Abstract
Background
Currently, tumor-node-metastasis stage and histologic type are the established prognostic factors for malignant pleural mesothelioma, whereas no prognostic markers have been established for clinical practice. We investigated the prognostic value of CD10, a metalloproteinase that can promote cancer aggressiveness through enzymatic degradation and intracellular signaling crosstalk, in malignant pleural mesothelioma.
Methods
CD10 immunostaining was performed for 176 cases of malignant pleural mesothelioma (epithelioid, 148; biphasic, 14; sarcomatoid, 14), and its expression was dichotomized as negative (no staining) or positive (any staining). Epithelioid tumors were classified as pleomorphic subtype when cytologic pleomorphism was ≥10 % of the tumor. Overall survival (OS) was analyzed by log-rank tests and Cox proportional hazard models.
Results
Tumoral CD10 expression was identified in 42 % of epithelioid non-pleomorphic tumors, 57 % of epithelioid pleomorphic tumors, 79 % of biphasic tumors, and 93 % of sarcomatoid tumors (p < 0.001). Positive CD10 expression was correlated with higher mitotic count (p = 0.002). Overall survival for patients with positive CD10 expression was significantly shorter than that for patients with negative CD10 expression in all patients (p = 0.001) and in patients with epithelioid tumor (p = 0.04). On multivariate analysis, CD10 expression was an independent prognostic factor for all patients (hazard ratio 1.48; p = 0.019).
Conclusions
Tumoral CD10 expression correlated with aggressive histologic types and higher mitotic activity and is an independent prognostic factor for patients with malignant pleural mesothelioma.
Malignant pleural mesothelioma is an uncommon but aggressive tumor. Despite improvements in surgical management, chemotherapy, and radiotherapy, the prognosis for malignant pleural mesothelioma remains poor, with a median survival of <2 years.1–3 Even though several prognostic markers have been proposed (including specific histologic patterns, tumor markers, immune cell infiltrates, and radiologic findings),4–9 at present, tumor-node-metastasis (TNM) stage and histologic type (epithelioid, biphasic, and sarcomatoid) are the most established factors for determination of clinical management.1–3 However, the prognostic utility of TNM staging is limited to differentiating between early- (I–II) and late-stage (III–IV) disease.1,2 Even among patients with epithelioid mesothelioma, survival outcomes remain variable. Therefore, further prognostic factors are necessary to optimize treatment options, as well as to better stratify patients in clinical trials.
CD10 (neutral endopeptidase), a zinc-dependent metalloproteinase, is expressed in various normal tissues10 and is capable of efficiently degrading various peptides and cytokines.11,12 CD10 is also expressed in malignant tumors and has been identified as a predictor of tumor biological aggressiveness through extracellular enzymatic degradation and intracellular signaling crosstalk.13–23 Although CD10 is expressed in malignant pleural mesothelioma,24 its prognostic significance for malignant pleural mesothelioma is not known.
In this study, we investigate whether CD10 expression can be used to stratify patients with respect to survival and whether it correlates with clinicopathologic factors in patients with malignant pleural mesothelioma.
MATERIALS AND METHODS
Patients
The current retrospective study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center. We reviewed all patients who were diagnosed with malignant pleural mesothelioma at our institution between 1989 and 2009. A total of 305 cases had tumor slides available for histologic evaluation. Of these, 198 had tumor blocks available for construction of tissue microarrays. Clinical data were collected from the prospectively maintained malignant pleural mesothelioma database. Disease stage was based on the reported imaging findings, the surgeon’s intraoperative findings, and the pathologic evaluation of the resected specimens, according to the 6th edition of the American Joint Committee on Cancer Staging Manual.25
The cases in this study have been included in previous reports from our group; the pathologic diagnosis of malignant mesothelioma was confirmed by histologic, histochemical, and immunohistochemical examination.4,5
Histologic Evaluation
All available hematoxylin and eosin (H&E)-stained tumor slides [median 9 slides/case (range 1–43 slides/ case)] were reviewed by two pathologists (KK and WDT) blinded to the patients’ clinical outcomes, by use of an Olympus BX51 microscope (Olympus Co., Tokyo, Japan) with a standard 22-mm diameter eyepiece. Epithelioid mesothelioma can be composed of one or more of the following five histologic patterns, which were recorded in 5 % increments: trabecular, tubulopapillary, micropapillary, solid, and pleomorphic, as previously reported.5 Tumors were classified as pleomorphic subtype when cytologic pleomorphism made up at least 10 % of the tumor. The remaining tumors were classified according to the predominant histologic pattern.5
Mitotic counts were determined with a high-power field (HPF) of 400× magnification (0.237 mm2), as previously reported.4,26 Mitoses were evaluated in 50 HPF areas, with the highest mitotic activity after scanning through all tumor slides being used, and were recorded as the average number of mitotic figures per 10 HPFs.
Tissue Microarray
Formalin-fixed, paraffin-embedded tumor blocks were used for construction of tissue microarrays. For epithelioid tumors, six representative tumor areas and three tumor-related stromal areas were marked on H&E-stained slides. For biphasic and sarcomatoid tumors, six representative areas of sarcomatoid morphologic pattern were marked on H&E-stained slides. Cylindrical 0.6-mm tissue cores were arrayed from the marked areas of corresponding paraffin blocks into a recipient block by use of an automated tissue arrayer (ATA-27; Beecher Instruments, Sun Prairie, WI, USA), resulting in five tissue microarray blocks.
Immunohistochemical Analysis and Scoring of CD10
In brief, 4-μm-thick sections from the blocks were deparaffinized. The standard avidin-biotin-complex per-oxidase technique was used for immunostaining of anti-CD10 antibody [56C6; diluted at 1:50] (Vector Laboratories, Burlingame, CA, USA). Sections were stained using a Ventana Discovery XT automated immunohistochemical stainer (Ventana, Tucson, AZ, USA) in accordance with the manufacturer’s guidelines.
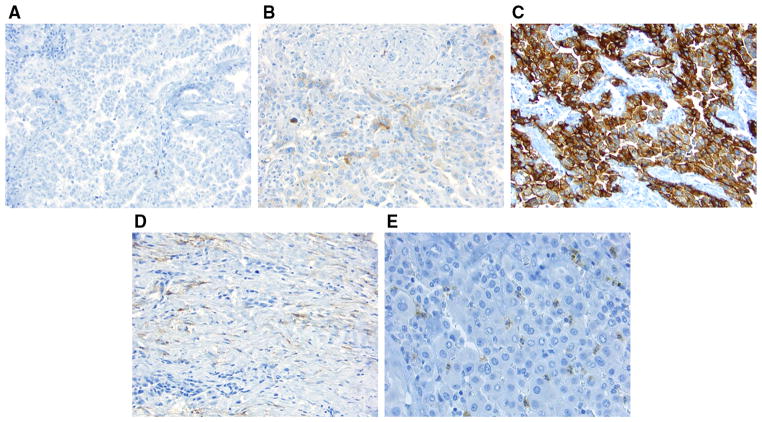
CD10 expression was observed in the cytoplasm of tumor cells, tumor-related stromal cells, and granulocytes in tumoral and stromal areas (Fig. 1), as previously observed in other solid tumors.17,18 In addition, CD10 was expressed in the interstitial stromal cells of normal lung tissue, which was used as an internal positive control.10 The intensity score (0, no expression; 1, mild; 2, intermediate; 3, strong) and distribution score (0, 0 %; 1, 1–50 %; 2, 51–100 %) for immunostaining were summed into a total score for each tumor and stromal core (total score, 0–5),27,28 which was dichotomized as negative (score 0) or positive (score >0). The prevalence of CD10-positive granulocytes was scored in tumoral and stromal cores semiquantitatively: score 0 (CD10-positive granulocytes = 0), score 1 (1–2), score 2 (3–9), score 3 (10–29), and score 4 (≥30). The average score for the tumor or stromal core was considered to be the score for each patient. In total, 176 tumors (epithelioid, 148; biphasic, 14; sarcomatoid, 14) had adequate cores available for immunohistochemical analysis of tumoral CD10.
FIG. 1.
CD10 immunohistochemical analysis using a tissue micro-array (original magnification, ×200). a Tumor cells are negative for CD10; b tumor cytoplasm is weakly positive for CD10; c tumor cytoplasm is strongly positive for CD10; d tumor-related stroma is positive for CD10; e granulocytes infiltrating in tumor cells are positive for CD10
Statistical Analysis
Associations between clinicopathologic factors and CD10 expression were analyzed using the Fisher’s exact test for categorical variables and the Wilcoxon test for continuous variables. Overall survival (OS) following surgery was estimated using the Kaplan–Meier method, with patients censored if they were alive at the time of the last follow-up. Non-parametric group comparisons were performed using the log-rank test. Multivariate analyses were performed using the Cox proportional hazard regression model to study the effects of different variables on OS. All p values were based on two-tailed statistical analysis, and p < 0.05 was considered to indicate statistical significance. All analyses were performed using SAS statistical software (version 9.2; SAS Institute, Cary, NC, USA).
RESULTS
Clinicopathologic Demographic Characteristics of Patients with Epithelioid Malignant Pleural Mesothelioma and Their Association with Overall Survival (OS)
The median age of all patients with epithelioid mesothelioma (n = 148) was 63 years (range 29–83 years). Most patients were men (n = 106), and most had stage III disease (n = 85). In total, 32 patients received preoperative chemotherapy, and 136 had undergone surgical resection (Table 1). Median OS was 15.4 months. On univariate analysis, nodal metastasis (p = 0.016), advanced disease stage (stage III–IV; p = 0.006), lymphatic invasion (p = 0.007), vascular invasion (p = 0.001), and pleomorphic subtype (p < 0.001) were associated with shorter OS (Table 1).
TABLE 1.
Associations between clinicopathologic factors and overall survival in patients with epithelioid tumors
| Variable | n | % | Median OS (months) | p value |
|---|---|---|---|---|
| Total | 148 | 100 | 15.4 | |
| Age (years) | 0.63 | |||
| ≤65 | 85 | 57 | 16.3 | |
| >65 | 63 | 43 | 15.1 | |
| Sex | 0.43 | |||
| Female | 42 | 28 | 18.9 | |
| Male | 106 | 72 | 15.0 | |
| Preoperative chemotherapy | ||||
| No | 116 | 78 | 15.2 | 0.55 |
| Yes | 32 | 22 | 16.3 | |
| Disease laterality | 0.82 | |||
| Left | 62 | 42 | 16.1 | |
| Right | 86 | 58 | 15.1 | |
| Surgical procedure | 0.17 | |||
| Extrapleural pneumonectomy | 81 | 55 | 14.5 | |
| Pleurectomy-decortication | 55 | 37 | 18.9 | |
| Other | 12 | 8 | 7.9 | |
| T stage | 0.050 | |||
| T1 + T2 | 6 + 60 | 45 | 16.3 | |
| T3 + T4 | 69 + 13 | 55 | 14.5 | |
| N stage | 0.016 | |||
| N0 | 89 | 60 | 18.1 | |
| N1 + N2 | 12 + 47 | 40 | 9.5 | |
| TNM stage | 0.006 | |||
| I + II | 5 + 41 | 31 | 19.2 | |
| III + IV | 85 + 17 | 69 | 12.5 | |
| Lymphatic invasion | 0.007 | |||
| Absence | 77 | 52 | 20.9 | |
| Presence | 71 | 48 | 12.3 | |
| Vascular invasion | 0.001 | |||
| Absence | 114 | 77 | 17.0 | |
| Presence | 34 | 23 | 9.7 | |
| Histologic subtype | < 0.001 | |||
| Non-pleomorphic | 125 | 84 | 16.3 | |
| Pleomorphic | 23 | 16 | 8.1 |
Significant p values (p <0.05) are shown in bold
OS overall survival, TNM tumor-node-metastasis
Association between CD10 Expression and OS in Patients with Epithelioid Malignant Pleural Mesothelioma
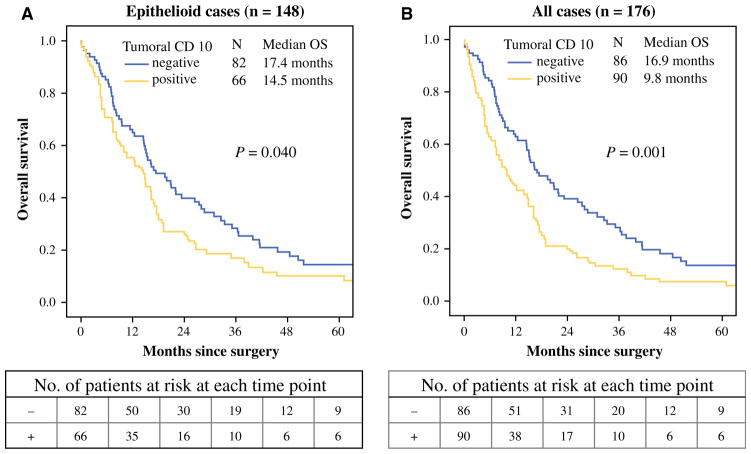
Positive tumoral CD10 expression was observed in 45 % (66/148) of patients with epithelioid malignant pleural mesothelioma. Among these patients, there were 22 cases with a total score of 0–1, 19 cases with a total score of 1–2, 14 cases with a total score of 2–3, 6 cases with a total score of 3–4, and 5 cases with a total score >4. The OS for patients with positive tumoral CD10 expression (n = 66) was significantly shorter (median OS 14.5 months) than that for patients with negative CD10 expression (n = 82; 17.4 months; p = 0.04) (Fig. 2a). Among the patients with early-stage disease (stage I–II; n = 46), OS was significantly shorter for patients with positive CD10 expression (n = 21; median OS 16.3 months) than for patients with negative CD10 expression (n = 25; 36.4 months; p = 0.008), although this result was determined from a small number of patients.
FIG. 2.
Association between CD10 expression and OS in patients with epithelioid and all malignant pleural mesotheliomas. a In patients with epithelioid malignant pleural mesothelioma, the OS for patients with positive tumoral CD10 expression (n = 66) was significantly shorter (median OS 14.5 months) than that for patients with negative CD10 expression (n = 82; 17.4 months; p = 0.04). b For all patients with epithelioid and non-epithelioid tumors, the OS for patients with positive tumoral CD10 expression (n = 90) was significantly shorter (median OS 9.8 months) than that for patients with negative CD10 expression (n = 86; 16.9 months; p = 0.001). OS overall survival
On multivariate analysis of patients with epithelioid mesothelioma, a trend was observed between tumoral CD10 expression and shorter OS [hazard ratio (HR) 1.41; p = 0.052]. Advanced disease stage (stage III–IV; HR 1.49; p = 0.045) and pleomorphic subtype (HR 1.89; p = 0.018) were independent predictors of OS (Table 2).
TABLE 2.
Multivariate model in patients with epithelioid tumors (n = 148)
| Variable | HR | 95 % CI | p value |
|---|---|---|---|
| Tumoral CD10: positive vs. negative | 1.41 | 0.99–2.00 | 0.052 |
| TNM stage: III–IV vs. I–II | 1.49 | 1.00–2.19 | 0.045 |
| Lymphatic invasion: presence vs. absence | 1.26 | 0.84–1.88 | 0.26 |
| Vascular invasion: presence vs. absence | 1.29 | 0.78–2.12 | 0.33 |
| Histologic subtype: pleomorphic vs. non-pleomorphic | 1.89 | 1.12–3.18 | 0.018 |
Significant p values (p <0.05) are shown in bold
HR hazard ratio, CI confidence interval, TNM tumor-node-metastasis
In total, 23 % of cases had CD10 expression in tumor-related stroma. Tumoral CD10 + granulocytes and stromal CD10 + granulocytes were observed in 57 % (10 % with a score ≥3) and 42 % (3 % with a score ≥3) of cases, respectively; however, these parameters had no association with clinicopathological factors, including OS.
Association between Tumoral CD10 Expression and Histologic Types or Clinicopathologic Factors in Patients with Malignant Pleural Mesothelioma
CD10 expression was positively correlated with higher-grade histologic types (p < 0.001); it was identified in 42 % (53/125) of epithelioid non-pleomorphic tumors, 57 % (13/23) of epithelioid pleomorphic tumors, 79 % (11/ 14) of biphasic tumors in the sarcomatoid area, and 93 % (13/14) of sarcomatoid tumors (Fig. 3a). Among epithelioid non-pleomorphic tumors, tumoral CD10 expression was identified in 37 % (11/30) of tubulopapillary tumors, 25 % (5/20) of trabecular tumors, 50 % (30/60) of solid tumors, and 47 % (7/15) of micropapillary tumors.
FIG. 3.
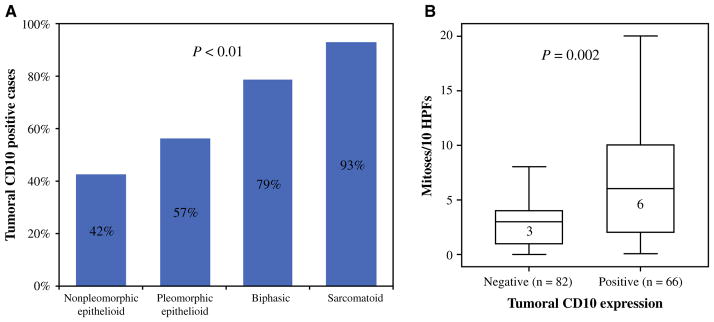
Association between CD10 expression and histologic types and mitotic count. a Tumoral CD10 expression was positive in 42 % (53/125) of epithelioid non-pleomorphic tumors, 57 % (13/23) of epithelioid pleomorphic tumors, 79 % (11/14) of biphasic tumors, and 93 % (13/14) of sarcomatoid tumors (p < 0.001). b Mitotic counts (mitoses per ten HPFs) were significantly higher in CD10-positive tumors than in CD10-negative tumors [median 6 (range 0–42) vs. 3 (range 0–64); p = 0.002]. HPFs high-power fields
Mitotic counts (mitoses per 10 HPFs) were significantly higher in CD10-positive tumors than in CD10-negative tumors [median 6 (range 0–42) vs. 3 (range 0–64); p = 0.002] (Fig. 3b); however, tumoral CD10 expression did not correlate with other clinicopathologic factors, including TNM stage.
Association between Tumoral CD10 Expression and OS in All Patients with Epithelioid and Non-Epithelioid Mesothelioma
Among all patients with epithelioid and non-epithelioid mesothelioma (n = 176), OS for patients with positive CD10 expression (n = 90) was significantly shorter (median 9.8 months) than that for patients with negative CD10 expression (n = 86; 16.9 months; p = 0.001) (Fig. 2b). On multivariate analysis of all patients, positive CD10 expression was an independent factor of prognosis (HR 1.48; p = 0.019) (Table 3).
TABLE 3.
Multivariate model in all patients (n = 176)
| Variable | HR | 95 % CI | p value |
|---|---|---|---|
| Tumoral CD10: positive vs. negative | 1.48 | 1.07–2.04 | 0.019 |
| TNM stage: III–IV vs. I–II | 1.45 | 1.00–2.09 | 0.046 |
| Lymphatic invasion: presence vs. absence | 1.32 | 1.93–1.85 | 0.12 |
| Vascular invasion: presence vs. absence | 1.34 | 0.90–1.99 | 0.15 |
| Histologic type: non-epithelioid vs. epithelioid | 2.27 | 1.43–3.62 | <0.001 |
Significant p values (p <0.05) are shown in bold
HR hazard ratio, CI confidence interval, TNM tumor-node-metastasis
DISCUSSION
We have demonstrated that tumoral CD10 expression correlates with aggressive histologic types and higher mitotic activity, and is an independent prognostic factor of OS in patients with malignant pleural mesothelioma.
Previous studies identified CD10 as a predictor of biological aggressiveness in various malignant tumors.13–23 In breast carcinoma, CD10 expression in tumor-related stroma is correlated with worse survival, with a greater risk of lymph node metastasis and higher tumor grade.13–15 In colorectal carcinoma, tumoral CD10 expression is correlated with a greater risk of liver metastasis.16,17 In addition, in colorectal carcinoma, CD10 expression was observed in tumor-infiltrating granulocytes, which was correlated with worse survival.18 In prostate carcinoma, tumoral CD10 expression identifies a subset of patients with a poor prognosis, with a higher risk of lymph node metastasis and a higher Gleason grade.19–21 In contrast, in hematopoietic tumors such as malignant lymphoma, tumoral CD10 expression is correlated with improved survival.22,23 In our study, tumoral CD10 expression identified a subset of patients with malignant pleural mesothelioma with a poor prognosis. CD10 was also expressed in tumor-related stroma and tumor-infiltrating immune cells (granulocytes) in patients with malignant pleural mesothelioma, although this finding did not have prognostic significance.
We identified that tumoral CD10 expression was more frequently observed in non-epithelioid tumors (79 % of biphasic tumors, 93 % of sarcomatoid tumors) than in epithelioid tumors (45 %). Among epithelioid tumors, the pleomorphic subtype, which we recently identified as the epithelioid mesothelioma subtype with the worst prognosis,5 was more likely to express CD10 (57 %) compared with non-pleomorphic tumors (42 %). Even though tumoral CD10 expression was significantly associated with the non-epithelioid histologic type, which has been considered a poor prognostic type, tumoral CD10 expression was an independent prognostic factor for survival in our multivariate model that adjusted for histologic subtype (epithelioid vs. non-epithelioid). Furthermore, even among patients with epithelioid tumors, CD10 expression showed potential as an independent prognostic factor.
At present, cisplatin combined with pemetrexed is the standard first-line regimen for the treatment of malignant pleural mesothelioma.29,30 Recently, CD10 was demonstrated to be a novel marker of cisplatin resistance and cancer stem cells using cell lines from other solid malignancies.31 In addition, CD10 has been reported to cleave and activate a peptidic prodrug of doxorubicin,32,33 and recent clinical trials suggest that chemotherapy with doxorubicin could be effective in patients with malignant pleural mesothelioma, with improvements in quality of life and an acceptable level of toxicity.34,35 Therefore, CD10 is a potential marker for investigating chemotherapy sensitivity or resistance in patients with malignant pleural mesothelioma.
A limitation of the present study is that we used a cohort that was heterogeneous in terms of TNM stage. However, tumoral CD10 expression remained a prognostic factor in patients with early-stage disease, and was an independent prognostic factor on multivariate analysis after adjustment for TNM stage (early vs. advanced). Another potential limitation in this study, which used a tissue microarray, is that CD10-negative tumors might be focally positive if they are stained with whole-tissue blocks; however, when whole-tissue blocks were used in a previous study, the total rate of CD10 positivity was 54 % for malignant mesothelioma.24 This is similar to that in the present study using tissue microarray analysis (51 % in all patients with mesothelioma). Therefore, we believe that our conclusions would not be significantly changed if a whole-tissue block was used to confirm tumoral CD10 positivity.
CONCLUSIONS
In malignant pleural mesothelioma, expression of CD10 correlates with aggressive histologic types and high mitotic activity, and is an independent predictor of patient survival. As immunohistochemical analysis has become routine clinical practice, prognostic stratification using CD10 immunostaining can be readily implemented to potentially select treatment options for patients with malignant pleural mesothelioma.
Acknowledgments
The authors’ laboratory work is supported in part by Grants from the National Institutes of Health (R21 CA164568-01A1, R21 CA164585-01A1, R01 CA136705-06, U54 CA137788, P30 CA008748, and P50 CA086438-13), the US Department of Defense (PR101053 and LC110202), Mr. William H. Goodwin and Mrs. Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center. We thank Joe Dycoco of the Memorial Sloan Kettering Thoracic Surgery Service for assisting with the Thoracic Surgery Service’s malignant pleural mesothelioma database; Irina Linkov, Avani Giri, and Louie Lopez of the Memorial Sloan Kettering Pathology Core Facility for their help in making the tissue microarray and for technical assistance with immunohisto-chemistry; and David Sewell and Alex Torres of the Memorial Sloan Kettering Thoracic Surgery Service for their editorial assistance.
Footnotes
DISCLOSURE: Kyuichi Kadota, Jonathan Villena-Vargas, Jun-ichi Nitadori, Camelia S. Sima, David R. Jones, William D. Travis, and Prasad S. Adusumilli do not have any commercial interests to disclose.
References
- 1.Flores RM, Zakowski M, Venkatraman E, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol. 2007;2(10):957–965. doi: 10.1097/JTO.0b013e31815608d9. [DOI] [PubMed] [Google Scholar]
- 2.Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the International Association For the Study of Lung Cancer mesothelioma database. J Thorac Oncol. 2012;7(11):1631–1639. doi: 10.1097/JTO.0b013e31826915f1. [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg. 1999;117(1):54–63. doi: 10.1016/s0022-5223(99)70469-1. discussion 63–55. [DOI] [PubMed] [Google Scholar]
- 4.Kadota K, Suzuki K, Colovos C, et al. A nuclear grading system is a strong predictor of survival in epithelioid diffuse malignant pleural mesothelioma. Mod Pathol. 2012;25(2):260–271. doi: 10.1038/modpathol.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadota K, Suzuki K, Sima CS, Rusch VW, Adusumilli PS, Travis WD. Pleomorphic epithelioid diffuse malignant pleural mesothelioma: a clinicopathological review and conceptual proposal to reclassify as biphasic or sarcomatoid mesothelioma. J Thorac Oncol. 2011;6(5):896–904. doi: 10.1097/JTO.0b013e318211127a. [DOI] [PubMed] [Google Scholar]
- 6.Righi L, Papotti MG, Ceppi P, et al. Thymidylate synthase but not excision repair cross-complementation group 1 tumor expression predicts outcome in patients with malignant pleural mesothelioma treated with pemetrexed-based chemotherapy. J Clin Oncol. 2010;28(9):1534–1539. doi: 10.1200/JCO.2009.25.9275. [DOI] [PubMed] [Google Scholar]
- 7.Burt BM, Bader A, Winter D, Rodig SJ, Bueno R, Sugarbaker DJ. Expression of interleukin-4 receptor alpha in human pleural mesothelioma is associated with poor survival and promotion of tumor inflammation. Clin Cancer Res. 2012;18(6):1568–1577. doi: 10.1158/1078-0432.CCR-11-1808. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Kadota K, Sima CS, et al. Chronic inflammation in tumor stroma is an independent predictor of prolonged survival in epithelioid malignant pleural mesothelioma patients. Cancer Immunol Immunother. 2011;60(12):1721–1728. doi: 10.1007/s00262-011-1073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadota K, Kachala SS, Nitadori J, et al. High SUVmax on FDG-PET indicates pleomorphic subtype in epithelioid malignant pleural mesothelioma: supportive evidence to reclassify pleomorphic as nonepithelioid histology. J Thorac Oncol. 2012;7(7):1192–1197. doi: 10.1097/JTO.0b013e3182519d96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McIntosh GG, Lodge AJ, Watson P, et al. NCL-CD10-270: a new monoclonal antibody recognizing CD10 in paraffin-embedded tissue. Am J Pathol. 1999;154(1):77–82. doi: 10.1016/S0002-9440(10)65253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shipp MA, Look AT. Hematopoietic differentiation antigens that are membrane-associated enzymes: cutting is the key! Blood. 1993;82(4):1052–1070. [PubMed] [Google Scholar]
- 12.Turner AJ, Tanzawa K. Mammalian membrane metallopeptidases: NEP, ECE, KELL, and PEX. FASEB J. 1997;11(5):355–364. doi: 10.1096/fasebj.11.5.9141502. [DOI] [PubMed] [Google Scholar]
- 13.Iwaya K, Ogawa H, Izumi M, Kuroda M, Mukai K. Stromal expression of CD10 in invasive breast carcinoma: a new predictor of clinical outcome. Virchows Arch. 2002;440(6):589–593. doi: 10.1007/s00428-002-0639-4. [DOI] [PubMed] [Google Scholar]
- 14.Kim HS, Kim GY, Kim YW, Park YK, Song JY, Lim SJ. Stromal CD10 expression and relationship to the E-cadherin/beta-catenin complex in breast carcinoma. Histopathology. 2010;56(6):708–719. doi: 10.1111/j.1365-2559.2010.03534.x. [DOI] [PubMed] [Google Scholar]
- 15.Desmedt C, Majjaj S, Kheddoumi N, et al. Characterization and clinical evaluation of CD10 + stroma cells in the breast cancer microenvironment. Clin Cancer Res. 2012;18(4):1004–1014. doi: 10.1158/1078-0432.CCR-11-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimoto Y, Nakanishi Y, Sekine S, et al. CD10 expression in colorectal carcinoma correlates with liver metastasis. Dis Colon Rectum. 2005;48(10):1883–1889. doi: 10.1007/s10350-005-0141-6. [DOI] [PubMed] [Google Scholar]
- 17.Kuniyasu H, Luo Y, Fujii K, et al. CD10 enhances metastasis of colorectal cancer by abrogating the anti-tumoural effect of methionine-enkephalin in the liver. Gut. 2010;59(3):348–356. doi: 10.1136/gut.2009.178376. [DOI] [PubMed] [Google Scholar]
- 18.Khanh do T, Mekata E, Mukaisho K, et al. Prognostic role of CD10(+) myeloid cells in association with tumor budding at the invasion front of colorectal cancer. Cancer Sci. 2011;102(9):1724–1733. doi: 10.1111/j.1349-7006.2011.01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dall’Era MA, True LD, Siegel AF, Porter MP, Sherertz TM, Liu AY. Differential expression of CD10 in prostate cancer and its clinical implication. BMC Urol. 2007;7(1):3. doi: 10.1186/1471-2490-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleischmann A, Rocha C, Saxer-Sekulic N, Zlobec I, Sauter G, Thalmann GN. High CD10 expression in lymph node metastases from surgically treated prostate cancer independently predicts early death. Virchows Arch. 2011;458(6):741–748. doi: 10.1007/s00428-011-1084-z. [DOI] [PubMed] [Google Scholar]
- 21.Ho ME, Quek SI, True LD, et al. Prostate cancer cell phenotypes based on AGR2 and CD10 expression. Mod Pathol. 2013;26(6):849–859. doi: 10.1038/modpathol.2012.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biasoli I, Morais JC, Scheliga A, et al. CD10 and Bcl-2 expression combined with the International Prognostic Index can identify subgroups of patients with diffuse large-cell lymphoma with very good or very poor prognoses. Histopathology. 2005;46(3):328–333. doi: 10.1111/j.1365-2559.2005.02099.x. [DOI] [PubMed] [Google Scholar]
- 23.Bilalovic N, Blystad AK, Golouh R, et al. Expression of bcl-6 and CD10 protein is associated with longer overall survival and time to treatment failure in follicular lymphoma. Am J Clin Pathol. 2004;121(1):34–42. doi: 10.1309/TNKL-7GDC-66R9-WPV5. [DOI] [PubMed] [Google Scholar]
- 24.Butnor KJ, Nicholson AG, Allred DC, et al. Expression of renal cell carcinoma-associated markers erythropoietin, CD10, and renal cell carcinoma marker in diffuse malignant mesothelioma and metastatic renal cell carcinoma. Arch Pathol Lab Med. 2006;130(6):823–827. doi: 10.5858/2006-130-823-EORCCM. [DOI] [PubMed] [Google Scholar]
- 25.Greene F, Page D, Fleming I, et al. AJCC cancer staging manual. 6. New York: Springer; 2002. [Google Scholar]
- 26.Kadota K, Suzuki K, Kachala SS, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Mod Pathol. 2012;25(8):1117–1127. doi: 10.1038/modpathol.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshizawa A, Fukuoka J, Shimizu S, et al. Overexpression of phospho-eIF4E is associated with survival through AKT pathway in non-small cell lung cancer. Clin Cancer Res. 2010;16(1):240–248. doi: 10.1158/1078-0432.CCR-09-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki K, Kadota K, Sima CS, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor inter-leukin-12 receptor beta2 (IL-12Rbeta2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol. 2013;31(4):490–498. doi: 10.1200/JCO.2012.45.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 30.Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol. 2009;27(18):3007–3013. doi: 10.1200/JCO.2008.20.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukusumi T, Ishii H, Konno M, et al. CD10 as a novel marker of therapeutic resistance and cancer stem cells in head and neck squamous cell carcinoma. Br J Cancer. 2014;111(3):506–514. doi: 10.1038/bjc.2014.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravel D, Dubois V, Quinonero J, et al. Preclinical toxicity, toxicokinetics, and antitumoral efficacy studies of DTS-201, a tumor-selective peptidic prodrug of doxorubicin. Clin Cancer Res. 2008;14(4):1258–1265. doi: 10.1158/1078-0432.CCR-07-1165. [DOI] [PubMed] [Google Scholar]
- 33.Pan C, Cardarelli PM, Nieder MH, et al. CD10 is a key enzyme involved in the activation of tumor-activated peptide prodrug CPI-0004Na and novel analogues: implications for the design of novel peptide prodrugs for the therapy of CD10(+) tumors. Cancer Res. 2003;63(17):5526–5531. [PubMed] [Google Scholar]
- 34.Scherpereel A, Berghmans T, Lafitte JJ, et al. Valproate-doxorubicin: promising therapy for progressing mesothelioma: a phase II study. Eur Respir J. 2011;37(1):129–135. doi: 10.1183/09031936.00037310. [DOI] [PubMed] [Google Scholar]
- 35.Arrieta O, Medina LA, Estrada-Lobato E, et al. First-line chemotherapy with liposomal doxorubicin plus cisplatin for patients with advanced malignant pleural mesothelioma: phase II trial. Br J Cancer. 2012;106(6):1027–1032. doi: 10.1038/bjc.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]