Abstract
With increasing age, the ability of the adaptive immune system to respond to vaccines and to protect from infection declines. In parallel, the production of inflammatory mediators increases. While cross-sectional studies have been successful in defining age-dependent immunological phenotypes, studies of accelerated immune aging in human subpopulations has been instrumental in obtaining mechanistic insights. The immune system depends on its regenerative capacity, however, the T cell repertoire, once established, is relatively robust to aging and only decompensates when additionally stressed. Such stressors include chronic infections such as CMV and HIV, even when viral replication is controlled, and autoimmune diseases. Reduced regenerative capacity, chronic immune activation in the absence of cell exhaustion, T cell memory inflation and accumulation of highly potent effector T cells in these patients synergize to develop an immune phenotype that is characteristic of the elderly. Studies of accelerated immune aging in autoimmune diseases have identified an unexpected link to chronic DNA damage responses that are known to be important in aging, but so far had not been implicated in immune aging.
Keywords: Immune aging, Thymic involution, HIV, CMV, Autoimmunity, Latency
Introduction
The immune system is a dynamic system that is highly dependent on the regenerative power of hematopoietic precursor cells and that is constantly challenged by external and internal forces threatening the homeostasis of the system. It is not surprising that the immune system undergoes dramatic changes with age. In particular, the adaptive immune system is affected because it relies on complicated selection mechanisms and it constantly has to find a balance between maintaining homeostasis and adapting to external stresses. Immune aging, therefore, includes the collective antigen experience of the organism stored as immune memory. At the same time, the major clinical manifestation of immune aging is an increased susceptibility to infections, both to new infections and also to some, but not all, chronic or latent infections. In immune aging research, it is, therefore, often difficult to decipher between adaptation such as memory formation and cell differentiation and defects such as cellular senescence. Frequently age-associated changes reflect a combination of both. Most studies rely on comparison between cohorts of different ages. While these can reliably identify differences, the mechanism leading to these differences and their implications for immune health are not very well understood. Animal models are only partially helpful to provide mechanistic insights. In addition to the usual challenge that the murine differs substantially from the human system and that the environmental forces shaping the immune system in different species are fundamentally different,1 animal models of immune aging face the additional challenge that for many questions it is not possible to telescope time. For example, it is not possible to extrapolate the consequences of thymic involution and homeostatic proliferation for cellular and organismal senescence from a species that has a lifespan of three years to humans living many decades.2 Longitudinal studies in humans are very difficult because most immune changes associated with aging develop slowly. Longtime studies are not only costly, but also cannot incorporate the rapid technological progress that is being made in developing new immunometric tools.3, 4 Human models of accelerated immune aging have been increasingly explored in the last years to get insights into the mechanisms and the consequences of the age-related changes in the human immune system. Studies of thymectomized individuals addressed the question what the true contribution of thymic involution is for some of the phenotypes of immune aging.5, 6 Is it truly necessary to have an active thymus after the age of 207, 8 or is the involution of the thymus evolutionary intended?9, 10 Latent CMV infection appears to accelerate immune aging.11, 12 What are the mechanisms and what is the difference to other chronic infections that either induce lymphocyte exhaustion rather than immune aging13 or that exhibit a fading T cell memory leading to reactivation of the latent viruses such as with varicella herpes zoster?14 HIV infection under HAART treatment has emerged as an interesting model to understand the influence of immune defects on morbidity and mortality of all causes.15 Finally, accelerated immune aging is seen in some of the autoimmune diseases.16-18 While it remains to be determined whether this accelerated immune aging is a cause or a consequence of the autoimmune disease, the elucidation of the mechanisms allows conclusions on common pathway of immune aging in the healthy adult.
Thymic Involution Accelerates Immune Aging
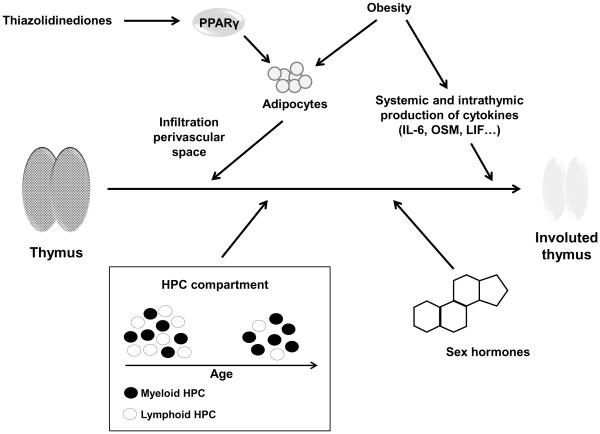
The thymus is known to undergo dramatic structural changes with age.19, 20 Relative thymic output is the highest during early life; absolute output starts to decline after puberty.21, 22 The structural changes are many-fold (Figure 1). Thymopoietic niches disappear, while the thymic perivascular space increases. These changes are associated with a loss of thymic epithelial cells and thymocytes, and the transformation of cells into adipocytes which infiltrate the perivascular space.23 Given that thymic T cell production essentially ceases after organism growth has been concluded, one could argue that thymic involution is not a typical marker of aging, but that thymic production is a developmental step that is no longer needed when the peripheral T cell compartment is seeded with naïve T lymphocytes.8 Indeed, even shortly before and after birth, thymic T cell generation only contributes 50% of the newly generated T cell population in humans, the remainder is produced by peripheral homeostatic proliferation.24 This initial clonal expansion may be necessary to develop clonal sizes of T lymphocytes sufficient to maintain a diverse repertoire in spite of stochastic loss and compensatory homeostatic proliferation.25 Studies in DiGeorge patients in the first two years after thymic transplantation suggested that TCR-independent are by far more important than TCR-dependent mechanisms in maintaining T cell compartment size under steady state conditions. The clonal sizes of naïve T lymphocytes have been estimated to be in the order of 1,000 to 10,000 cells per clonotype.26-28 Diversity is similar, but clonal sizes are much smaller in small species, such as the mouse, where holes in the repertoire may develop. Indeed, the majority of naïve T cells expressing one particular T cell receptor are recruited in an antigen-specific response in the mouse,29 causing a hole in the naïve repertoire.30 Data in humans are not available, but this is unlikely to be the case in larger species. Consistent with this assessment, the diversity of the naïve T cell repertoire is relatively stable with age up to the age of 65 years in spite of only minimal influx of new thymocytes during adulthood.31, 32 Homeostatic proliferation, recruitment of naïve cells into antigen-specific responses and transition to the memory compartment may, therefore, increase the variance in clonal sizes, but is not sufficient to induce a significant contraction in repertoire diversity. Preliminary results using deep sequencing of T cell receptor genes are supportive of this interpretation;33 formal and more detailed analyzes are still pending.
Figure 1. Mechanisms in thymic involution.
Traditionally, increased production of sex hormones, intrathymic production of inflammatory mediators and the age-dependent change in lineage commitment of hematopoietic stem cells have been associated with thymic involution. Recently identified accelerators of thymic involution are obesity and possibly PPARγ (peroxisome proliferator-activated receptor gamma) stimulators that are routinely used in the treatment of type II diabetes mellitus and that support adipocyte differentiation and invasion into the perivascular space.
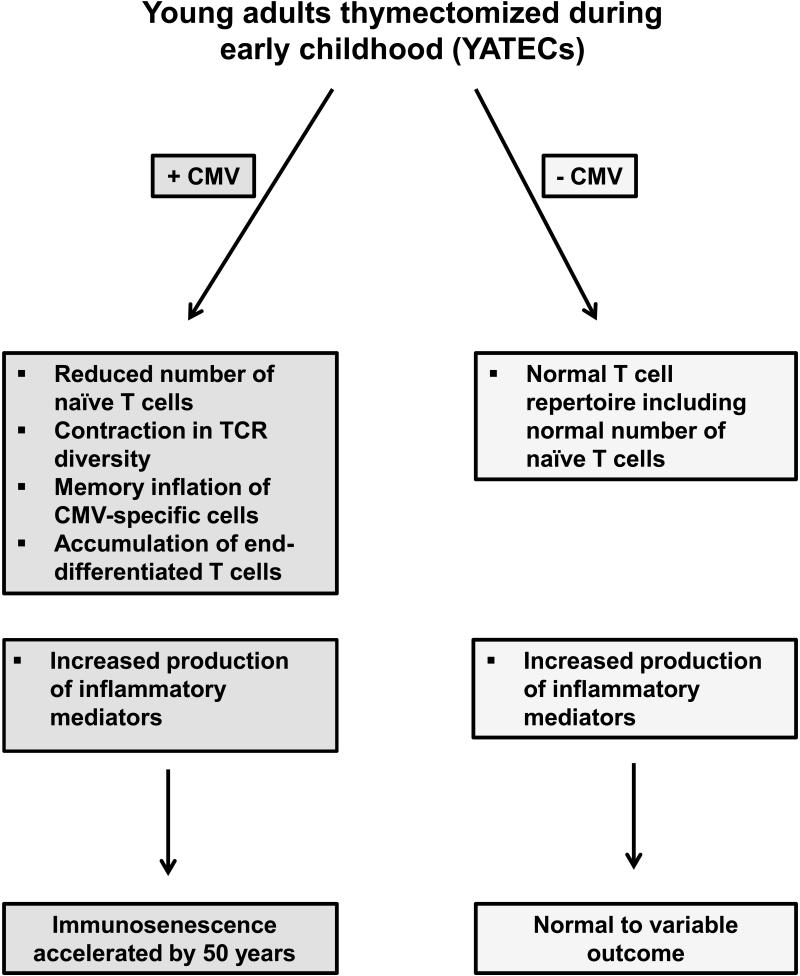
To address the question whether early thymic demise accelerates immune aging, Appay and colleagues examined young adults thymectomized during early childhood as part of surgical corrections of life-threatening congenital heart defects.6 Thymectomy during these surgical procedures has been common to improve access to the surgical field, although it is unlikely to be complete and may be quite variable in different individuals. Because there may be some residual tissue, the study provides a lower estimate of the effect of thymic depletion to accelerate immune aging over a 20+ year period. Consistent with other previous studies,29, 32 the authors showed that the absolute CD4 and CD8 T cell counts decline, mainly due to loss of naïve T cells, and that oligoclonal expansions, mainly within memory CD8 T cells, emerge. These findings were quite variable in different individuals. A subgroup of individuals had a T cell repertoire characteristic of 50 years older than their biological age including accumulation of end-differentiated and clonally expanded CD8 T cells with shortened telomeres.6 This phenotype was highly associated with seropositivity for previous CMV infection, suggesting that thymic involution alone was not sufficient to destabilize the repertoire even in a young individual who at time of thymic removal had not established a full T cell repertoire. In synergy with chronic CMV infection, early thymus removal recapitulated the immune phenotype in young adults that is characteristic of the elderly (Figure 2). In contrast, thymectomized young individuals had a normal T cell repertoire including a normal number of naïve T cells. Intriguingly, the authors also found an increased production of inflammatory mediators in their cohort similar to elderly individuals. From their data, it remains undetermined whether this increased production requires a co-infection with CMV or is also found in CMV-negative thymectomized individuals; nevertheless, the data support the notion that a T cell defect contributes to the activation of the innate immune system and the increased production of inflammatory cytokines in the elderly.
Figure 2. CMV infection accelerates immune aging after thymectomy.
In individuals thymectomized in early childhood, T cell repertoire changes consistent with accelerated immune aging are mostly limited to those individuals who acquired a CMV infection. Increased production of inflammatory mediators is common in thymectomized individuals supporting the notion that T cell defects contribute to age-associated inflammation. In the studies so far, the relationship between CMV infection and increased production of inflammatory factors was not examined.
It is well established that aging of the hematopoietic stem cells, serological factor as well as thymic microenvironment all contribute to thymic involution.34-36 In spite of their ability to express telomerase, telomeres in hematopoietic stem cells erode with age. However, a shift in lineage commitment from lymphoid to myeloid potential appears to be more important than classical senescence pathways induced by DNA damage accumulation or induction of tumor suppressor pathways in the stem cell.37 Age-dependent genetic programming may in part account for this shift. Clonal expansion of intrinsically myeloid-based stem cells with robust renewal potential in the aging host may even be more important (Figure 1). In further support of this interpretation, accelerated thymic involution in the telomere – dysfunctional mouse was conferred by the environment, but not by the stem cells expressing shortened telomeres. B and T cell development was only minimally impaired with mTERC(−/−) hematopoietic stem cells. Moreover, transplantation of dysfunctional thymi from mTERC(−/−) into wild-type mice restored thymic structure and rejuvenated its function.38
One major structural change in thymic organ involution is the infiltration of adipocytes into the perivascular space, which at least in part appears to be caused by a transformation of thymic resident cells into adipocytes.35, 39 Adipocyte development is under the control of peroxisome proliferator-activated receptor gamma (PPARγ) activity, which is targeted by thiazolidinediones, a class of compounds used in the treatment of diabetes mellitus.40 Rosiglitazone, a member of this family, reduces thymic cellularity, lowers the frequency of T cell receptor excision circles (TREC)+ and naïve T cells and reduced receptor diversity of the peripheral T cell repertoire in a murine model, consistent with the model that it accelerates T cell aging (Figure 1). Studies in human diabetic patients are not yet available. This action of rosiglitazone may be a concern for diabetic patients as well as an opportunity to better study the consequences for thymic involution for immune health and aging in an adult human population.
Accelerated Immune Aging in CMV-Infected Individuals
The finding that CMV infection acts in concert with thymic involution to accelerate aging builds on cumulative evidence assembled over the last years.41 One of the most striking features of immune aging are shifts in T cell subset distributions with a decline in naïve T cell population and an increase in effector T cells. In particular, in the CD8 compartment, end-differentiated CD45RA+CD8+CD28− T cells accumulate with age.42, 43 Initial studies showed that this phenotype was characteristic for CMV-specific T cells even in young individuals, drawing a comparison between the phenotypes found in immune aging and in chronic CMV infection.44 Subsequent studies have provided evidence for a cross-relationship of CMV infection and immune aging converging into the hypothesis that chronic CMV infection accelerates immune aging (Figure 3). This hypothesis was built on the following observations. The compartment of CMV-specific T cells in some individuals is huge and comprises a large proportion of the CD4, as well as the CD8, compartment leading to the hypothesis that memory inflation during chronic infection outcompetes other specificities.45 In support of this hypothesis, oligoclonal expansions in animal models were found to correlate with defective immune responses to third party antigens.46 Moreover, increased frequencies of the effector cell population lacking CD28 inversely correlated with vaccine responses to influenza antigen.47, 48 Inversion of the CD4:CD8 ratio, mainly induced by an expansion of CD28−CD8 effector T cells, is the key determinant of an immune risk profile that has been shown to correlate with mortality and morbidity in a Swedish study of octogenarians.49
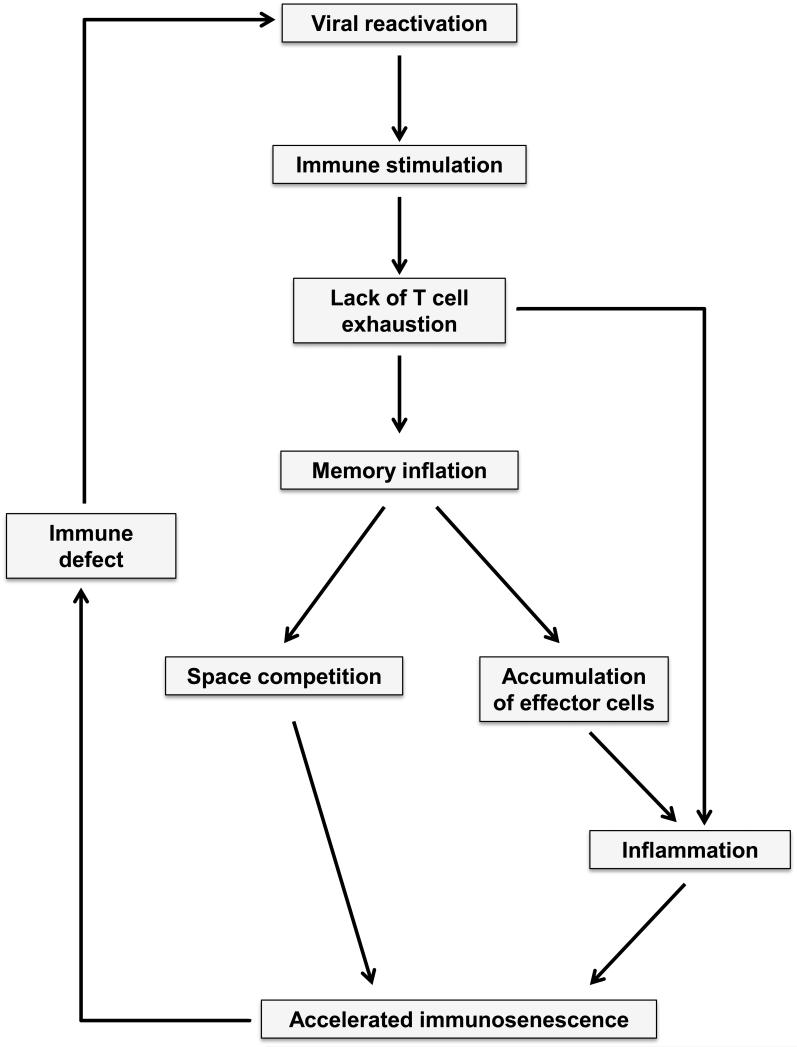
Figure 3. CMV-induced acceleration of immune aging.
The current model of CMV-induced accelerated immune aging proposes that CMV infection induces T cell memory inflation over time due to chronic stimulation and lack of T cell exhaustion. In addition, increased production of inflammatory mediators due to innate immune stimulation as well as accumulation of T effector cells further compromises immune competence. Defects in the immune control mechanisms of viral latency will feed into a positive feedback loop of accelerated immune aging.
The prevailing hypothesis how chronic CMV infection may accelerate immune aging is that the virus induces an inflation in CMV-specific T cell memory. The first evidence for this hypothesis were HLA-A2 tetramer studies with the pp65 and eventually the IE-1 peptide of the CMV virus that showed CD8 T cell frequencies in the range of several percents of the total repertoire, significantly higher than for any other viral peptides.44, 50-53 In a more comprehensive study, Picker et al characterized the CMV response using approximately 20,000 overlapping peptides and concluded that the CMV-specific response is very broad and can nearly comprise half of the total CD8 T cell repertoire in selected individuals.54 In general, the CD4 T cell response to CMV is smaller; however, the Picker group also found a large percentage of the CD4 population being specific for CMV in selected individuals. In a study by Akbar et al, expansion of CD4+CD28− effector cell populations, either CD45RA+ or CD45RA−, were only found in anti-CMV antibody positive individuals.55 Moreover, many of these effector cell populations were specific for CMV antigens, suggesting that qualitatively CD4, as well as CD8, T cells are involved in the chronic CMV response although the magnitude is higher for CD8 cells. The mechanism underlying these unopposed expansions of CMV-specific T cells to clonal sizes that may compromise the overall immune competence are unclear. Molecular profiling after initial CMV infection showed enhanced expression of genes associated with cell cycle and metabolic activity only in the acute phase while at all later stages a gene expression signature consistent with a Th1 response including the expression of Th1-associated transcription factors, eomesodermin and T-bet prevailed.56 CMV-specific cells express high amounts of IFN-γ, perforin and granzyme B and the fractalkine receptor that is frequently found on cytotoxic effector cells. Such an expression signature is not only limited to CD8 cells, but is also typical for CMV-specific CD4 T cell responses. The gene expression profile is completely different from that of exhausted T cells that usually develop in the presence of highly replicating tumors or viruses57-59 and that are characterized by the expression of a number of inhibitory receptors, such as PD-1 or TIM-3, that impair T cell responses.60, 61 In contrast to exhausted T cells, the CMV-specific T cell response is fully functional although CMV-specific effector cell populations also frequently express negative regulatory receptors, mostly of the MHC class I recognizing receptor family such as KIR (killer-cell immunoglobulin-like receptor), KLR (killer-cell lectin-like receptor) and ILT (immunoglobulin-like transcript)62 and not of the CD28 family including PD-1. This feature sets CMV-specific and age-associated T cell repertoire changes clearly apart from those of chronic active viral diseases.63 In the latter case, CD8 T cells are getting increasingly dysfunctional, in part due to the expression of negative regulatory receptors PD-1 and TIM-3.61 In the absence of chronic active infection with highly replicating viruses, PD-1 expression or other features of clonal exhaustion are not a typical marker for immune aging and do not explain the immune defects that are obvious with old age.
Mechanisms underlying the memory inflation to a latent virus are unclear. Memory inflation is not a typical finding for all herpes viruses, for example, T cell responses to VZV tend to decline with age rather than to increase64 and responses to EBV inversely correlate with the size of the CMV-specific compartment.65 It is possible that the expansion of a highly competent CMV response is responsible for the lasting control of these viruses. Latent VZV infection tends to relapse in patients of old age who are at increased risk of shingles.66 Animal models that would allow to further mechanistically examine this unique behavior of the CMV response do not exist. Murine gamma herpes viruses, such as gamma HV68, establish latency, which is effectively controlled by the CD8 T cell response over time.67 Gamma herpes virus-specific CD8 T cells remained functionally active, without evidence of clonal exhaustion, and also neither show a decrease nor an increase in frequency with age.68
Memory inflation of CMV-specific T cells is a finding of some, but not all, CMV-infected individuals. One of the epidemiological characteristics of CMV infection is that it can be acquired at any age.69, 70 The question whether the age at the time of primary CMV infection may determine the size and nature of the memory T cell response has not been studied. Nikolich-Zugich and colleagues have shown in mice that the global distinctive features of a primary CD8 T cell response to an immunodominant herpes simplex virus epitope, glycoprotein B 495-502, was highly conserved independent of what age the mice were first infected.71 It remains to be seen whether this holds up for the CMV infection in humans.
While most studies at this time have focused on the hypothesis that chronic CMV infection accelerates aging and have tried to elucidate the mechanism of this acceleration, as well as the reasons for the variable contribution of CMV infection for immune aging in different individuals, the hypothesis cannot be excluded that memory inflation of the CMV-specific response is a consequence rather than the cause of age-related immune dysfunction. Studies in individuals of long-lived families in the Leiden longevity study have shown that compared to the age-matched control group, the individuals who presumably carry longevity genes are less susceptible for the characteristic CMV-induced repertoire changes including the accumulation of CMV-specific large effector cell populations.72 While this initial finding was consistent with the hypothesis that CMV is an accelerator of immune aging, but only in genetic-susceptible individuals, subsequent studies yielded the surprising result that the incidence of CMV infection as determined by a positive antibody titer was much lower in members of long-lived families. These studies indicate that longevity genes protect from infection or allow for clearance of the infection. Consistent with this overall concept, patients with chronic lymphocytic leukemia who are immunocompromised due to their disease have a markedly expanded CMV-specific CD4 T cell population, more than twice that of CMV-positive controls.73 The magnitude of the CMV-specific CD4 T cell response correlated with disease stage and also with chemotherapy. Expansion of the CMV response was associated with the phenotypic marker profile that is also characteristic for the subset distribution in CMV-infected elderly individuals.74 Possibly, immune defects in the innate or NK cell repertoire and their ability to control the CMV infection may lead to an expansion of the adaptive immune response to CMV. Alternatively, increased T cell death generating space may allow for the expansion of CMV-specific T cells. Such a concept would also be consistent with the findings that expansion of CMV-specific cells and intensity of the CMV-specific immune responses are correlated with overall increased morbidity in elderly individuals such as in a recent study by Sansoni et al who found an inverse correlation of CD4 and of antibody responses to CMV with impaired health and physical and cognitive functional status.75
Accelerated Immune Aging and HIV Infection
Many of the T cell abnormalities associated with aging are similar to those observed in untreated HIV patients.76-78 In fact, several of the biomarkers in T cell defects that are now considered as hallmarks of immunosenescence have been first and in more depth studied in the context of HIV infection.79 Compared to age-matched controls, HIV-infected patients have reduced thymic function as determined by TREC frequencies,80 a loss in naïve T cells, a reduced T cell telomere length, an expansion of end-differentiated effector T cells that have lost the expression of CD28 and a contracted T cell repertoire.78, 79, 81, 82 All of these changes are also characteristic of the aging immune system in healthy individuals older than 70 years old. Clinically, this accelerated immunosenescence in HIV patients has gained increasing relevance now that HIV-infected patients can be treated with antiretroviral therapy and their overall prognosis shifted from years to decades. Antiretroviral therapy can completely suppress viral replication and prevent AIDS-related complications; nevertheless, HIV-infected patients age faster and prematurely develop age-associated diseases ranging from frailty to cardiovascular disease, central nervous system complications and renal disease.83 The increased morbidity and mortality from all causes in HIV-infected patients has been proposed to be causally linked to accelerated immunosenescence. Although lymphocyte counts recover in many patients after the institution of antiretroviral therapy, not all HIV-associated immune defects can be restored.84, 85 Several lines of evidence suggest that the aging of the immune system continues to be accelerated even though viral replication is suppressed. Accelerated immune senescence features in treated HIV patients include impaired hematopoiesis, impaired T cell generation and homeostasis, functional defects in vaccine responses, and increased constitutive production of inflammatory mediators comparable to what is observed in healthy elderly individuals.76, 78, 86 Understanding immune aging in treated HIV-infected patients is, therefore, not only important to improve quality of life and life expectancy in these patients, but also provides a unique model to identify pathways that are central to normal immune aging (Figure 4). Accelerated immune aging in HIV-infected patients is certainly not caused by a single mechanism. Obviously, the treated HIV patient needs to rebuild its T cell repertoire at an age when thymic function is compromised by age and even more so by the infection.87 Even if normal lymphocyte numbers are being reached, T cell diversity and subset distribution may be compromised. Homeostatic proliferation to rebuild the repertoire will not only increase replicative stress to the system, but will also be associated with proliferation-induced differentiation even in the absence of antigen recognition as have been shown in the murine system. Similar to the situation in the thymectomized children, co-occurrence with latent CMV infection in HIV-infected patients is likely a confounding cofactor that accelerates immune aging in a host who has a compromised T cell regenerative system and a repertoire stability.88 Restoring thymic activity for this rebuilding process will be more important than it is for normal immune aging, but similar needs can be envisioned for a number of medical conditions other than HIV infection such as the condition after chemotherapeutic therapy. Strategies developed mostly in preclinical models to improve thymic output include sex hormone ablation, inhibition of c-kit and administration of various growth factors, in particular growth hormone, keratinocyte growth factor, acetylated ghrelin and interleukin (IL)-7.89, 90 In a clinical study of 22 HIV-infected patients with a mean age of 50 years and antiretroviral treatment of two years, daily subcutaneous injection of growth hormone increased thymic size, T cell output and peripheral numbers of naïve CD4 T cells.91
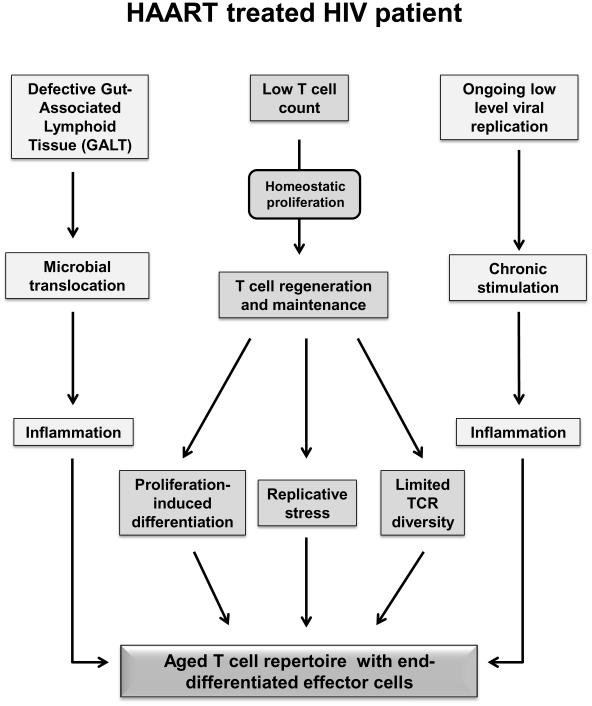
Figure 4. Accelerated immunosenescence in HAART treated HIV patient.
The schematic diagram depicts possible defects and mechanisms that contribute to accelerate immune aging in HIV patients in whom viral replication is controlled on HAART treatment.
Accelerated aging in treated HIV patients provides an opportunity to understand how the senescent immune system contributes to major dysfunction in organ systems that are generally considered to not be dependent on immune function, such as the cardiovascular and nervous systems, lungs and kidneys. Constitutively increased production of inflammatory mediators is likely an important link between immunosenescence and the accelerated dysfunction in several non-immune organ systems. Deregulation of this inflammatory process in the treated HIV patients, as well as in the aging host, appears to be complex, and several pathways are currently being explored for their relevance. Expansion and activation of CD8 effector cell populations appear to contribute, in particular, when the immune system is not able to fully control latent viral infections and when features of T cell exhaustion are absent as they are in the aging host. Cellular senescence by itself induces the activation of gene regulatory pathways that control the production of inflammatory pathways not only in hematopoietic lineages, but also in non-immune cells. Loss of the gut-associated lymphoid tissue is one of the hallmarks of HIV infection and is not easily reversible with antiretroviral therapy. Loss of gut mucosal integrity will lead to increased microbial translocation, which will stimulate the innate immune system and lead to the production of inflammatory mediators. How well the gut-associated lymphoid tissue is preserved in the healthy elderly host is not known.
Accelerated Immune Aging and Autoimmunity
Several autoimmune diseases have been associated with phenotypic changes that can be interpreted as accelerated immune aging. Many studies have focused on rheumatoid arthritis (RA), with a frequent disease onset in the second half of adult life. In initial studies, patients with RA have been found to have expanded effector CD4 and CD8 T cell populations that have lost the expression of CD28, a phenotypic marker that has been closely associated with immune aging.92 Loss of CD28 was particularly evident in patients who had major organ complications in addition to the usual inflammation of small and large joints. Expansion of CD28− effector subpopulations has also been observed in other autoimmune diseases, such as Wegener’s granulomatosis.93 While the expansion of CD28− effector cell populations could be interpreted as a consequence of a chronic adaptive immune response to an autoantigen as part of the disease process, these clonal populations in the autoimmune disease have also been shown to correlate with CMV infection or even be CMV-specific, suggesting that the autoimmune-prone environment favors clonal expansions to CMV. Some other findings are also supportive of this concept. Telomeric erosion in hematopoietic lineages, including the hematopoietic stem cells, serves as a typical finding in immune senescence. Patients with RA have telomere lengths that are comparable to healthy individuals 20 years older in biological age.94 This telomeric erosion is not limited to memory and effector T cell populations, but also includes the naïve CD4 T cell population, as well as the population of circulating hematopoietic precursor cells, clearly demonstrating that T cell erosion is not a consequence of the expansion and increased replicative history of T cell population responding to the recognition of autoantigen.95, 96 In part, this premature telomeric erosion in T cells is due to a reduced activation-induced expression of telomerase in naïve T cells consistent with the interpretation that telomeric erosion is not only the reflection of an increased replicative history.97 Several lines of additional evidence suggest that T cell generation and T cell homeostasis are fundamentally disturbed in patients with RA. Patients with RA have a reduced number of TREC in their CD4 population, suggesting an age disproportional decline in thymic output and/or an increased peripheral replication and T cell loss.94 Similarly, the repertoire of T cell receptor diversity in the naïve T cell populations from patients with RA is contracted, patients have a smaller number of different T cell receptor β-chain sequences with compensatory increase in clonal sizes, again a finding that could be induced by increased peripheral loss and compensatory homeostatic clonal expansion.98
If premature telomeric erosion and immune aging in RA is not a consequence of increased replicative stress due to the inflammatory milieu or the increased proliferative activity of cells involved in the disease process, the underlying mechanism may be a primary defect and precede autoimmunity. Indeed, T lymphocytes from patients with RA have defects in their DNA repair machinery that result in increased susceptibility for apoptosis, in particular, in the naïve T cell population (Figure 5). Increased lymphocyte loss and the associated relative lymphopenia may be a risk factor for autoimmunity as has been shown in several animal models.16 In these models, lymphopenia induces IL-7- and/or IL-15-driven homeostatic proliferation, which lowered the level of T cell responsiveness and impaired tolerance to peripheral antigens. At least two mechanisms of accelerated cell loss have been described for naïve T cells from patients with RA. First, naïve RA T cells are compromised in expressing telomerase in the first days after antigen stimulation. The defective induction of telomerase not only accelerated telomere loss, but also rendered these cells more susceptible to undergo apoptosis. This increased apoptosis susceptibility was independent of telomeric erosion, but correlated with repressed transcription of Bcl-2.99 Expression of Bcl-2, mainly induced by common gamma chain cytokines such as IL-7, is important in preventing clonal downsizing after T cell activation and the transition into memory cells. Moreover, Bcl-2 expression is also important in determining T cell survival in homeostatic proliferation.
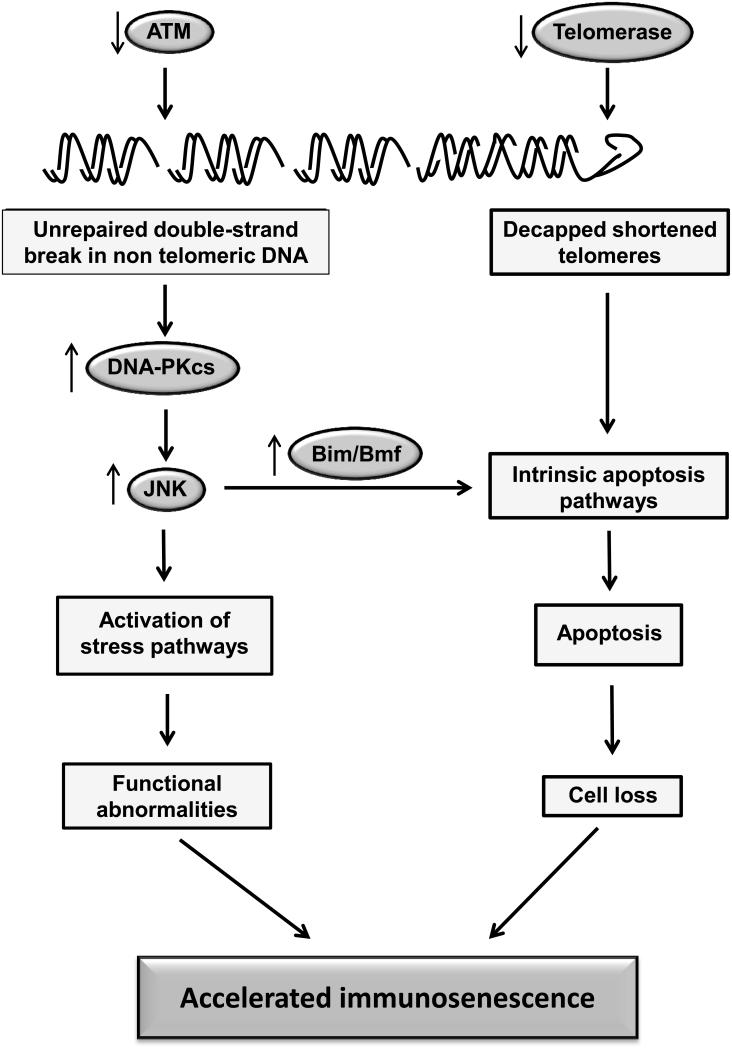
Figure 5. Defective DNA damage responses as a cause of accelerated immune aging in patients with rheumatoid arthritis.
T cells from patients with RA exhibit shortened telomeres, increased number of DNA double-strand breaks and chronic DNA damage responses due to defects in telomerase expression and ATM activity. As a consequence, cells undergo apoptosis or enter cellular senescence accelerating the aging process of the global immune system.
Telomerase deficiency, possibly independent of telomere elongation, is not the only defective DNA repair mechanism found in RA patients. RA T cells, both naïve and memory cells, have increased number of double-strand breaks in non-telomeric DNA. The underlying defect is a reduced production of the DNA damage-inducible protein kinase, ataxia telangiectasia mutated kinase (ATM).100 The accumulation of fragmented DNA is associated with increased susceptibility to apoptosis. In parallel, and most likely as a compensatory mechanism to deal with the increased DNA damage, RA T cells show a robust induction of the DNA-dependent protein kinase catalytic subunit (DNA-PKcs).101 The deficiency in ATM transcription and phosphorylation leads to the induction of p53-independent pro-apoptotic pathway. In parallel, chronic PKcs activation activates downstream signaling pathways including stress kinase pathways, as well as the inflammasome. The net effect of these changes is a reduced threshold to undergoing activation and increased susceptibility to enduring apoptosis, leading to increased attrition of peripheral T cells. Defective DNA maintenance is known to accelerate aging; in its most extreme forms genetic polymorphisms of molecules involved in nuclear stability or DNA repair induce progeroid syndromes.102, 103 Similar mechanisms are likely to also contribute to the aging of cells of the immune system. In RA, the increased attrition of T cells accelerates immune aging and possibly also sets the stage for tolerance defects that eventually manifest in the form of peripheral arthritis and possibly also other autoimmune manifestations. This role of ATM defects in accelerating immune aging has been recently confirmed in patients with classical and mild variant ataxia telangiectasia.104 Cellular phenotypes characteristic of an aged host were present disproportionate to the calendar age, in this model independent of the absence or presence of CMV infection.
Synopsis
Immune aging is a multi-faceted process that is obviously very heterogeneous in a population depending on genetic background and environmental factors. Identifying factors and pathways that accelerate or delay immune aging hold the promise to understand this process and to define interventions that influence the process. These may at least in part be different from interventions that delay the aging process in general. For example, while calorie restriction promotes longevity in all model systems including mammals, studies in non-human primates have shown its effects on immune aging to be very context-dependent.105 Rapamycin, which recently has been shown to mimic calorie restrictions in its effect and probably its mechanisms,106, 107 compromises the immune system at least as much as immune senescence does. We do not know much on how to prolong immune competence. Conversely, we have increasing understanding of accelerated aging in models of chronic infections and autoimmunity. The mechanisms that drive immune aging in HIV-infected individuals certainly include the need to rebuild a functional CD4 T cell repertoire that has been destroyed by the viral infection, but appears to be more complex. Cytomegalovirus infection is a very instructive model different from most other viral infections based on its ability to induce memory inflation without much T cell exhaustion. The finding that defective DNA repair mechanisms accelerate immune aging in autoimmune diseases raises the question whether inflammation and autoimmunity drive DNA damage or whether DNA damage drives inflammation and autoimmunity, analogous to the Campisi model of cellular senescence associated production of inflammatory mediators.108 Of particular interest, in all of these models the accelerated aging process is not limited to the immune system, but involves other organ systems in particular the cardiovascular system.
Acknowledgement
This work was funded in part by grants from the National Institutes of Health, U19 AI57266, U19 AI090019, RC4 AG039014, R01 AR42527, R01 AI44142, R01 EY11916 and HL P01 HL58000.
REFERENCES
- 1.Davis MM. A prescription for human immunology. Immunity. 2008;29:835–838. doi: 10.1016/j.immuni.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goronzy JJ, Lee WW, Weyand CM. Aging and T-cell diversity. Exp. Gerontol. 2007;42:400–406. doi: 10.1016/j.exger.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendall SC, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332:687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakaya HI, et al. Systems biology of vaccination for seasonal influenza in humans. Nat. Immunol. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prelog M, et al. Thymectomy in early childhood: significant alterations of the CD4(+)CD45RA(+)CD62L(+) T cell compartment in later life. Clin. Immunol. 2009;130:123–132. doi: 10.1016/j.clim.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Sauce D, et al. Evidence of premature immune aging in patients thymectomized during early childhood. J. Clin. Invest. 2009;119:3070–3078. doi: 10.1172/JCI39269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aspinall R, Andrew D. Thymic involution in aging. J. Clin. Immunol. 2000;20:250–256. doi: 10.1023/a:1006611518223. [DOI] [PubMed] [Google Scholar]
- 8.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 9.George AJ, Ritter MA. Thymic involution with ageing: obsolescence or good housekeeping? Immunol. Today. 1996;17:267–272. doi: 10.1016/0167-5699(96)80543-3. [DOI] [PubMed] [Google Scholar]
- 10.Montecino-Rodriquez E, Min H, Dorshkind K. Reevaluating current models of thymic involution. Semin. Immunol. 2005;17:356–361. doi: 10.1016/j.smim.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Pawelec G, et al. Human immunosenescence: is it infectious? Immunol. Rev. 2005;205:257–268. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 12.Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr. Opin. Immunol. 2005;17:480–485. doi: 10.1016/j.coi.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr. Opin. Immunol. 2010;22:223–230. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arvin A. Aging, immunity, and the varicella-zoster virus. N. Engl. J. Med. 2005;352:2266–2267. doi: 10.1056/NEJMp058091. [DOI] [PubMed] [Google Scholar]
- 15.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goronzy JJ, Shao L, Weyand CM. Immune aging and rheumatoid arthritis. Rheum. Dis. Clin. North Am. 2010;36:297–310. doi: 10.1016/j.rdc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weyand CM, Goronzy JJ. Stem cell aging and autoimmunity in rheumatoid arthritis. Trends Mol. Med. 2004;10:426–433. doi: 10.1016/j.molmed.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Goronzy JJ, Weyand CM. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity - catalysts of autoimmunity and chronic inflammation. Arthritis Res. Ther. 2003;5:225–234. doi: 10.1186/ar974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinmann GG. Changes in the human thymus during aging. Curr. Top. Pathol. 1986;75:43–88. doi: 10.1007/978-3-642-82480-7_2. [DOI] [PubMed] [Google Scholar]
- 20.Rodewald HR. Thymus organogenesis. Annu. Rev. Immunol. 2008;26:355–388. doi: 10.1146/annurev.immunol.26.021607.090408. [DOI] [PubMed] [Google Scholar]
- 21.Lynch HE, et al. Thymic involution and immune reconstitution. Trends Immunol. 2009;30:366–373. doi: 10.1016/j.it.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haynes BF, et al. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu. Rev. Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 23.Dixit VD. Thymic fatness and approaches to enhance thymopoietic fitness in aging. Curr. Opin. Immunol. 2010;22:521–528. doi: 10.1016/j.coi.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schonland SO, et al. Homeostatic control of T-cell generation in neonates. Blood. 2003;102:1428–1434. doi: 10.1182/blood-2002-11-3591. [DOI] [PubMed] [Google Scholar]
- 25.Dowling MR, Hodgkin PD. Modelling naive T-cell homeostasis: consequences of heritable cellular lifespan during ageing. Immunol. Cell. Biol. 2009;87:445–456. doi: 10.1038/icb.2009.11. [DOI] [PubMed] [Google Scholar]
- 26.Ciupe SM, et al. The dynamics of T-cell receptor repertoire diversity following thymus transplantation for DiGeorge anomaly. PLoS Comput. Biol. 2009;5:e1000396. doi: 10.1371/journal.pcbi.1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arstila TP, et al. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286:958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- 28.Casrouge A, et al. Size estimate of the alpha beta TCR repertoire of naive mouse splenocytes. J. Immunol. 2000;164:5782–5787. doi: 10.4049/jimmunol.164.11.5782. [DOI] [PubMed] [Google Scholar]
- 29.Hale JS, et al. Thymic output in aged mice. Proc. Natl. Acad. Sci. U S A. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed M, et al. Clonal expansions and loss of receptor diversity in the naive CD8 T cell repertoire of aged mice. J. Immunol. 2009;182:784–792. doi: 10.4049/jimmunol.182.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr. Opin. Immunol. 2005;17:468–475. doi: 10.1016/j.coi.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Naylor K, et al. The influence of age on T cell generation and TCR diversity. J. Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 33.Robins HS, et al. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114:4099–4107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zoller AL, Kersh GJ. Estrogen induces thymic atrophy by eliminating early thymic progenitors and inhibiting proliferation of beta-selected thymocytes. J. Immunol. 2006;176:7371–7378. doi: 10.4049/jimmunol.176.12.7371. [DOI] [PubMed] [Google Scholar]
- 35.Manley NR, et al. Structure and function of the thymic microenvironment. Front. Biosci. 2011;17:2461–2477. doi: 10.2741/3866. [DOI] [PubMed] [Google Scholar]
- 36.Min H, Montecino-Rodriguez E, Dorshkind K. Reduction in the developmental potential of intrathymic T cell progenitors with age. J. Immunol. 2004;173:245–250. doi: 10.4049/jimmunol.173.1.245. [DOI] [PubMed] [Google Scholar]
- 37.Beerman I, et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl. Acad. Sci. U S A. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song Z, et al. Alterations of the systemic environment are the primary cause of impaired B and T lymphopoiesis in telomere-dysfunctional mice. Blood. 2010;115:1481–1489. doi: 10.1182/blood-2009-08-237230. [DOI] [PubMed] [Google Scholar]
- 39.Yang H, Youm YH, Dixit VD. Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. J. Immunol. 2009;183:3040–3052. doi: 10.4049/jimmunol.0900562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Youm YH, et al. Thiazolidinedione treatment and constitutive-PPARgamma activation induces ectopic adipogenesis and promotes age-related thymic involution. Aging Cell. 2010;9:478–489. doi: 10.1111/j.1474-9726.2010.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derhovanessian E, Larbi A, Pawelec G. Biomarkers of human immunosenescence: impact of Cytomegalovirus infection. Curr. Opin. Immunol. 2009;21:440–445. doi: 10.1016/j.coi.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Kuijpers TW, et al. Frequencies of circulating cytolytic, CD45RA+CD27-, CD8+ T lymphocytes depend on infection with CMV. J. Immunol. 2003;170:4342–4348. doi: 10.4049/jimmunol.170.8.4342. [DOI] [PubMed] [Google Scholar]
- 43.Czesnikiewicz-Guzik M, et al. T cell subset-specific susceptibility to aging. Clin. Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Looney RJ, et al. Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clin. Immunol. 1999;90:213–219. doi: 10.1006/clim.1998.4638. [DOI] [PubMed] [Google Scholar]
- 45.Nikolich-Zugich J, Rudd BD. Immune memory and aging: an infinite or finite resource? Curr. Opin. Immunol. 2010;22:535–540. doi: 10.1016/j.coi.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brien JD, et al. Key role of T cell defects in age-related vulnerability to West Nile virus. J. Exp. Med. 2009;206:2735–2745. doi: 10.1084/jem.20090222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goronzy JJ, et al. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J. Virol. 2001;75:12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saurwein-Teissl M, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J. Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 49.Wikby A, et al. Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study. Mech. Ageing Dev. 1998;102:187–198. doi: 10.1016/s0047-6374(97)00151-6. [DOI] [PubMed] [Google Scholar]
- 50.Khan N, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J. Immunol. 2002;169:1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 51.Chidrawar S, et al. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin. Exp. Immunol. 2009;155:423–432. doi: 10.1111/j.1365-2249.2008.03785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Leeuwen EM, et al. Human virus-specific CD8+ T cells: diversity specialists. Immunol. Rev. 2006;211:225–235. doi: 10.1111/j.0105-2896.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 53.Palendira U, et al. Selective accumulation of virus-specific CD8+ T cells with unique homing phenotype within the human bone marrow. Blood. 2008;112:3293–3302. doi: 10.1182/blood-2008-02-138040. [DOI] [PubMed] [Google Scholar]
- 54.Sylwester AW, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fletcher JM, et al. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J. Immunol. 2005;175:8218–8225. doi: 10.4049/jimmunol.175.12.8218. [DOI] [PubMed] [Google Scholar]
- 56.Hertoghs KM, et al. Molecular profiling of cytomegalovirus-induced human CD8+ T cell differentiation. J. Clin. Invest. 2010;120:4077–4090. doi: 10.1172/JCI42758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fourcade J, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J. Exp. Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakuishi K, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. U S A. 2009;106:8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 61.Jin HT, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. U S A. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat. Rev. Immunol. 2011;11:289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 64.Levin MJ, et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J. Infect. Dis. 2003;188:1336–1344. doi: 10.1086/379048. [DOI] [PubMed] [Google Scholar]
- 65.Khan N, et al. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J. Immunol. 2004;173:7481–7489. doi: 10.4049/jimmunol.173.12.7481. [DOI] [PubMed] [Google Scholar]
- 66.Gershon AA, et al. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J. Clin. Virol. 2010;48(Suppl 1):S2–7. doi: 10.1016/S1386-6532(10)70002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freeman ML, et al. Two kinetic patterns of epitope-specific CD8 T-cell responses following murine gammaherpesvirus 68 infection. J. Virol. 2010;84:2881–2892. doi: 10.1128/JVI.02229-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yager EJ, et al. Differential impact of ageing on cellular and humoral immunity to a persistent murine gamma-herpesvirus. Immun. Ageing. 2010;7:3. doi: 10.1186/1742-4933-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Staras SA, et al. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin. Infect. Dis. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 70.Stadler LP, et al. Seroprevalence of cytomegalovirus (CMV) and risk factors for infection in adolescent males. Clin. Infect. Dis. 2010;51:e76–81. doi: 10.1086/656918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rudd BD, et al. Evolution of the antigen-specific CD8+ TCR repertoire across the life span: evidence for clonal homogenization of the old TCR repertoire. J. Immunol. 2011;186:2056–2064. doi: 10.4049/jimmunol.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Derhovanessian E, et al. Hallmark features of immunosenescence are absent in familial longevity. J. Immunol. 2010;185:4618–4624. doi: 10.4049/jimmunol.1001629. [DOI] [PubMed] [Google Scholar]
- 73.Pourgheysari B, et al. The number of cytomegalovirus-specific CD4+ T cells is markedly expanded in patients with B-cell chronic lymphocytic leukemia and determines the total CD4+ T-cell repertoire. Blood. 2010;116:2968–2974. doi: 10.1182/blood-2009-12-257147. [DOI] [PubMed] [Google Scholar]
- 74.Pita-Lopez ML, et al. Effect of ageing on CMV-specific CD8 T cells from CMV seropositive healthy donors. Immun. Ageing. 2009;6:11. doi: 10.1186/1742-4933-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vescovini R, et al. Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. J. Immunol. 2010;184:3242–3249. doi: 10.4049/jimmunol.0902890. [DOI] [PubMed] [Google Scholar]
- 76.Desai S, Landay A. Early immune senescence in HIV disease. Curr. HIV/AIDS Rep. 2010;7:4–10. doi: 10.1007/s11904-009-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Justice AC. HIV and aging: time for a new paradigm. Curr. HIV/AIDS Rep. 2010;7:69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- 78.Appay V, et al. Accelerated immune senescence and HIV-1 infection. Exp. Gerontol. 2007;42:432–437. doi: 10.1016/j.exger.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 79.Kalayjian RC, et al. Age-related immune dysfunction in health and in human immunodeficiency virus (HIV) disease: association of age and HIV infection with naive CD8+ cell depletion, reduced expression of CD28 on CD8+ cells, and reduced thymic volumes. J. Infect. Dis. 2003;187:1924–1933. doi: 10.1086/375372. [DOI] [PubMed] [Google Scholar]
- 80.Dion ML, et al. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity. 2004;21:757–768. doi: 10.1016/j.immuni.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 81.Effros RB. Telomere/telomerase dynamics within the human immune system: effect of chronic infection and stress. Exp. Gerontol. 2011;46:135–140. doi: 10.1016/j.exger.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Effros RB, et al. The role of CD8+ T-cell replicative senescence in human aging. Immunol. Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 83.Rickabaugh TM, et al. The dual impact of HIV-1 infection and aging on naive CD4 T-cells: additive and distinct patterns of impairment. PLoS One. 2011;6:e16459. doi: 10.1371/journal.pone.0016459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Douek DC, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 85.Dion ML, et al. Slow disease progression and robust therapy-mediated CD4+ T-cell recovery are associated with efficient thymopoiesis during HIV-1 infection. Blood. 2007;109:2912–2920. doi: 10.1182/blood-2006-09-047308. [DOI] [PubMed] [Google Scholar]
- 86.Sauce D, et al. HIV disease progression despite suppression of viral replication is associated with exhaustion of lymphopoiesis. Blood. 2011;117:5142–5151. doi: 10.1182/blood-2011-01-331306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Teixeira L, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS. 2001;15:1749–1756. doi: 10.1097/00002030-200109280-00002. [DOI] [PubMed] [Google Scholar]
- 88.Appay V, et al. Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS. 2011;25:1813–1822. doi: 10.1097/QAD.0b013e32834640e6. [DOI] [PubMed] [Google Scholar]
- 89.Hollander GA, Krenger W, Blazar BR. Emerging strategies to boost thymic function. Curr. Opin. Pharmacol. 2010;10:443–453. doi: 10.1016/j.coph.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taub DD, Murphy WJ, Longo DL. Rejuvenation of the aging thymus: growth hormone-mediated and ghrelin-mediated signaling pathways. Curr. Opin. Pharmacol. 2010;10:408–424. doi: 10.1016/j.coph.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Napolitano LA, et al. Growth hormone enhances thymic function in HIV-1-infected adults. J. Clin. Invest. 2008;118:1085–1098. doi: 10.1172/JCI32830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goronzy JJ, Weyand CM. Thymic function and peripheral T-cell homeostasis in rheumatoid arthritis. Trends Immunol. 2001;22:251–255. doi: 10.1016/s1471-4906(00)01841-x. [DOI] [PubMed] [Google Scholar]
- 93.Morgan MD, et al. CD4+CD28− T cell expansion in granulomatosis with polyangiitis (Wegener's) is driven by latent cytomegalovirus infection and is associated with an increased risk of infection and mortality. Arthritis Rheum. 2011;63:2127–2137. doi: 10.1002/art.30366. [DOI] [PubMed] [Google Scholar]
- 94.Koetz K, et al. T cell homeostasis in patients with rheumatoid arthritis. Proc. Natl. Acad. Sci. U S A. 2000;97:9203–9208. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Colmegna I, et al. Defective proliferative capacity and accelerated telomeric loss of hematopoietic progenitor cells in rheumatoid arthritis. Arthritis Rheum. 2008;58:990–1000. doi: 10.1002/art.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schonland SO, et al. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc. Natl. Acad. Sci. U S A. 2003;100:13471–13476. doi: 10.1073/pnas.2233561100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andrews NP, et al. Telomeres and immunological diseases of aging. Gerontology. 2010;56:390–403. doi: 10.1159/000268620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wagner UG, et al. Perturbation of the T cell repertoire in rheumatoid arthritis. Proc. Natl. Acad. Sci. U S A. 1998;95:14447–14452. doi: 10.1073/pnas.95.24.14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fujii H, et al. Telomerase insufficiency in rheumatoid arthritis. Proc. Natl. Acad. Sci. U S A. 2009;106:4360–4365. doi: 10.1073/pnas.0811332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shao L, et al. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J. Exp. Med. 2009;206:1435–1449. doi: 10.1084/jem.20082251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shao L, Goronzy JJ, Weyand CM. DNA-dependent protein kinase catalytic subunit mediates T-cell loss in rheumatoid arthritis. EMBO Mol. Med. 2010;2:415–427. doi: 10.1002/emmm.201000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu Y, et al. DNA damage responses in progeroid syndromes arise from defective maturation of prelamin A. J. Cell Sci. 2006;119:4644–4649. doi: 10.1242/jcs.03263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen T, et al. A functional single nucleotide polymorphism in promoter of ATM is associated with longevity. Mech. Ageing Dev. 2010;131:636–640. doi: 10.1016/j.mad.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 104.Exley AR, et al. Premature ageing of the immune system underlies immunodeficiency in ataxia telangiectasia. Clin. Immunol. 2011;140:26–36. doi: 10.1016/j.clim.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 105.Messaoudi I, et al. Optimal window of caloric restriction onset limits its beneficial impact on T-cell senescence in primates. Aging Cell. 2008;7:908–919. doi: 10.1111/j.1474-9726.2008.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miller RA, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rodier F, Campisi J. Four faces of cellular senescence. J. Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]