Abstract
Background
Quality of life (QoL) impairment and fatigue are frequently experienced during treatment for recurrent high-grade glioma (HGG). Fatigue and QoL impairments can be due to primary neurological dysfunction, cytotoxic treatments, mood disturbances, and supportive medications. We now seek to understand how QoL and fatigue impacts survival in recurrent HGG.
Patients and Methods
Using a prospective observational design, 237 patients with recurrent HGG and KPS≥70 completed a self-administered questionnaire that evaluated QoL and fatigue. QoL was assessed with Functional Assessment of Cancer Therapy-General (FACT-G) and FACT-Brain (FACT-Br) scales while fatigue was assessed using Functional Assessment of Chronic Illness Therapy (FACIT-F) scale. Cox proportional hazard models were utilized to evaluate the association between QoL and fatigue and survival.
Results
Seventy-three (31%) subjects had recurrent WHO grade III gliomas and 164 (69%) had recurrent WHO grade IV gliomas. Median follow-up analysis was 27.60 months. In univariate Cox analyses, the FACT-Br specific subscale (HR=0.88; CI 95%, 0.77-1; p=0.048) and FACIT-F (HR=0.82; CI 95%, 0.68-0.99; p=0.045) were both significant predictors of survival. Fatigue added prognostic information beyond that provided by KPS, age, sex, tumor grade, and number of prior progressions (HR=0.80; CI 95%, 0.68-0.9; p=0.031). A greater degree of fatigue was associated with poorer survival in recurrent HGG patients.
Conclusion
In multivariable analyses, FACT-G and FACT-Br are not independent predictors of prognosis. Fatigue is a strong independent predictor of survival that provides incremental prognostic value to the traditional markers of prognosis in recurrent HGG. Pharmacological or non-pharmacological strategies to treat fatigue warrant investigation.
Keywords: Recurrent glioma, fatigue, quality of life
Introduction
High grade gliomas (HGG) represent a heterogeneous but aggressive group of primary brain tumors. Despite multimodal therapy, these cancers can recur with a median progression-free survival after recurrence of only 9 weeks for WHO grade IV tumors (glioblastoma) and 12 weeks for WHO grade III tumors (anaplastic astrocytoma (AA), anaplastic oligodendroglioma (AO), and anaplastic oligoastrocytoma (AOA)).[1, 2] There is no standardized method of treatment for recurrent HGG, but most patients continue chemotherapy and may even pursue further surgery and radiotherapy.[3, 4] While direct treatment of the recurrent disease with further chemotherapy, radiation, and/or surgery is paramount, recognition and maintenance of quality of life (QoL) is equally important and is now starting to be utilized as a secondary outcome measure in clinical trials for treatment of recurrent disease.[5, 6]
QoL impairment, defined as dysfunction in one's perception of personal well-being in life and community, is evident in both the newly-diagnosed and recurrent HGG population and is measured using patient-reported questionnaires.[7-10] Examples of impairment in brain tumor patients include neurological dysfunction, cognitive dysfunction, fatigue, mood disturbances, physical limitations, and insomnia. Since QoL impairment appears ubiquitous for this population and other cancer types, evaluation of QoL and its significance on prognosis is now being utilized in clinical trials.[11] Meta-analysis from EORTC clinical trials report that baseline QoL is a prognostic factor for survival in patients with many types of cancer;[11] however, for newly diagnosed high grade malignant gliomas, general QoL measures do not add to the prediction of survival beyond traditional measures of prognosis (age, sex, KPS, etc.).[8, 12] In a study by Brown and colleagues, survival is not impacted by patient-reported outcomes of QoL in newly diagnosed HGG patients.[9, 13] However, fatigue, when measured independently, is a significant independent predictor of survival.[9, 13] Indeed with the dynamic changes seen in recurrence such as worsening neurological, cognitive, and functional decline, QoL could be impacted and fatigue could be more prevalent. Logically, this could potentially impact treatment considerations and ultimately survival.
Meyers and colleagues further have studied HGG patients and QoL with a focus on the recurrent disease population.[14] Per this study of 80 patients, there is no association between measures of QoL and survival, but there is an association between neurocognitive function and survival in recurrent HGG patients.[14] While it has been well-documented that QoL is impaired during treatment for recurrent malignant glioma,[10] more evaluation with a larger cohort of patients at different points in the disease trajectory is warranted to understand about how it impacts treatment outcome and survival in patients with recurrent HGG. Moreover, fatigue, as a specific component of QoL impairment in HGG also deserves a particular evaluation and understanding in regards to its association with treatment outcome and survival. First we can potentially utilize these tools that quantify QoL and fatigue to aid clinicians and prognosis. Next, we can devise interventions to improve QoL and mitigate symptoms such as fatigue that could, in turn, improve overall treatment tolerance and perhaps survival. We now seek to identify whether impaired QoL and fatigue is associated with a poorer prognosis in recurrent HGG patients.
Methods
Patients and Setting
Patients with histologically confirmed WHO grades III to IV malignant glioma (e.g. glioblastoma, anaplastic astrocytoma, anaplastic oligodendroglioma, and anaplastic oligoastrocytoma) with recurrent disease on magnetic resonance imaging (MRI), who had received or were receiving salvage therapy, and who were presenting to The Preston Robert Tisch Brain Tumor Center at Duke University Medical Center were eligible. Patients were eligible regardless of treatment (receiving versus not receiving treatment) and disease (active versus stable disease) status. All eligible patients had to be at least 6 months from original diagnosis and have a documented diagnosis of recurrent HGG per Revised Assessment in Neuro-Oncology (RANO) criteria which is standard objective criteria for radiographic assessment of tumor response in HGGs.[15] Additional eligibility criteria included the following: age ≥ 18 yrs, KPS ≥ 70, and ability to read and understand English. The Duke University Medical Center institutional review board approved the study, and written informed consent was obtained from all participants before evaluation.
Quality of Life and Fatigue Measures
QoL and fatigue were assessed using patient-reported standardized questionnaires. These included Functional Assessment of Cancer Therapy for Brain Tumors (FACT-Br) and Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F). The FACT‐BR (version 4) contained the Functional Assessment of Cancer Therapy-General (FACT-G), which has subscales for physical well-being (7-items), functional well-being (7-items), emotional well-being (6-items), and social/family well-being (7-items), as well as a 23-item brain cancer subscale (BrCS) that assesses symptoms commonly reported by brain cancer patients.[16] FACT-BR Trial Outcome Index (TOI) was reported as the sum of the physical well-being score from FACT-G, functional well-being score from FACT-G, and BrCS score. FACT-Br total score was based upon all components of the FACT-G plus the BrCS score. FACT-Br total score, along with BrCS and TOI, were scored separately. Cancer related fatigue was assessed by the 13-item Fatigue Scale using the FACIT-F, version 4.[17] Score range for FACIT-F was from 0-52 with scores less than 30 indicated severe fatigue. Use of questionnaires was registered per the FACT website (www.FACIT.org). Higher scores were associated with better QoL and less symptoms of fatigue for the associated scales.
The questionnaires were administered during a patient visit to the Preston Robert Tisch Brain Tumor Center. They were administered at any stage after a diagnosis of recurrent disease per RANO criteria and as determined by their treating neuro-oncologist. Trained research administrative assistants were responsible for administering the testing and patients completed the questionnaires on paper with pens.
Clinical Characteristics and Survival Data
Data in regards to patient clinical characteristics and survival outcome were obtained from the medical record. Patient characteristics included age, gender, tumor grade, progression number, type of salvage treatment, use of supportive medication (anti-epileptic drugs (AEDs) and corticosteroids), and KPS. Overall survival data was obtained from the medical record through July 2010.
Statistical Analysis
Descriptive statistics were reported for clinical parameters and study outcomes. The Cox proportional hazards model was used to examine the effect of QoL (measured by subscales of the FACT-G and FACT-Br) and fatigue on survival. A likelihood ratio (LR) test was used in the context of the Cox model to assess the contribution of QoL and fatigue in predicting survival beyond that provided by KPS (<90 vs. ≥90) alone as well as the combination of age (<45 vs. ≥45), gender, grade (III vs. IV), the number of prior disease progressions (<2 vs. ≥2), and KPS. Of note, we used time from diagnosis as a covariate to control for variable time from diagnosis to death. QoL and fatigue were analyzed as continuous variables. Unadjusted hazard ratios were obtained. Further analysis included hazard ratios adjusted for age, gender, tumor grade, number of prior tumor progressions, and KPS. Fatigue was also categorized using a cut-off score of 35 based on prior work.[18, 19] A fatigue score of 35 corresponded to a score that was approximately one standard deviation (15 points) worse than the general population. Survival time was defined as the time between questionnaire completion and death; for patients remaining alive, survival was censored at the time of last follow-up. A two-sided significance level of 0.05 was used for all statistical tests. All statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Patient Characteristics
Study recruitment was conducted from May 2007 to April 2009. For this study, 244 patients were consented. Full patient characteristics have been published previously.[20] Of the 244 subjects, 237 (95%) subjects completed the patient-reported outcomes measures for QoL including FACT-Br and FACIT-F and had valid scoring available. Initially 1,528 patients were screened for eligibility with 374 (24%) meeting inclusion criteria and 237 (65%) agreeing to participate, consent, and complete questionnaires. Participant characteristics can be seen in Table 1. For this analysis, 73 (31%) patients had recurrent WHO grade III gliomas and 164 (69%) had recurrent WHO grade IV glioma. Mean age was 50 years (range 20-77). Most patients were males at 161 patients (68%). Functional status as measured by KPS ranged from 70 to 100 with a majority at 90. Median scores for the FACT-G total, FACT-Br total, and FACIT-F were 84 (range 34-108), 136 (range 46-182), and 36 (range 10-46), respectively (Table 1).
Table 1. Demographic and Clinical Characteristics of Participants.
| Grade of Disease | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| All Patients | III | IV | ||||
|
|
||||||
| Variable | No. | % | No. | % | No. | % |
| Total patients | 237 | 100 | 73 | 31 | 164 | 69 |
|
| ||||||
| Age, years | ||||||
| Mean | 50 | 44 | 52 | |||
| SD | 12 | 11 | 11 | |||
| < 45 | 79 | 33 | 38 | 52 | 41 | 25 |
| ≥ 45 | 158 | 67 | 35 | 48 | 123 | 75 |
|
| ||||||
| Male sex | 161 | 68 | 50 | 68 | 111 | 68 |
|
| ||||||
| Karnofsky performance status | ||||||
| 70 | 11 | 5 | 4 | 5 | 7 | 4 |
| 80 | 74 | 31 | 20 | 27 | 54 | 33 |
| 90 | 121 | 51 | 41 | 56 | 80 | 49 |
| 100 | 31 | 13 | 8 | 11 | 23 | 14 |
|
| ||||||
| Status of disease at diagnosis | ||||||
| Active | 42 | 18 | 12 | 16 | 30 | 18 |
| Stable | 195 | 82 | 61 | 84 | 134 | 82 |
|
| ||||||
| No. of prior treatments | ||||||
| 1 | 22 | 9 | 4 | 5 | 18 | 11 |
| 2 | 74 | 31 | 25 | 34 | 49 | 30 |
| 3 | 76 | 32 | 19 | 26 | 57 | 35 |
| ≥ 4 | 65 | 27 | 25 | 34 | 40 | 24 |
|
| ||||||
| No. of prior progressions | ||||||
| 0 | 17 | 7 | 7 | 10 | 10 | 6 |
| 1 | 132 | 56 | 37 | 51 | 95 | 58 |
| 2 | 58 | 24 | 12 | 16 | 46 | 28 |
| ≥ 3 | 30 | 13 | 17 | 23 | 13 | 8 |
|
| ||||||
| Receiving salvage therapy at testing | ||||||
| No | 31 | 13 | 9 | 12 | 22 | 13 |
| Yes | 206 | 87 | 64 | 88 | 142 | 87 |
|
| ||||||
| Receiving bevacizumab | ||||||
| No | 120 | 51 | 38 | 52 | 82 | 50 |
| Yes | 117 | 49 | 35 | 48 | 82 | 50 |
|
| ||||||
| Receiving dexamethasone | ||||||
| No | 167 | 70 | 55 | 75 | 112 | 68 |
| Yes | 68 | 29 | 16 | 22 | 52 | 32 |
| Unknown | 2 | 1 | 2 | 3 | 0 | 0 |
|
| ||||||
| Receiving anti-epileptic medication | ||||||
| No | 57 | 24 | 16 | 22 | 41 | 25 |
| Yes | 180 | 76 | 57 | 78 | 123 | 75 |
|
| ||||||
| FACT-G: Physical well-being (range 0-28) | ||||||
| Median | 23 | 22 | 24 | |||
| Minimum – Maximum | 4 - 28 | 5 - 28 | 4 - 28 | |||
|
| ||||||
| FACT-G: Functional well-being (range 0-28) | ||||||
| Median | 20 | 19 | 20 | |||
| Minimum – Maximum | 4 - 28 | 5 - 28 | 4 - 28 | |||
|
| ||||||
| FACT-G: Emotional well-being (range 0-24) | ||||||
| Median | 19 | 19 | 19 | |||
| Minimum – Maximum | 4 - 24 | 4 - 24 | 9 - 24 | |||
|
| ||||||
| FACT-G: Social well-being (range 0-28) | ||||||
| Median | 24 | 23 | 24 | |||
| Minimum – Maximum | 6 - 28 | 6 - 28 | 9 - 28 | |||
|
| ||||||
| FACT-G: Total score (range 0-108) | ||||||
| Median | 84 | 83 | 85 | |||
| Minimum – Maximum | 34 - 108 | 34 - 105 | 37 - 108 | |||
|
| ||||||
| FACT-Br: Brain Cancer Subscale (range 0-76) | ||||||
| Median | 52 | 53 | 52 | |||
| Minimum – Maximum | 12 - 76 | 12 - 76 | 24 - 76 | |||
|
| ||||||
| FACT-Br: Total score (range 0-184) | ||||||
| Median | 136 | 131 | 138 | |||
| Minimum – Maximum | 46 - 182 | 46 - 176 | 72 - 182 | |||
|
| ||||||
| FACT-Br: Trial Outcome Index (range 0-132) | ||||||
| Median | 94 | 94 | 95 | |||
| Minimum – Maximum | 25 - 130 | 25 - 128 | 43 - 130 | |||
|
| ||||||
| FACIT-Fatigue Subscale (range 0-52) | ||||||
| Median | 36 | 34 | 36 | |||
| Minimum – Maximum | 10 - 46 | 10 - 46 | 11 - 45 | |||
Association between QoL and Survival
The median follow-up was 27.60 months. In follow-up, 145 deaths were recorded (61% of the patients completing questionnaires). Median overall survival time for the entire sample was 15.76 month (95% CI, 12.70 to 21.55 months).
The association between QoL and survival can be seen in Table 2. There were no independent associations between survival and the FACT-G physical, emotional, functional, or social well-being subscale scores, the FACT-G total score, the FACT-Br total score, or the Trial Outcome Index (TOI) (Table 2). Though the unadjusted FACT-G and FACT-Br total scores did not achieve a significant association with survival, there was a significant association between the FACT-Br brain cancer subscale and overall survival in recurrent glioma patients in an unadjusted analysis (HR=0.88; CI 95% 0.77-1.00; p=0.048). In the model where the hazard ratio was adjusted for traditional measures of prognosis including age, gender, number of prior progressions, and KPS, the effect of the FACT-Br brain cancer subscale was not statistically significant (p=0.100). Other adjusted analyses did not show that FACT-G and FACT-BR subscales provided incremental prognostic information beyond the traditional markers of prognosis (Table 2).
Table 2. Association between Quality of Life Measures and Survival.
| Unadjusted* | Adjusted*† | |||
|---|---|---|---|---|
|
|
||||
| QoL Measure | Hazard Ratio (95% CI) | Likelihood ratio P | Hazard Ratio (95% CI) | Likelihood ratio P |
| FACT-G: Physical well-being | 0.91 (0.66 - 1.24) | 0.541 | 0.95 (0.67 - 1.34) | 0.758 |
|
| ||||
| FACT-G: Functional well-being | 0.79 (0.60 - 1.03) | 0.086 | 0.82 (0.62 - 1.08) | 0.148 |
|
| ||||
| FACT-G: Emotional well-being | 0.89 (0.59 - 1.36) | 0.592 | 0.87 (0.57 - 1.32) | 0.513 |
|
| ||||
| FACT-G: Social well-being | 0.91 (0.65 - 1.29) | 0.612 | 0.93 (0.63 - 1.37) | 0.702 |
|
| ||||
| FACT-G: Total score | 0.94 (0.84 - 1.04) | 0.242 | 0.95 (0.84 - 1.06) | 0.329 |
|
| ||||
| FACT-Br: Brain cancer subscale | 0.88 (0.77 - 1.00) | 0.048 | 0.89 (0.77 - 1.02) | 0.100 |
|
| ||||
| FACT-Br: Total score | 0.95 (0.89 - 1.01) | 0.090 | 0.95 (0.88 - 1.02) | 0.154 |
|
| ||||
| FACT-Br: Trial Outcome Index (TOI) | 0.93 (0.86 - 1.00) | 0.062 | 0.93 (0.85 - 1.02) | 0.124 |
|
| ||||
| FACIT-Fatigue Subscale Score | 0.82 (0.68 - 0.99) | 0.045 | 0.80 (0.65 - 0.98) | 0.031 |
All hazard ratios are associated with a 10 unit change in each QoL measure.
Adjusted for age, sex, grade, number of prior progressions, and KPS.
Association between Fatigue and Survival
The association between fatigue and survival can be seen in Tables 2 and 3. In the univariate model, FACIT-F score was an independent predictor of survival (p=0.045) with an unadjusted hazard ratio of 0.82 (95% CI, 0.68-0.99) (Table 2). Patients reporting more fatigue symptoms on the FACIT-F scale demonstrated a poorer survival than patients who reported less fatigue. When adjusted for traditional markers of prognosis, the adjusted hazard ratio of 0.80 (95% CI, 0.65-0.98) remained statistically significant.
Table 3. Association between FACIT-Fatigue (≤ 35, >35) and Survival.
| FACIT-Fatigue Score | Likelihood ratio P | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Analysis | ≤ 35 | >35 | ||||
| No. of events | 82 | 63 | ||||
|
| ||||||
| No. at risk | 117 | 120 | ||||
|
| ||||||
| Survival, months | ||||||
| Median (95% CI) | 12.50 (10.63 - 18.06) | 20.59 (14.31 - ∞*) | ||||
|
| ||||||
| Unadjusted HR (95% CI) | Referent | 1.60 (1.15 - 2.22) | 0.005 | |||
|
| ||||||
| HR (95% CI) adjusted for KPS | Referent | 1.45 (1.04 - 2.04) | 0.029 | |||
|
| ||||||
| Adjusted HR (95% CI)† | Referent | 1.66 (1.18 - 2.33) | 0.003 | |||
Upper value was out of range.
Adjusted for age, sex, grade, number of prior progressions, and KPS.
Abbreviations: HR, hazard ratio; CI, confidence interval; KPS, Karnofsky performance scale.
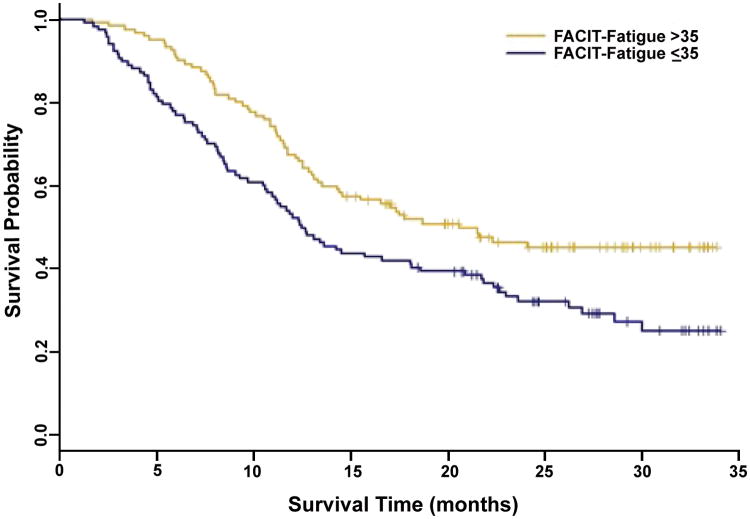
A previously described cut point score of 35 on the FACIT-F score was used to categorize patients.[19] In adjusted analyses, the categorized fatigue subscale score (≤ 35 vs. > 35) was a significant predictor of survival (Figure 1 and Table 3, p=0.005), and it provided incremental prognostic information beyond KPS alone (<90 vs. ≥90) (Table 3, p=0.029). Patients reporting a fatigue score ≤ 35 exhibited significantly poorer survival than those patients reporting a score greater than 35 after adjustment for traditional markers of prognosis (HR=1.66; 95% CI, 1.18 to 2.33; p=0.003) (Table 3). Median survival was 12.50 months(95% CI, 10.62 to 18.06) for patients reporting a fatigue score ≤ 35 compared with 20.59 months (95% CI, 14.31 to ∞) in those reporting a score greater than 35 (Figure 1).
Figure 1.
Association between FACIT-Fatigue and survival.
Discussion
Patients with HGG are likely to experience recurrent disease and outcomes continue to be poor. Extensive clinical research is being applied to improve these outcomes for this challenging population. While therapeutic clinical interventions are paramount, improvement in prognostication can aid clinicians to provide the best care for recurrent HGG patients. To improve outcomes, researchers have sought to understand the factors associated with survival beyond traditional measures. Traditional characteristics of poor prognosis in the recurrent HGG population have included grade, older age, male gender, KPS< 90, and increased number of progressions. Patient-reported measures of QoL have become increasingly used in clinical trials not only as a secondary outcome but also as a marker of prognosis in patients with cancer.[21] QoL is routinely impaired in patients with HGG, but associations with survival in this population have not necessarily been supported. In our study, we have evaluated the role of QoL and fatigue in patients with recurrent HGG and our findings include that subjective measures of fatigue, using the FACIT-F, are an independent predictor of survival in this population. This represents the first study to measure specificly fatigue using FACIT-F in the recurrent HGG and associate measures from this questionnaire with survival in recurrent HGG.
One study of 273 newly diagnosed HGG patients have shown that changes in QoL measures have not been found to be associated with survival, but a second study of 194 newly diagnosed HGG patients has shown that fatigue is a significant predictor of survival but other measures of QoL did not add prognostic value. In a study of 80 recurrent high-grade patients, QoL measures (in particular FACT-Br) do not add prognostic value, but presence of cognitive impairment does have an association with a poorer survival.[14] Our study of 237 recurrent patients shows that the FACT-Br specific subscale does correlate with survival in univariate analysis, but in multivariable analysis does not add to the prognostic value of traditional markers. Furthermore, other measures of QoL (FACT-Br Total Score and Trial Outcome Index) do not provide prognostic information in unadjusted and adjusted models. One concern raised in our analysis is that multiple analyses have been performed on our single data set and that this could increase the odds of yielding a false positive result. In future analyses, this could be controlled for with analysis using the Bonferroni correction.[22]
In our study, the key finding is that fatigue, as measured by the FACIT-F, is a strong independent predictor of survival in recurrent HGG patients (Tables 2 and 3, Figure 1). Fatigue has been identified as an significant predictor of survival in other cancers such as metastatic prostate cancer,[23] breast cancer,[24] and non-small cell lung cancer.[25] Measures used have included FACIT-F, European Organization for Research and Treatment of Cancer Quality of Life Core Questionnaire (EORTC QLQ-C30), Symptom Distress Scale (SDS). Because this occurs in other cancers, it would suggest that there could be a common etiology to fatigue and a common intervention for the alleviation of fatigue in cancer patients. Our findings and similar findings in other cancers indicate that specific measures of fatigue should be included in studies involving outcomes in health-related QoL.
Fatigue, in the context of QoL, impacts most patients struggling with a cancer diagnosis.[26-30] Diagnostic criteria for cancer-related fatigue are as follows: “diminished energy and mental capacity and increased need to rest that is disproportionate to any recent change in activity level and is evident nearly every day during any 2-week period in the past month.”[31] Invariably, it is measured in a subjective fashion using patient-report outcomes. Etiology of cancer-related fatigue is multifactorial and includes comorbid disorders, chemotherapy, radiotherapy, overall frailty, and other medications. Mood disorders such as depression and anxiety often are associated with fatigue.[32] Endocrine dysfunction, in particular adrenal insufficiency, testosterone deficiency, and thyroid hormone deficiency, can occur in the context of cancer, particularly for patients with brain tumors that have received radiotherapy and this can produce fatigue.[33-35] Universally, chemotherapy and radiotherapy, by virtue of affecting bone marrow and blood counts, causes fatigue.[12, 19, 36] For patients with cancer that are receiving chemotherapy actively, fatigue ranks as the most significant symptom in comparison to other symptoms and problems.[36] For brain tumor patients, use of supportive medications such as corticosteroids and anti-epileptics are associated with fatigue.[37] Recent research in low-grade glioma show that QoL impairment is associated with the presence of seizures and use of anti-epileptics.[38] In brain tumor patients, fatigue severity and risk factors for fatigue have been studied; female sex, poor KPS, disease status, use of opioids and AEDs are predictors of fatigue.[39] Other symptoms associated with fatigue are sleep disturbance, weakness, pain, and psychiatric distress.[39] Fatigue, with its broad associations and causations, remains an important marker of QoL in brain tumor patients and now a predictor of survival in the recurrent HGG population.
In light of its importance, fatigue appears to be an obvious avenue for intervention. Unfortunately, the multifocal nature of this symptom could make identification of one single intervention challenging. Recognition of endocrine dysfunction (e.g. thyroid deficiency and testosterone deficiency) and mitigation of severe bone marrow toxicity must first be undertaken in this population to treat fatigue.[37, 40] Close management of other medications such as anti-epileptics is recommended to help lower side effects such as fatigue.[39] Novel interventions, whether pharmacologic and non-pharmacologic, are now being studied in this population. Psychostimulants, such as methylphenidate and modafinil, have been used to improve cognition and to reduce fatigue in patients with brain tumors,[41, 42] but initial results have not shown significant improvements over placebo.[43] Work from our group has focused on the role of exercise behavior and functional capacity outcomes in patients with primary brain tumors. As part of a companion study in the same set of patients, exercise behavior (as measured in MET-h/week) was an independent predictor of survival in recurrent high-grade glioma with median survival of 13.03 months for patients reporting < 9 MET-h/week relative to 21.84 months for those patient reporting > 9-MET-h/week.[20] Thus, exercise interventions for particular patient populations with cancer could abrogate fatigue and in turn improve survival as these are likely associated. Previous meta-analyses showed the exercise training indeed improves QoL outcomes and fatigue.[44] Exercise in the non-neurological cancer population could, at first glance, appear to be more readily applicable, but further work is needed to evaluate exercise in the cancer population with neurological dysfunction such as cognitive and functional impairments. From our study observations, these pharmacological or non-pharmocological interventions on fatigue, if taken separately or in combination, could potential impact survival and further studies are warranted into this particular aspect.
Primary drawbacks of this current study include the focus on one measurement of self-reported QoL and fatigue rather than a series of assessments as well as the variability of the patient population. Since only one time point is evaluated, our conclusions are limited and cannot be generalized to all recurrent HGG patients. For future therapeutic clinical trials involving recurrent HGG patients, QoL and fatigue should be assessed throughout treatment course. Our study population includes both patients with active progressive and stable disease in the context of recurrent HGG. While this heterogeneity could force a dichotomization, we feel that the presence of this heterogeneity, in fact, reflects the true population of recurrent HGG. Furthermore, it provides large patient numbers to improve the power in this study. Another potential drawback is that QoL can be measured using a variety of different instruments. In this study, we chose to use FACT-Br and FACIT-F, but other studies, such as the study by Meyers and colleagues, have utilized European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire (EORTC QlQ-30).[45] While it could be concluded that different instruments could lead to different results, we suggest that our use of a specific brain tumor instrument such as the FACT-Br provides our study with increased relevance.
In conclusion, fatigue, as measured by the FACIT-F, is an independent predictor of survival in recurrent HGG. Moreover, the addition of this measure to traditional measures of prognosis improves the prognostic value to a greater degree than with traditional measures alone. Additional studies are warranted to investigate the etiology of fatigue in recurrent HGG patients and to develop interventions to alleviate and/or prevent fatigue.
Footnotes
Conflict of interest: The authors have no conflicts of interest to disclose.
Contributor Information
Katherine B. Peters, Email: katherine.peters@duke.edu, Department of Neurology, Duke University Medical Center, Durham, NC 27710, USA, Phone: 919-684-5301, Fax: 919-684-6674.
Miranda J. West, Department of Pediatrics, Duke University Medical Center, Durham, NC 27710, USA
Whitney E. Hornsby, Department of Radiation Oncology, Duke University Medical Center, Durham, NC 27710, USA
Emily Waner, Department of Radiation Oncology, Duke University Medical Center, Durham, NC 27710, USA.
April D. Coan, Department of Biostatistics, St. Jude Children's Research Hospital, Memphis, TN 38105, USA
Frances McSherry, Department of Biostatistics, Duke University Medical Center, Durham, NC 27710, USA.
James E. Herndon, II, Department of Biostatistics, Duke University Medical Center, Durham, NC 27710, USA.
Henry S. Friedman, Department of Surgery, Duke University Medical Center, Durham, NC 27710, USA
Annick Desjardins, Department of Neurology, Duke University Medical Center, Durham, NC 27710, USA.
Lee W. Jones, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY 10065, USA
References
- 1.Ohgaki H. Epidemiology of brain tumors. Methods Mol Biol. 2009;472:323–342. doi: 10.1007/978-1-60327-492-0_14. [DOI] [PubMed] [Google Scholar]
- 2.Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, Levin VA, Yung WK. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 3.Kyritsis AP, Levin VA. An algorithm for chemotherapy treatment of recurrent glioma patients after temozolomide failure in the general oncology setting. Cancer chemotherapy and pharmacology. 2011;67:971–983. doi: 10.1007/s00280-011-1617-9. [DOI] [PubMed] [Google Scholar]
- 4.Wen PY, Brandes AA. Treatment of recurrent high-grade gliomas. Curr Opin Neurol. 2009;22:657–664. doi: 10.1097/WCO.0b013e32833229e3. [DOI] [PubMed] [Google Scholar]
- 5.Heimans JJ, Taphoorn MJ. Impact of brain tumour treatment on quality of life. Journal of neurology. 2002;249:955–960. doi: 10.1007/s00415-002-0839-5. [DOI] [PubMed] [Google Scholar]
- 6.Taphoorn MJ, Sizoo EM, Bottomley A. Review on quality of life issues in patients with primary brain tumors. The oncologist. 2010;15:618–626. doi: 10.1634/theoncologist.2009-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng JX, Liu BL, Zhang X, Lin W, Zhang YQ, Liu WP, Zhang JN, Lin H, Wang R, Yin H. Health-related quality of life in glioma patients in China. BMC cancer. 2010;10:305. doi: 10.1186/1471-2407-10-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mauer M, Stupp R, Taphoorn MJ, Coens C, Osoba D, Marosi C, Wong R, de Witte O, Cairncross JG, Efficace F, Mirimanoff RO, Forsyth P, van den Bent MJ, Weller M, Bottomley A. The prognostic value of health-related quality-of-life data in predicting survival in glioblastoma cancer patients: results from an international randomised phase III EORTC Brain Tumour and Radiation Oncology Groups, and NCIC Clinical Trials Group study. British journal of cancer. 2007;97:302–307. doi: 10.1038/sj.bjc.6603876. doi:6603876 [pii] 10.1038/sj.bjc.6603876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown PD, Maurer MJ, Rummans TA, Pollock BE, Ballman KV, Sloan JA, Boeve BF, Arusell RM, Clark MM, Buckner JC. A prospective study of quality of life in adults with newly diagnosed high-grade gliomas: the impact of the extent of resection on quality of life and survival. Neurosurgery. 2005;57:495–504. doi: 10.1227/01.neu.0000170562.25335.c7. discussion 495-504. [DOI] [PubMed] [Google Scholar]
- 10.Giovagnoli AR, Silvani A, Colombo E, Boiardi A. Facets and determinants of quality of life in patients with recurrent high grade glioma. Journal of neurology, neurosurgery, and psychiatry. 2005;76:562–568. doi: 10.1136/jnnp.2004.036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinten C, Coens C, Mauer M, Comte S, Sprangers MA, Cleeland C, Osoba D, Bjordal K, Bottomley A. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. The lancet oncology. 2009;10:865–871. doi: 10.1016/S1470-2045(09)70200-1. [DOI] [PubMed] [Google Scholar]
- 12.Mauer ME, Taphoorn MJ, Bottomley A, Coens C, Efficace F, Sanson M, Brandes AA, van der Rijt CC, Bernsen HJ, Frenay M, Tijssen CC, Lacombe D, van den Bent MJ. Prognostic value of health-related quality-of-life data in predicting survival in patients with anaplastic oligodendrogliomas, from a phase III EORTC brain cancer group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:5731–5737. doi: 10.1200/JCO.2007.11.1476. [DOI] [PubMed] [Google Scholar]
- 13.Brown PD, Ballman KV, Rummans TA, Maurer MJ, Sloan JA, Boeve BF, Gupta L, Tang-Wai DF, Arusell RM, Clark MM, Buckner JC. Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. Journal of neuro-oncology. 2006;76:283–291. doi: 10.1007/s11060-005-7020-9. [DOI] [PubMed] [Google Scholar]
- 14.Meyers CA, Hess KR, Yung WK, Levin VA. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18:646–650. doi: 10.1200/JCO.2000.18.3.646. [DOI] [PubMed] [Google Scholar]
- 15.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 16.Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA. The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75:1151–1161. doi: 10.1002/1097-0142(19950301)75:5<1151::aid-cncr2820750515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.Van Belle S, Paridaens R, Evers G, Kerger J, Bron D, Foubert J, Ponnet G, Vander Steichel D, Heremans C, Rosillon D. Comparison of proposed diagnostic criteria with FACT-F and VAS for cancer-related fatigue: proposal for use as a screening tool. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2005;13:246–254. doi: 10.1007/s00520-004-0734-y. [DOI] [PubMed] [Google Scholar]
- 18.Cella D. Factors influencing quality of life in cancer patients: anemia and fatigue. Seminars in oncology. 1998;25:43–46. [PubMed] [Google Scholar]
- 19.Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 20.Ruden E, Reardon DA, Coan AD, Herndon JE, 2nd, Hornsby WE, West M, Fels DR, Desjardins A, Vredenburgh JJ, Waner E, Friedman AH, Friedman HS, Peters KB, Jones LW. Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2918–2923. doi: 10.1200/JCO.2011.34.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health and quality of life outcomes. 2009;7:102. doi: 10.1186/1477-7525-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. Bmj. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan PW, Nelson JB, Mulani PM, Sleep D. Quality of life as a potential predictor for morbidity and mortality in patients with metastatic hormone-refractory prostate cancer. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2006;15:1297–1306. doi: 10.1007/s11136-006-0003-2. [DOI] [PubMed] [Google Scholar]
- 24.Groenvold M, Petersen MA, Idler E, Bjorner JB, Fayers PM, Mouridsen HT. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast cancer research and treatment. 2007;105:209–219. doi: 10.1007/s10549-006-9447-x. [DOI] [PubMed] [Google Scholar]
- 25.Brown DJ, McMillan DC, Milroy R. The correlation between fatigue, physical function, the systemic inflammatory response, and psychological distress in patients with advanced lung cancer. Cancer. 2005;103:377–382. doi: 10.1002/cncr.20777. [DOI] [PubMed] [Google Scholar]
- 26.Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, Johnson DH, Miaskowski C, Scherr SL, Portenoy RK, Vogelzang NJ. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. The oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 27.Mock V, Atkinson A, Barsevick A, Cella D, Cimprich B, Cleeland C, Donnelly J, Eisenberger MA, Escalante C, Hinds P, Jacobsen PB, Kaldor P, Knight SJ, Peterman A, Piper BF, Rugo H, Sabbatini P, Stahl C. NCCN Practice Guidelines for Cancer-Related Fatigue. Oncology. 2000;14:151–161. [PubMed] [Google Scholar]
- 28.Wagner LI, Cella D. Fatigue and cancer: causes, prevalence and treatment approaches. British journal of cancer. 2004;91:822–828. doi: 10.1038/sj.bjc.6602012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piper BF, Cella D. Cancer-related fatigue: definitions and clinical subtypes. Journal of the National Comprehensive Cancer Network : JNCCN. 2010;8:958–966. doi: 10.6004/jnccn.2010.0070. [DOI] [PubMed] [Google Scholar]
- 30.Berger AM, Abernethy AP, Atkinson A, Barsevick AM, Breitbart WS, Cella D, Cimprich B, Cleeland C, Eisenberger MA, Escalante CP, Jacobsen PB, Kaldor P, Ligibel JA, Murphy BA, O'Connor T, Pirl WF, Rodler E, Rugo HS, Thomas J, Wagner LI. Cancer-related fatigue. Journal of the National Comprehensive Cancer Network : JNCCN. 2010;8:904–931. doi: 10.6004/jnccn.2010.0067. [DOI] [PubMed] [Google Scholar]
- 31.Cella D, Peterman A, Passik S, Jacobsen P, Breitbart W. Progress toward guidelines for the management of fatigue. Oncology. 1998;12:369–377. [PubMed] [Google Scholar]
- 32.Brown LF, Kroenke K. Cancer-related fatigue and its associations with depression and anxiety: a systematic review. Psychosomatics. 2009;50:440–447. doi: 10.1176/appi.psy.50.5.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morton A, King D. Fatigue and endocrine disorders. Causes and comorbidities. Aust Fam Physician. 2003;32:895–900. [PubMed] [Google Scholar]
- 34.Duffner PK. Long-term effects of radiation therapy on cognitive and endocrine function in children with leukemia and brain tumors. Neurologist. 2004;10:293–310. doi: 10.1097/01.nrl.0000144287.35993.96. [DOI] [PubMed] [Google Scholar]
- 35.Constine LS, Woolf PD, Cann D, Mick G, McCormick K, Raubertas RF, Rubin P. Hypothalamic-pituitary dysfunction after radiation for brain tumors. The New England journal of medicine. 1993;328:87–94. doi: 10.1056/NEJM199301143280203. [DOI] [PubMed] [Google Scholar]
- 36.Butt Z, Rosenbloom SK, Abernethy AP, Beaumont JL, Paul D, Hampton D, Jacobsen PB, Syrjala KL, Von Roenn JH, Cella D. Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. Journal of the National Comprehensive Cancer Network : JNCCN. 2008;6:448–455. doi: 10.6004/jnccn.2008.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drappatz J, Schiff D, Kesari S, Norden AD, Wen PY. Medical management of brain tumor patients. Neurol Clin. 2007;25:1035–1071, ix. doi: 10.1016/j.ncl.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Aaronson NK, Taphoorn MJ, Heimans JJ, Postma TJ, Gundy CM, Beute GN, Slotman BJ, Klein M. Compromised health-related quality of life in patients with low-grade glioma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4430–4435. doi: 10.1200/JCO.2011.35.5750. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong TS, Cron SG, Bolanos EV, Gilbert MR, Kang DH. Risk factors for fatigue severity in primary brain tumor patients. Cancer. 2010;116:2707–2715. doi: 10.1002/cncr.25018. [DOI] [PubMed] [Google Scholar]
- 40.Pruitt AA. Medical management of patients with brain tumors. Curr Treat Options Neurol. 2011;13:413–426. doi: 10.1007/s11940-011-0132-y. [DOI] [PubMed] [Google Scholar]
- 41.Gehring K, Patwardhan SY, Collins R, Groves MD, Etzel CJ, Meyers CA, Wefel JS. A randomized trial on the efficacy of methylphenidate and modafinil for improving cognitive functioning and symptoms in patients with a primary brain tumor. Journal of neuro-oncology. 2011 doi: 10.1007/s11060-011-0723-1. [DOI] [PubMed] [Google Scholar]
- 42.Meyers CA, Weitzner MA, Valentine AD, Levin VA. Methylphenidate therapy improves cognition, mood, and function of brain tumor patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998;16:2522–2527. doi: 10.1200/JCO.1998.16.7.2522. [DOI] [PubMed] [Google Scholar]
- 43.Butler JM, Jr, Case LD, Atkins J, Frizzell B, Sanders G, Griffin P, Lesser G, McMullen K, McQuellon R, Naughton M, Rapp S, Stieber V, Shaw EG. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. International journal of radiation oncology, biology, physics. 2007;69:1496–1501. doi: 10.1016/j.ijrobp.2007.05.076. [DOI] [PubMed] [Google Scholar]
- 44.Jones LW, Liang Y, Pituskin EN, Battaglini CL, Scott JM, Hornsby WE, Haykowsky M. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. The oncologist. 2011;16:112–120. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]