. An increasing number of case reports and pilot studies exist on the therapy of uveitis and episcleritis; however, only few reports focus on patients with IBD.42,114–124
Episcleritis and Scleritis
Episcleritis is more common in CD than in UC.1 It is characterized by acute hyperemia, irritation, burning, and tenderness. Episcleritis usually does not need specific treatment other than those for the underlying disease. Scleritis affects the deeper layers of the eye and can cause visual impairment if not diagnosed early. Patients often complain of severe pain associated with tenderness to palpation.125 Recurrent scleritis can lead to scleromalacia, retinal detachment, or optic nerve swelling. If therefore mandates aggressive treatment. Disease-specific treatment and topical steroid therapy usually provide prompt relief of symptoms (Table 3).
In case of impairment of vision, the presence of scleritis must be suspected, and prompt referral to an ophthalmologist is mandatory to avoid vision loss.
Uveitis
Uveitis is less common than episcleritis and occurs in 0.5% to 3% of patients with IBD.4 When associated with UC, it is frequently bilateral, insidious in onset, and long-lasting.4 It presents as ocular pain, blurred vision, photophobia, and headaches. In contrast to episcleritis, the temporal correlation of uveitis with IBD is less predictable, and its occurrence may precede the diagnosis of IBD. On slit-lamp examination, uveitis presents as a perilimbic edema and inflammatory flare in the anterior chamber.125 Prompt diagnosis and treatment with topical and systemic corticosteroids is necessary to prevent progression to blindness. Steroid refractory cases are treated with cyclosporine A (Table 3). Successful use of infliximab for IBD-associated uveitis was demonstrated in a patient with CD with uveitis and sacroileitis.42
HEPATOBILIARY EIM
Up to 50% of patients with IBD are affected by hepatobiliary manifestations during the course of their disease.5 PSC, small-duct PSC, fatty liver disease, granulomatous hepatitis, autoimmune liver and pancreas disease, cholestasis, gallstone formation, and liver injury are hepatobiliary manifestations of IBD.126
PSC Is the Most Frequent Biliary Manifestation of IBD
It is more common in patients with UC than in CD. Approximately, 2.4% to 7.5% of patients with UC are diagnosed with PSC.127,128 Conversely, 75% of patients with PSC suffer from IBD, typically UC.129,130 PSC manifests with inflammation and fibrosis of the biliary system that presents clinically with a chronic cholestatic disease. A cholestatic biochemical profile is seen, and characteristic features are frequently found on cholangiography. These include multifocal bile duct strictures and segmental dilatation. PSC can precede the diagnosis of IBD; however, some patients are even diagnosed with PSC several years after proctocolectomy due to UC.3
Patients with PSC should undergo colonoscopy to evaluate concomitant IBD. Extensive involvement of the colon with rectal sparing, backwash ileitis in UC, and predominance in male patients are typical features of PSC.3,130 Patients with PSC can develop bouts of acute cholangitis and ultimately progress to cirrhosis, portal hypertension, and acute decompensation.131 Interestingly, the diagnosis of PSC seems to influence the course of IBD, as patients with both PSC and UC are suggested to have a milder course of their colitis with less histological inflammation of the colon than patients without PSC.132 Nevertheless, the presence of PSC is an independent risk factor for the development of colorectal dysplasia and/or cancer in patients with IBD, leading to the recommendation for annual surveillance colonoscopies in affected patients from the time of first diagnosis of IBD.133–135 The natural course of PSC is independent of IBD, and the bile duct damage is irreversible and nonresponsive to medication. Ursodeoxycholic acid is used widely in patients with PSC; however, only limited effect has been shown. Ursodeoxycholic acid is reported to improve liver enzymes; however, the disease course of PSC is not changed.136 Some patients with dominant strictures on endoscopic retrograde cholangiography might improve with dilatation. The majority of patients with PSC ultimately require liver transplantation.
CONCLUSIONS
IBD is a systemic disease, and EIM are the proof that IBD is not only limited to the gut. Those EIM may affect multiple organs beyond the intestine. Sometimes, these EIM can even be more debilitating than the intestinal disease. Careful screening for EIMs in these patients and early appropriate diagnosis are imperative to prevent morbidity. In EIM responding to the underlying IBD, sufficient IBD therapy and careful monitoring of the EIM is often enough to improve symptoms of the EIM. In EIM not after the activity of the underlying IBD, a multidisciplinary approach is often needed. Clinicians who care for patients with IBD must recognize those various systemic manifestations, as failure to diagnose and treat them early may result in major morbidity.
Footnotes
Supported by research grants from the Swiss National Science Foundation (33CSC0_134274 [Swiss IBD Cohort Study] to G. Rogler, 320000-114009/3 and 32473B_135694/1 to S. R. Vavricka, and 314730-146204 and CRSII3_154488/1 to M. Scharl) and the Zurich Center for Integrative Human Physiology of the University of Zurich (to G. Rogler, M. Scharl, and S. R. Vavricka).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Rothfuss KS, Stange EF, Herrlinger KR. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J Gastroenterol. 2006;12:4819–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trikudanathan G, Venkatesh PG, Navaneethan U. Diagnosis and therapeutic management of extra-intestinal manifestations of inflammatory bowel disease. Drugs. 2012;72:2333–2349. [DOI] [PubMed] [Google Scholar]
- 3.Navaneethan U, Shen B. Hepatopancreatobiliary manifestations and complications associated with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1598–1619. [DOI] [PubMed] [Google Scholar]
- 4.Orchard TR, Chua CN, Ahmad T, et al. Uveitis and erythema nodosum in inflammatory bowel disease: clinical features and the role of HLA genes. Gastroenterology. 2002;123:714–718. [DOI] [PubMed] [Google Scholar]
- 5.Danese S, Semeraro S, Papa A, et al. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol. 2005;11:7227–7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein CN, Blanchard JF, Rawsthorne P, et al. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2001;96:1116–1122. [DOI] [PubMed] [Google Scholar]
- 7.Ricart E, Panaccione R, Loftus EV, Jr, et al. Autoimmune disorders and extraintestinal manifestations in first-degree familial and sporadic inflammatory bowel disease: a case-control study. Inflamm Bowel Dis. 2004;10:207–214. [DOI] [PubMed] [Google Scholar]
- 8.Mendoza JL, Lana R, Taxonera C, et al. [Extraintestinal manifestations in inflammatory bowel disease: differences between Crohn's disease and ulcerative colitis [Article in Spanish]. Med Clin (Barc). 2005;125:297–300. [DOI] [PubMed] [Google Scholar]
- 9.Rankin GB, Watts HD, Melnyk CS, et al. National Cooperative Crohn's Disease Study: extraintestinal manifestations and perianal complications. Gastroenterology. 1979;77:914–920. [PubMed] [Google Scholar]
- 10.Su CG, Judge TA, Lichtenstein GR. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:307–327. [DOI] [PubMed] [Google Scholar]
- 11.Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn's disease and ulcerative colitis: a study of 700 patients. Medicine. 1976;55:401–412. [DOI] [PubMed] [Google Scholar]
- 12.Farmer RG, Hawk WA, Turnbull RB., Jr Clinical patterns in Crohn's disease: a statistical study of 615 cases. Gastroenterology. 1975;68:627–635. [PubMed] [Google Scholar]
- 13.Veloso FT, Carvalho J, Magro F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol. 1996;23:29–34. [DOI] [PubMed] [Google Scholar]
- 14.Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011;106:110–119. [DOI] [PubMed] [Google Scholar]
- 15.Vavricka SR, Gantenbein C, Spoerri M, et al. Chronological order of appearance of extraintestinal manifestations relative to the time of IBD diagnosis in the Swiss Inflammatory Bowel Disease Cohort. Inflamm Bowel Dis. 2015;21:1794–1800. [DOI] [PubMed] [Google Scholar]
- 16.Ardizzone S, Puttini PS, Cassinotti A, et al. Extraintestinal manifestations of inflammatory bowel disease. Dig Liver Dis. 2008;40(suppl 2):S253–S259. [DOI] [PubMed] [Google Scholar]
- 17.Biancone L, Mandal A, Yang H, et al. Production of immunoglobulin G and G1 antibodies to cytoskeletal protein by lamina propria cells in ulcerative colitis. Gastroenterology. 1995;109:3–12. [DOI] [PubMed] [Google Scholar]
- 18.Das KM, Sakamaki S, Vecchi M, et al. The production and characterization of monoclonal antibodies to a human colonic antigen associated with ulcerative colitis: cellular localization of the antigen by using the monoclonal antibody. J Immunol. 1987;139:77–84. [PubMed] [Google Scholar]
- 19.Geng X, Biancone L, Dai HH, et al. Tropomyosin isoforms in intestinal mucosa: production of autoantibodies to tropomyosin isoforms in ulcerative colitis. Gastroenterology. 1998;114:912–922. [DOI] [PubMed] [Google Scholar]
- 20.Bhagat S, Das KM. A shared and unique peptide in the human colon, eye, and joint detected by a monoclonal antibody. Gastroenterology. 1994;107:103–108. [DOI] [PubMed] [Google Scholar]
- 21.Das KM, Vecchi M, Sakamaki S. A shared and unique epitope(s) on human colon, skin, and biliary epithelium detected by a monoclonal antibody. Gastroenterology. 1990;98:464–469. [DOI] [PubMed] [Google Scholar]
- 22.Satsangi J, Grootscholten C, Holt H, et al. Clinical patterns of familial inflammatory bowel disease. Gut. 1996;38:738–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roussomoustakaki M, Satsangi J, Welsh K, et al. Genetic markers may predict disease behavior in patients with ulcerative colitis. Gastroenterology. 1997;112:1845–1853. [DOI] [PubMed] [Google Scholar]
- 24.Ott C, Scholmerich J. Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol. 2013;10:585–595. [DOI] [PubMed] [Google Scholar]
- 25.Orchard TR, Thiyagaraja S, Welsh KI, et al. Clinical phenotype is related to HLA genotype in the peripheral arthropathies of inflammatory bowel disease. Gastroenterology. 2000;118:274–278. [DOI] [PubMed] [Google Scholar]
- 26.Mallas EG, Mackintosh P, Asquith P, et al. Histocompatibility antigens in inflammatory bowel disease. Their clinical significance and their association with arthropathy with special reference to HLA-B27 (W27). Gut. 1976;17:906–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smale S, Natt RS, Orchard TR, et al. Inflammatory bowel disease and spondylarthropathy. Arthritis Rheum. 2001;44:2728–2736. [DOI] [PubMed] [Google Scholar]
- 28.Gravallese EM, Kantrowitz FG. Arthritic manifestations of inflammatory bowel disease. Am J Gastroenterol. 1988;83:703–709. [PubMed] [Google Scholar]
- 29.Orchard TR, Wordsworth BP, Jewell DP. Peripheral arthropathies in inflammatory bowel disease: their articular distribution and natural history. Gut. 1998;42:387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Reyna TS, Martinez-Reyes C, Yamamoto-Furusho JK. Rheumatic manifestations of inflammatory bowel disease. World J Gastroenterol. 2009;15:5517–5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brakenhoff LK, van der Heijde DM, Hommes DW. IBD and arthropathies: a practical approach to its diagnosis and management. Gut. 2011;60:1426–1435. [DOI] [PubMed] [Google Scholar]
- 32.Generini S, Giacomelli R, Fedi R, et al. Infliximab in spondyloarthropathy associated with Crohn's disease: an open study on the efficacy of inducing and maintaining remission of musculoskeletal and gut manifestations. Ann Rheum Dis. 2004;63:1664–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herfarth H, Obermeier F, Andus T, et al. Improvement of arthritis and arthralgia after treatment with infliximab (Remicade) in a German prospective, open-label, multicenter trial in refractory Crohn's disease. Am J Gastroenterol. 2002;97:2688–2690. [DOI] [PubMed] [Google Scholar]
- 34.Atzeni F, Ardizzone S, Bertani L, et al. Combined therapeutic approach: inflammatory bowel diseases and peripheral or axial arthritis. World J Gastroenterol. 2009;15:2469–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarzi-Puttini P, Antivalle M, Marchesoni A, et al. Efficacy and safety of anti-TNF agents in the Lombardy rheumatoid arthritis network (LORHEN). Reumatismo. 2008;60:290–295. [DOI] [PubMed] [Google Scholar]
- 36.Kaufman I, Caspi D, Yeshurun D, et al. The effect of infliximab on extraintestinal manifestations of Crohn's disease. Rheumatol Int. 2005;25:406–410. [DOI] [PubMed] [Google Scholar]
- 37.Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Regueiro M, Valentine J, Plevy S, et al. Infliximab for treatment of pyoderma gangrenosum associated with inflammatory bowel disease. Am J Gastroenterol. 2003;98:1821–1826. [DOI] [PubMed] [Google Scholar]
- 39.Tanida S, Inoue N, Kobayashi K, et al. Adalimumab for the treatment of Japanese patients with intestinal Behcet's disease. Clin Gastroenterol Hepatol. 2015;13:940–948. [DOI] [PubMed] [Google Scholar]
- 40.Vanbiervliet G, Anty R, Schneider S, et al. Sweet's syndrome and erythema nodosum associated with Crohn's disease treated by infliximab [Article in French]. Gastroenterol Clin Biol. 2002;26:295–297. [PubMed] [Google Scholar]
- 41.Singh S, Khanna S, Pardi DS, et al. Effect of ursodeoxycholic acid use on the risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2013;19:1631–1638. [DOI] [PubMed] [Google Scholar]
- 42.Fries W, Giofre MR, Catanoso M, et al. Treatment of acute uveitis associated with Crohn's disease and sacroileitis with infliximab. Am J Gastroenterol. 2002;97:499–500. [DOI] [PubMed] [Google Scholar]
- 43.Calvo-Rio V, Blanco R, Beltran E, et al. Anti-TNF-alpha therapy in patients with refractory uveitis due to Behcet's disease: a 1-year follow-up study of 124 patients. Rheumatology (Oxford). 2014;53:2223–2231. [DOI] [PubMed] [Google Scholar]
- 44.Lakatos PL, Lakatos L, Kiss LS, et al. Treatment of extraintestinal manifestations in inflammatory bowel disease. Digestion. 2012;86(suppl 1):28–35. [DOI] [PubMed] [Google Scholar]
- 45.Clegg DO, Reda DJ, Abdellatif M. Comparison of sulfasalazine and placebo for the treatment of axial and peripheral articular manifestations of the seronegative spondylarthropathies: a Department of Veterans Affairs cooperative study. Arthritis Rheum. 1999;42:2325–2329. [DOI] [PubMed] [Google Scholar]
- 46.Bjarnason I, Hayllar J, MacPherson AJ, et al. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology. 1993;104:1832–1847. [DOI] [PubMed] [Google Scholar]
- 47.Kaufmann HJ, Taubin HL. Nonsteroidal anti-inflammatory drugs activate quiescent inflammatory bowel disease. Ann Intern Med. 1987;107:513–516. [DOI] [PubMed] [Google Scholar]
- 48.Miner PB., Jr Factors influencing the relapse of patients with inflammatory bowel disease. Am J Gastroenterol. 1997;92:1S–4S. [PubMed] [Google Scholar]
- 49.Kefalakes H, Stylianides TJ, Amanakis G, et al. Exacerbation of inflammatory bowel diseases associated with the use of nonsteroidal anti-inflammatory drugs: myth or reality? Eur J Clin Pharmacol. 2009;65:963–970. [DOI] [PubMed] [Google Scholar]
- 50.Takeuchi K, Smale S, Premchand P, et al. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:196–202. [DOI] [PubMed] [Google Scholar]
- 51.Sandborn WJ, Stenson WF, Brynskov J, et al. Safety of celecoxib in patients with ulcerative colitis in remission: a randomized, placebo-controlled, pilot study. Clin Gastroenterol Hepatol. 2006;4:203–211. [DOI] [PubMed] [Google Scholar]
- 52.Reinisch W, Miehsler W, Dejaco C, et al. An open-label trial of the selective cyclo-oxygenase-2 inhibitor, rofecoxib, in inflammatory bowel disease-associated peripheral arthritis and arthralgia. Aliment Pharmacol Ther. 2003;17:1371–1380. [DOI] [PubMed] [Google Scholar]
- 53.Mahadevan U, Loftus EV, Jr, Tremaine WJ, et al. Safety of selective cyclooxygenase-2 inhibitors in inflammatory bowel disease. Am J Gastroenterol. 2002;97:910–914. [DOI] [PubMed] [Google Scholar]
- 54.El Miedany Y, Youssef S, Ahmed I, et al. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective cox-2 inhibitor in inflammatory bowel diseases. Am J Gastroenterol. 2006;101:311–317. [DOI] [PubMed] [Google Scholar]
- 55.Monsen U, Sorstad J, Hellers G, et al. Extracolonic diagnoses in ulcerative colitis: an epidemiological study. Am J Gastroenterol. 1990;85:711–716. [PubMed] [Google Scholar]
- 56.Fornaciari G, Salvarani C, Beltrami M, et al. Muscoloskeletal manifestations in inflammatory bowel disease. Can J Gastroenterol. 2001;15:399–403. [DOI] [PubMed] [Google Scholar]
- 57.Brewerton DA, James DC. The histocompatibility antigen (HL-A 27) and disease. Semin Arthritis Rheum. 1975;4:191–207. [DOI] [PubMed] [Google Scholar]
- 58.Braun J, Sieper J. The sacroiliac joint in the spondyloarthropathies. Curr Opin Rheumatol. 1996;8:275–287. [DOI] [PubMed] [Google Scholar]
- 59.Breban M, Gombert B, Amor B, et al. Efficacy of thalidomide in the treatment of refractory ankylosing spondylitis. Arthritis Rheum. 1999;42:580–581. [DOI] [PubMed] [Google Scholar]
- 60.Keyser FD, Mielants H, Veys EM. Current use of biologicals for the treatment of spondyloarthropathies. Expert Opin Pharmacother. 2001;2:85–93. [DOI] [PubMed] [Google Scholar]
- 61.Van den Bosch F, Kruithof E, De Vos M, et al. Crohn's disease associated with spondyloarthropathy: effect of TNF-alpha blockade with infliximab on articular symptoms. Lancet. 2000;356:1821–1822. [DOI] [PubMed] [Google Scholar]
- 62.Ellman MH, Hanauer S, Sitrin M, et al. Crohn's disease arthritis treated with infliximab: an open trial in four patients. J Clin Rheumatol. 2001;7:67–71. [DOI] [PubMed] [Google Scholar]
- 63.Zochling J, van der Heijde D, Dougados M, et al. Current evidence for the management of ankylosing spondylitis: a systematic literature review for the ASAS/EULAR management recommendations in ankylosing spondylitis. Ann Rheum Dis. 2006;65:423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vavricka SR, Scharl M, Gubler M, et al. Which extraintestinal manifestations of IBD respond to biologics? Curr Drug Targets. 2014;15:1064–1073. [DOI] [PubMed] [Google Scholar]
- 65.Farhi D, Cosnes J, Zizi N, et al. Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine. 2008;87:281–293. [DOI] [PubMed] [Google Scholar]
- 66.Freeman HJ. Erythema nodosum and pyoderma gangrenosum in 50 patients with Crohn's disease. Can J Gastroenterol. 2005;19:603–606. [DOI] [PubMed] [Google Scholar]
- 67.Timani S, Mutasim DF. Skin manifestations of inflammatory bowel disease. Clin Dermatol. 2008;26:265–273. [DOI] [PubMed] [Google Scholar]
- 68.Kugathasan S, Miranda A, Nocton J, et al. Dermatologic manifestations of Crohn disease in children: response to infliximab. J Pediatr Gastroenterol Nutr. 2003;37:150–154. [DOI] [PubMed] [Google Scholar]
- 69.Ortego-Centeno N, Callejas-Rubio JL, Sanchez-Cano D, et al. Refractory chronic erythema nodosum successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2007;21:408–410. [DOI] [PubMed] [Google Scholar]
- 70.Quin A, Kane S, Ulitsky O. A case of fistulizing Crohn's disease and erythema nodosum managed with adalimumab. Nat Clin Pract Gastroenterol Hepatol. 2008;5:278–281. [DOI] [PubMed] [Google Scholar]
- 71.Clayton TH, Walker BP, Stables GI. Treatment of chronic erythema nodosum with infliximab. Clin Exp Dermatol. 2006;31:823–824. [DOI] [PubMed] [Google Scholar]
- 72.Jorizzo JL, Solomon AR, Zanolli MD, et al. Neutrophilic vascular reactions. J Am Acad Dermatol. 1988;19:983–1005. [DOI] [PubMed] [Google Scholar]
- 73.Bennett ML, Jackson JM, Jorizzo JL, et al. Pyoderma gangrenosum. A comparison of typical and atypical forms with an emphasis on time to remission. Case review of 86 patients from 2 institutions. Medicine. 2000;79:37–46. [DOI] [PubMed] [Google Scholar]
- 74.Nguyen GC, Torres EA, Regueiro M, et al. Inflammatory bowel disease characteristics among African Americans, Hispanics, and non-Hispanic Whites: characterization of a large North American cohort. Am J Gastroenterol. 2006;101:1012–1023. [DOI] [PubMed] [Google Scholar]
- 75.Orchard T. Extraintestinal complications of inflammatory bowel disease. Curr Gastroenterol Rep. 2003;5:512–517. [DOI] [PubMed] [Google Scholar]
- 76.Callen JP. Pyoderma gangrenosum. Lancet. 1998;351:581–585. [DOI] [PubMed] [Google Scholar]
- 77.Sheldon DG, Sawchuk LL, Kozarek RA, et al. Twenty cases of peristomal pyoderma gangrenosum: diagnostic implications and management. Arch Surg. 2000;135:564–568; discussion 568–569. [DOI] [PubMed] [Google Scholar]
- 78.Hughes AP, Jackson JM, Callen JP. Clinical features and treatment of peristomal pyoderma gangrenosum. JAMA. 2000;284:1546–1548. [DOI] [PubMed] [Google Scholar]
- 79.Gellert A, Green ES, Beck ER, et al. Erythema nodosum progressing to pyoderma gangrenosum as a complication of Crohn's disease. Postgrad Med J. 1983;59:791–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chow RK, Ho VC. Treatment of pyoderma gangrenosum. J Am Acad Dermatol. 1996;34:1047–1060. [DOI] [PubMed] [Google Scholar]
- 81.Powell RJ, Holbrook MR, Stevens A. Pyoderma gangrenosum and its treatment. Lancet. 1997;350:1720–1721. [DOI] [PubMed] [Google Scholar]
- 82.Trost LB, McDonnell JK. Important cutaneous manifestations of inflammatory bowel disease. Postgrad Med J. 2005;81:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Friedman S, Marion JF, Scherl E, et al. Intravenous cyclosporine in refractory pyoderma gangrenosum complicating inflammatory bowel disease. Inflamm Bowel Dis. 2001;7:1–7. [DOI] [PubMed] [Google Scholar]
- 84.Wollina U, Haroske G. Pyoderma gangraenosum. Curr Opin Rheumatol. 2011;23:50–56. [DOI] [PubMed] [Google Scholar]
- 85.Arnott ID, McDonald D, Williams A, et al. Clinical use of Infliximab in Crohn's disease: the Edinburgh experience. Aliment Pharmacol Ther. 2001;15:1639–1646. [DOI] [PubMed] [Google Scholar]
- 86.Batres LA, Mamula P, Baldassano RN. Resolution of severe peristomal pyoderma gangrenosum with infliximab in a child with Crohn disease. J Pediatr Gastroenterol Nutr. 2002;34:558–560. [DOI] [PubMed] [Google Scholar]
- 87.Ferkolj I, Hocevar A, Golouh R, et al. Infliximab for treatment of resistant pyoderma gangrenosum associated with Crohn's disease. Acta Dermatovenerol Alp Pannonica Adriat. 2006;15:173–177. [PubMed] [Google Scholar]
- 88.Grange F, Djilali-Bouzina F, Weiss AM, et al. Corticosteroid-resistant pyoderma gangrenosum associated with Crohn's disease: rapid cure with infliximab. Dermatology. 2002;205:278–280. [DOI] [PubMed] [Google Scholar]
- 89.Juillerat P, Christen-Zach S, Troillet FX, et al. Infliximab for the treatment of disseminated pyoderma gangrenosum associated with ulcerative colitis. Case report and literature review. Dermatology. 2007;215:245–251. [DOI] [PubMed] [Google Scholar]
- 90.Kiran RP, O'Brien-Ermlich B, Achkar JP, et al. Management of peristomal pyoderma gangrenosum. Dis Colon Rectum. 2005;48:1397–1403. [DOI] [PubMed] [Google Scholar]
- 91.Kouklakis G, Moschos J, Leontiadis GI, et al. Infliximab for treatment of pyoderma gangrenosum associated with clinically inactive Crohn's disease. A case report. Rom J Gastroenterol. 2005;14:401–403. [PubMed] [Google Scholar]
- 92.Ljung T, Staun M, Grove O, et al. Pyoderma gangrenosum associated with crohn disease: effect of TNF-alpha blockade with infliximab. Scand J Gastroenterol. 2002;37:1108–1110. [DOI] [PubMed] [Google Scholar]
- 93.Fonder MA, Cummins DL, Ehst BD, et al. Adalimumab therapy for recalcitrant pyoderma gangrenosum. J Burns Wounds. 2006;5:e8. [PMC free article] [PubMed] [Google Scholar]
- 94.Martin D, Handler T, McDermott J. Leucocytoclastic vasculitis in severe ulcerative colitis. Mil Med. 2011;176:581–583. [DOI] [PubMed] [Google Scholar]
- 95.Lopez San Roman A, Bermejo F, Aldanondo I, et al. Pyoderma gangrenosum associated with ulcerative colitis: response to infliximab. Rev Esp Enferm Dig. 2004;96:420–422; 422-424. [DOI] [PubMed] [Google Scholar]
- 96.Sapienza MS, Cohen S, Dimarino AJ. Treatment of pyoderma gangrenosum with infliximab in Crohn's disease. Dig Dis Sci. 2004;49:1454–1457. [DOI] [PubMed] [Google Scholar]
- 97.Singh M, Andrew SM, Lear JT. Infliximab as a treatment for recalcitrant pyoderma gangrenosum. Clin Exp Dermatol. 2004;29:196–197. [DOI] [PubMed] [Google Scholar]
- 98.Tan MH, Gordon M, Lebwohl O, et al. Improvement of Pyoderma gangrenosum and psoriasis associated with Crohn disease with anti-tumor necrosis factor alpha monoclonal antibody. Arch Dermatol. 2001;137:930–933. [PubMed] [Google Scholar]
- 99.Triantafillidis JK, Cheracakis P, Sklavaina M, et al. Favorable response to infliximab treatment in a patient with active Crohn disease and pyoderma gangrenosum. Scand J Gastroenterol. 2002;37:863–865. [PubMed] [Google Scholar]
- 100.Zaccagna A, Bertone A, Puiatti P, et al. Anti-tumor necrosis factor alpha monoclonal antibody (infliximab) for the treatment of Pyoderma gangrenosum associated with Crohn's disease. Eur J Dermatol. 2003;13:258–260. [PubMed] [Google Scholar]
- 101.Alkhouri N, Hupertz V, Mahajan L. Adalimumab treatment for peristomal pyoderma gangrenosum associated with Crohn's disease. Inflamm Bowel Dis. 2009;15:803–806. [DOI] [PubMed] [Google Scholar]
- 102.Pomerantz RG, Husni ME, Mody E, et al. Adalimumab for treatment of pyoderma gangrenosum. Br J Dermatol. 2007;157:1274–1275. [DOI] [PubMed] [Google Scholar]
- 103.Zold E, Nagy A, Devenyi K, et al. Successful use of adalimumab for treating fistulizing Crohn's disease with pyoderma gangrenosum: two birds with one stone. World J Gastroenterol. 2009;15:2293–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mimouni D, Anhalt GJ, Kouba DJ, et al. Infliximab for peristomal pyoderma gangrenosum. Br J Dermatol. 2003;148:813–816. [DOI] [PubMed] [Google Scholar]
- 105.Thrash B, Patel M, Shah KR, et al. Cutaneous manifestations of gastrointestinal disease: part II. J Am Acad Dermatol. 2013;68:211 e211-233; quiz 244–216. [DOI] [PubMed] [Google Scholar]
- 106.Becuwe C, Delaporte E, Colombel JF, et al. Sweet's syndrome associated with Crohn's disease. Acta Derm Venereol. 1989;69:444–445. [PubMed] [Google Scholar]
- 107.Benton EC, Rutherford D, Hunter JA. Sweet's syndrome and pyoderma gangrenosum associated with ulcerative colitis. Acta Derm Venereol. 1985;65:77–80. [PubMed] [Google Scholar]
- 108.Banet DE, McClave SA, Callen JP. Oral metronidazole, an effective treatment for Sweet's syndrome in a patient with associated inflammatory bowel disease. J Rheumatol. 1994;21:1766–1768. [PubMed] [Google Scholar]
- 109.Treton X, Joly F, Alves A, et al. Azathioprine-induced Sweet's syndrome in Crohn's disease. Inflamm Bowel Dis. 2008;14:1757–1758. [DOI] [PubMed] [Google Scholar]
- 110.Ali M, Duerksen DR. Ulcerative colitis and Sweet's syndrome: a case report and review of the literature. Can J Gastroenterol. 2008;22:296–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kemmett D, Hunter JA. Sweet's syndrome: a clinicopathologic review of twenty-nine cases. J Am Acad Dermatol. 1990;23:503–507. [DOI] [PubMed] [Google Scholar]
- 112.Vavricka SR, Manser CN, Hediger S, et al. Periodontitis and gingivitis in inflammatory bowel disease: a case-control study. Inflamm Bowel Dis. 2013;19:2768–2777. [DOI] [PubMed] [Google Scholar]
- 113.Petrelli EA, McKinley M, Troncale FJ. Ocular manifestations of inflammatory bowel disease. Ann Ophthalmol. 1982;14:356–360. [PubMed] [Google Scholar]
- 114.Foeldvari I, Nielsen S, Kummerle-Deschner J, et al. Tumor necrosis factor-alpha blocker in treatment of juvenile idiopathic arthritis-associated uveitis refractory to second-line agents: results of a multinational survey. J Rheumatol. 2007;34:1146–1150. [PubMed] [Google Scholar]
- 115.Hale S, Lightman S. Anti-TNF therapies in the management of acute and chronic uveitis. Cytokine. 2006;33:231–237. [DOI] [PubMed] [Google Scholar]
- 116.Joseph A, Raj D, Dua HS, et al. Infliximab in the treatment of refractory posterior uveitis. Ophthalmology. 2003;110:1449–1453. [DOI] [PubMed] [Google Scholar]
- 117.Lindstedt EW, Baarsma GS, Kuijpers RW, et al. Anti-TNF-alpha therapy for sight threatening uveitis. Br J Ophthalmol. 2005;89:533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rajaraman RT, Kimura Y, Li S, et al. Retrospective case review of pediatric patients with uveitis treated with infliximab. Ophthalmology. 2006;113:308–314. [DOI] [PubMed] [Google Scholar]
- 119.Sfikakis PP, Theodossiadis PG, Katsiari CG, et al. Effect of infliximab on sight-threatening panuveitis in Behcet's disease. Lancet. 2001;358:295–296. [DOI] [PubMed] [Google Scholar]
- 120.Murphy CC, Ayliffe WH, Booth A, et al. Tumor necrosis factor alpha blockade with infliximab for refractory uveitis and scleritis. Ophthalmology. 2004;111:352–356. [DOI] [PubMed] [Google Scholar]
- 121.Saurenmann RK, Levin AV, Rose JB, et al. Tumour necrosis factor alpha inhibitors in the treatment of childhood uveitis. Rheumatology. 2006;45:982–989. [DOI] [PubMed] [Google Scholar]
- 122.Kahn P, Weiss M, Imundo LF, et al. Favorable response to high-dose infliximab for refractory childhood uveitis. Ophthalmology. 2006;113:860–864.e862. [DOI] [PubMed] [Google Scholar]
- 123.Biester S, Deuter C, Michels H, et al. Adalimumab in the therapy of uveitis in childhood. Br J Ophthalmol. 2007;91:319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Braun J, Baraliakos X, Listing J, et al. Decreased incidence of anterior uveitis in patients with ankylosing spondylitis treated with the anti-tumor necrosis factor agents infliximab and etanercept. Arthritis Rheum. 2005;52:2447–2451. [DOI] [PubMed] [Google Scholar]
- 125.Mintz R, Feller ER, Bahr RL, et al. Ocular manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:135–139. [DOI] [PubMed] [Google Scholar]
- 126.Yarur AJ, Czul F, Levy C. Hepatobiliary manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:1655–1667. [DOI] [PubMed] [Google Scholar]
- 127.Olsson R, Danielsson A, Jarnerot G, et al. Prevalence of primary sclerosing cholangitis in patients with ulcerative colitis. Gastroenterology. 1991;100:1319–1323. [PubMed] [Google Scholar]
- 128.Fausa O, Schrumpf E, Elgjo K. Relationship of inflammatory bowel disease and primary sclerosing cholangitis. Semin Liver Dis. 1991;11:31–39. [DOI] [PubMed] [Google Scholar]
- 129.Wiesner RH, LaRusso NF. Clinicopathologic features of the syndrome of primary sclerosing cholangitis. Gastroenterology. 1980;79:200–206. [PubMed] [Google Scholar]
- 130.Broome U, Bergquist A. Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer. Semin Liver Dis. 2006;26:31–41. [DOI] [PubMed] [Google Scholar]
- 131.Tischendorf JJ, Hecker H, Kruger M, et al. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102:107–114. [DOI] [PubMed] [Google Scholar]
- 132.Joo M, Abreu-e-Lima P, Farraye F, et al. Pathologic features of ulcerative colitis in patients with primary sclerosing cholangitis: a case-control study. Am J Surg Pathol. 2009;33:854–862. [DOI] [PubMed] [Google Scholar]
- 133.Soetikno RM, Lin OS, Heidenreich PA, et al. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48–54. [DOI] [PubMed] [Google Scholar]
- 134.Loftus EV, Jr, Harewood GC, Loftus CG, et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Talwalkar JA, Lindor KD. Primary sclerosing cholangitis. Inflamm Bowel Dis. 2005;11:62–72. [DOI] [PubMed] [Google Scholar]
- 136.Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336:691–695. [DOI] [PubMed] [Google Scholar]