Abstract
Background
Persistent maternal smoking during pregnancy, reduction or cessation during pregnancy, and smoking initiation or resumption postpartum impel further research to understand these behavioral patterns and opportunities for intervention.
Objectives
We investigated heterogeneous longitudinal patterns of smoking quantity to determine if these patterns vary across three maternal age groups, and whether the influence of individual and contextual risk factors varies by maternal age.
Methods
Separate general growth mixture models were estimated for mothers ages 15–25, 26–35, and 36+, allowing different empirical patterns of an ordinal measure of smoking behavior at six time points, from preconception through child entry to kindergarten.
Results
We identify five classes for mothers ages 15–25, four classes for ages 26–35, and three classes for ages 36+. Each age group presents classes of nonsmokers and persistent heavy smokers. Intermediate to these ends of the spectrum, each age group exhibited its own smoking classes characterized by the extent of pregnancy smoking reductions and postpartum behavior. In all three age groups, class membership can be distinguished by individual sociodemographic and behavioral characteristics. Co-resident smokers predicted nearly all smoking classifications across age groups, and selected neighborhood characteristics predicted classification of younger (15–25) and older (36+) mothers.
Conclusions
The design, timing, and delivery of smoking prevention and cessation services, for women seeking to become pregnant and for women presenting for prenatal or pediatric care, are best guided by individual characteristics, particularly maternal age, preconception alcohol consumption, and postpartum depression, but neighborhood characteristics merit further attention for mothers at different ages.
Introduction
Given the documented harmful effects of maternal smoking to women themselves (U.S. DHHS, 2001) and their children (U.S. DHHS, 2006), prenatal and postpartum smoking abstinence and cessation are important targets for women (e.g., Healthy People 2020). Although smoking rates decline from 22% in the first trimester to 14% by the second trimester of pregnancy, by the time the child is 18 months old maternal smoking rates (30%) rebound close to rates of their childless peers (33%) (Substance Abuse and Mental Health Services Administration, 2009). Further, while the smoking rate among all women of reproductive age (WRA) fell from 30.7% to 26.7% between 2002 and 2010, the rate among pregnant women failed to decline significantly (from 18.0% to 16.3%) over the same period (Substance Abuse and Mental Health Services Administration, 2011).
Recent studies have enhanced our understanding of longitudinal profiles of maternal smoking during and following pregnancy. Among British mothers in the early 1990s (with child surviving to one year of age), approximately 33% were smokers from 3 months prior to conception through 33 months postpartum (Munafo, Heron, & Araya, 2008). Nearly 18% of the sample persisted in smoking throughout the study period, and about 10% who smoked prior to conception quit at some point during pregnancy and then resumed smoking by 8 months post-delivery. Less than 4% quit during pregnancy and stayed quit through the study period. Mothers of a 2001 U.S. birth cohort showed similar patterns, although the initial smoking rate was slightly lower (23%), and 5% of the sample initiated smoking following the pregnancy (Mumford, Hair, Yu, & Liu, 2013). While efforts to effect behavioral change are often concentrated during pregnancy when smoking cessation interventions are moderately successful (Lumley et al., 2009), the rate of relapse post-delivery requires further investigation to inform effective design and targeting of ongoing prevention and cessation efforts (Colman & Joyce, 2003; Phillips et al., 2010).
Overall, consistent with life course theory (Elder, 1998) as well as empirical evidence for maternal alcohol consumption (Jagodzinski & Fleming, 2007; Meschke, Holl, & Messelt, 2013), the literature indicates that maternal age is correlated with different patterns of adult mothers’ perinatal smoking. Younger pregnant women and recent mothers exhibit more instability of smoking behavior (characterized by quitting and relapsing) than older mothers. Younger mothers are more likely to smoke before (Tong et al., 2011) and during pregnancy (Crozier et al., 2009; Lu, Tong, & Oldenburg, 2001; Pevalin, Wade, Brannigan, & Sauve, 2001; Substance Abuse and Mental Health Services Administration, 2007), and are more likely to smoke a greater amount than older mothers (Martin et al., 2002). Further, mothers ages 20–29 are more likely to quit during pregnancy than mothers ages 30 and older (Colman & Joyce, 2003; Kahn, Certain, & Whitaker, 2002), and postpartum resumption of smoking behavior is more likely for mothers ages 20–24 (Tong et al., 2009). Current smoking among recent mothers ages 18–25 exceeds that of recent mothers ages 26–44 (Substance Abuse and Mental Health Services Administration, 2007). Despite this evidence, there has been no research investigating differences in developmental patterns of maternal smoking by age group, nor regarding how relevant personal and contextual characteristics associated with mothers’ smoking behavior may differ between maternal age groups. These are gaps that are critical to designing effective smoking prevention and cessation services.
In addition to targeting maternal age groups, prevention and cessation efforts benefit from identifying malleable risk factors. Maternal behaviors that predict perinatal smoking include limited or no breastfeeding (Hauge, Torgersen, & Vollrath, 2012; Kendzor et al., 2010; L. T. Martin et al., 2008; Page, Padilla, & Hamilton, 2012) and alcohol consumption (Holtrop et al., 2010; Martin et al., 2008). Maternal depression predicts smoking during pregnancy (Hauge et al., 2012; Linares Scott, Heil, Higgins, Badger, & Bernstein, 2009) and postpartum (Munafo et al., 2008). While some research has found current maternal employment to be a protective characteristic (Havens, Simmons, Shannon, & Hansen, 2009), other studies found no association with smoking during pregnancy (Gilman, Breslau, Subramanian, Hitsman, & Koenen, 2008). Additional correlates (documented in a multitude of studies) before, during and/or after pregnancy include education, income, marital status, and race/ethnicity (Holtrop et al., 2010; Lu et al., 2001; Page et al., 2012).
While the accepted wisdom is that individual characteristics are key proximal determinants of health essential to ecological models, contextual characteristics likely play some role (Macintyre, Ellaway, & Cummins, 2002; Pickett & Pearl, 2001). Glass and McAtee (2006) characterize the development of health actions or behaviors over the stream of time — potentially marked and influenced by turning points such as pregnancy and the transition to parenthood (Rutter, 1996) as well as individual age (Pickett & Pearl, 2001) — with social structures influencing the quality, direction, and intensity of health behaviors. Stressful economic conditions such as individual and area unemployment and poverty are fundamental mechanisms by which neighborhood contexts may influence smoking behavior (Kendzor et al., 2012; Lantz & Pritchard, 2010; Nkansah-Amankra, 2010; Schempf, Strobino, & O’Campo, 2009). Although maternal smoking during pregnancy moderates the effect of neighborhood variables on child outcomes (Nkansah-Amankra, 2010; Rich-Edwards, Buka, Brennan, & Earls, 2003), few neighborhood studies of pregnant women are designed to focus on maternal smoking behavior as a risk factor in their own health profiles. Embedded within the broader community context, household context is known to shape individual smoking profiles (Gage, Everett, & Bullock, 2007). Smoking by a partner or other household members (Gage et al., 2007; Kahn et al., 2002; Martin et al., 2008; Page et al., 2012; Park, Tudiver, Schultz, & Campbell, 2004) has consistently been shown to increase the likelihood of smoking during pregnancy.
While the above cited body of literature is informative, there remain crucial research gaps. First, studies of maternal smoking behavior usually focus on smoking during pregnancy, with a few studies including the postpartum period immediately after the delivery of the child. Despite the secondhand smoke risks for children (Datta et al., 2006; DiFranza, Masaquel, Barrett, Colosia, & Mahadevia, 2012), only a few studies extend examination of maternal smoking into the early parenting years (Kahn et al., 2002; Mumford et al., 2013; Munafo et al., 2008). Further, past studies have tended to examine all adult mothers as a group (Holtrop et al., 2010; Mumford et al., 2013) and/or included age as a covariate (e.g., Maxson, Edwards, Ingram, & Miranda, 2012; Page et al., 2012), limiting the ability to detect important differences in the developmental patterns of maternal smoking as well as risk factors of perinatal smoking by maternal age. Additionally, no studies to our knowledge have examined the ecological context of mothers’ longitudinal smoking behavior. Moreover, only a handful of studies examine quantity smoked during pregnancy (Kandel, Griesler, & Schaffran, 2009; Pickett, Wakschlag, Dai, & Leventhal, 2003; Pickett, Wakschlag, Rathouz, Leventhal, & Abrams, 2002; Schempf et al., 2009; Troe et al., 2008), none of which examined preconception or postpartum smoking behavior. Studies that have included postpartum smoking did not examine patterns of quantity smoked (Colman & Joyce, 2003; Fingerhut, Kleinman, & Kendrick, 1990; Kahn et al., 2002; Mumford et al., 2013; Munafo et al., 2008). Finally, most studies use a convenience sample, limiting the generalizability of study results.
In an attempt to address these gaps, the purpose of the current study is to provide a broader picture of women’s longitudinal smoking patterns (taking into account quantity smoked), and the individual and contextual correlates thereof, from preconception through entry of the child to kindergarten separately for three groups of mothers who gave birth at different ages, with the benefit of a large nationally representative study sample. The separation of maternal age groups not only allows us to study how patterns of maternal smoking differ, but also facilitates detection of how individual and neighborhood characteristics differentially distinguish longitudinal maternal smoking patterns across age groups.
Methods
Data and Sample
The study sample comes from the Early Childhood Longitudinal Study (ECLS-B), nationally representative of the 2001 U.S. birth cohort. Data on 10,700 children and their parents were collected when the child was approximately 9 months old (baseline) and follow-up surveys were conducted at 2 years (2003–04), 4 years (2005–06, preschool sample), and 5 or 6 years of age (2006–07, samples allowing for staggered entry into kindergarten). At baseline, respondents were queried about their behavior in the three months prior to conception and during the third trimester of pregnancy. A sample of 9,150 cases (counts rounded to the nearest 50 in compliance with the ECLS-B confidentiality rules per National Center for Education Statistics, accessed June 8, 2012) were selected based on the following criteria. First, to collect pre-childbirth smoking behavior, only biological mothers were included. Second, the respondent had a valid measure on at least one of the six smoking measures. Third, the respondent had valid measures on all the covariates included in the analysis. We grouped respondents by baseline maternal age as follows: ages 15 to 25, 26 to 35, and age 36 or older. This categorization was motivated by theoretical reasoning, closely matches the typical categorization used in past empirical studies on maternal perinatal substance use (Meschke et al., 2013; Turney, 2012), and considered the empirical distribution of the sample. An attrition analysis conducted separately for each age group revealed no substantial differences between selected and unselected subjects on self-reported smoking behavior or covariates included in the analysis (detailed results available upon request).
Outcome variables: Longitudinal measures of maternal smoking
Those respondents who reported having ever smoked 100 cigarettes at the baseline interview were asked about average daily quantity smoked in terms of cigarettes per day (CPD) for the six time periods of the study. For the preconception and third trimester retrospective measures, respondents were prompted to think about the specified three-month periods. Post-delivery of the child, smoking measures are current reports of ‘how many cigarettes or packs per day’ the respondent reports smoking. To manage the low response frequency of higher smoking quantities likely to pose problems for model estimation (e.g. over 90% of women in the youngest age group smoked zero or only one CPD during pregnancy, and this percentage is larger for the two older age groups) while still maintaining an ordinal scale, a four-level categorical ordinal variable was created for each of the six time points: never smokers (0); very light smokers (Husten, 2009) who smoked ≤5 CPD (1); smokers of 6–10 CPD (2); and smokers of >10 CPD (3).
Individual-level covariates
Mother’s race was coded as white (0); black (1); Hispanic (2); Asian-American or other (3) (Ebrahim & Gfroerer, 2003; Yang, Shoff, Noah, Black, & Sparks). Mother’s household income was coded as <100% of the federal poverty threshold (0); 100–130% of the federal poverty threshold (1); 130–185% of the threshold (2); and greater than 185% of the federal poverty threshold (3), reflecting common federal aid eligibility requirements and prior maternal smoking research (Mumford et al., 2013). Mother’s education at baseline was coded as less than a high school diploma (0); high school graduate (1); or some college (2); with a college or graduate degree (3) (Kandel et al., 2009; Patterson, Seravalli, Hanlon, & Nelson, 2012). Mother’s marital status at baseline was coded as married or living together (1) or other relationship status (0) (Carmichael & Ahluwalia, 2000). We distinguished past year employment status as not employed (0) compared to part-time (1) and full-time (2) schedules (Cooklin, Donath, & Amir, 2008). Maternal postpartum depression at baseline is measured by a modified 12-item version of the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977). Following Paulson et al. (2009), a binary depression indicator was coded “moderate to severe depression” (1) if a total score (the average of all responses for individuals who answered at least nine out of 12 items) was at least 10. Preconception alcohol use was coded as an indicator of consumption (1) or not (0) (Pevalin et al., 2001). A categorical variable distinguishes having breastfed the child for 6 months or less (1); >6 months (2); or not at all (0) (Ogbuanu, Glover, Probst, Liu, & Hussey, 2011).
Contextual covariates
We included an indicator for the presence of one or more additional household smokers (coded as 1) as identified by CDC’s maternal health indicators for pregnancy (Prevention, 2011). Regarding community context, ECLS-B respondents are coded by zip code. To access summary Census measures (consistent with neighborhood research of birth outcomes (Messer et al., 2006)), we assigned a 2000 U.S. Census tract ID to each sample respondent. Using MapInfo Pro, we determined the geographic center of each ZIP code and applied a geographic merge of the ZIP code centroid and the 2000 Census Tract boundaries. Our primary focus was the economic conditions of the neighborhood, captured through Census tract-level measures of the percentage of (a) households reporting income below the federal poverty level; (b) residents age 25 or older reporting a high school education or less; and (c) unemployment among residents age 16 or older. As additional proxies of household economic constraints, we also include the percentage (e) of female-headed households and (f) reporting a residential move within the past year. These measures were dichotomized at the weighted median for each maternal age group. Individual and contextual sample characteristics are reported in Table 1.
Table 1.
Weighted distribution of individual and contextual covariates, by maternal age Early Childhood Longitudinal Study-Birth Cohort (ECLS-B)
| Age 15–25 (N=3400) | Age 26–35 (N=4400) | Age 36+ (N=1300) | p-value | |
|---|---|---|---|---|
|
|
||||
| White | 49.4% | 64.1% | 70.1% | <0.001 |
| Black | 20.3% | 10.5% | 9.8% | <0.001 |
| Hispanic | 25.4% | 18.8% | 14.7% | <0.001 |
| Asian-American or other | 4.9% | 6.6% | 5.3% | <0.001 |
| Married or living together | 65.3% | 90.2% | 92.6% | <0.001 |
| Less than a high school diploma | 33.0% | 9.8% | 6.3% | <0.001 |
| High school gradute | 38.9% | 23.3% | 17.3% | <0.001 |
| Some college | 24.2% | 32.5% | 26.7% | <0.001 |
| Having a college degree or above | 3.9% | 34.4% | 49.7% | <0.001 |
| Not employed | 52.9% | 43.8% | 43.1% | <0.001 |
| Part-time employment | 18.3% | 21.6% | 22.0% | <0.001 |
| Full-time employment | 28.8% | 34.6% | 34.9% | <0.001 |
| Moderately/severely depressed | 22.4% | 13.6% | 12.7% | <0.001 |
| Houshold income <100% poverty line | 38.2% | 14.9% | 10.7% | <0.001 |
| Household income 100–130% poverty line | 17.2% | 8.8% | 6.0% | <0.001 |
| Household income 130–185% poverty line | 15.9% | 10.9% | 8.1% | <0.001 |
| Household income above 185% poverty line | 28.8% | 65.4% | 75.3% | <0.001 |
| Ever breast fed child | 59.0% | 74.0% | 78.4% | <0.001 |
| Drink alcohol before pregnancy | 31.3% | 42.0% | 49.9% | <0.001 |
| Other smokers in household | 37.4% | 19.2% | 18.2% | <0.001 |
| % below poverty in neighborhood | 50.3% | 50.0% | 50.0% | <0.001 |
| % high school or less in neighborhood | 50.0% | 49.9% | 50.2% | <0.001 |
| % moved within past year in neighborhood | 50.0% | 50.0% | 50.0% | <0.001 |
| % female headed households in neighborhood | 49.8% | 50.0% | 49.9% | <0.001 |
| % unemployed in neighborhood | 50.0% | 50.0% | 50.2% | <0.001 |
Analytic strategy
We used general growth mixture modeling (GGMM) (Muthén, 2004) to empirically identify developmental trajectories of maternal cigarette smoking before, during and after pregnancy. GGMM uses a categorical latent class variable in combination with continuous growth factors to explore population heterogeneity in the change process of the outcome of interest, i.e., whether the study population consists of two or more discrete classes of individuals with varying growth trajectories (Muthén, 2004; Petras & Masyn, 2010). Analyses were conducted separately for each age group.
In order to determine the functional form for individual change in our ordered categorical smoking outcome (Masyn, Petras, & Liu, 2013), we first estimated a series of growth models with fixed or random intercept, linear and nonlinear slopes (Petras & Masyn, 2010). The origin of time was set at the baseline (i.e., 9 months after delivery) interview in order to accommodate inclusion of covariates measured at that time point. Thus, the methods support the association of baseline covariates with subsequent smoking measures (Biesanz, Deeb-Sossa, Papadakis, Bollen, & Curran, 2004). We compared these preliminary models for model fit, parsimony and interpretability, with final model selection based on theoretical reasoning as well as standard criteria recommended by Masyn et al. (2013) such as the Bayesian Information Criterion (BIC) (Schwarz, 1978) and the Lo-Mendell-Rubin (LMR) likelihood ratio chi-square test (Lo, Mendell, & Rubin, 2001). We found that a functional form with a random intercept and a random slope achieved optimal fit for all three age groups. Heterogeneity in the longitudinal development of maternal smoking was then explored by estimating models with increasing numbers of classes. Once the best fitting GGMM model was selected, we modeled the association of individual and contextual covariates with class membership, via categorical logistic regression (Long & Cheng, 2004).
We accounted for missing data on smoking measures over time through full information maximum likelihood (FIML) estimation (Arbuckle, 1996; Schafer & Graham, 2002). Among the approximately 9,050 mothers included in the final analysis, over 60% had valid information on all six smoking measures and 20% missed only one smoking measure. Complex sampling design was accounted for by computing robust standard errors using a sandwich estimator (White, 1980). Results were weighted to represent the 2001 birth cohort. Separate analyses for each age group were conducted using Mplus version 6.12 (Muthén & Muthén, 1998–2012).
Results
Patterns of smoking across three age groups
As shown in Table 2, older mothers were less likely to smoke than younger mothers across the study period. Mothers of all three age groups reduced smoking substantially during pregnancy, but relapsed to various degrees after child delivery. For mothers 15–25, the percentage of very light smokers (≤5 CPD) 5–6 years post-delivery is as high as their preconception rates, while the percentage smoking >5 CPD never fully rebounded postpartum. Likewise, the rates of mothers ages 26+ smoking >5 CPD also never fully returned to preconception rates. The rate of very light smoking among mothers ages 36+ fluctuated within a tight range over the full study period. For the most part, declines in the proportion of mothers smoking >10 CPD from preconception through the study period were greater than declines in the proportion of mothers smoking 6–10 CPD. For example, the proportion of mothers ages 26–35 reporting 6–10 CPD declined 15% between preconception and the child’s entry to preschool, whereas the proportion of this age group reporting >10 CPD declined 32% over the same period, suggesting overall reductions in quantity smoked.
Table 2.
Weighted distribution of smoking behavior (6 time points), by maternal age Early Childhood Longitudinal Study-Birth Cohort (ECLS-B)
| Age 15–25 (N=3400) | Age 26–35 (N=4400) | Age 36+ (N=1300) | p-value | |
|---|---|---|---|---|
|
|
||||
| 3 months before pregnancy | ||||
| Non-smoker | 64.7% | 81.2% | 86.5% | <0.001 |
| Smoking ≤ cigarettes per day (CPD) | 8.5% | 4.9% | 3.6% | <0.001 |
| Smoking 6–10 CPD | 13.4% | 6.0% | 4.6% | <0.001 |
| Smoking >10 CPD | 13.4% | 7.9% | 5.3% | <0.001 |
| Last 3 months of pregnancy | ||||
| Non-smoker | 84.1% | 91.0% | 93.4% | <0.001 |
| Smoking ≤5 CPD | 8.0% | 4.0% | 2.8% | <0.001 |
| Smoking 6–10 CPD | 5.1% | 2.9% | 3.1% | <0.001 |
| Smoking >10 CPD | 2.9% | 2.1% | 0.7% | <0.001 |
| 9 months post-partum | ||||
| Non-smoker | 70.7% | 84.5% | 89.2% | <0.001 |
| Smoking ≤5 CPD | 8.1% | 4.7% | 3.4% | <0.001 |
| Smoking 6–10 CPD | 11.6% | 5.3% | 3.2% | <0.001 |
| Smoking >10 CPD | 9.6% | 5.5% | 4.2% | <0.001 |
| 2 years post-partum | ||||
| Non-smoker | 70.6% | 85.1% | 90.1% | <0.001 |
| Smoking ≤5 CPD | 8.2% | 4.3% | 2.4% | <0.001 |
| Smoking 6–10 CPD | 12.1% | 5.0% | 3.9% | <0.001 |
| Smoking >10 CPD | 9.1% | 5.7% | 3.6% | <0.001 |
| Child in Preschool | ||||
| Non-smoker | 70.1% | 86.0% | 90.2% | <0.001 |
| Smoking ≤5 CPD | 8.0% | 3.5% | 1.6% | <0.001 |
| Smoking 6–10 CPD | 11.9% | 5.1% | 3.7% | <0.001 |
| Smoking >10 CPD | 10.1% | 5.4% | 4.5% | <0.001 |
| Child in Kindergarten | ||||
| Non-smoker | 70.5% | 88.3% | 90.8% | <0.001 |
| Smoking ≤5 CPD | 8.7% | 3.1% | 3.0% | <0.001 |
| Smoking 6–10 CPD | 11.7% | 3.5% | 2.8% | <0.001 |
| Smoking >10 CPD | 9.2% | 5.2% | 3.4% | <0.001 |
Profiles of smoking behavior for three age groups
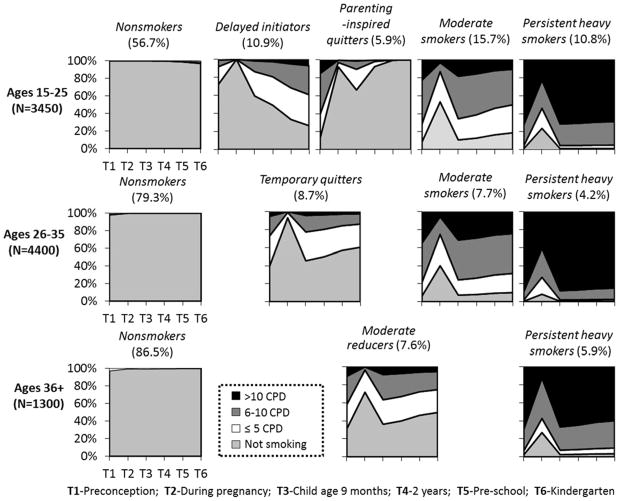
Fit indices for models with different number of classes are presented in Table 3. The selection of the best solution is based on statistical criteria (e.g. the diminishing return of reduction in BIC with additional parameters) as well as substantive considerations. A 5-class solution was selected for age group 15–25 (LL=−10890.987 (31), BIC=22034.504); a 4-class solution was selected for age group 26–35 (LL=−7946.09 (25), BIC=16102.04); and a 3-class solution was selected for age group 36+ (LL=−1827.227 (19), BIC=3790.788). As shown in Figure 1, a “nonsmoker” class was identified for all three age groups, with different class proportions (ages 15–25: 56.7%; ages 26–35: 79.3%; ages 36+: 86.5%). In each age group, mothers with a significant likelihood of ongoing heavy smoking were classified as “persistent heavy smokers” (with a tendency for reduced smoking during pregnancy). Across age groups, about 70–80% of the members in the persistent heavy smoker class smoked >10 CPD and an additional 10–30% smoked between 6 and 10 CPD preconception and after delivery (lower percentages were observed during pregnancy).
Table 3.
Determining the number of classes in GGMM, by maternal age
| Model | Log Likelihood | # of free parameters | BICa | VLMR-LRTb P-value |
|---|---|---|---|---|
| Ages 15–25, N=3450 | ||||
| 1-class | −15745.173 | 7 | 31547.369 | N/A |
| 2-class | −11713.949 | 13 | 23533.797 | 0.3333 |
| 3-class | −11164.301 | 19 | 22483.379 | 0 |
| 4-class | −10993.005 | 25 | 22189.663 | 0 |
| 5-class | −10890.987 | 31 | 22034.504 | 0.7602 |
| 6-class | −10817.664 | 37 | 21936.734 | 0.2398 |
| Age 26–35, N=4400 | ||||
| 1-class | −13361.81 | 7 | 26782.39 | N/A |
| 2-class | −8603.746 | 13 | 17316.62 | 0 |
| 3-class | −8090.027 | 19 | 16339.55 | 0 |
| 4-class | −7946.09 | 25 | 16102.04 | 0 |
| 5-class | −7896.197 | 31 | 16052.62 | 0.2873 |
| 6-class | −7859.964 | 37 | 16030.52 | 0 |
| Age 36+, N=1300 | ||||
| 1-class | −3088.367 | 7 | 6226.963 | N/A |
| 2-class | −1957.712 | 13 | 4008.705 | 0.0007 |
| 3-class | −1827.227 | 19 | 3790.788 | 0.6765 |
| 4-class | −1797.673 | 25 | 3774.734 | 0.1176 |
| 5-class | −1770.275 | 31 | 3762.99 | 0.7602 |
| 6-class | −1749.586 | 37 | 3764.664 | 0.0668 |
Bayesian Information Criterion.
Lo-Mendell-Rubin likelihood ratio chi-square test.
Figure 1.
Predictive smoking behavior conditional class solutions, by maternal age
Between nonsmokers and persistent heavy smokers, each age group exhibit related intermediate smoking patterns with slight variations characterized by the extent of their smoking reductions during pregnancy and the patterns followed postpartum. “Moderate smokers” (with a strong tendency to reduce smoking during pregnancy) constituted 15.7% of mothers ages 15–25 and 7.7% of mothers ages 26–35. Among mothers ages 36+, this class is termed “moderate reducers” (7.6%) to reflect a tendency for smoking reduction and cessation throughout the study period, following significant reductions during pregnancy. The postpartum pattern of moderate reducers (ages 36+) is mirrored by a class of “temporary quitters” (8.7%) among mothers ages 26–35, whose cessation during pregnancy is much more prominent. For the youngest age group exhibiting very little smoking during pregnancy, two opposing patterns are evident: “delayed initiators” (10.9%) who for the most part adopt smoking behavior after the child is born, and “motherhood-inspired quitters” (5.9%) who largely quit during pregnancy, may smoke lightly while the child is very young, but tend to have fully quit smoking by the time the child is entering preschool.
Covariate effects for three age groups
Table 4 presents the role of individual and neighborhood covariates associated with being assigned to the smoking classes versus the non-smokers class as the reference on an odds ratio scale. Among the individual sociodemographic characteristics, as expected, a college education, being married or cohabiting with a partner, and being non-White tends to be protective in terms of smoking classification. An indicator of adolescent mothers (ages 15–17) illustrates that this characteristic is protective for moderate smoking compared to nonsmoking (AOR=0.34), but does not distinguish the other smoking classes from nonsmokers. Household income effects are apparent only for the youngest mothers, in the expected direction. And among these youngest mothers, part-time employment predicts moderate smoking behavior (AOR=1.48). Moderate/severe depression, by contrast, exhibited stronger effects for older mothers. Depression increases the odds of being classified as persistent heavy smokers for mothers ages 36+ and as moderate smokers for mothers ages 15–35, although the size of these effects varies. Being depressed at baseline for the two younger age groups increased the odds of being in the moderate smoker class by less than two-fold (age 15–25: AOR=1.48; age 26–35: AOR=1.94), while the strength of this association between depression and persistent heavy smoking is more than twice that for the oldest age group (AOR=4.5).
Table 4.
Adjusted Odds Ratios of Maternal Smoking Class Membership for Individual and Contextual Covariates, ECLS-B
| Ages 15–25,
N=3450 |
Ages 26–35,
N=4400 |
Ages 36+,
N=1300 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
Delayed initiators (10.9%) |
Parenting- inspired quitters (5.9%) |
Moderate smokers (15.7%) |
Persistent heavy smokers (10.8%) |
Temp- orary quitters (8.7%) |
Moderate smokers (7.7%) |
Persistent heavy smokers (10.8%) |
Moderate reducers (7.6%) |
Persistent heavy smokers (5.9%) |
|
| Age 15–17 | – | – | 0.34* | – | n/a | n/a | n/a | n/a | n/a |
| Some college | – | – | 0.32** | 0.28** | – | 0.35* | 0.27** | – | – |
| College or graduate degree | fixed | – | 0.12** | Fixed | 0.41** | 0.12** | 0.03** | – | – |
| Married or living together | 0.62* | 0.48* | – | 0.54* | 0.49* | 0.28** | 0.34* | – | 0.00** |
| Part-time employed | – | – | 1.58* | – | – | – | – | – | – |
| Full-time employed | – | – | – | – | – | – | – | – | – |
| Black | 0.46* | – | 0.22** | 0.03** | – | 0.30* | 0.02** | – | – |
| Hispanic | 0.23** | 0.32** | 0.12** | 0.03** | – | 0.05** | 0.00** | – | 0.01** |
| Asian or Other | – | – | – | 0.37** | – | – | – | – | – |
| Depression | – | – | 1.48* | – | – | 1.94** | – | – | 4.50* |
| Income 100–130% poverty | 0.61* | – | – | – | – | – | – | – | – |
| Income 130–185% poverty | – | – | – | – | – | – | – | – | – |
| Income >185% poverty | – | – | – | 0.43** | – | – | – | – | – |
| Breastfed child <6 months | – | – | – | – | – | – | 0.46** | – | – |
| Breastfed child >6 months | 0.42** | 0.40* | 0.27** | 0.19** | – | 0.33** | – | – | 0.03** |
| Drinking before pregnancy | 2.90** | 4.00** | 2.83** | 5.29** | 3.06** | 1.93** | 2.20** | 2.80* | – |
| Others smoking in household | 1.75* | – | 4.55** | 5.21** | 5.13** | 8.53** | 9.10** | 4.94** | 583.47** |
| % below poverty | – | – | – | – | – | – | – | 0.20* | – |
| % high school or less | – | – | – | – | – | – | – | 9.05** | – |
| % moved in past year | – | – | – | – | – | – | – | – | – |
| % female headed households | – | – | – | – | – | – | – | – | 0.42* |
| % unemployed | – | – | – | 0.60* | – | – | – | – | – |
Non-significant results not displayed.
<0.05
<0.01
Behavioral covariate effects are consistently in the expected direction. Reported breastfeeding for more than six months was a significant protective characteristic for maternal smoking for all three age groups with various levels of strength. Likewise, preconception alcohol consumption was a consistent risk factor for maternal smoking in all age groups. However, while younger mothers were less likely to drink alcohol before pregnancy compared to older mothers (see Table 2), preconception drinking tended to have a stronger negative influence on their smoking behavior. Mothers ages 15–25 who drank before pregnancy were over five times as likely to be assigned to the persistent smokers class compared to those who did not (AOR=5.29). This association is weaker for mothers 26–35 (AOR=2.2) and non-significant for those who gave birth at age 36 or older.
The contextual predictors can be interpreted and compared across age groups in a similar pattern. For all three age groups, living with one or more other smokers in the home significantly increases the odds of being classified in any smoking class, except for the parenting-inspired quitters ages 15–25. The strength of the association varies across age groups, with effects generally larger for persistent smokers. Significant neighborhood-level covariates distinguish the youngest mothers and the oldest mothers, but not mothers ages 26–35. Mothers ages 15–25 living in neighborhoods with more unemployment were less likely to be heavy smokers (AOR=0.6). For mothers ages 36+, living in neighborhoods with more poverty and female-headed households were less likely to be moderate (AOR=0.2) or heavy smokers (AOR=0.42), respectively. By contrast for these older mothers, living in a neighborhood with a higher proportion of residents having at most a high school education increases the odds of being a persistent heavy smoker over nine-fold (AOR=9.05).
Discussion
Overall, the aggregate smoking rates among mothers of a national representative birth cohort show higher rates of smoking at younger ages over a 6-year span from preconception to child’s kindergarten entry, consistent with preconception data from the Pregnancy Risk Assessment Monitoring System (Tong et al., 2011). Our analyses revealed different class structures across maternal age groups. Younger mothers are more likely to smoke before pregnancy and to return to preconception level after child delivery, as shown in other research (Crozier et al., 2009; Lu et al., 2001; Pevalin et al., 2001; Substance Abuse and Mental Health Services Administration, 2007; Tong et al., 2011). While population trends indicate declines in smoking among WRA over the past decade (Hayes, Fan, Smith, & Bombard, 2011), tobacco marketing targeting different age groups may further impact age-specific trends (Tong et al., 2009).
The difference in class structures by maternal age groups is also reflected in the percentage of the sample assigned to each class across the three age groups. The probability of mothers ages 15–25 being classified in a smoking pattern was at least twice that of mothers ages 26 and older. In addition, two unique classes emerged for mothers ages 15–25: those who appeared inspired by pregnancy and parenting an infant to successfully stay quit as the child develops, and those who initiated smoking following childbirth. Nearly 11% of younger mothers who tended to be nonsmokers prior to the pregnancy adopted smoking behavior postpartum, a finding consistent with past studies of their peers (Webb, Culhane, Mathew, Bloch, & Goldenberg, 2011). This pattern has important implications for primary prevention services during pregnancy and early pediatric care contacts, as well as for extending the evaluation of smoking cessation interventions beyond pregnancy and the six-month postpartum period.
Second, our results indicate that in some instances individual predictors of heterogeneous patterns vary across maternal age groups. For example, we found that drinking alcohol before pregnancy has a stronger relationship with persistent smoking for younger mothers than for older mothers. While prior research has linked alcohol consumption with perinatal smoking (Holtrop et al., 2010; Martin et al., 2008), our finding of an age difference is consistent with general population data on the diminishing association between smoking and drinking with advancing age (Falk, Yi, & Hiller-Sturmhofel, 2006). Conversely, we found that the association between persistent smoking and postpartum depression became stronger as mothers age. A positive relationship between depression and smoking is consistent with past research (Hauge et al., 2012; Linares Scott et al., 2009; Munafo et al., 2008), but our study extends the literature by distinguishing the differential strength of association across age groups.
Third, we found that neighborhoods with low average educational attainment were associated with persistent smoking behavior before, during and after pregnancy for mothers ages 36 and older. This is consistent with the general finding that disadvantaged neighborhoods are associated with significantly greater probability of smoking in a middle-aged Australian population and reduced likelihood of smoking cessation over time (Turrell, Hewitt, & Miller, 2012). However, some neighborhood characteristics that might be considered to be disadvantageous had protective effects for smoking behavior among mothers ages 15–25 (neighborhoods with more unemployment) and mothers ages 36+ (neighborhoods with more poverty and female-headed households). It is important to note that these relationships hold while controlling for an array of individual covariates including personal SES as well as other neighborhood contextual variables. These results bear further investigation, principally in terms of personal economic constraints, as well as potentially different cultural norms within neighborhoods of these descriptions. For example, research has shown associations between neighborhood walkability and interpersonal civility (Messer, Vinikoor-Imler, & Laraia, 2012) as well as perceived safety (Patterson et al., 2012) with maternal smoking behaviors. Our results highlight the importance of avoiding assumptions about the nature of perceived advantages and disadvantages in neighborhood research.
Limitations
Although the ECLS-B self-reported measures of smoking have no biological verification, reports of perinatal and maternal smoking behavior are highly correlated with smokers’ urinary cotinine levels (Ashford et al., 2010; Patterson et al., 2012; Pickett, Kasza, Biesecker, Wright, & Wakschlag, 2009) as well as urinary cotinine measures from co-resident children (Ino, Ohtani, & Yoshimi, 2011). However, data for the preconception and pregnancy time points (collected at the baseline interview, or approximately 9 months post-childbirth) may be subject to recall bias. Our data did not support linkage to block-level Census data; however, the distinction of area measurement at the tract vs. block level did not impact the positive relationship between neighborhood and pregnancy smoking (Messer et al., 2012). Further research to distinguish the impact of block-level measurements is warranted.
Implications
The finding that maternal smoking behavior in the perinatal period varies with maternal age indicates that prevention and treatment efforts need to reflect the different life stages of mothers. Several additional implications can be drawn from this research. First, it is concerning that a sizable group of women smoke persistently (with a high likelihood of smoking 6–10 or >10 CPD) across the perinatal and early parenthood period, especially given the consistent dose-response relationship for both maternal and child health outcomes (Clifford, Lang, & Chen, 2012; U.S. DHHS, 2006) and the risks of even light smoking for numerous poor health outcomes (Schane, Ling, & Glantz, 2010). The individual and contextual variables found to predict class membership (with variation in significance and strength across age groups) inform the early identification of these women. Further, prevention and cessation for women seeking to get pregnant and those presenting for prenatal or pediatric care can be guided by these individual and contextual characteristics to inform the design, timing, and delivery. Of particular interest to these goals are preconception alcohol consumption and postpartum depression, which distinguish mothers’ smoking behavior at different ages and serve both as identifying characteristics to target mothers at risk of persistent smoking and malleable characteristics for direct interventions. Although national rates of postpartum depression are similar for adult mothers across the reproductive age span (Vesga-Lopez et al., 2008), pregnancy rates have been increasing among older mothers (Ventura, Curtin, Abma, & Henshaw, 2012). Thus, cessation treatment for older mothers in particular may benefit from a comprehensive care approach involving mental health services for the benefit of both maternal and infant outcomes (Goler et al., 2012).
Second, it would be fruitful to identify and to develop effective prevention strategies for the group of young mothers (age 15–25) who initiated smoking after delivery. Multiple characteristics (race, marital status, income, preconception drinking, breastfeeding behavior, and co-resident smokers) distinguish delayed initiators from non-smokers. Young mothers who drank alcohol before pregnancy were three times as likely, and those who breastfed their child longer than 6 months were half as likely, to follow the pattern of delayed initiation. In addition, whites were at a much higher risk to follow this pattern than nonwhites. Such information may help clinicians identify likely members of this class during gynecological or perinatal care visits. While the development of postpartum cessation services is important and encouraging (Winickoff et al., 2005; Winickoff et al., 2010), attention to postpartum prevention is also warranted.
Third, living with smoking household members consistently increased the likelihood of following a persistent smoking trend and leads to a higher risk of returning to smoking after delivery. The literature on interventions for partners of pregnant women, which include a broad array of designs, shows mixed results (Hemsing, Greaves, O’Leary, Chan, & Okoli, 2012). Refined approaches that access co-resident smokers’ motivation to support their partner’s and their own cessation efforts, in addition to extending the timeframe of efforts through early childhood parenting, may make greater headway (Duckworth & Chertok, 2012; Gage et al., 2007). Overall, while we found that individual characteristics are consistent correlates of maternal perinatal smoking behavior — suggesting the importance of continued public health efforts targeting individual behavior and clinical support tailored to the individual — household level and neighborhood level contextual effects merit more attention for distinct maternal age groups.
Contributor Information
Elizabeth A. Mumford, Email: mumford-elizabeth@norc.org.
Weiwei Liu, Email: liu-weiwei@norc.org.
References
- Arbuckle JL. Full information estimation in the presence of incomplete data. Advanced structural equation modeling: Issues and techniques. 1996:243–277. doi: 10.1177/01466216980221008. [DOI] [Google Scholar]
- Ashford KB, Hahn E, Hall L, Rayens MK, Noland M, Collins R. Measuring prenatal secondhand smoke exposure in mother–baby couplets. Nicotine & Tobacco Research. 2010;12(2):127–135. doi: 10.1093/ntr/ntp185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesanz JC, Deeb-Sossa N, Papadakis AA, Bollen KA, Curran PJ. The role of coding time in estimating and interpreting growth curve models. Psychological methods. 2004;9(1):30–52. doi: 10.1037/1082-989x.9.1.30. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Ahluwalia IB. Correlates of postpartum smoking relapse. Results from the Pregnancy Risk Assessment Monitoring System (PRAMS) Am J Prev Med. 2000;19(3):193–196. doi: 10.1016/s0749-3797(00)00198-7. S0749379700001987 [pii] [DOI] [PubMed] [Google Scholar]
- Clifford A, Lang LD, Chen RL. Effects of maternal cigarette smoking during pregnancy on cognitive parameters of children and young adults: A literature review. Neurotoxicology And Teratology. 2012;34(6):560–570. doi: 10.1016/j.ntt.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Colman GJ, Joyce T. Trends in smoking before, during, and after pregnancy in ten states. Am J Prev Med. 2003;24(1):29–35. doi: 10.1016/s0749-3797(02)00574-3. S0749379702005743 [pii] [DOI] [PubMed] [Google Scholar]
- Cooklin AR, Donath SM, Amir LH. Maternal employment and breastfeeding: results from the longitudinal study of Australian children. Acta Pædiatrica. 2008;97(5):620–623. doi: 10.1111/j.1651-2227.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- Crozier SR, Robinson SM, Borland SE, Godfrey KM, Cooper C, Inskip HM. Do women change their health behaviours in pregnancy? Findings from the Southampton Women’s Survey. Paediatr Perinat Epidemiol. 2009;23(5):446–453. doi: 10.1111/j.1365-3016.2009.01036.x. PPE1036 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta GD, Subramanian S, Colditz GA, Kawachi I, Palmer JR, Rosenberg L. Individual, neighborhood, and state-level predictors of smoking among US Black women: a multilevel analysis. Social Science & Medicine. 2006;63(4):1034–1044. doi: 10.1016/j.socscimed.2006.03.010. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Masaquel A, Barrett AM, Colosia AD, Mahadevia PJ. Systematic literature review assessing tobacco smoke exposure as a risk factor for serious respiratory syncytial virus disease among infants and young children. Bmc Pediatrics. 2012;12 doi: 10.1186/1471-2431-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth AL, Chertok IRA. Review of Perinatal Partner-Focused Smoking Cessation Interventions. Mcn-the American Journal of Maternal-Child Nursing. 2012;37(3):174–181. doi: 10.1097/NMC.0b013e31824921b4. [DOI] [PubMed] [Google Scholar]
- Ebrahim SH, Gfroerer J. Pregnancy-related substance use in the United States during 1996–1998. Obstetrics & Gynecology. 2003;101:374–379. doi: 10.1016/s0029-7844(02)02588-7. [DOI] [PubMed] [Google Scholar]
- Elder GH., Jr The Life Course as Developmental Theory. Child Development. 1998;69(1):1–12. [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health. 2006;29(3):162–171. [PMC free article] [PubMed] [Google Scholar]
- Fingerhut LA, Kleinman JC, Kendrick JS. Smoking before, during, and after pregnancy. American Journal of Public Health. 1990;80(5):541–544. doi: 10.2105/ajph.80.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage JD, Everett KD, Bullock L. A review of research literature addressing male partners and smoking during pregnancy. Jognn-Journal of Obstetric Gynecologic and Neonatal Nursing. 2007;36(6):574–580. doi: 10.1111/J.1552-6909.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Breslau J, Subramanian SV, Hitsman B, Koenen KC. Social factors, psychopathology, and maternal smoking during pregnancy. Am J Public Health. 2008;98(3):448–453. doi: 10.2105/AJPH.2006.102772. AJPH.2006.102772 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass TA, McAtee MJ. Behavioral science at the crossroads in public health: extending horizons, envisioning the future. Soc Sci Med. 2006;62(7):1650–1671. doi: 10.1016/j.socscimed.2005.08.044. S0277-9536(05)00462-4 [pii] [DOI] [PubMed] [Google Scholar]
- Goler NC, Armstrong MA, Osejo VM, Hung YY, Haimowitz M, Caughey AB. Early Start A Cost-Beneficial Perinatal Substance Abuse Program. Obstetrics and gynecology. 2012;119(1):102–110. doi: 10.1097/AOG.0b013e31823d427d. [DOI] [PubMed] [Google Scholar]
- Hauge LJ, Torgersen L, Vollrath M. Associations between maternal stress and smoking: findings from a population-based prospective cohort study. Addiction. 2012;107(6):1168–1173. doi: 10.1111/j.1360-0443.2011.03775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens JR, Simmons LA, Shannon LM, Hansen WF. Factors associated with substance use during pregnancy: results from a national sample. Drug & Alcohol Dependence. 2009;99(1–3):89–95. doi: 10.1016/j.drugalcdep.2008.07.010. S0376-8716(08)00255-X [pii] [DOI] [PubMed] [Google Scholar]
- Hayes DK, Fan AZ, Smith RA, Bombard JM. Trends in Selected Chronic Conditions and Behavioral Risk Factors Among Women of Reproductive Age, Behavioral Risk Factor Surveillance System, 2001–2009. Preventing Chronic Disease. 2011;8(6) [PMC free article] [PubMed] [Google Scholar]
- Hemsing N, Greaves L, O’Leary R, Chan K, Okoli C. Partner Support for Smoking Cessation During Pregnancy: A Systematic Review. Nicotine & Tobacco Research. 2012;14(7):767–776. doi: 10.1093/ntr/ntr278. [DOI] [PubMed] [Google Scholar]
- Holtrop JS, Meghea C, Raffo J, Biery L, Chartkoff SB, Roman L. Smoking Among Pregnant Women with Medicaid Insurance: Are Mental Health Factors Related? Maternal and Child Health Journal. 2010;14(6):971–977. doi: 10.1007/s10995-009-0530-x. [DOI] [PubMed] [Google Scholar]
- Husten CG. How should we define light or intermittent smoking? Does it matter? Nicotine & Tobacco Research. 2009;11(2):111–121. doi: 10.1093/ntr/ntp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino T, Ohtani T, Yoshimi I. Urinary Biomarkers for Secondhand Smoke. Journal of Clinical Laboratory Analysis. 2011;25(5):354–358. doi: 10.1002/jcla.20485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagodzinski T, Fleming MF. Correlates of Postpartum Alcohol Use. Wisconsin Medical Journal. 2007;106:319–325. [PubMed] [Google Scholar]
- Kahn RS, Certain L, Whitaker RC. A reexamination of smoking before, during, and after pregnancy. American Journal of Public Health. 2002;92(11):1801–1808. doi: 10.2105/ajph.92.11.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Griesler PC, Schaffran C. Educational attainment and smoking among women: risk factors and consequences for offspring. Drug & Alcohol Dependence. 2009;104:S24–S33. doi: 10.1016/j.drugalcdep.2008.12.005. S24–33. S0376-8716(08)00436-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendzor DE, Businelle MS, Costello TJ, Castro Y, Reitzel LR, Vidrine JI, Wetter DW. Breast feeding is associated with postpartum smoking abstinence among women who quit smoking due to pregnancy. Nicotine & Tobacco Research. 2010;12(10):983–988. doi: 10.1093/ntr/ntq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendzor DE, Reitzel LR, Mazas CA, Cofta-Woerpel LM, Cao YM, Ji LY, Wetter DW. Individual- and area-level unemployment influence smoking cessation among African Americans participating in a randomized clinical trial. Social Science & Medicine. 2012;74(9):1394–1401. doi: 10.1016/j.socscimed.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz PM, Pritchard A. Socioeconomic Indicators That Matter for Population Health. Preventing Chronic Disease. 2010;7(4):A74. [PMC free article] [PubMed] [Google Scholar]
- Linares Scott TJ, Heil SH, Higgins ST, Badger GJ, Bernstein IM. Depressive symptoms predict smoking status among pregnant women. Addictive Behaviors. 2009;34(8):705–708. doi: 10.1016/j.addbeh.2009.04.003. S0306-4603(09)00087-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YT, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. doi: 10.1093/biomet/88.3.767. [DOI] [Google Scholar]
- Long JS, Cheng S. Regression models for categorical outcomes. Thousand Oaks, CA: Sage Publications Ltd; 2004. [Google Scholar]
- Lu Y, Tong SL, Oldenburg B. Determinants of smoking and cessation during and after pregnancy. Health Promotion International. 2001;16(4):355–365. doi: 10.1093/heapro/16.4.355. [DOI] [PubMed] [Google Scholar]
- Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews. 2009;(3):Cd001055. doi: 10.1002/14651858.CD001055.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalise and measure them? Social Science & Medicine. 2002;55(1):125–139. doi: 10.1016/s0277-9536(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Ventura SJ, Menacker F, Park MM, Sutton PD. Births: Final data for 2001. Hyattsville, MD: 2002. Retrieved from http://www.cdc.gov/nchs/data/nvsr/nvsr51/nvsr51_02.pdf. [PubMed] [Google Scholar]
- Martin LT, McNamara M, Milot A, Bloch M, Hair EC, Halle T. Correlates of smoking before, during, and after pregnancy. Am J Health Behav. 2008;32(3):272–282. doi: 10.5555/ajhb.2008.32.3.272. [DOI] [PubMed] [Google Scholar]
- Masyn K, Petras H, Liu W. Growth Curve Models and the Study of Change with Categorical Indicators. In: Gerben Bruinsma DW, editor. Encyclopedia of Criminology and Criminal Justice. Springer; 2013. [Google Scholar]
- Maxson PJ, Edwards SE, Ingram A, Miranda ML. Psychosocial differences between smokers and non-smokers during pregnancy. Addictive Behaviors. 2012;37(2):153–159. doi: 10.1016/j.addbeh.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Meschke LL, Holl J, Messelt S. Older Not Wiser: Risk of Prenatal Alcohol Use by Maternal Age. Maternal & Child Health Journal. 2013;17:147–155. doi: 10.1007/s10995-012-0953-7. [DOI] [PubMed] [Google Scholar]
- Messer LC, Laraia BA, Kaufman JS, Eyster J, Holzman C, Culhane J, O’Campo P. The development of a standardized neighborhood deprivation index. Journal of Urban Health-Bulletin of the New York Academy of Medicine. 2006;83(6):1041–1062. doi: 10.1007/s11524-006-9094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer LC, Vinikoor-Imler LC, Laraia BA. Conceptualizing neighborhood space: Consistency and variation of associations for neighborhood factors and pregnancy health across multiple neighborhood units. Health & Place. 2012;18(4):805–813. doi: 10.1016/j.healthplace.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford E, Hair E, Yu T-C, Liu W. Women’s Longitudinal Smoking Patterns from Preconception Through Child’s Kindergarten Entry: Profiles of Biological Mothers of a 2001 US Birth Cohort. Maternal and Child Health Journal. 2013:1–11. doi: 10.1007/s10995-013-1305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo M, Heron J, Araya R. Smoking patterns during pregnancy and postnatal period and depressive symptoms. Nicotine & Tobacco Research. 2008;10(11):1609–1620. doi: 10.1080/14622200802412895. [DOI] [PubMed] [Google Scholar]
- Muthén B. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of quantitative methodology for the social sciences. Newbury Park, CA: Sage Publications; 2004. pp. 345–368. [Google Scholar]
- Muthén LK, Muthén BO. Mplus (Version 6.12) Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- National Center for Education Statistics (Producer) [accessed June 8, 2012];Early Childhood Longitudinal Program. Retrieved from http://nces.ed.gov/ecls/birthdatainformation.asp.
- Nkansah-Amankra S. Neighborhood Contextual Factors, Maternal Smoking, and Birth Outcomes: Multilevel Analysis of the South Carolina PRAMS Survey, 2000–2003. Journal of Womens Health. 2010;19(8):1543–1552. doi: 10.1089/jwh.2009.1888. [DOI] [PubMed] [Google Scholar]
- Ogbuanu C, Glover S, Probst J, Liu JH, Hussey J. The Effect of Maternity Leave Length and Time of Return to Work on Breastfeeding. Pediatrics. 2011;127(6):E1414–E1427. doi: 10.1542/peds.2010-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RL, Padilla YC, Hamilton ER. Psychosocial Factors Associated with Patterns of Smoking Surrounding Pregnancy in Fragile Families. Maternal and Child Health Journal. 2012;16(1):249–257. doi: 10.1007/s10995-010-0735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EW, Tudiver F, Schultz JK, Campbell T. Does enhancing partner support and interaction improve smoking cessation? A meta-analysis. Annals Of Family Medicine. 2004;2(2):170–174. doi: 10.1370/afm.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Seravalli L, Hanlon A, Nelson DB. Neighborhood safety as a correlate of tobacco use in a sample of urban, pregnant women. Addictive Behaviors. 2012;37(10):1132–1137. doi: 10.1016/j.addbeh.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petras H, Masyn K. General Growth Mixture Analysis with Antecedents and Consequences of Change. In: Piquero AR, Weisburd D, editors. Handbook of Quantitative Criminology. 1. New York: Springer; 2010. pp. 69–100. [Google Scholar]
- Pevalin DJ, Wade TJ, Brannigan A, Sauve R. Beyond biology: the social context of prenatal behaviour and birth outcomes. Soz Praventivmed. 2001;46(4):233–239. doi: 10.1007/BF01593178. [DOI] [PubMed] [Google Scholar]
- Phillips RM, Merritt TA, Goldstein MR, Deming DD, Job JS, Rudatsikira EM. Increasing the Duration of Breastfeeding by Preventing Postpartum Smoking Relapse in Mothers of Infants in the Neonatal Intensive Care Unit. Paper presented at the American Academy of Pediatrics (AAP) National Conference and Exhibition; San Francisco. 2010. http://aap.confex.com/aap/2010/webprogram/Paper10842.html. [Google Scholar]
- Pickett KE, Kasza K, Biesecker G, Wright RJ, Wakschlag LS. Women who remember, women who do not: a methodological study of maternal recall of smoking in pregnancy. Nicotine Tob Res. 2009;11(10):1166–1174. doi: 10.1093/ntr/ntp117. ntp117 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett KE, Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. Journal of Epidemiology and Community Health. 2001;55(2):111–122. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett KE, Wakschlag LS, Dai L, Leventhal BL. Fluctuations of maternal smoking during pregnancy. Obstet Gynecol. 2003;101(1):140–147. doi: 10.1016/s0029-7844(02)02370-0. S0029784402023700. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Wakschlag LS, Rathouz PJ, Leventhal BL, Abrams B. The working-class context of pregnancy smoking. Health Place. 2002;8(3):167–175. doi: 10.1016/s1353-8292(01)00042-9. S1353829201000429. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. PNSS Health Indicators. Pediatric and Pregnancy Nutrition Surveillance System. 2011 Mar 08; from http://www.cdc.gov/pednss/what_is/pnss_health_indicators.htm.
- Rich-Edwards JW, Buka SL, Brennan RT, Earls F. Diverging associations of maternal age with low birthweight for black and white mothers. International Journal of Epidemiology. 2003;32(1):83–90. doi: 10.1093/ije/dyg008. [DOI] [PubMed] [Google Scholar]
- Rutter M. Transitions and turning points in developmental psychopathology: As applied to the age span between childhood and mid-adulthood. International Journal of Behavioral Development. 1996;19:603–626. doi: 10.1177/016502549601900309. [DOI] [Google Scholar]
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychological Methods. 2002;7(2):147. [PubMed] [Google Scholar]
- Schane RE, Ling PM, Glantz SA. Health effects of light and intermittent smoking a review. Circulation. 2010;121(13):1518–1522. doi: 10.1161/CIRCULATIONAHA.109.904235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schempf A, Strobino D, O’Campo P. Neighborhood effects on birthweight: An exploration of psychosocial and behavioral pathways in Baltimore, 1995–1996. Social Science & Medicine. 2009;68(1):100–110. doi: 10.1016/j.socscimed.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G. Estimating the Dimension of a Model. The Annals of Statistics. 1978;6(2):461–464. doi: 10.2307/2958889. citeulike-article-id:90008. [DOI] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. The NSDUH Report. Rockville, MD: Office of Applied Studies; 2007. Cigarette use among pregnant women and recent mothers. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. The NSDUH Report. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2009. Substance Use among Women During Pregnancy and Following Childbirth. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. Retrieved from http://www.samhsa.gov/data/NSDUH/2k10NSDUH/2k10Results.pdf. [Google Scholar]
- Tong, Dietz P, England L, Farr S, Kim S, D’Angelo D, Bombard J. Age and Racial/Ethnic Disparities in Prepregnancy Smoking Among Women Who Delivered Live Births. Preventing Chronic Disease. 2011;8(6) [PMC free article] [PubMed] [Google Scholar]
- Tong, Jones J, Dietz P, D’Angelo D, Bombard J, CfDCaP Trends in smoking before, during, and after pregnancy - Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000–2005. MMWR Surveillance Summary. 2009;58(4):1–29. [PubMed] [Google Scholar]
- Troe EJWM, Raat H, Jaddoe VWV, Hofman A, Steegers EAP, Verhulst FC, Mackenbach JP. Smoking during pregnancy in ethnic populations: the Generation R study. Nicotine & Tobacco Research. 2008;10(8):1373–1384. doi: 10.1080/14622200802238944. [DOI] [PubMed] [Google Scholar]
- Turney K. Prevalence and Correlates of Stability and Change in Maternal Depression: Evidence from the Fragile Families and Child Wellbeing Study. Plos One. 2012;7(9):e45709. doi: 10.1371/journal.pone.0045709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrell G, Hewitt BA, Miller SA. The influence of neighbourhood disadvantage on smoking cessation and its contribution to inequalities in smoking status. Drug and Alcohol Review. 2012;31(5):645–652. doi: 10.1111/j.1465-3362.2012.00452.x. [DOI] [PubMed] [Google Scholar]
- U.S. DHHS. Women and smoking: A report of the Surgeon General. Atlanta, GA: 2001. Retrieved from http://www.cdc.gov/tobacco/data_statistics/sgr/2001/complete_report/index.htm. [Google Scholar]
- U.S. DHHS. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: 2006. Retrieved from http://www.surgeongeneral.gov/library/reports/secondhandsmoke/fullreport.pdf. [PubMed] [Google Scholar]
- Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated Pregnancy Rates and Rates of Pregnancy Outcomes for the United States, 1990–2008. National Vital Statistics Reports. 2012;60(7) [PubMed] [Google Scholar]
- Vesga-Lopez O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Archives of General Psychiatry. 2008;65(7):805–815. doi: 10.1001/archpsyc.65.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DA, Culhane JF, Mathew L, Bloch JR, Goldenberg RL. Incident Smoking During Pregnancy and the Postpartum Period in a Low-Income Urban Population. Public Health Reports. 2011;126(1):50–59. doi: 10.1177/003335491112600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. A heteroskedasticity-consistent covariance-matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48(4):817–838. doi: 10.2307/1912934. [DOI] [Google Scholar]
- Winickoff JP, Berkowitz AB, Brooks K, Tanski SE, Geller A, Thomson C, Pbert L. State-of-the-art interventions for office-based parental tobacco control. Pediatrics. 2005;115(3):750–760. doi: 10.1542/peds.2004-1055. 115/3/750 [pii] [DOI] [PubMed] [Google Scholar]
- Winickoff JP, Healey EA, Regan S, Park ER, Cole C, Friebely J, Rigotti NA. Using the Postpartum Hospital Stay to Address Mothers’ and Fathers’ Smoking: The NEWS Study. Pediatrics. 2010;125(3):518–525. doi: 10.1542/peds.2009-0356. [DOI] [PubMed] [Google Scholar]
- Yang T-C, Shoff C, Noah AJ, Black N, Sparks CS. Racial segregation and maternal smoking during pregnancy: a multilevel analysis using the racial segregation interaction index. Social Science & Medicine. 2014 doi: 10.1016/j.socscimed.2014.01.030. http://dx.doi.org/10.1016/j.socscimed.2014.01.030. [DOI] [PMC free article] [PubMed]