From 2010 to 2013, 5 cases of nontuberculous mycobacteria infective endocarditis (IE), exclusively from bioprosthesis, were diagnosed in three hospitals out of 370 blood culture-negative-suspected IE. The porcine origin of this underestimated etiology is questioned.
Keywords: bioprosthetic valve, infective endocarditis, nontuberculous mycobacteria
Abstract
Background. Atypical mycobacteria, or nontuberculous mycobacteria (NTM), have been barely reported as infective endocarditis (IE) agents.
Methods. From January 2010 to December 2013, cardiac valve samples sent to our laboratory as cases of blood culture-negative suspected IE were analyzed by 16S rDNA polymerase chain reaction (PCR). When positive for NTM, hsp PCR allowed species identification. Demographic, clinical, echocardiographic, histopathological, and Ziehl-Neelsen staining data were then collected.
Results. Over the study period, 6 of 370 cardiac valves (belonging to 5 patients in 3 hospitals) were positive for Mycobacterium chelonae (n = 5) and Mycobacterium lentiflavum (n = 1) exclusively on bioprosthetic material. The 5 patients presented to the hospital for heart failure without fever 7.1–18.9 months (median 13.1 months) after biological prosthetic valve implantation. Echocardiography revealed paravalvular regurgitation due to prosthesis dehiscence in all patients. Histopathological examination of the explanted material revealed inflammatory infiltrates in all specimens, 3 of which were associated with giant cells. Gram staining and conventional cultures remained negative, whereas Ziehl-Neelsen staining showed acid-fast bacilli in all patients. Allergic etiology was ruled out by antiporcine immunoglobulin E dosages. These 5 cases occurred exclusively on porcine bioprosthetic material, revealing a statistically significant association between bioprosthetic valves and NTM IE (P < .001).
Conclusions. The body of evidence confirmed the diagnosis of prosthetic IE. The statistically significant association between bioprosthetic valves and NTM IE encourages systematic Ziehl-Neelsen staining of explanted bioprosthetic valves in case of early bioprosthesis dysfunction, even without an obvious sign of IE. In addition, we strongly question the cardiac bioprosthesis conditioning process after animal sacrifice.
Infective endocarditis (IE) is a rare but serious disease with a high mortality rate, even in Western countries [1]. Appropriate antibiotic treatment is the cornerstone of therapeutic care and relies on identification of the causative microorganism. At present, approximately 5% of IE cases remain non-microbiologically documented, mainly due to prior antibiotic administration or to fastidious organisms [2, 3]. It is thus essential to improve the diagnosis and management of such blood culture-negative IE. Of the approximately 174 Mycobacterium species that have been described and classified into 2 major groups, or “rapidly growing” and “slowly growing” nontuberculous mycobacteria (NTM), also known as atypical mycobacteria [4], approximately 80 species have been described in human disease. These species have been mainly reported in lung or skin and soft-tissue infections and in immunocompetent patients or patients with impaired immunity [5]. These organisms are also involved in waterborne nosocomial outbreaks [6]. Very few have been reported as agents of IE, which are mostly Mycobacterium fortuitum, Mycobacterium abscessus, and Mycobacterium chelonae [7–10]. Since the first reports in the late 1970s [10–12], approximately 40 papers have been published on this topic. Our laboratory performs bacteriological analysis of cardiac valves, vegetations, and prostheses for a cardiac teaching hospital (Hôpital Louis Pradel) and surrounding private heart surgery clinics (Clinique du Tonkin and Infirmerie Protestante) in Lyon, France. In case of IE suspicion, 16S rDNA polymerase chain reaction (PCR) is performed when cardiac samples and blood cultures remain negative on conventional media. Between January 2010 and December 2013, 5 cases of bioprosthetic valve failure were found to be NTM IE. The strong association of NTM with bioprosthetic valve IE that we put in evidence alerted us and urged us to report those cases so that the medical community can be warned and public health authorities can get involved.
METHODS
Study Population and Study Design
Polymerase chain reaction data for cardiac samples analyzed from January 1, 2010 to December 31, 2013 were retrospectively collected. Demographic data on the related patients, the valve type, and its anatomic localization were compiled. Samples with 16S rDNA PCR positive for NTM underwent Mycobacterium-specific hsp PCR for species identification. The clinical records of the patients were collected, including their medical history, time of disease occurrence, and clinical symptoms at presentation, and echocardiographic signs, the types of lesions observed during surgery, antibiotic treatment, and outcomes were obtained from their medical files. Histopathological observations were collected. Ziehl-Neelsen staining and a specific culture on Löwenstein-Jensen medium (Bio-Rad, Marnes-la-Coquette, France), Coletsos (Bio-Rad, Marnes-la-Coquette, France), and Supplemented Mycobacteria Growth Indicator Tubes (MGIT, Becton Dickinson, Le Pont-de-Claix, France) were performed.
Molecular Analysis
DNA was extracted from cardiac sample fragments after mechanical cell disruption (MagNA Lyser, Roche Diagnostics, Meylan, France), proteinase K enzymatic digestion, and DNA isolation using a MagNA Pure Compact Nucleic Acid Isolation Kit according to the manufacturer's recommendations (Roche Diagnostics, Meylan, France). Next, 16S rDNA was amplified using the primers 13BS (GCCCGGGAACGTATTCAC) and 91E (TCAAAKGAATTGACGGGGGC), as previously described [13]. Propidium MonoAzide (Interchim, Montlucon, France) was added to the 16S rDNA PCR mix (2.5 µM final) to remove unspecific DNA background, as described by Hein et al [14]. The mycobacterial hsp65 (gro-EL2)-specific sequence was amplified by nested PCR using the primers hsp1 (ACCAACGATGGTGTGTCCATC) and hsp2 (CTTGTCGAACCGCATACCC), and the internal primers hsp3 (CCAAGGAGATCGAGCTGG) and hsp4 (TCGGTGAGCTCSAGCTG) were adapted from Telenti et al [15]. After double-stranded sequencing of the 16S rDNA and hsp amplicons (Biofidal, Vaulx-en-Velin, France) with the primers 13BS and 91E or hsp3 and hsp4, respectively, bacterial identification was performed by analyzing the obtained sequences using the leBIBI gene database, a software environment for sequence-based phylogenetic identification of prokaryotes (https://umr5558-bibiserv.univ-lyon1.fr/lebibi/lebibi.cgi) [16]. In brief, the method involves a conventional phylogenetic approach combining a supervised consensus extraction from the chromatograph, the construction of an ad hoc reference data set, alignment, selection of the best evolution model, and phylogenetic reconstruction by using a maximum likelihood method [17].
Antiporcine Epithelium Immunoglobulin E Dosages
Antiporcine epithelium immunoglobulin (IgE) was quantified in sera by fluoro-enzyme immunoassay (ImmunoCAP 250, Thermo Fischer, Illkrich, France) according to the manufacturer's recommendations.
Ethics Committee Approval
According to French law and the Helsinki declaration, this retrospective observational study did not require local institutional review board approval.
RESULTS
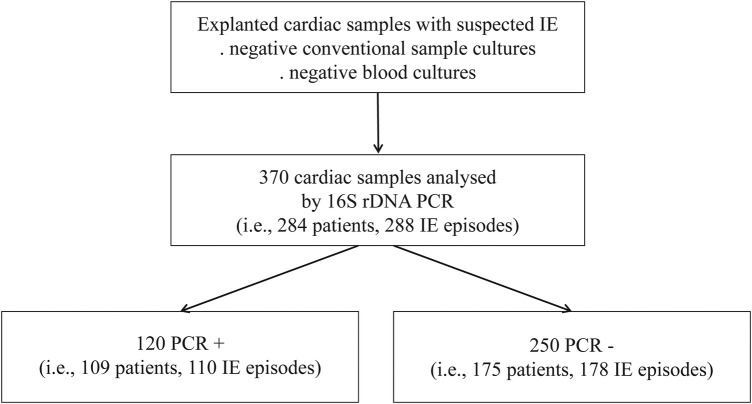
From January 2010 to December 2013, 284 patients undergoing surgery for possible or definite IE episodes [18] had their 370 cardiac valves analyzed by 16S rDNA PCR because of a lack of documentation by conventional bacteriology (Figure 1). These 370 samples belonging to 284 patients (288 IE episodes) involved 221 native valves (181 IE episodes), 70 mechanical prostheses (51 IE episodes), and 79 biological prostheses (56 IE episodes) and were further examined.
Figure 1.
Flow chart of sample inclusion.
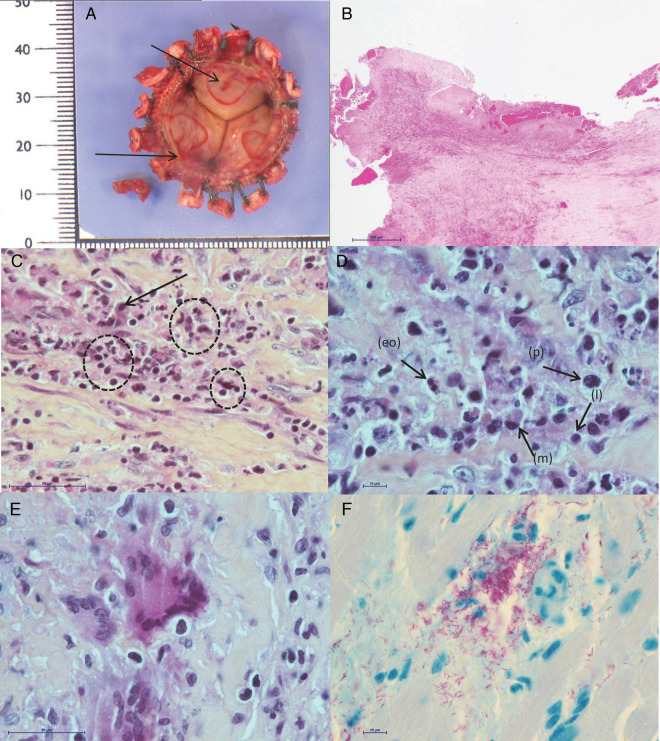
Figure 2.
Histological examinations and Ziehl-Neelsen staining of explanted bioprostheses. A, Macroscopic aspect. Endothelial ulcerations (black arrows) with fibrin replacement. B, Vegetation with leukocytes and fibrin matrix at the surface of the prosthesis (hematoxylin-eosin staining) (scale bar = 500 μm) (low magnification). C, Enlargement of (B): focus on the inflammatory infiltrate with macrophages (black arrow) and foci of necrotic neutrophils (dotted circles) (scale bar = 50 μm). D, Enlargement of (C): focus on plasma cells (p), lymphocytes (l), macrophages (m), and rare eosinophils (eo) (scale bar = 10 μm). E, Enlargement of (C): focus on few scattered multinucleated giant cells (scale bar = 50 μm). F, Ziehl-Neelsen staining revealing numerous acid-fast bacilli (in pink) (scale bar = 10 μm).
Of the 284 patients, 200 (70.4%) were men. The patients' median age was 62 years old (range, 7 months to 86 years old). Of the 370 samples, 120 (related to 110 IE episodes) yielded a positive 16S rDNA PCR. Streptococci accounted for 52% of cases (n = 57) (52% of specimens, n = 63), among which oral streptococci were predominant (18 cases, 20 specimens), followed by Streptococcus gallolyticus (12 cases, 12 specimens) and enterococci (8 cases, 9 specimens) (Table 1). In addition, 16S rDNA PCR was positive for staphylococci in 16% of cases (n = 17) (15% of specimens, n = 18), most of which involved non-aureus staphylococci (11 cases, 12 specimens). Cases of Whipple disease (n = 2), Q fever (n = 1), Streptobacillus moniliformis (n = 1), and HACEK group pathogens (ie, Haemophilus species, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae) (n = 2) were also diagnosed. Over the study period, 16S rDNA PCR was positive for Mycobacterium species in 5 samples, all bioprostheses, from 5 patients (Tables 1 and 2). Mycobacterium-specific hsp65 PCR was performed on these 5 samples and confirmed the presence of M chelonae (patient nos. 1, 2, 3, and 5) and Mycobacterium lentiflavum (patient no. 4) (Supplementary Figure 1 and 2). Patient no. 1 also had his bioprosthetic mitral valve explanted at the same time, which remained 16S rDNA PCR negative but was positive for M chelonae by Mycobacterium-specific hsp PCR (Supplementary Figure 1). The clinical, microbiological, and histopathological data of the 5 patients were reviewed.
Table 2.
continued.
| Histopathology | Ziehl-Neelsen Staining | Mycobacterial Culture | 16S rDNA PCR | hsp65 PCR | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Necrosis, fibrin with neutrophils infiltrates. Few giant cells | Negative | Negative | Positive: Mycobacterium spp | Positive: M chelonae | Aortic valvular replacement (mechanical prosthesis) | Death. (Postoperative hemorrhage) |
| Necrosis, fibrin with neutrophils infiltrates. Few giant cells and rare eosinophils | Rare acid-fast bacilli | Negative | Negative | Positive: M chelonae | Mitral valvular replacement (mechanical prosthesis) | |
| Necrosis, fibrin with neutrophils infiltrates. Few giant cells and rare eosinophils | Numerous acid-fast bacilli | Negative | Positive: Mycobacterium spp | Positive: M chelonae | Aortic valvular replacement (mechanical prosthesis). Cefoxitin (8 wk) + clarithromycin (6 wk) + moxifloxacin (6 wk) |
Recovery (44-mo follow-up) |
| Neutrophils infiltrates in conjunctive tissue. Few macrophages and rare eosinophils | Numerous acid-fast bacilli | Negative | Positive: Mycobacterium spp | Positive: M chelonae | Aortic valvular replacement (bioprosthesis). Ceftriaxon + vancomycin (4 wk) |
Recovery (32-mo follow-up) |
| Neutrophils infiltrates in conjunctive tissue. Few macrophages | Numerous acid-fast bacilli | Positive: M abscessus | Positive: Mycobacterium spp | Positive: M lentiflavum | Aortic valvular replacement (bioprosthesis). Imipenem + tobramycin, ofloxacin + clarithromycin (4 wk). Switch to moxifloxacin + azithromycin per os |
Recovery. Ongoing treatment |
| Necrosis, fibrin with neutrophils infiltrates. Few giant cells | Numerous acid-fast bacilli | Negative | Positive: Mycobacterium spp | Positive: M chelonae | Imipenem + ofloxacin + azithromycin (3 mo) then switch to azithromycin per os | Recovery. Ongoing treatment |
Abbreviations: CT, Clinique du Tonkin; HLP, Hôpital Louis Pradel; IE, infective endocarditis; IP, Infirmerie Prostestante; rNTM, nontuberculous mycobacteria; EE,CliniquHLPHpital lymerase haireaction; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
a Follow-up duration was defined from surgical treatment to the end of the study.
Table 1.
The 16S rDNA Polymerase Chain Reaction (PCR) Results of Culture-Negative Valves in Infective Endocarditis
| Identified Microorganism | No. of Samples (Episodes) n = 120 (110) | % of Episodes |
|---|---|---|
| Staphylococci | 18 (17) | 16 |
| Non-aureus staphylococci | 12 (11) | 10 |
| Staphylococcus aureus | 6 (6) | 5 |
| Streptococci | 63 (57) | 52 |
| Oral streptococci | 20 (18) | 17 |
| Streptococcus gallolyticus | 12 (12) | 11 |
| Enterococci | 9 (8) | 7 |
| Group B streptococci | 4 (4) | 4 |
| Streptococcus pneumoniae | 3 (2)a | |
| Otherb | 15 (13) | 12 |
| Enterobacteriaceae | 9 (7) | 6 |
| HACEK groupc | 3 (2) | 2 |
| Propionibacterium acnes | 6 (6) | 5 |
| Mycobacteria | 5 (5) | 5 |
| Mycobacterium chelonaed | 4 (4)a | |
| Mycobacterium lentiflavumd | 1 (1)a | |
| Coxiella burnetii | 1 (1)a | |
| Tropheryma whipplei | 2 (2)a | |
| Actinomyces oris | 1 (1)a | |
| Streptobacillus moniliformis | 1 (1)a | |
| Othere | 11 (11) | 10 |
a Percentages were not reported because of very small numbers.
b Including Streptococcus lutetiensis (n = 1), Streptococcus gordonii (n = 4), Streptococcus species (n = 4), Gemella haemolysans (n = 2), Gemella morbillorum (n = 2), Gemella bergeri (n = 1), and Gemella species (n = 1).
c Including Aggregatibacter actinomycetemcomitans (n = 1) and Cardiobacterium hominis (n = 2).
d Obtained by Mycobacterium-specific hsp PCR.
e Acinetobacter species (n = 1), Bacillus species (n = 1), Brevibacterium species (n = 1), Burkholderia species (n = 1), Corynebacterium species (n = 1), Friedmanniella capsulata (n = 1), Hyphomicrobium denitrificans (n = 1), Micrococcus species (n = 1), Neisseria flava (n = 1), Paracoccus species (n = 1), or contaminated DNA sequence (n = 1).
Table 2.
Clinical and Laboratory Data of the 5 NTM IE Casesa
| Patient | Cardiology Center | Age (y)/Sex | Valve Localization | Valve Type | Interval Between Valve Implant and First Suspect Symptoms (Months) | Clinical Symptoms | TTE/TEE Vegetation/Abscess | TTE/TEE Cardiac Dysfunction |
|---|---|---|---|---|---|---|---|---|
| 1. | CT | 78/M | Aortic | Biological (porcine Labcor) | 17.1 | Acute valve failure | Vegetation | Severe paravalvular leak. Prosthetic valve perforation |
| Mitral | Biological (porcine Medtronic) | 17.1 | Acute valve failure | Vegetation | Severe paravalvular leak | |||
| 2. | IP | 76/M | Aortic | Biological (porcine Labcor) | 7.1 | Acute pulmonary edema due to valve failure | Perivalvular abscess | Severe paravalvular leak |
| 3. | CT | 81/M | Aortic | Biological (porcine Labcor) | 18.9 | Acute valve failure | Periannular abscess | Severe paravalvular leak |
| 4. | HLP | 76/M | Aortic | Biological (porcine Vaskutek) | 13.1 | Acute valve failure | No | Severe paravalvular leak |
| 5. | HLP | 73/M | Aortic | Biological (porcine Vaskutek) | 8.9 | Progressive aortic and mitral failure | No | Moderate to progressive severe paravalvular leak |
The 5 patients had undergone aortic valve bioprosthesis implantation in the past months or years for severe calcific aortic stenosis. One patient (patient no. 1) had undergone associated mitral valve bioprosthesis implantation because of mitral regurgitation. The 5 patients were admitted to the hospital 7.1–18.9 months after surgery (median 13.1 months) for an impaired general condition and moderate to severe heart failure (Table 2). None of them had fever or reported having fever episodes. Transthoracic and/or transesophageal echocardiography revealed paravalvular regurgitation due to prosthesis dehiscence in all patients, plus perforation of the aortic bioprosthesis in one (patient no. 1). A typical vegetation was detected on echocardiography in only 1 patient. A periannular abscess was found in 2 others, and the last 2 patients did not show additional echocardiographic abnormalities beyond a valvular leak (Table 2). These echocardiographic findings in the 5 patients were confirmed during surgery. Macroscopic examination of the explanted material by the pathologist-microbiologist team revealed multifocal ulcerated lesions of the tissue in all 6 samples (Figure 2A). Gram staining and conventional cultures of the cardiac material remained negative in all cases, as did the routine blood cultures that were concomitantly sampled. Mycobacteria-specific culture was positive for only 1 of 6 samples (patient no. 4) on Löwenstein-Jensen medium with M abscessus.
Infective endocarditis was histologically proven for all patients: inflammatory infiltrates composed of neutrophils, mononuclear cells, and fibrin layers associated with several giant cells were observed in 3 of 6 specimens (patient nos. 1, 2, and 5) (Figure 2B–E). In patient nos. 3 and 4, histology revealed neutrophil infiltrates in conjunctive tissue, with few macrophages and without giant cells. Examination by polarized light microscopy revealed the absence of a foreign body. Ziehl-Neelsen staining of the histological sections revealed the presence of numerous acid-fast bacilli in all patients (Figure 2F). No eosinophil infiltrates were observed. In 3 of the 5 patients, sera were available, and antiporcine epithelium IgE dosages were performed. Patient no. 3 showed no sensitization to the antigen, and patient nos. 4 and 5 had subnormal antibody titers.
Because the NTM IE cases exclusively occurred on bioprosthetic material, the hypothesis of a significant association was posed. Indeed, we found that NTM IE was statistically significantly associated with biological prosthetic valves compared with native valves (P < .001) and all other valve types (P < .001) (Fischer's exact test) (Table 3).Because antibiotic susceptibility testing was not available, treatment consisted in empirical combined therapy with cefoxitin or imipenem and clarithromycin followed by a fluoroquinolone regimen for a minimum of 6 weeks; 2 patients were still under treatment at the time of manuscript submission. One patient died from a postoperative hemorrhage. The other 4 patients had a favorable clinical evolution.
Table 3.
PCR-Analyzed IE Episodes
| IE Episodes |
||
|---|---|---|
| NTM-Positive | NTM-Negativea | |
| Native valve | 0 | 181 |
| Bioprosthetic valve | 5 | 51 |
| Mechanical valve | 0 | 51 |
Abbreviations: IE, infective endocarditis; NTM, nontuberculous mycobacteria; PCR, polymerase chain reaction.
a Includes 16S rDNA negative PCR or positive for another bacterium. P values: P < .001 between bioprosthetic and native valves, P < .001 between bioprosthetic valves and both native and mechanical valves. Statistical association was estimated using Fisher's exact test.
Overall, the presence of significant histopathological patterns associated with positive Ziehl-Neelsen staining, positive PCR results, and relevant clinical symptoms allowed the diagnosis of NTM prosthetic valve IE.
DISCUSSION
From January 2010 to December 2013, 6 bioprostheses of 370 cardiac samples analyzed by PCR for possible or definite blood culture-negative IE were positive for NTM. The combination of clinical and molecular evidence and the results of Ziehl-Neelsen staining and histopathological analysis ascertained a diagnosis of bioprosthetic valve IE. All of the patients had undergone valvular replacement 13.1 months earlier (median) with a biological prosthesis, revealing a significant association between such prosthesis and NTM IE (P < .001). We think that systematic Ziehl-Neelsen staining of explanted bioprosthetic valves, in case of early bioprosthesis dysfunction, could help NTM IE diagnosis and that the bioprosthesis disinfection process after animal explantation should be explored as a route of contamination.
Histopathological analysis revealed the association of neutrophil infiltrates with either giant cells or macrophages. The diagnosis of foreign body granuloma was excluded by examination by polarized light microscopy. The presence of giant cells and macrophages associated with acute inflammation is very unusual and should alert one to mycobacterial infection. Such association was previously noted in a case of Mycobacterium chimaera IE with multiple septic emboli [19]. In the current study, specific mycobacterial culture was positive only in 1 patient (patient no. 4), which could have been related to the detrimental effect of peroperative antibiotic prophylaxis or the presence of metabolic variants associated with viable but noncultivable (VBNC) mycobacteria, as previously observed in latent infections [20].
In our study, for the first time, we showed a significant association between NTM IE and biological prosthetic valves (P < .001). This association is all the more significant because bioprostheses accounted for only 20% of the 370 total analyzed samples and because none of the 7084 other 16S rDNA PCRs performed in the same time period on diverse sample types was positive for NTM. The 5 cases occurred on porcine bioprostheses only, but no significant association with porcine or bovine origin could be inferred because of missing information for 19 bioprosthetic samples. Only possibly minimal sensitization to porcine antigens was suggested in 2 of 3 patients in our study, excluding allergy as a differential diagnosis [21, 22]. In addition, the time of appearance of symptoms (median 13.1 months, range 7.1–18.9 months) is inconsistent with both mechanical dysfunction, which usually occurs after 10–15 years [23], and with postoperative IE due to common pathogens such as Staphylococcus aureus and non-aureus staphylococci, which develop earlier and in a more aggressive way. The timeline here suggests a very indolent infectious process that could thus be misdiagnosed as noninfectious, leading to an underestimation of NTM prosthetic IE.
The aforementioned body of evidence confirmed the diagnosis of NTM prosthetic valve IE, which is of great importance because NTM infections are known to be difficult to treat, requiring long-lasting multitherapy in most cases [24]. Moreover, no recommendation concerning NTM IE treatment is included in current guidelines, most likely due to the extremely low incidence of this entity. Addition of a second antimicrobial agent, tailored on NTM species, beyond macrolides is recommended [24, 25]. Tobramycin is the recommended aminoglycoside for the treatment of M chelonae infections [26]. Of importance, M abscessus is known to be resistant to tobramycin and is generally susceptible to cefoxitin, unlike M chelonae [27]. Thus, imipenem is preferred to cefoxitin for M chelonae infections [26]. Oxazolidinone and glycylcyclines could be valuable and have already been tested as rescue therapy. However, we still lack data for a possible use as first-line therapy [28]. Less is known about the treatment of M lentiflavum-disseminated infection, and some reports state variable susceptibilities for ethambutol and rifampicin. Treatment duration is irregularly mentioned in reported cases but, when specified, extends from 10 to 17 weeks [29, 30]. There are no data on the optimal duration of antibiotic therapy for NTM IE, but treatment lasting several months could be considered, as recommended for disseminated infections [24]. However, the use of antibiotic therapy needs to be balanced because we cannot exclude the fact that a proportion of NTM IE, misdiagnosed as mechanical dehiscence, can be cured solely by surgical replacement. Regarding the potential contamination sources, preoperative contamination of implanted valves or peroperative surgical inoculation have been proposed [7, 9, 31, 32]. The latter hypothesis is very unlikely because the 6 valves involved in our study were implanted at 3 different hospitals and by different surgical teams. In addition, in case of peroperative contamination, mycobacteria would theoretically affect all patients undergoing valve replacement, and the global incidence of NTM IE would thus be much higher. Our series shows a strong and significant association of NTM IE with bioprosthetic valves only, suggesting contamination of these devices before their implantation, as already proposed by Strabelli et al [9] and pointed out by the Centers for Disease Control and Prevention several decades ago [32]. The valves analyzed in our study belonged to different manufacturer's brands, originating from different pig-breeding farms, as in previously published studies [33, 34]. Regardless of the brand and the breeding farm, the decontamination process of bioprosthetic valves after explantation from animals uses chemicals such as glutaraldehyde [32]. However, mycobacteria, which are highly prevalent in the environment, are known to be resistant to those disinfectants (including 8% aqueous formaldehyde, 2% alkaline glutaraldehyde, and free chlorine), and the disinfection step has recently been suspected in NTM infections [35, 36]. Thus, one can speculate that in very rare instances, deficient decontamination following explantation from animals could lead to bacterial survival and proliferation—although in a very slow process associated with VBNC conditions [20]—eventually causing IE. Such environmental contamination at the time of sacrifice is supported by case no. 4, in which the valve contained both M lentiflavum (PCR) and M abscessus (culture). In this last case, we postulate an initial environmental contamination with both species, the dominant M lentiflavum being detected by PCR and the subdominant M abscessus being detected by culture.
At present, precision and clarification about the bioprosthetic valve manufacturing process are needed. Thus, it is of great concern to mobilize public health authorities. In addition, NTM bioprosthetic IE may be considerably underdiagnosed because it mimics mechanical prosthesis dehiscence and thus is not considered for histological and bacteriological analyses. Finally, we could not rule out the possibility that infection of the bioprosthesis occurred postoperatively; to confirm such a hypothesis, the specificity of superinfection for bioprosthestic valve remains to be elucidated, along with the identification of a route of entry.
CONCLUSIONS
In this study, we report 5 cases of NTM bioprosthetic IE diagnosed from 2010 to 2013 in our laboratory. Regardless of the mechanism of contamination, the strong association between NTM and bioprosthetic material demands specific attention to a possible diagnosis of mycobacterial IE in the presence of prosthesis dysfunction with or without obvious signs of IE. In this context, careful histological and microbial examination of the explanted material, including at least a systematic Ziehl-Neelsen staining, followed by a molecular technique whenever possible, should be considered.
Supplementary Material
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank Viviane Delorme, Catherine Jacquet, Laurence Rodier, Valérie Portrat, and Nicole Tacca for valuable technical support, Dr. Françoise Bienvenu for contribution of immunologic analyses, and Emmanuelle Cambau for fruitful discussions. Bastien Michon and Maxime Gaillet were of great help for the medical file investigations and statistical analyses.
Financial support. O. D. received grants from BioMérieux but outside any purpose of this study. F. V. received grants from BioMérieux and personal fees from Astra Zeneca but outside the submitted work.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Moreillon P, Que YA. Infective endocarditis. Lancet 2004; 363:139–49. [DOI] [PubMed] [Google Scholar]
- 2.Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center. Medicine 2005; 84:162–73. [DOI] [PubMed] [Google Scholar]
- 3.Selton-Suty C, Celard M, Le Moing V et al. . Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis 2012; 54:1230–9. [DOI] [PubMed] [Google Scholar]
- 4.Tortoli E. Impact of genotypic studies on mycobacterial taxonomy: the new mycobacteria of the 1990s. Clin Microbiol Rev 2003; 16:319–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marras TK, Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med 2002; 23:553–67. [DOI] [PubMed] [Google Scholar]
- 6.Wallace RJ, Brown BA, Griffith DE. Nosocomial outbreaks/pseudo-outbreaks caused by nontuberculous mycobacteria. Annu Rev Microbiol 1998; 52:453–90. [DOI] [PubMed] [Google Scholar]
- 7.Rumisek JD, Albus RA, Clarke JS. Late Mycobacterium chelonei bioprosthetic valve endocarditis: activation of implanted contaminant? Ann Thorac Surg 1985; 39:277–9. [DOI] [PubMed] [Google Scholar]
- 8.Wallace RJ, Musser JM, Hull SI et al. . Diversity and sources of rapidly growing mycobacteria associated with infections following cardiac surgery. J Infect Dis 1989; 159:708–16. [DOI] [PubMed] [Google Scholar]
- 9.Strabelli TM, Siciliano RF, Castelli JB et al. . Mycobacterium chelonae valve endocarditis resulting from contaminated biological prostheses. J Infect 2010; 60:467–73. [DOI] [PubMed] [Google Scholar]
- 10.Repath F, Seabury JH, Sanders CV, Domer J. Prosthetic valve endocarditis due to Mycobacterium chelonei. South Med J 1976; 69:1244–6. [DOI] [PubMed] [Google Scholar]
- 11.Norenberg RG, Sethi GK, Scott SM, Takaro T. Opportunistic endocarditis following open-heart surgery. Ann Thorac Surg 1975; 19:592–604. [DOI] [PubMed] [Google Scholar]
- 12.Robicsek F, Hoffman PC, Masters TN et al. . Rapidly growing nontuberculous mycobacteria: a new enemy of the cardiac surgeon. Ann Thorac Surg 1988; 46:703–10. [DOI] [PubMed] [Google Scholar]
- 13.Gauduchon V, Chalabreysse L, Etienne J et al. . Molecular diagnosis of infective endocarditis by PCR amplification and direct sequencing of DNA from valve tissue. J Clin Microbiol 2003; 41:763–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hein I, Schneeweiss W, Stanek C, Wagner M. Ethidium monoazide and propidium monoazide for elimination of unspecific DNA background in quantitative universal real-time PCR. J Microbiol Methods 2007; 71:336–9. [DOI] [PubMed] [Google Scholar]
- 15.Telenti A, Marchesi F, Balz M, Bally F et al. . Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol 1993; 31:175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devulder G, Perrière G, Baty F, Flandrois JP. BIBI, a bioinformatics bacterial identification tool. J Clin Microbiol 2003; 41:1785–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramulu HG, Groussin M, Talla E et al. . Ribosomal proteins: toward a next generation standard for prokaryotic systematics? Mol Phylogenet Evol 2014; 75:103–17. [DOI] [PubMed] [Google Scholar]
- 18.Li JS, Sexton DJ, Mick N et al. . Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30:633–8. [DOI] [PubMed] [Google Scholar]
- 19.Achermann Y, Rössle M, Hoffmann M et al. . Prosthetic valve endocarditis and bloodstream infection due to Mycobacterium chimaera. J Clin Microbiol 2013; 51:1769–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barry CE, Boshoff HI, Dartois V et al. . The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 2009; 7:845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loyens M, Thuny F, Grisoli D et al. . Link between endocarditis on porcine bioprosthetic valves and allergy to pork. Int J Cardiol 2013; 167:600–2. [DOI] [PubMed] [Google Scholar]
- 22.Fournier PE, Thuny F, Grisoli D et al. . A deadly aversion to pork. Lancet 2011; 377:1542. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui RF, Abraham JR, Butany J. Bioprosthetic heart valves: modes of failure. Histopathology 2009; 55:135–44. [DOI] [PubMed] [Google Scholar]
- 24.Griffith DE, Aksamit T, Brown-Elliott BA et al. . An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416. [DOI] [PubMed] [Google Scholar]
- 25.Tebas P, Sultan F, Wallace RJ, Fraser V. Rapid development of resistance to clarithromycin following monotherapy for disseminated Mycobacterium chelonae infection in a heart transplant patient. Clin Infect Dis 1995; 20:443–4. [DOI] [PubMed] [Google Scholar]
- 26.Swenson JM, Wallace RJ, Silcox VA, Thornsberry C. Antimicrobial susceptibility of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob Agents Chemother 1985; 28:807–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown-Elliott BA, Wallace RJ. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev 2002; 15:716–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace RJ, Dukart G, Brown-Elliott BA et al. . Clinical experience in 52 patients with tigecycline-containing regimens for salvage treatment of Mycobacterium abscessus and Mycobacterium chelonae infections. J Antimicrob Chemother 2014; 69:1945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai WC, Hsieh HC, Su HM et al. . Mycobacterium abscessus endocarditis: a case report and literature review. Kaohsiung J Med Sci 2008; 24:481–6. [DOI] [PubMed] [Google Scholar]
- 30.Jönsson G, Rydberg J, Sturegård E, Christensson B. A case of Mycobacterium goodii prosthetic valve endocarditis in a non-immunocompromised patient: use of 16S rDNA analysis for rapid diagnosis. BMC Infect Dis 2012; 12:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy C, Curtin JA, Watkins A et al. . Mycobacterium chelonei infection of porcine heart valves. N Engl J Med 1977; 297:667–8. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. Isolation of mycobacteria species from porcine heart valve prostheses. MMWR Morb Mortal Wkly Rep 1977; 26:42–3. [Google Scholar]
- 33.Kunin M, Salamon F, Weinberger M et al. . Conservative treatment of prosthetic valve endocarditis due to Mycobacterium fortuitum. Eur J Clin Microbiol Infect Dis 2002; 21:539–41. [DOI] [PubMed] [Google Scholar]
- 34.Bosio S, Leekha S, Gamb SI et al. . Mycobacterium fortuitum prosthetic valve endocarditis: a case for the pathogenetic role of biofilms. Cardiovasc Pathol 2012; 21:361–4. [DOI] [PubMed] [Google Scholar]
- 35.Soman R, Gupta N, Suthar M et al. . Intravascular stent-related endocarditis due to rapidly growing mycobacteria: a new problem in the developing world. J Assoc Physicians India 2015; 63:19. [PubMed] [Google Scholar]
- 36.Carson LA, Petersen NJ, Favero MS, Aguero SM. Growth characteristics of atypical mycobacteria in water and their comparative resistance to disinfectants. Appl Environ Microbiol 1978; 36:839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.