Abstract
Objective
To determine efficacy of a protocol for managing urethral obstruction (UO) in male cats without urethral catheterization.
Design
Clinical trial.
Animals
15 male cats with UO in which conventional treatment had been declined.
Procedures
Laboratory testing and abdominal radiography were performed, and cats with severe metabolic derangements or urinary calculi were excluded. Treatment included administration of acepromazine (0.25 mg, IM, or 2.5 mg, PO, q 8 h), buprenorphine (0.075 mg, PO, q 8 h), and medetomidine (0.1 mg, IM, q 24 h) and decompressive cystocentesis and SC administration of fluids as needed. Cats were placed in a quiet, dark environment to minimize stress. Treatment success was defined as spontaneous urination within 72 hours and subsequent discharge from the hospital.
Results
Treatment was successful in 11 of the 15 cats. In the remaining 4 cats, treatment was considered to have failed because of development of uroabdomen (n = 3) or hemoabdomen (1). Cats in which treatment failed had significantly higher serum creatinine concentrations than did cats in which treatment was successful. Necropsy was performed on 3 cats in which treatment had failed. All 3 had severe inflammatory disease of the urinary bladder, but none had evidence of bladder rupture.
Conclusions and Clinical Relevance
Results suggested that in male cats, a combination of pharmacological treatment, decompressive cystocentesis, and a low-stress environment may allow for resolution of UO without the need for urethral catheterization. This low-cost protocol could serve as an alternative to euthanasia when financial constraints prevent more extensive treatment.
Urethral obstruction is a relatively common condition in domestic male cats that typically requires emergency treatment.1 The pathophysiology of UO, expected physical examination findings, biochemical and acid-base abnormalities, and typical treatment course have been reviewed.1–8 Standard treatment for UO in male cats includes stabilization of cardiovascular and metabolic derangements, correction of electrolyte abnormalities through IV administration of fluids, and relief of the obstruction through urethral catheterization.2,3 Following relief of the obstruction, affected cats may require maintenance of an indwelling urinary catheter and intensive monitoring until the catheter is removed and sustained spontaneous urination is demonstrated. Treatment for UO may involve several days of hospitalization and considerable expense. As a result, affected cats may be euthanatized because of financial constraints of the owners, especially given the potentially recurrent nature of this disease process.
Although it has generally been accepted that the physical presence of a mucous plug or calculus within the urethra plays a primary role in the pathogenesis of UO,9–11 a recent study12 found that the cause was idiopathic in > 50% of cats and that urolithiasis and urethral plugs were less common (29% and 18%, respectively, of affected cats). In addition, urethral spasm and edema have been shown to play an important role in UO.7,13 Given these findings, we speculated that pharmacological manipulation of stress, urethral tone, and discomfort could help alleviate some of the functional component of the obstructive process and might preclude the need for catheterization. If this treatment were successful in restoring spontaneous urination, the cost of treatment could be decreased as well as the risk of complications associated with urethral catheterization, such as exacerbation of urethral inflammation, urinary tract infection, and urethral trauma.14–16
The purpose of the study reported here was to determine the efficacy of a protocol for managing UO in male cats that involved pharmacological manipulation, intermittent cystocentesis, and provision of a low-stress environment. We hypothesized that this protocol would allow for spontaneous resolution of UO without the need for urethral catheterization and without a significant increase in recurrence rate, compared with conventional treatment.
Materials and Methods
Case selection
Male cats brought to The Ohio State University Veterinary Teaching Hospital for treatment of naturally occurring UO between June 2007 and June 2008 were considered for inclusion in the study. Cats were eligible for inclusion in the study if a diagnosis of UO had been made on the basis of history and physical examination findings and the owners had declined conventional treatment (ie, urethral catheterization and intensive care) because of financial considerations and were considering euthanasia. Cats with clinically important physical examination abnormalities (ie, heart rate < 120 beats/min, rectal temperature < 35.6°C [96°F], or unresponsive mentation), severe metabolic derangements (venous pH < 7.1 or serum potassium concentration > 8.0 mEq/L), or radiographic evidence of cystic or urethral calculi were excluded from the study. Severity of azotemia at the time of initial examination was not used as an inclusion or exclusion criterion. Owner consent was obtained prior to initiation of treatment. The treatment protocol used in the study was approved by The Ohio State University Veterinary Teaching Hospital’s Executive Committee. At the time this study was performed, clinical research projects performed at the Veterinary Teaching Hospital and involving client-owned subjects did not require approval from The Ohio State University’s Institutional Animal Care and Use Committee.
Procedures
Owners of cats enrolled in the study were required to pay a set fee ($350) to cover hospital expenses associated with administering the protocol, a cost that was substantially less than the estimated cost of standard treatment ($1,200 to $1,800). Cats were given acepromazine (0.25 mg, IM) and buprenorphine (0.075 mg, IM) to provide sedation and analgesia and help minimize stress. Approximately 10 minutes later, the penis was extruded, inspected, and gently massaged in an attempt to dislodge any obstructions in the distal portion of the penis. A single attempt to gently express the bladder was then made. If no urine was produced, cystocentesis was performed with a 22-gauge, 1.5-inch needle connected to extension tubing, a 3-way stopcock, and a 20-mL syringe to alleviate bladder distention and discomfort. A venous blood sample was obtained, and venous pH and serum sodium, potassium, chloride, urea nitrogen, and creatinine concentrations were measured with a commercial analyzer.a Packed cell volume was determined by means of centrifugation, and plasma TP concentration was determined by means of refractometry.b A single lateral radiographic view of the abdomen was obtained and assessed for evidence of cystic and urethral calculi; care was taken to include the entire lower urinary tract to the tip of the penis. Cats were allowed to continue in the study only if results of this initial diagnostic testing did not meet any of the exclusion criteria for the study. For cats that continued in the study, saline (0.9% NaCl) solution was administered SC (100 to 200 mL, depending on hydration status and severity of azotemia). Neither urinary nor IV catheterization was performed.
Cats were then placed in a low-stress environment consisting of a darkened, low-traffic treatment ward that did not house any dogs. General status and presence of spontaneous urination were assessed every 8 hours. Additional doses of acepromazine (0.25 mg, IM, or 2.5 mg, PO) and buprenorphine (0.075 mg, PO or IM) were administered every 8 hours to provide continued sedation and analgesia. After medications were given, the urinary bladder was palpated to determine size and firmness, and cystocentesis was performed as needed (up to 3 times/d) to alleviate urinary bladder distention. A physical examination was performed each morning while cats were hospitalized. Fresh food (based on the cat’s typical diet) and water were offered every 8 hours. Medetomidine (0.1 mg, IM, q 24 h) was administered beginning 24 hours after initial examination to provide additional sedation and urethral relaxation, and additional fluids were administered SC once or twice daily at the attending clinician’s discretion.
Treatment was continued as described for up to 3 days (72 hours) to allow for spontaneous urination to occur. Treatment was discontinued if the cat developed clinically important complications (eg, uroabdomen or a worsening of the cat’s clinical condition) or failed to respond within 3 days. If the cat urinated spontaneously, observation and administration of acepromazine and buprenorphine were continued for an additional 24 hours but administration of medetomidine was discontinued. The cat was discharged from the hospital if spontaneous urination continued during this period. On the day of discharge, serum biochemical testing was repeated and PCV and plasma TP concentration were measured to assess for resolution of electrolyte abnormalities and azotemia. Acepromazine (2.5 mg, PO, q 8 h) and buprenorphine (0.075 mg, PO, q 8 h) were dispensed for continued administration for 5 days after discharge. In addition, owners were given uniform instructions on methods to increase water intake, the appropriate number of litter boxes for the household, and implementation of environmental enrichment.17 Follow-up telephone calls were made 3 days, 3 weeks, and 1 year after discharge to determine the incidence of reobstruction.
Treatment success was defined as spontaneous urination within 72 hours and subsequent discharge from the hospital. Treatment failure was defined as development of clinically important complications (eg, uroabdomen or hemoabdomen) or failure to have spontaneous urination within 3 days after initiation of treatment.
Data collected for cats included in the study consisted of age; weight; rectal temperature, heart rate, respiratory rate, results of serum biochemical testing (sodium, potassium, chloride, urea nitrogen, and creatinine concentrations), venous pH, PCV, and TP concentration measured at the time of initial examination and at the time of discharge; the number of times cystocentesis was performed; time to spontaneous urination; and total duration of hospitalization. Complete necropsies were performed on all cats that were euthanatized.
Statistical analysis
Data were summarized as mean ± SD. The D’Agostino and Pearson omnibus normality test was used to determine whether data were normally distributed, and 2-tailed Student t tests (normally distributed data) or 1-tailed Mann-Whitney tests (nonnormally distributed data) were used to compare values obtained at the time of initial examination with values obtained at the time of discharge. These tests also were used to compare data values obtained from the treatment success group and the treatment failure group. Standard softwarec was used for all analyses; values of P < 0.05 were considered significant.
Results
Cats
Urethral obstruction was diagnosed in 33 cats during the study period. Fifteen of these cats met the criteria for inclusion and were enrolled in the study. Mean ± SD age was 3.3 ± 2.3 years (range, 1.0 to 7.5 years), and mean body weight was 6.1 ± 1.6 kg (13.4 ± 3.5 lb). All 15 cats were of mixed breeding (10 domestic shorthairs, 3 domestic medium hairs, and 2 domestic longhairs). Two were sexually intact, and 13 were castrated. Eight had reportedly previously had signs consistent with feline idiopathic cystitis, but none had a history of previous episodes of UO.
Physical examination findings
All 15 cats were responsive on initial examination, and mean rectal temperature (mean ± SD, 38.2 ± 1.1°C [100.8 ± 2.0°F]) was within reference limits. At the time of initial examination, mean heart rate was 211 ± 33 beats/min (range, 160 to 276 beats/min) and mean respiratory rate was 48 ± 26 breaths/min (range, 20 to 124 breaths/min). In all cats, the urinary bladder appeared to be of moderate to large size during abdominal palpation and attempts to express urine were unsuccessful.
Laboratory data
Results of laboratory testing at the time of initial examination were available for all 15 cats included in the study (Table 1). Although mean serum potassium concentration was within reference limits, some cats had clinically important hyperkalemia (potassium concentration as high as 8 mEq/L). Severity of azotemia and acidemia also varied among cats. Mean PCV was 46 ± 8%, and mean plasma TP concentration was 7.2 ± 0.6 g/dL.
Table 1.
Laboratory data at the time of initial examination and at the time of hospital discharge for 15 cats with UO treated with a combination of pharmacological manipulation, intermittent cystocentesis, and provision of a low-stress environment but without urethral catheterization.
| Variable | Reference range | Initial examination
|
Discharge (n = 11) | ||
|---|---|---|---|---|---|
| All cats (n = 15) | Treatment success (n = 11) | Treatment failure (n = 4) | |||
| Venous pH | 7.24–7.39 | 7.34 ± 0.06 (7.26–7.43) | 7.36 ± 0.06 (7.26–7.43) | 7.30 ± 0.06 (7.26–7.32) | 7.38 ± 0.06 (7.27–7.49) |
| Serum potassium (mmol/L) | 2.8–5.9 | 4.8 ± 1.4 (3.4–8.0) | 4.4 ± 1.0 (3.4–7.0) | 5.8 ± 1.3 (4.1–8.0) | 4.0 ± 0.5 (3.4–4.8) |
| SUN (mg/dL) | 5–30 | 74 ± 39 (19–140) | 62 ± 38 (19–128) | 105 ± 24 (83–140) | 35 ± 27 (14–100)* |
| Serum creatinine (mg/dL) | 0.6–2.1 | 5.9 ± 4.9 (1.1–19.5) | 4.4 ± 3.6 (1.1–12.7) | 10.10 ± 6.3 (6.3–19.5)† | 2.4 ± 2.1 (1.1–8.2)* |
Data are given as mean ± SD (range). The analyzer did not report SUN concentrations > 140 mg/dL; therefore, samples for which SUN concentration was > 140 mg/dL were assigned a value of 140 mg/dL.
Significantly (P < 0.05) different from value for cats at the time of initial examination.
Significantly (P < 0.05) different from value for cats in which treatment was successful.
Results of laboratory testing at the time of hospital discharge were available for all 11 cats that survived to discharge (Table 1). Serum urea nitrogen and creatinine concentrations were significantly lower at the time of discharge, compared with values obtained at the time of initial examination; however, mean values at the time of discharge were higher than the upper reference limit. No significant differences in venous pH, serum potassium concentration, plasma TP concentration, or PCV were identified between the time of initial examination and the time of discharge.
Radiography
In all 15 cats, a lateral abdominal radiographic view was obtained after initial administration of acepromazine and buprenorphine and initial cystocentesis. None of the cats had radiographic evidence of cystic or urethral calculi, but 7 of the 15 did have evidence of mineralized material, presumed a urethral plug, within the urethra (Figure 1). In addition, 8 cats had radiographic evidence of mild to moderate caudal abdominal effusion, including 6 of the 11 cats in which treatment was successful and 2 of the 4 cats in which treatment failed.
Figure 1.
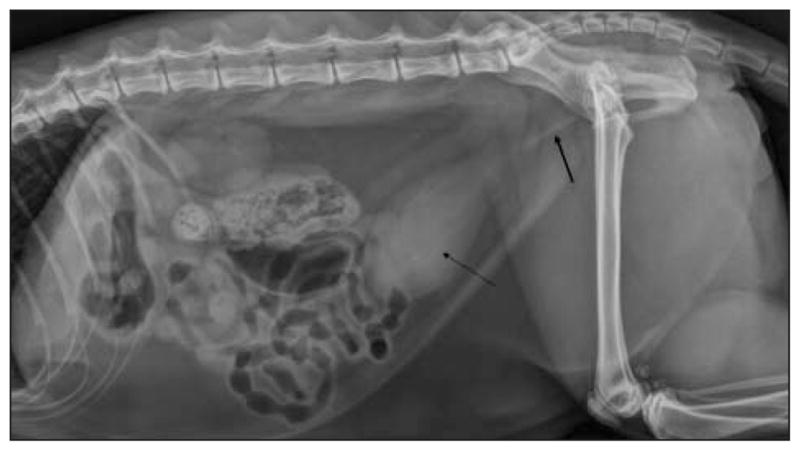
Left lateral radiographic view of the abdomen of a male cat examined because of UO. There is moderate distention of the urinary bladder with faint, linear opacities superimposed (thin arrow). Notice the linear opacity within the proximal portion of the urethra, which likely represented a mineralized urethral plug (thick arrow).
Outcome
Eleven of the 15 cats had a successful outcome, defined as spontaneous urination within 3 days and subsequent discharge from the hospital. In the remaining 4 cats, treatment was considered to have failed because of development of uroabdomen (n = 3) or hemoabdomen (1). Three of the cats in which treatment failed were euthanatized and submitted for necropsy. One cat was adopted by a veterinary student and was subsequently treated successfully.
For the cats in which treatment was successful, mean ± SD time to spontaneous urination after initiation of treatment was 34.6 ± 21.6 hours (range, 4 to 69 hours), with 9 of the 11 cats urinating spontaneously within 48 hours after treatment was initiated. Mean number of times cystocentesis was performed was 3 (range, 1 to 10), and mean duration of hospitalization was 67 ± 23 hours (range, 36 to 96 hours). In the 4 cats in which treatment failed, uroabdomen or hemoabdomen was identified between 48 and 72 hours after treatment was initiated. The diagnosis was made on the basis of worsening of the cats’ clinical condition and analysis of fluid obtained by means of abdominocentesis, and was confirmed at necropsy in the 3 cats that were euthanatized. Mean number of times cystocentesis was performed in cats in which treatment failed was 7 (range, 4 to 11). This was significantly higher than the number of times cystocentesis was performed in cats in which treatment was successful.
No significant differences were identified between cats in which treatment was successful and cats in which treatment failed with regard to age, weight, or rectal temperature, heart rate, or respiratory rate at the time of initial examination. Serum creatinine concentration at the time of initial examination was significantly higher among cats in which treatment failed than among cats in which treatment was successful (Table 1), but no other significant differences between groups were identified.
Necropsy findings
Two of the 3 cats that were euthanatized had approximately 70 to 100 mL of reddish cloudy fluid that appeared to be urine in the abdomen at the time of necropsy. The urinary bladder was diffusely thickened and dark red to black in appearance. Histologic examination of the urethra and urinary bladder revealed severe congestion, hemorrhage, and edema, along with neutrophilic urethritis and cystitis. The third cat had 250 mL of hemorrhagic fluid in the abdominal cavity at the time of necropsy. The serosal surface of the urinary bladder was diffusely dark red, and the bladder wall was thickened. The bladder mucosa was diffusely dark red to black; numerous strands of fibrin were loosely adhered to the mucosa. No urethral abnormalities were observed on gross inspection. Histologic examination of the kidneys revealed mild hydronephrosis with coagulation necrosis, acute tubular necrosis, and hemorrhage. The urinary bladder was found to have severe widespread transmural hemorrhage with necrosis and reactive fibrosis. Diffuse loss of the urothelium also was noted. The urethra was found to have severe multifocal fibronectrotizing urethritis with urethral plugs still present. The source of the hemoabdomen was not apparent. No evidence of overt rupture of the bladder or visible defects in the bladder wall that could have produced the uroabdomen or hemoabdomen in these cats were identified.
Follow-up information
Of the 11 cats successfully treated and discharged, none had had a recurrence of UO by 3 days after discharge. However, by 3 weeks after discharge, 2 of the 11 cats had had an additional episode of obstruction. One of these cats was returned to the Veterinary Teaching Hospital and was again successfully treated by use of the study protocol. The other was examined by the owner’s regular veterinarian and was successfully treated with the same protocol. However, the cat had another episode of UO immediately afterward and was euthanatized. Owners of 2 cats could not be reached by telephone 3 weeks after discharge; owners of the remaining 7 cats reported no recurrence of signs.
Owners of 7 cats were available for long-term (1-year) follow-up. None of the 7 cats had had additional episodes of UO, although 2 of them had reported signs consistent with feline idiopathic cystitis. Despite repeated attempts to contact the owners, the remaining 3 cats were lost to follow-up.
Discussion
Results of the present study suggested that in male cats, a combination of pharmacological treatment, decompressive cystocentesis, and a low-stress environment may result in resolution of UO without the need for urethral catheterization. Urethral obstruction was successfully treated in 11 of 15 cats with this protocol, and cats in which treatment was successful did not appear to have a greater risk of recurrence with this protocol, compared with perceived recurrence rates for cats that receive conventional treatment.
Treatment recommendations for cats with UO usually include placement of a urinary catheter and flushing of the urethra to relieve the presumed physical obstruction.2,3,7 However, in cats with idiopathic cystitis, urethral obstruction may be functional, rather than physical, developing secondary to inflammation-induced urethral spasm and edema. Environmental stress, pain, and agitation could potential exacerbate the autonomic imbalance associated with feline idiopathic cystitis and contribute to the development of UO.18,19 Thus, interventions that serve to reduce stress could facilitate resolution of functional obstruction. One aspect of stress reduction incorporated in the treatment protocol evaluated in the present study was placement of the cats in a dark, quiet, secluded environment free from dogs. In addition, we sought to provide analgesia and sedation and possibly reduce urethral tone through the use of medications. A previous study20 involving 20 male cats with UO found that administration of amitriptyline, a tricyclic antidepressant, was associated with a high rate of spontaneous resolution, but the methodology used in that study was unclear. In the present study, we chose to use a combination of acepromazine and buprenorphine. Acepromazine appears to cause sedation, thereby reducing stress responsiveness, by decreasing the activity of dopamine in the CNS. Acepromazine also exerts α1-adrenergic receptor antagonistic effects, which could result in urethral sphincter relaxation and has been shown to cause a significant reduction in intraurethral pressures, as measured by means of urethral pressure profilometry, in anesthetized male cats.21 Buprenorphine is a partial μ-opioid receptor agonist that provides mild to moderate analgesia, thereby helping address discomfort associated with UO and underlying idiopathic cystitis.22 Medetomidine, an α2-adrenergic receptor agonist, also was used once daily if spontaneous urination did not occur within 24 hours to provide additional sedation and analgesia and to help reduce the catecholamine excess documented in cats with idiopathic cystitis.19,23,24 The presynaptic α2-adrenergic receptors serve to decrease sympathetic outflow; thus, stimulation of them might result in decreased stress response and promote urethral relaxation.23 Despite the potential benefits of these medications, we have no direct evidence that urethral relaxation occurred or that use of these medications had any impact on outcome.
Because urinary catheterization was not performed in cats enrolled in the present study, it was necessary to perform intermittent cystocentesis to decompress the bladder until spontaneous urination occurred. Although its use is controversial, there are potential benefits to performing cystocentesis in cats with UO, even when catheterization is to be performed. Potential benefits include allowing more immediate decompression of the bladder, reducing urethral backpressure, and obtaining an unadulterated diagnostic sample for urinalysis and bacterial culture.25 The major concern in performing cystocentesis in a cat with UO is that the needle might cause damage to or rupture of the bladder wall because of bladder wall distension and friability, which could lead to uroabdomen. The risk of complications is most likely related to the extent of disease in the urinary bladder and the technique used.25
In the present study, 4 of 15 cats developed uroabdomen (n = 3) or hemoabdomen (1). The obvious concern in these patients is that repeated cystocentesis was responsible for these complications. This is potentially supported by the fact that cats in which treatment failed underwent cystocentesis a significantly higher number of times than did cats in which treatment was successful. However, 1 cat in which treatment was successful underwent cystocentesis 10 times without complications. Furthermore, there was no gross evidence of a bladder rupture or defect in the 3 cats that underwent necropsy, although it is possible that a defect had sealed or was not apparent at the time of necropsy. It also is possible that a combination of severe, diffuse cystic mural disease and high intramural pressure could have resulted in leakage of fluid or blood across the bladder wall. Cats in which treatment failed also were generally sicker, with more severe azotemia, higher serum potassium concentrations, and lower venous pH. These cats may have had UO for a longer time, which could have resulted in more severe bladder wall disease and predisposed them to develop complications.
Eight cats in the present study had radiographic evidence of mild to moderate caudal abdominal effusion. Because cystocentesis was performed prior to radiography, it was not possible to determine whether abdominal effusion was present before cystocentesis was performed or only developed afterward. The presence of effusion on an abdominal radiograph at the time of initial examination did not seem to reflect the likelihood of treatment failure, in that the incidence for cats in which treatment failed was similar to the incidence for cats in which treatment was successful. Of the 15 cats considered for enrollment in the study, none were excluded because of urethral calculi, although 7 had radiopaque urethral plugs. Although it is generally recommended that abdominal radiography be performed in cats with UO to rule out the presence of uroliths, the overall low incidence of uroliths as the cause of physical obstruction (reportedly between 5% and 12%10,11) could support the omission of this step in the application of this protocol to further reduce costs.
The overall success rate in the present study (11/15) was lower than the reported survival rate associated with standard treatment of cats with UO (91% to 94%1,12). In addition, given the minimalist approach to treatment dictated by this protocol, we excluded the sickest patients, including cats with profound physiologic and metabolic derangements. Patients with severe hypothermia (rectal temperature < 35.6°C), bradycardia (heart rate < 120 beats/min), signs of depression, or severe hyperkalemia (serum potassium concentration > 8 mmol/L) or acidemia (venous pH < 7.1) would typically be at a high risk of death without emergency intervention and intensive care and so were excluded. Physical examination parameters used to screen cats for inclusion in the study were selected on the basis of their ability to predict the presence of severe hyperkalemia,4 and none of the cats that were considered qualified for inclusion on the basis of physical examination findings had to be excluded later on the basis of serum potassium concentration or venous pH. The severity of azotemia at the time of initial examination was not used as an exclusion criterion because accumulation of uremic toxins, although deleterious, is not immediately life threatening. As previously stated, cats in which treatment failed had significantly higher creatinine concentrations at the time of initial examination than did cats in which treatment was successful. Although a significant difference between groups was not identified with regard to SUN concentration, this may have been because the analyzer that was used did not report concentrations higher than 140 mg/dL. All 4 cats in which treatment failed had an SUN concentration > 80 mg/dL and serum creatinine concentration > 6.0 mg/dL at the time of initial examination. This may have reflected a longer duration of obstruction and greater compromise to the integrity of the bladder wall. In addition, these cats may have been more likely to have postobstructive diuresis, leading to more rapid distention of the bladder following cystocentesis. There were, however, 3 cats in which treatment was successful that had SUN and serum creatinine concentrations higher than these values, including 1 cat that still had severe azotemia at the time of hospital discharge (SUN concentration > 140 mg/dL and serum creatinine concentration of 8.2 mg/dL) but recovered without complications and did not have recurrences of UO. Unfortunately, there were not enough patients in the study to perform regression analysis to determine whether specific values of SUN or serum creatinine concentration could be used to predict the likelihood of success. Nevertheless, clients should be made aware of the greater risk of complications and lower chance of success in patients with severe azotemia when this protocol is used as an alternative to euthanasia.
Another potential concern related to the protocol used in the present study was whether cats would have a substantial risk for reobstruction, given that the lack of urethral catheterization meant that there was not a sustained conduit for egress of any further debris, mucous, or clots that might have resulted in urethral plugging. Even if euthanasia were the only other alternative, frequent reobstruction might have precluded the use of this protocol. However, cats in which treatment was successful in the present study had no episodes of reobstruction within 3 days after hospital discharge. In contrast, the reported rate of reobstruction after catheter removal is 14%.1 Given the limited number of cases in the present study, no conclusions can be drawn. However, it is possible that less urethral injury and inflammation occurred in these cats because of the lack of urethral catheterization. Only 2 cats in the present study had a recurrence within 3 weeks after hospital discharge (although 2 cats were lost to follow-up at that time), and there were no further episodes of UO in the 7 cats for which the owners could be contacted 1 year after discharge. Compared with reported recurrence rates of 35% and 36% following conventional management,12,26 this suggests that the treatment protocol used in the present study was not associated with a greater risk of recurrence.
There were several limitations to the present study. In particular, the small sample size prevented accurate estimation of the short- and long-term efficacy of this protocol and made it impossible to identify factors that could be used to predict outcome or identify cats in which this protocol should not be considered. Further, because the treatment protocol involved multiple components, we were unable to determine which aspects of treatment (ie, sedation, analgesia, cystocentesis, and low-stress environment) played a role in having a successful outcome.
Finally, findings of the present study lend support to the hypothesis that a substantial portion of the obstructive process in male cats with UO is functional in nature (ie, a result of urethral spasm and edema), rather than physical (ie, a result of a urethral plug or calculi). The protocol used in the present study could allow for treatment of UO at a reduced cost, compared with conventional management, thereby serving as an alternative to euthanasia owing to financial constraints. However, no direct comparison to conventional management was made, so this protocol cannot be recommended as an alternative to conventional management at this time. Further investigation, including a prospective comparison, is warranted to determine the optimal use of this protocol.
Abbreviations
- TP
Total protein
- UO
Urethral obstruction
Footnotes
Critical Care Xpress, Nova Biomedical, Waltham, Mass.
Clinical Refractometer, Jorgensen Laboratories Inc, Loveland, Colo.
GraphPad Prism, version 4.00 for Windows, GraphPad Software, San Diego, Calif.
Presented in abstract form at the International Veterinary Emergency and Critical Care Symposium, San Antonio, Tex, September 2008.
References
- 1.Lee JA, Drobatz KJ. Characterization of the clinical characteristics, electrolytes, acid-base, and renal parameters in male cats with urethral obstruction. J Vet Emerg Crit Care. 2003;13:227–233. [Google Scholar]
- 2.Osborne CA, Kruger JM, Lulich JP, et al. Medical management of feline urethral obstruction. Vet Clin North Am Small Anim Pract. 1996;26:483–498. doi: 10.1016/s0195-5616(96)50079-3. [DOI] [PubMed] [Google Scholar]
- 3.Rieser TM. Urinary tract emergencies. Vet Clin North Am Small Anim Pract. 2005;35:359–373. doi: 10.1016/j.cvsm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Lee JA, Drobatz KJ. Historical and physical parameters as predictors of severe hyperkalemia. J Vet Emerg Crit Care. 2006;16:104–111. [Google Scholar]
- 5.Burrows CF, Bovée KC. Characterization and treatment of acid-base and renal defects due to urethral obstruction in cats. J Am Vet Med Assoc. 1978;172:801–805. [PubMed] [Google Scholar]
- 6.Finco DR, Cornelius LM. Characterization and treatment of water, electrolyte, and acid-base imbalances of induced urethral obstruction in the cat. Am J Vet Res. 1977;38:823–830. [PubMed] [Google Scholar]
- 7.Osborne CA, Lees GE, Polzin DJ, et al. Immediate relief of feline urethral obstruction. Vet Clin North Am Small Anim Pract. 1984;14:599–608. doi: 10.1016/s0195-5616(84)50064-3. [DOI] [PubMed] [Google Scholar]
- 8.Bartges JW, Finco DR, Polzin DJ, et al. Pathophysiology of urethral obstruction. Vet Clin North Am Small Anim Pract. 1996;26:255–264. [PubMed] [Google Scholar]
- 9.Lulich JP, Osborne CA. Overview of diagnosis of feline lower urinary tract disorders. Vet Clin North Am Small Anim Pract. 1996;26:339–348. [PubMed] [Google Scholar]
- 10.Kruger JM, Osborne CA, Goyal SM, et al. Clinical evaluation of cats with lower urinary tract disease. J Am Vet Med Assoc. 1991;199:211–216. [PubMed] [Google Scholar]
- 11.Barsanti JA, Brown J, Marks A, et al. Relationship of lower urinary tract signs to seropositivity for feline immunodeficiency virus in cats. J Vet Intern Med. 1996;10:34–38. doi: 10.1111/j.1939-1676.1996.tb02021.x. [DOI] [PubMed] [Google Scholar]
- 12.Gerber B, Eichenberger S, Reusch CE. Guarded long-term prognosis in male cats with urethral obstruction. J Feline Med Surg. 2008;10:16–23. doi: 10.1016/j.jfms.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Straeter-Knowlen IM, Marks SL, Rishniw M, et al. Urethral pressure response to smooth and skeletal muscle relaxants in anesthetized, adult male cats with naturally acquired urethral obstruction. Am J Vet Res. 1995;56:919–923. [PubMed] [Google Scholar]
- 14.Smith CW, Schiller AG, Smith AR, et al. Effects of indwelling urinary catheters in male cats. J Am Anim Hosp Assoc. 1981;17:427–433. [Google Scholar]
- 15.Corgozinho KB, de Souza HJ, Pereira AN, et al. Catheter-induced urethral trauma in cats with urethral obstruction. J Feline Med Surg. 2007;9:481–486. doi: 10.1016/j.jfms.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lees GE, Osborne CA. Use and misuse of indwelling urinary catheters in cats. Vet Clin North Am Small Anim Pract. 1984;14:599–608. doi: 10.1016/s0195-5616(84)50065-5. [DOI] [PubMed] [Google Scholar]
- 17.Buffington CAT, Westropp JL, Chew DJ, et al. Clinical evaluation of multimodal environmental modification (MAMO) in the management of cats with idiopathic cystitis. J Feline Med Surg. 2006;8:261–268. doi: 10.1016/j.jfms.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westropp JL, Buffington CAT. Feline idiopathic cystitis: current understanding of pathophysiology and management. Vet Clin Small Anim Pract. 2004;34:1043–1055. doi: 10.1016/j.cvsm.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Westropp JL, Kass PH, Buffington CAT. Evaluation of the effects of stress in cats with idiopathic cystitis. Am J Vet Res. 2006;67:731–736. doi: 10.2460/ajvr.67.4.731. [DOI] [PubMed] [Google Scholar]
- 20.Achar E, Achar RA, Paiva TB, et al. Amitriptyline eliminates calculi through urinary tract smooth muscle relaxation. Kidney Int. 2003;64:1356–1364. doi: 10.1046/j.1523-1755.2003.00222.x. [DOI] [PubMed] [Google Scholar]
- 21.Marks SL, Straeter-Knowlen IM, Moore M, et al. Effects of acepromazine maleate and phenoxybenzamine on urethral pressure profiles of anesthetized, healthy, sexually intact male cats. Am J Vet Res. 1996;57:1497–1500. [PubMed] [Google Scholar]
- 22.Westropp J, Buffington CAT, Chew DJ. Feline lower urinary tract diseases. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine. St Louis: Elsevier-Saunders; 2005. pp. 1828–1850. [Google Scholar]
- 23.Westropp JL, Kass PH, Buffington CAT. In vivo evaluation of α2-adrenoceptors in cats with idiopathic cystitis. Am J Vet Res. 2007;68:203–207. doi: 10.2460/ajvr.68.2.203. [DOI] [PubMed] [Google Scholar]
- 24.Buffington CAT, Pacack K. Increased plasma norepinephrine concentration in cats with interstitial cystitis. J Urol. 2001;165:2051–2054. doi: 10.1097/00005392-200106000-00068. [DOI] [PubMed] [Google Scholar]
- 25.Kruger JM, Osborne CA, Ulrich LK. Cystocentesis. Vet Clin North Am Small Anim Pract. 1996;26:353–361. doi: 10.1016/s0195-5616(96)50215-9. [DOI] [PubMed] [Google Scholar]
- 26.Bovée KC, Reif JS, Maguire TG, et al. Recurrence of feline urethral obstruction. J Am Vet Med Assoc. 1979;174:93–96. [PubMed] [Google Scholar]


