Introduction
Type 2 diabetes has become an enormous public health burden, making diabetes prevention a pressing issue. While lifestyle modification is the most effective preventive strategy, it is resource intensive and not universally sustainable. We review the evidence on pharmacological options for diabetes prevention, in search for a medication that is efficacious, easy to adhere to, well tolerated, and cost-effective. With the exception of metformin, most other drugs have either limited efficacy or costly side effects.
The burden of type 2 diabetes
Diabetes has emerged as one of the most burdensome chronic diseases and is increasing in alarming proportions in the US and worldwide. In 2012, the total number of people with diabetes in the US was estimated to be 29.1 million people or 9.3% of the population (1). This number is predicted to double or triple by 2050 with 1 in 3 to 1 in 5 people estimated to have diabetes by that time (2). In addition to numerous complications involving the eyes, kidneys and nerves, individuals with diabetes are at increased risk for cardiovascular disease, peripheral vascular disease and stroke. Also, diabetes is not just a significant cause of morbidity and mortality; it is also a costly disease. In 2012 in the US, direct medical costs as well as costs due to disability and work loss from diabetes were estimated at 245 billion dollars. Pre-diabetes is the term used for individuals who are at high risk for future development of diabetes and includes individuals with elevated blood sugars that do not meet the diagnostic cutoff for diabetes, such as those with impaired fasting glucose (fasting glucose of 100 to 125 mg/dl or 5.6 to 6.9 mmol/l), or impaired glucose tolerance (2 hour glucose on the 75 g oral glucose tolerance test of 140 to 199 mg/dl or 7.8 to 11.0 mmol/l). There is increasing evidence to show that individuals with pre-diabetes are also at increased risk for cardiovascular disease independent of associated risk factors (3). Between 2009 and 2012, over one third of U.S. adults and over half of adults aged 65 years and older had pre-diabetes (1).
Given the expected burden of these comorbidities, diabetes prevention seems to be a naturally pressing issue. While clinical trials have convincingly demonstrated that diabetes can be delayed in some individuals, it is less clear how this should be done or whether the benefits are sustained. The goals of treatment include prevention or delay of the onset of diabetes but also critically reduction in the risk of long-term microvascular and cardiovascular complications. Preservation of beta-cell function is also essential, given its fundamental role in the pathogenesis of diabetes. Also, the question arises as to whether we are in essence attempting to lower the treatment threshold for diabetes by introducing therapeutics in prevention at the pre-diabetes stage. Various therapeutic options have been trialed for diabetes prevention in the recent past, with few drugs satisfactorily meeting the mark in terms of sustained effectiveness, low cost and long-term benefits on cardiovascular outcomes.
Lifestyle intervention is the most effective strategy to prevent or delay type 2 diabetes
Several well-designed randomized clinical trials have demonstrated that lifestyle intervention aimed at weight loss is effective at preventing or delaying the onset of diabetes (Figure 1, Table 1). Furthermore the benefits of lifestyle intervention appear to be sustained. Early trials including the Da Qing trial in China and the Finnish Diabetes Prevention Program demonstrated that lifestyle intervention was effective at preventing the onset of diabetes when compared tocontrols.. Furthermore, these trials indicated that the effects of lifestyle intervention appeared to persist after the studies were discontinued with a lower cumulative incidence of type 2 diabetes in the participants originally assigned to lifestyle intervention, though higher than the rates observed during theactive interventions.
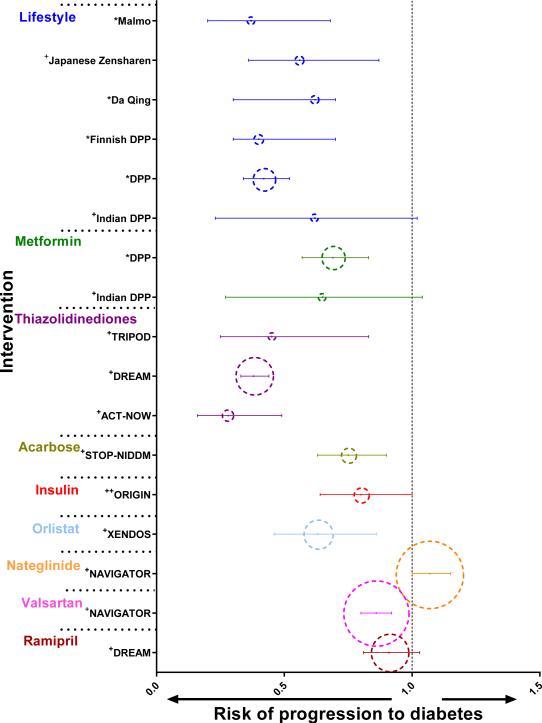
Figure 1. Risk of progression to diabetes compared to controls for various interventions that have been tested for diabetes prevention.
This figure illustrates the risk of progression to diabetes compared to controls with corresponding 95% confidence intervals of various studies grouped by intervention. * denotes relative risk ratio; + denotes hazard ratio; ++ denotes odds ratio. In each group, the studies are listed in chronological order according to the years in which the original interventions were conducted. The dotted circles for each study have been scaled to be proportional to the sample size of the study.
Table 1.
Interventions that have been tested in clinical trials for diabetes prevention
| Intervention | Study | Sample size for intervention of interest | Average study duration (years) | Average age of participants (years) | Average BMI of participants (kg/m2) | Risk of progression to diabetes compared to controls (95 % CI) |
|---|---|---|---|---|---|---|
| Lifestyle | Malmo feasibility study | 260 | 6 | 48 | 26.7 | 0.37(0.2-0.68)* |
| Japanese Zensharen Study for Prevention of Lifestyle Diseases | 458 | 3 | 49 | 23.9 | 0.56 (0.36-0.87)+ | |
| China Da Qing Diabetes Prevention study | 259 | 6 | 45 | 26.3 | 0.62 (0.3-0.7)* | |
| Finnish DPP | 522 | 3.2 | 55 | 31.2 | 0.40 (0.30-0.70)* | |
| DPP | 2161 | 2.8 | 51 | 34.0 | 0.42 (0.34-0.52)* | |
| Indian DPP | 269 | 2.5 | 46 | 25.8 | 0.62 (0.23-1.02)+ | |
| Metformin | DPP | 2155 | 2.8 | 51 | 34.0 | 0.69 (0.57-0.83)* |
| Indian DPP | 269 | 2.5 | 46 | 25.8 | 0.65 (0.27-1.04)+ | |
| Troglitazone | TRIPOD | 266 | 2.5 | 35 | 30.5 | 0.45 (0.25-0.83)+ |
| Rosiglitazone | DREAM | 5269 | 3 | 55 | 30.9 | 0.38 (0.33-0.44)+ |
| Pioglitazone | ACT-NOW | 602 | 2.4 | 52.3 | 33.8 | 0.28 (0.16-0.49)+ |
| Acarbose | STOP-NIDDM | 1429 | 3.3 | 54 | 31.0 | 0.75 (0.63-0.90)+ |
| Insulin | ORIGIN | 1452 | 6.2 | 63.5 | 29.9 | 0.80 (0.64-1.00)++ |
| Orlistat | XENDOS | 3305 | 4 | 43 | 37.4 | 0.63 (0.46-0.83)+ |
| Nateglinide | NAVIGATOR | 9518 | 6.5 | 63.8 | 30.5 | 1.07 (1.0-1.15)+ |
| Valsartan | NAVIGATOR | 9306 | 5 | 63.8 | 30.5 | 0.86(0.80-0.92)+ |
| Ramipril | DREAM | 5269 | 3.0 | 55 | 30.9 | 0.91(0.81-1.03)+ |
denotes relative risk ratio
denotes hazard ratio
denotes odds ratio
The Diabetes Prevention Program (DPP) was a landmark clinical trial in the United States and the first multi-ethnic randomized control trial involving adults with pre-diabetes. In this study, 3,234 participants at high risk for the development of diabetes were randomly assigned to a metformin arm, an intensive lifestyle intervention arm or a placebo arm. The study initially included a fourth intervention arm with troglitazone, which had to be discontinued because of liver toxicity concerns. The lifestyle intervention program was intensive with the goals of at least 7% weight loss and at least 150 minutes of physical activity per week. The average period of follow up in the study was 2.8 years. The results showed that lifestyle intervention reduced the incidence of diabetes by 58% (95% CI, 48% - 66%) and metformin by 31% (95% CI, 17% - 43%) as compared with placebo. The incidence of diabetes was 39% lower (95% CI, 24% -51%) in the lifestyle group than in the metformin group demonstrating that although both metformin and lifestyle intervention were more effective than placebo in preventing diabetes, the effects of lifestyle intervention were significantly better than metformin. Lifestyle intervention was shown to be effective in all age groups and in both men and women but was significantly more effective in older participants (4).
The Diabetes Prevention Program Outcomes Study (DPPOS) investigated the persistence of these effects in the long term with a 10-year follow-up study in 88% of the original participants. Based on the benefits from the intensive lifestyle intervention seen in the DPP, all three groups were offered group implemented lifestyle interventions. The original metformin group was continued on metformin and the original lifestyle intervention group was offered additional lifestyle support. The incidence of diabetes in the 10-year period after DPP randomization was reduced by 34% (95% CI, 24% –42%) in the lifestyle group and by 18% (95% CI, 7%–28%) in the metformin group compared with placebo. Overall, the incidence of diabetes in the former placebo and metformin groups fell to equal the rates in the former lifestyle group, but the cumulative incidence of diabetes still remained lowest in the lifestyle group. These results indicate that prevention of diabetes with both lifestyle intervention and metformin could persist for at least 10 years (5).
Similarly the Indian Diabetes Prevention program compared lifestyle intervention and metformin use with placebo in the Asian Indian population, a seemingly high-risk population with a younger age of onset of diabetes at relatively lower body mass indices, with high rates of insulin resistance and lower thresholds for the risk factors for diabetes. The results of the study showed that lifestyle intervention and metformin were both effective at preventing the onset of diabetes with lifestyle intervention being more effective than metformin (6).
Therefore lifestyle intervention targeting weight loss has proven to be effective in preventing diabetes across varying age groups and ethnicities. Additionally, it seems to have sustained effects and very few side effects, undoubtedly making it a highly promising strategy for diabetes prevention. However, the putative benefit of lifestyle intervention on microvascular and macrovascular outcomes, which are ultimately the critical health-related endpoints, still remain in question. Look AHEAD (Action for Health in Diabetes) was a multi-center clinical trial conducted in the US designed to examine the effect of intensive lifestyle intervention on cardiovascular outcomes. In this study subjectswith type 2 diabetes were randomly assigned to receive either intensive lifestyle intervention or standardcare .. The results showed that there was no significant difference in the incidence of cardiovascular outcomes between the two groups (HR 0.95; 95% confidence interval, 0.83 to 1.09; p=0.51) (7). Nonetheless, Look AHEAD demonstrated that the positive effects of lifestyle intervention extended beyond glycemic control. Intensive lifestyle intervention caused weight loss, improvement in cardiovascular risk factors including lipid parameters and blood pressure (with concomitant reduction in related medications), and many non cardiac benefits including improvements in quality of life and well being, fitness, sleep apnea, physical mobility, depression and sexual functioning.
However, lifestyle interventions as done in the DPP and Look AHEAD studies are resource intensive, and implementation may not be feasible across all levels in a real world setting. Also, intensive lifestyle changes may not be sustainable for everyone leading to reduced adherence, and do not work for everyone as shown by the diabetes incidence rates in the DPP and other trials. Although an analysis of costs in the DPP did demonstrate that lifestyle intervention was extremely cost-effective compared to placebo (8), our current healthcare system is not set up to regularly reimburse for individualized lifestyle intervention programs in the full population at risk. With all these challenges of intensive lifestyle intervention, adherence to a pill, if effective, cheap and free of side effects, might be easier to achieve.
So far, lifestyle intervention has demonstrated the greatest effectiveness in diabetes prevention with the fewest side effects, and can therefore be considered as setting the benchmark in diabetes prevention. In evaluating therapeutic options for diabetes prevention, our standards should be to achieve results that are at the very least on par with lifestyle intervention, if not better.
Pharmacological options for diabetes prevention: Is there an “exercise pill”?
Over the years, several drug classes have been considered as potential options for diabetes prevention and have been tested in clinical trials (Figure 1, Table 1). Of these drugs, not many can be seen in a positive light for the prevention of diabetes, based on demonstrating only modest effectiveness or because of side effects that outweigh the benefits of prevention. There is also a dearth of long-term follow-up data on most drugs questioning the persistence of effects. When interpreting the results from different trials, readers should recognize that varying subject characteristics can influence diabetes incidence rates.
Metformin
Metformin is a biguanide which acts by decreasing hepatic glucose output by inhibiting gluconeogenesis. This is done by altering the energy metabolism in the cell, primarily by mitochondrial inhibition. The molecular mechanism of action of metformin is not completely understood but is thought to be mediated through activation of the enzyme 5’AMP kinase, which in addition to modulating insulin signaling is also thought to have positive downstream effects on lipid metabolism (9).
As described earlier, the DPP demonstrated that metformin at a dose of 850 mg twice daily was effective at preventing the onset of diabetes in participants with elevated fasting and post-load plasma glucose concentrations , although it was less effective than lifestyle intervention. The effects of metformin were similar in both men and women and across all racial and ethnic groups. In certain groups such as the younger, more obese and in women with a history of gestational diabetes, metformin was found to be relatively more effective. The most common side effects reported in the study were gastrointestinal and included diarrhea, flatulence, nausea and vomiting, which were significantly increased in the metformin group compared to placebo, with no serious adverse effects attributed to metformin treatment (4). The DPPOS also demonstrated that long-term metformin use was associated with a small increase in the prevalence of vitamin B12 deficiency, although not accompanied by increases in the prevalence of anemia. Along similar lines, the Indian DPP demonstrated similar results for metformin treatment in the Asian Indian population. The study also demonstrated that there was no added benefit of combining lifestyle intervention to metformin, suggesting that both interventions might exert their effect on overlapping pathways. (6).
It is possible that the reduced incidence rates of diabetes in the metformin arm of the studies described could be attributed to the therapeutic effects of metformin itself on newly developed diabetes, thereby masking its onset. Thus, to address this question and to verify whether participants truly remained diabetes-free as a result of metformin treatment, the DPP conducted a follow up study by obtaining glucose measurements after an appropriate washout period. In this study, to test whether the observed effect of metformin on the development of diabetes persisted after metformin was withdrawn, 1,274 participants who did not develop diabetes at the end of the study period participated in a washout study in which a repeat oral glucose tolerance test was done after discontinuing metformin for 1 to 2 weeks. After the washout period, the incidence of diabetes increased from the original study but was still reduced by 25% in the participants. When the diabetes conversion rates during the DPP and the washout period were combined, the odds of developing diabetes was still reduced by 25% in the metformin group when compared to placebo (OR, 0.75; 95% CI, 0.62–0.92; p=0.005). The results demonstrated that only around 25% of this effect could be accounted for by an acute pharmacological effect of metformin that did not persist when the drug was stopped; thereby postulating that the benefit obtained from metformin towards the prevention of diabetes was not transient. The washout period was short but a longer washout period could not be performed as it could be considered adverse to the well being of the participants (10).
In a much smaller study of 40 Finnish participants with impaired glucose tolerance who were first-degree relatives of patients with diabetes mellitus, with 20 participants on 500 mg twice daily of metformin and 20 on placebo, metformin therapy resulted in an improvement in glucose tolerance that lasted for up to 12 months after cessation of treatment (11). Although these results are promising, studies with larger sample sizes and longer drug-free periods are needed to conclude that metformin has a sustained effect on diabetes prevention.
Overall, it can be seen that metformin is effective in preventing or delaying diabetes, perhaps with sustained effects, and has a good safety profile. In addition to effects on glucose metabolism, metformin also causes modest improvement in weight and positive changes on lipid metabolism altogether making it a favorable choice for the prevention of diabetes. A cost-effectiveness analysis over 10 years using data from the both the DPP and the DPPOS showed that though lifestyle intervention was cost-effective compared with placebo, metformin was actually cost-saving (8), proving that intervening in high risk individuals represented a good value for the money spent.
Thiazolidinediones
The thiazolidinediones are a group of drugs that act primarily by increasing hepatic and peripheral insulin sensitivity and by preserving insulin secretion. Thiazolidinediones are thought to act by binding and activating the nuclear transcription factor PPAR – γ (peroxisome proliferator activated receptor - gamma), thereby regulating transcription of several genes involved in glucose and lipid metabolism (12). Pioglitazone also exerts some PPAR-α effects that might account for some differences in pharmacological effects compared to the other thiazolidinediones. Thiazolidinediones have been found to be effective in delaying the onset of diabetes but at the cost of significant side effects.
Troglitazone
Troglitazone has been withdrawn from the market in the US because of concerns related to severe hepatic injury leading to need for liver transplantation or causing death. Troglitazone was originally used in one of the intervention arms of the DPP but was discontinued for safety reasons after these liver toxicity concerns arose. On analysis of effects, it was found that during the mean 0.9 years of troglitazone treatment the diabetes incidence rate was less than in the other arms. The incidence rate was 3.0 cases per 100 person-years, compared with 12.0, 6.7, and 5.1 cases per 100 person-years in the placebo, metformin, and lifestyle intervention arms (p<0.001, troglitazone vs. placebo; p=0.02, troglitazone vs. metformin; p=0.18, troglitazone vs. lifestyle). In follow up during the 3 years after troglitazone withdrawal, the diabetes incidence rate was almost identical to that of the placebo group indicating that during its limited period of use, the action of reducing diabetes incidence did not persist (13).
Another clinical trial using troglitazone was the TRIPOD (Troglitazone in Prevention of Diabetes) trial in which 266 Hispanic women with a history of gestational diabetes were randomized to receive either placebo or 400 mg per day of troglitazone. During a median follow-up of 30 months, the average annual diabetes incidence rates were 12.1% and 5.4% in the placebo and troglitazone groups respectively (p<0.01) (14). We do not have long term follow up data to indicate whether the effects persisted.
Rosiglitazone
Rosiglitazone was tested for diabetes prevention in the DREAM (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication) trial in which 5269 adults with either impaired fasting glucose or impaired glucose tolerance, or both, and no previous history of cardiovascular disease were randomly assigned to receive either 8 mg of rosiglitazone daily or placebo. The groups were followed for a median of 3 years and the incidence of diabetes was reduced in the rosiglitazone group at 10.6% versus 25.0% in the placebo group (p<0.0001). While the trial did explicitly exclude individuals with previously diagnosed cardiovascular disease, new cardiovascular event rates were similar between the two groups. However, there were more cases of heart failure in the rosiglitazone group (15). In a follow-up study, the 3366 DREAM subjects at the end of the trial who had not developed diabetes were transferred to a single-blind placebo study to induce washout for 2 to 3 months. Diabetes incidence during the washout phase alone was identical in those previously allocated to either rosiglitazone or placebo, demonstrating that the beneficial effect of rosiglitazone did not appear to be sustained (16).
In 2010, based on data from a meta-analysis of placebo-controlled randomized trials that suggested an elevated risk of cardiovascular events in association with rosiglitazone use, the FDA restricted its use to patients who could not control their diabetes on other medications. In 2013, this restriction was lifted based on reevaluation of the RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycemia in Diabetes) trial that did not demonstrate any difference in the composite primary endpoint of cardiovascular death or hospitalization in patients on rosiglitazone.
Pioglitazone
Pioglitazone has also been tested for diabetes prevention. The ACT-NOW (Actos Now for Prevention of Diabetes) trial had 602 patients with impaired glucose tolerance who were randomly assigned to receive either 30 to 45 mg daily of pioglitazone or placebo. After a median follow-up period of 2.4 years, the annual incidence rates of diabetes were lower at 2.1% in the pioglitazone group compared to 7.6% in the placebo group, and the hazard ratio for conversion to diabetes in the pioglitazone group was 0.28 (95% CI, 0.16 - 0.49; p<0.001). Pioglitazone treatment was also associated with lower diastolic blood pressure, higher levels of HDL cholesterol, and reduced rates of carotid intima–media thickening when compared to placebo. However, pioglitazone treatment was associated with significant weight gain and edema. It is also significant to note that quite a large number of participants in this study, 24% in the placebo group and 30% in the pioglitazone group were lost to follow up (17).
The side effects associated with all thiazolidinediones include weight gain, fluid retention, decrease in bone density and increase in fracture risk, particularly in women, and increased risk of heart failure. As mentioned earlier, troglitazone has been associated with fatal hepatotoxicity and pioglitazone with bladder cancer.
Alpha- glucosidase inhibitors
These drugs act by competitively inhibiting alpha-glucosidases, which are enzymes at the brush border of the small intestine that break down complex polysaccharides into monosaccharides. These drugs slow the absorption of glucose and blunt the post prandial rise in blood glucose levels. In addition, these drugs also appear to have an effect on increasing insulin sensitivity (18). Acarbose and voglibose are two drugs in this class that have been tested for diabetes prevention.
Acarbose
The effect of acarbose on diabetes prevention was tested in STOP-NIDDM (Study To Prevent Non-Insulin-Dependent Diabetes Mellitus), which was a multicenter trial in which 1,429 participants with impaired glucose tolerance were randomly assigned to receive either 100 mg of acarbose three times a day or placebo with a mean follow up of 3.3 years. There was a lower rate of progression to diabetes in the acarbose arm with 32% of patients developing diabetes compared to 42% in the placebo arm (relative hazard ratio, 0.75; 95% CI, 0.63–0.90; p=0.0015). There was a high rate of gastrointestinal side effects in the acarbose arm with 83% of participants reporting symptoms, most commonly flatulence and diarrhea. This likely contributed to the high discontinuation rate of 19% in the acarbose group compared to 5% in the placebo arm (19).
Voglibose
Voglibose, another alpha glucosidase inhibitor, demonstrated reduction in the development of type 2 diabetes in high-risk Japanese individuals with impaired glucose tolerance in addition to lifestyle modification (20). However, voglibose is not available in the US.
Insulin
Conventionally, insulin has been used in the treatment of diabetes that is not controlled on oral antihyperglycemic agents. However, as beta-cell function and preservation are now known to be important in preventing diabetes, insulin has been considered for this use. There are very few studies looking at insulin treatment for diabetes prevention.
In the ORIGIN trial, 12,537 adults with cardiovascular risk factors plus impaired fasting glucose, impaired glucose tolerance, or type 2 diabetes were randomly assigned to receive either long acting insulin glargine or standard care with a median follow up of 6 years. In the 1,456 participants without diabetes at baseline, new onset diabetes was diagnosed in 30% in the insulin glargine group versus 35% in the standard care group (OR, 0.80; 95% CI, 0.64-1.00; p=0.05). There were no differences in the rates of incident cardiovascular outcomes. Insulin glargine was associated with more episodes of severe hypoglycemia and increased weight in comparison to standard care (21). Overall, based on the risk for hypoglycemia, potential to cause weight gain and lack of benefit on cardiovascular outcomes, a favorable argument cannot be made for insulin therapy in the treatment of pre-diabetes.
Orlistat
Orlistat is a weight loss drug that acts by altering the digestion of fat by inhibiting pancreatic lipases. Since obesity and type 2 diabetes are closely linked, orlistat has been tested for the prevention of diabetes. As part of a prospective study, 3,305 obese persons with normal or impaired glucose tolerance were randomized to receive lifestyle changes in addition to either 120 mg of orlistat three times daily or placebo. After 4 years of treatment, the cumulative incidence of diabetes was higher at 9.0% in the placebo group compared to 6.2% in the orlistat group, corresponding to a risk reduction of 37.3% (p=0.0032). Exploratory analyses by the study group indicated that the preventive effect of orlistat on the development of diabetes could be explained by the beneficial effect observed in the subjects with impaired glucose tolerance. The majority of patients on orlistat had gastrointestinal side effects that were more pronounced in the early phase of treatment. This study had a high withdrawal rate similar to many obesity related studies, with only 52% of subjects receiving orlistat and 34% receiving placebo completing treatment (22).
Nateglinide
Nateglinide, a meglitinide, increases insulin secretion in a glucose-dependent fashion by regulating ATP-dependent potassium channels on the beta-cell membrane. Its mechanism of action is similar to that of sulfonylureas, but the meglitinides are structurally different and do not bind the same receptors (23). In the NAVIGATOR (Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research) trial, 9,306 participants with impaired glucose tolerance and either cardiovascular disease or cardiovascular risk factors were randomly assigned to receive 60 mg three times daily of nateglinide or placebo, and in a 2×2 factorial design were assigned to valsartan or placebo. In addition, all subjects participated in a lifestyle modification program. After a median follow up of 5 years, nateglinide did not significantly reduce the cumulative incidence of diabetes or cardiovascular outcomes, but did increase the risk of hypoglycemia when compared to placebo. The rates of diabetes incidence were 36% and 34% in the nateglinide and placebo arms respectively. (HR, 1.07; 95% CI, 1.00-1.15; p=0.05) (24). Overall, there is lack of proven efficacy for the use of nateglinide in diabetes prevention.
Valsartan
Possible interactions are thought to exist between renin-angiotensin system blockade and glucose metabolism. Some potential mechanisms explaining this effect include impairment of insulin signaling by the action of angiotensin II, vasodilation in the skeletal muscle improving insulin sensitivity and a decrease in insulin resistance by an increase in circulating adiponectin levels, though the exact mechanism by which this occurs is unclear (25). The NAVIGATOR trial also tested Valsartan, a direct angiotensin II receptor blocker, for the prevention of diabetes. After a median follow-up period of 5 years, the cumulative incidence of diabetes was lower at 33.1% in the valsartan group, as compared with 36.8% in the placebo group ( HR, 0.86; 95% CI, 0.80-0.92; p<0.001). However, when compared to placebo, valsartan did not significantly reduce the incidence of cardiovascular outcomes (26).
Ramipril
Ramipril, an angiotensin converting enzyme inhibitor, has also been evaluated for the purpose of diabetes prevention. The DREAM trial, described above in reference to rosiglitazone, also evaluated the use of ramipril in the prevention of diabetes. In a 2×2 factorial design, the 5,269 participants without cardiovascular disease but with impaired fasting glucose levels or impaired glucose tolerance were randomly assigned to receive 15 mg a day of ramipril or placebo. After a median follow-up period of 3 years, the incidence of diabetes did not differ significantly between the groups at 17.1% and 18.5% in the ramipril and placebo groups respectively (HR, 0.91 (0.80–1.03); p=0.15). Fasting glucose levels were not different between the 2 groups but the 2 hour OGTT levels were lower in the ramipril group, suggesting some positive effects on glucose metabolism (27).
Overall, it can be said that the effects of angiotensin inhibition on glucose regulation are at best modest and not convincing enough for use in diabetes prevention.
Newer drugs for prevention of diabetes
Emerging therapeutic agents are being trialed in combination therapy for the treatment of uncontrolled type 2 diabetes. Although these drugs have not been specifically tested for use in diabetes prevention they have certain advantages that make them potential considerations for the future. The glucagon-like peptide-1 (GLP-1) receptor agonists mimic or enhance the actions of naturally occurring GLP-1, an incretin hormone that is released from the intestinal L cells in response to nutrients. GLP-1 is beneficial to glucose homeostasis by stimulating insulin secretion from pancreatic beta cells in a glucose dependent manner and by slowing gastric emptying, suppressing appetite and reducing inappropriate glucagon secretion. GLP-1 has also been shown to promote beta-cell proliferation and differentiation in animal models. (28) Another drug class in the incretin pathway includes dipeptidyl peptidase –4 (DPP-4) inhibitors; DPP-4 is an enzyme that inactivates GLP-1. GLP-1 receptor agonists have the benefit of promoting weight loss but have GI side effects and can only be used as injectable agents. Another newer class of drugs comprises the sodium-glucose cotransporter 2 (SGLT2) inhibitors. SGLT2 is expressed in the renal proximal tubule and SGLT2 inhibitors promote the renal excretion of glucose. (29) In addition to modest effects on improving glycemic control, these drugs have also been shown to promote weight loss and improvement in systolic blood pressure. However, side effects include predisposition to vulvovaginal candidiasis and urinary tract infections. Given the mechanism of action of these newer drugs, it is hard to imagine that they could eventually become the ‘exercise pill’ for diabetes prevention; however, by inducing weight loss and potentially improving beta-cell health they may have beneficial disease-modifying effects. Finally, long-term safety data for these drugs is lacking, even in the treatment of type 2 diabetes, and their high cost undermines the justification for their use in the context of disease prevention.
Diabetes prevention does not imply cardiovascular protection
While we know that some drugs have been shown to delay the onset of diabetes in certain individuals, there is a paucity of data on the effect of these drugs on the key outcomes of cardiovascular events or mortality. The impact of the DPP interventions on actual cardiovascular events has not been analyzed yet due to statistical power considerations, given the small number of events that have accrued in the study thus far. However in the short term, intensive lifestyle intervention was shown to beneficially modify known risk factors for cardiovascular disease including hypertension and lipid levels when compared to placebo and metformin therapy (30). Longer term analysis from the DPPOS showed that intensive lifestyle intervention achieved comparable effects on cardiovascular risk factors to that seen in the metformin and placebo groups, though favorably with lesser medication use (31). Further studies with relevant endpoints are needed to better elucidate the long term cardiovascular effects of these drugs.
The role of bariatric surgery in diabetes prevention
Bariatric surgical procedures, in particular Roux-en-Y Gastric Bypass, in addition to medical care, have been shown to treat diabetes in the short term. Surgery is a particularly relevant consideration in the severely obese who are at very high risk for the development of diabetes. The rapid improvement in metabolic control following surgery suggests endocrine mediated glycemic alterations that are not just related to weight loss, with involvement of GLP-1 implicated as a potential mechanism (32). The Swedish Obese Subjects study was a clinical trial to assess whether bariatric surgery can prevent diabetes in obese individuals. This study included 1,658 subjects who underwent bariatric surgery and 1,771 obese matched controls with none of the participants having diabetes at baseline. The subjects in the bariatric surgery cohort underwent procedures including banding (19%), vertical banded gastroplasty (69%), or gastric bypass (12%). During a median follow up period of 10 years, the incidence rates for diabetes were 28.4 cases per 1000 person-years in the control group and 6.8 cases per 1000 person-years in the bariatric surgery group (33). While these are promising results, there is the lack of long- term follow-up data and the risks and benefits of surgery have to be weighed carefully, especially in the context of diabetes prevention. Nevertheless, when recommended for another clinical indication in a pre-diabetic patient it is clear that bariatric surgery does have the additional benefit of diabetes prevention. Understanding the molecular and physiological mechanisms by which such a benefit is conferred may illuminate specific pathways that could be harnessed pharmacologically.
Diabetes prevention in children
Type 2 diabetes, once thought to be a disease of adulthood, is now increasing at an alarming rate in children, parallel to the rise in obesity. This was demonstrated in the SEARCH for Diabetes in Youth Study, a population-based study that was developed to assess the prevalence and incidence of diabetes in youth. The study estimated that the overall prevalence of type 2 diabetes was 0.34 per 1000 in 2001 and 0.46 per 1000 in 2009, representing a relative increase of 35% (34). As the risk of complications from diabetes increases with the duration of disease, children represent a population where preventative strategies seem most crucial. Metformin and insulin are currently the only two medications approved for the treatment of type 2 diabetes in children. Although there are no studies that have evaluated the use of medications in diabetes prevention in children, the TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) study deserves mention when discussing type 2 diabetes in children. TODAY was a multicenter trial in children with type 2 diabetes between the ages of 10 and 17 years. All participants were initially treated with 1000 mg twice a day of metformin to attain a glycated hemoglobin level of less than 8% and subsequently 699 children were randomized to continue treatment with metformin alone, metformin combined with 4 mg twice a day of rosiglitazone, or a lifestyle intervention program focusing on weight loss. The mean follow-up period for the study was 3.8 years and the primary outcome was loss of glycemic control. . The results of the study showed that metformin plus rosiglitazone was superior to metformin alone (p=0.006) and that metformin plus lifestyle intervention was not significantly different from metformin alone, with failure rates of 51.7%, 38.6% and 46.6% in the metformin, metformin plus rosiglitazone and metformin plus lifestyle intervention arms respectively (35).
With the limited evidence base that we have related to diabetes prevention in children, lifestyle intervention with the goal to prevent weight loss should be recommended to all children at risk for the development of type 2 diabetes. There is insufficient evidence and lack of long term safety data in children to recommend the use of metformin or other medications to prevent or delay the onset of diabetes.
What does the ADA recommend?
Based on current evidence, the American Diabetes Association (ADA) recommends that people at high risk for diabetes, including persons with impaired glucose tolerance, impaired fasting glucose, or a glycated hemoglobin of 5.7 to 6.4% be referred to a lifestyle intervention program. The goals of lifestyle intervention should be similar to those set in the DPP, targeting weight loss of 7% of body weight and moderate physical activity of at least 150 minutes a week. The guidelines also mention the importance of follow up counseling for treatment success. Based on its efficacy, low cost and safety, the ADA recommends considering metformin therapy in high-risk individuals and especially in individuals who showed the most benefit in the DPP. These were the more obese patients with a BMI greater than 35 kg/m2, the younger subjects less than 60 years of age, and women with a history of gestational diabetes. The ADA does not recommend the use of other drugs based on limited efficacy, side effects or cost (36).
We still have ways to go in our ability to prevent or delay the onset of diabetes. Though lifestyle intervention offers the most promise, large scale feasibility and adherence are major obstacles to contend with. With the exception of metformin, most of the existing drug classes tested for diabetes prevention have either demonstrated limited efficacy or costly side effects. At the end of the day, we still haven’t discovered the mythical ‘exercise pill’, a drug that would be effective, easy to adhere to and cost effective. So where do we go from here? In this rapidly evolving era of genetic advances and genomic medicine there is hope that we will have a better understanding of drug mechanisms and differential response to medications. Genome-wide association studies (GWAS) and large-scale sequencing studies have prompted an exciting ongoing research journey to unravel the genetic architecture of type 2 diabetes. Through genomic exploration we also hope to identify genes associated with favorable response to therapies and discover potential new drug targets. We aim to tailor therapies based on pharmacogenetics, or at least stratify the population into subgroups that are most likely to benefit from specific agents. A GWAS for lifestyle modification might help us identify genes associated with a favorable response to diet and/or exercise, so that this effective yet resource-intensive therapy can be targeted at the most benefiting populations. This is the hope that ‘precision medicine’ brings as an emerging approach that takes into account variability in genetic makeup, as well as the interactions between genes and the environment or lifestyle choices. Genetic advances might change the way we practice medicine and increase our ability to prevent burdensome diseases like type 2 diabetes.
Acknowledgments
S.S. is supported by NIH training grant T32-GM007748. We thank Ayesha Muhammad for her help with figure 1 as well as the other members of the Florez lab for helpful discussion and feedback.
Footnotes
Disclosures
J.C.F. has received consulting honoraria from Pfizer and PanGenX.
REFERENCES
- 1.CDC's National Diabetes Statistics Report 2014, derived from 2009–2012 National Health and Nutrition Examination Survey (NHANES) estimates applied to 2012 U.S. Census data [Google Scholar]
- 2.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Population Health Metrics. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Subclinical states of glucose intolerance and risk of death in the U.S. Diabetes Care. 2001;24:447–453. doi: 10.2337/diacare.24.3.447. [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England Journal of Medicine. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 7.Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. The New England Journal of Medicine. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012;35:723–730. doi: 10.2337/dc11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia. 2013;56:1898–1906. doi: 10.1007/s00125-013-2991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care. 2003;26:977–980. doi: 10.2337/diacare.26.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehtovirta M, Forsen B, Gullstrom M, Haggblom M, Eriksson JG, Taskinen MR, Groop L. Metabolic effects of metformin in patients with impaired glucose tolerance. Diabetic Medicine : A Journal of the British Diabetic Association. 2001;18:578–583. doi: 10.1046/j.1464-5491.2001.00539.x. [DOI] [PubMed] [Google Scholar]
- 12.Yki-Jarvinen H. Thiazolidinediones. The New England Journal of Medicine. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 13.Knowler WC, Hamman RF, Edelstein SL, Barrett-Connor E, Ehrmann DA, Walker EA, Fowler SE, Nathan DM, Kahn SE. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54:1150–1156. doi: 10.2337/diabetes.54.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high- risk hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 15.Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 16.Incidence of diabetes following ramipril or rosiglitazone withdrawal. Diabetes Care. 2011;34:1265–1269. doi: 10.2337/dc10-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Henry RR, Hodis HN, Kitabchi AE, Mack WJ, Mudaliar S, Ratner RE, Williams K, Stentz FB, Musi N, Reaven PD. Pioglitazone for diabetes prevention in impaired glucose tolerance. The New England Journal of Medicine. 2011;364:1104–1115. doi: 10.1056/NEJMoa1010949. [DOI] [PubMed] [Google Scholar]
- 18.Meneilly GS, Ryan EA, Radziuk J, Lau DC, Yale JF, Morais J, Chiasson JL, Rabasa-Lhoret R, Maheux P, Tessier D, Wolever T, Josse RG, Elahi D. Effect of acarbose on insulin sensitivity in elderly patients with diabetes. Diabetes Care. 2000;23:1162–1167. doi: 10.2337/diacare.23.8.1162. [DOI] [PubMed] [Google Scholar]
- 19.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 20.Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K. Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet. 2009;373:1607–1614. doi: 10.1016/S0140-6736(09)60222-1. [DOI] [PubMed] [Google Scholar]
- 21.Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, Ryden LE, Yusuf S. Basal insulin and cardiovascular and other outcomes in dysglycemia. The New England Journal of Medicine. 2012;367:319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 22.Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 23.Fuhlendorff J, Rorsman P, Kofod H, Brand CL, Rolin B, MacKay P, Shymko R, Carr RD. Stimulation of insulin release by repaglinide and glibenclamide involves both common and distinct processes. Diabetes. 1998;47:345–351. doi: 10.2337/diabetes.47.3.345. [DOI] [PubMed] [Google Scholar]
- 24.Holman RR, Haffner SM, McMurray JJ, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM, Chacra AR, Chiang FT, Charbonnel B, Chow CC, Davies MJ, Deedwania P, Diem P, Einhorn D, Fonseca V, Fulcher GR, Gaciong Z, Gaztambide S, Giles T, Horton E, Ilkova H, Jenssen T, Kahn SE, Krum H, Laakso M, Leiter LA, Levitt NS, Mareev V, Martinez F, Masson C, Mazzone T, Meaney E, Nesto R, Pan C, Prager R, Raptis SA, Rutten GE, Sandstroem H, Schaper F, Scheen A, Schmitz O, Sinay I, Soska V, Stender S, Tamas G, Tognoni G, Tuomilehto J, Villamil AS, Vozar J, Califf RM. Effect of nateglinide on the incidence of diabetes and cardiovascular events. The New England Journal of Medicine. 2010;362:1463–1476. doi: 10.1056/NEJMoa1001122. [DOI] [PubMed] [Google Scholar]
- 25.Vermes E, Ducharme A, Bourassa MG, Lessard M, White M, Tardif JC. Enalapril reduces the incidence of diabetes in patients with chronic heart failure: insight from the Studies Of Left Ventricular Dysfunction (SOLVD). Circulation. 2003;107:1291–1296. doi: 10.1161/01.cir.0000054611.89228.92. [DOI] [PubMed] [Google Scholar]
- 26.McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM, Chacra AR, Chiang FT, Charbonnel B, Chow CC, Davies MJ, Deedwania P, Diem P, Einhorn D, Fonseca V, Fulcher GR, Gaciong Z, Gaztambide S, Giles T, Horton E, Ilkova H, Jenssen T, Kahn SE, Krum H, Laakso M, Leiter LA, Levitt NS, Mareev V, Martinez F, Masson C, Mazzone T, Meaney E, Nesto R, Pan C, Prager R, Raptis SA, Rutten GE, Sandstroem H, Schaper F, Scheen A, Schmitz O, Sinay I, Soska V, Stender S, Tamas G, Tognoni G, Tuomilehto J, Villamil AS, Vozar J, Califf RM. Effect of valsartan on the incidence of diabetes and cardiovascular events. The New England Journal of Medicine. 2010;362:1477–1490. doi: 10.1056/NEJMoa1001121. [DOI] [PubMed] [Google Scholar]
- 27.Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, Dagenais G, Diaz R, Avezum A, Lanas F, Probstfield J, Fodor G, Holman RR. Effect of ramipril on the incidence of diabetes. The New England Journal of Medicine. 2006;355:1551–1562. doi: 10.1056/NEJMoa065061. [DOI] [PubMed] [Google Scholar]
- 28.Lee YS, Jun HS. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta- cells. Metabolism: Clinical and Experimental. 2014;63:9–19. doi: 10.1016/j.metabol.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Clar C, Gill JA, Court R, Waugh N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratner R, Goldberg R, Haffner S, Marcovina S, Orchard T, Fowler S, Temprosa M. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care. 2005;28:888–894. doi: 10.2337/diacare.28.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orchard TJ, Temprosa M, Barrett-Connor E, Fowler SE, Goldberg RB, Mather KJ, Marcovina SM, Montez M, Ratner RE, Saudek CD, Sherif H, Watson KE. Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabetic Medicine : A Journal of the British Diabetic Association. 2013;30:46–55. doi: 10.1111/j.1464-5491.2012.03750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhee NA, Vilsboll T, Knop FK. Current evidence for a role of GLP-1 in Roux-en-Y gastric bypass-induced remission of type 2 diabetes. Diabetes, Obesity & Metabolism. 2012;14:291–298. doi: 10.1111/j.1463-1326.2011.01505.x. [DOI] [PubMed] [Google Scholar]
- 33.Carlsson LM, Peltonen M, Ahlin S, Anveden A, Bouchard C, Carlsson B, Jacobson P, Lonroth H, Maglio C, Naslund I, Pirazzi C, Romeo S, Sjoholm K, Sjostrom E, Wedel H, Svensson PA, Sjostrom L. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. The New England Journal of Medicine. 2012;367:695–704. doi: 10.1056/NEJMoa1112082. [DOI] [PubMed] [Google Scholar]
- 34.Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, Bell R, Badaru A, Talton JW, Crume T, Liese AD, Merchant AT, Lawrence JM, Reynolds K, Dolan L, Liu LL, Hamman RF. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, Arslanian S, Cuttler L, Nathan DM, Tollefsen S, Wilfley D, Kaufman F. A clinical trial to maintain glycemic control in youth with type 2 diabetes. The New England Journal of Medicine. 2012;366:2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]