Abstract
Laboratory studies suggest that vitamin D (VD) supplementation inhibits skin carcinogenesis. However, epidemiologic studies report mixed findings in the association between circulating VD levels and skin cancer risk. We conducted a clinical study to determine whether oral cholecalciferol supplementation would exert direct bioactivity in human skin through modulation of the VD-receptor.
We enrolled 25 individuals with serum 25-hydroxyvitamin-D levels <30 ng/mL and with skin photodamage to take 50,000 IU of VD3 biweekly for 8–9 weeks. Then, we obtained baseline and end-of-study skin biopsies from photodamaged (PD) and photoprotected (PP) skin, and from benign nevi (BN) and tested for mRNA expression of VD-receptor (VDR) and cytochrome P450-24 (CYP24), and markers of keratinocytic differentiation. High dose cholecalciferol supplementation significantly elevated circulating levels of 25-hydroxyvitamin-D (p<0.0001) and 1,25-dihydroxyvitamin-D (p<0.0001). VDR expression in PD- and PP-skin showed minimum changes after supplementation. CYP24 expression in PD- and PP-skin was increased after supplementation by 186%, p=0.08, and 134%, p=0.07, respectively. In BNs from 11 participants, a trend for higher VDR and CYP24 expression was observed (average of 20%, p=0.08, and 544%, p=0.09, respectively). Caspase-14 expression at the basal layer in PD skin samples was the only epidermal differentiation marker that was significantly increased (49%, p<0.0001). High-dose cholecalciferol supplementation raised serum VD metabolite levels concurrently with CYP24 mRNA and Caspase-14 levels in the skin. Our findings of significant variability in the range of VDR and CYP24 expression across study samples represent an important consideration in studies evaluating the role of VD as a skin cancer chemopreventive agent.
Keywords: skin cancer, chemoprevention, vitamin D, vitamin D receptor, keratinocytes, melanocytic nevi
Introduction
Recent epidemiologic studies have found an association between low levels of 25-hydroxyvitamin D (25(OH)D) and the increased risk of several types of cancer (1–6), although the data are inconsistent (7, 8).
There is an apparent controversy in the association of VD status and skin cancer risk, and the benefit of UV in skin as the primary source of VD generation. Solar UV-exposure is the most important environmental risk factor for the development of both, cutaneous melanoma (CM) and non-melanoma, skin cancers (NMSC) (1, 5, 9–12) while in contrast, 90% of the body’s VD requirements are generated in the skin through sun exposure. In a nested case-control study performed as part of the Osteoporotic Fractures in Men Study, men in the highest quintile of 25-(OH)D (>30 ng/mL) had a 47% lower odds of non-melanoma skin cancer (95% CI: 0.30–0.93, p=0.026) compared to those in the lowest quintile (1). In contrast, another nested case-control study at Kaiser Permanente Northern California, higher serum of 25-(OH)D levels were associated with an increased risk of basal cell carcinoma in fully adjusted models (OR=1.02, 95% CI: 1.00–1.05, p<0.05; OR=3.61, 95% CI: 1.00–13.10, deficient vs. sufficient, t-trend p=0.03) (2).
A post hoc analyses of the Women’s Health Initiative randomized controlled trial concluded that cholecalciferol supplementation at a relatively low dose plus calcium (CaD) did not reduce the overall incidence of NMSC or melanoma (HR:1.02; 95% CI:0.95–1.07) (7, 11). However, in women with history of NMSC, CaD supplementation reduced the melanoma risk (HR:0.43; 95% CI:0.21–0.90; Pinteraction = .038), suggesting a potential role for calcium and cholecalciferol supplements in this high-risk group (10). This large placebo controlled trial of 36,282 postmenopausal women age 50 to 79 years were randomly assigned to receive 1,000 mg of elemental calcium plus 400 IU of cholecalciferol (CaD) daily or placebo for a mean follow-up period of 7 years. The strengths of the study included: the large sample size, assessment of adherence to medication intake, ascertainment of sun exposure, and the breath of demographic and medical information which controlled for potential confounders. The limitations included the fact that it was based on a post hoc analysis of a study initially designed to evaluate hip fracture and colorectal cancer, serum 25 (OH)D was only measured once in a relatively small subset of participants, the control group was allowed to take daily Ca and VD supplementation, and the NMSC were ascertained by annual self-report in contrast to melanoma skin cancers which underwent physician adjudication. Taking together the epidemiological studies indicate a potential but possibly selective effect of VD in skin cancer prevention.
Experimental studies suggest a beneficial effect of VD mediated events in keratinocytes (13, 14) and in some instances in melanocyte’s biology (15). Specifically, 1,25-dihydroxyvitamin D3, the biological active metabolite of VD, reduces keratinocytes growth and promotes differentiation, and consequently has already been successfully used in the therapy of hyperproliferative skin disorders (15). At high levels, 1,25(OH)2D inhibits keratinocyte proliferation in vitro and interacts with calcium to regulate keratinocyte differentiation (16). Keratinocytes lacking VDR are hyperproliferative and exhibit decreased apoptosis (17). Genetically engineered mice lacking the VDR (VDR knockout mice) demonstrate a variety of phenotypes according to the experimental model including reduced alopecia, abnormal hair follicles, dermal cysts and more skin tumors (primarily BCCs) when exposed to a carcinogen (oral 7,12- dimethylbenz[a] anthracene) (18) indicating a role of VDR in keratinocyte differentiation. Mice lacking VDR are predisposed to SCC formation when exposed to high and prolonged doses of UVB (19).
Like keratinocytes, melanocytes also have the capacity for autonomous local production of 1,25(OH)2D and harbor VDR (20). Such locally produced 1,25(OH)2D may play a role in innate and acquired cutaneous immunity (21). In vitro, 1,25(OH)2D stimulates melanocyte maturation, possibly through the stimulation of tyrosinase activity (22, 23). It also protects cells from apoptosis (24) and upregulates VDR expression (15).
To date a controversial association between low levels of serum 25(OH)D/1,25(OH)2D and increased risk of skin cancer development has been proposed by epidemiological studies. In addition, experimental studies as the ones mentioned above, support the notion that VD plays an important role in keratinocyte and melanocyte differentiation state and proliferative capability. These studies combined have fueled the scientific community with the rationale to further consider interventional studies to assess the role or cholecalciferol supplementation for the prevention of primary skin cancers and further recurrences.
Before such studies can be undertaken it is imperative that effective and reproducible biomarkers of VD bioactivity in the skin can be identified in the setting of oral supplementation. Understanding the net effect of oral VD in the biology of the targeted organ, in this case the skin, is critical to the justification and design of future interventional studies.
To determine the potential role of VD for skin cancer prevention, we performed a pilot clinical study of oral cholecalciferol supplementation to determine whether oral supplementation activates the VD pathway in the target skin tissue and whether serum 25(OH)D and 1,25(OH)2D correlates with a modulatory effect in skin differentiation markers.
Materials and Methods
Study Design
The study was an open label, single-arm intervention trial of high-dose cholecalciferol (vitamin D3) in healthy individuals who were considered vitamin D insufficient or deficient (serum 25-hydroxyvitamin D <30 ng/mL) with evidence of moderate to severe skin photodamage on the forearms. The study endpoints include intervention-induced change in the expression of vitamin D receptor (VDR) and cytochrome P450 24 (CYP24) in keratinocytes, skin layers thickness, markers of keratinocytic differentiation (caspase 14 and loricrin), and serum levels of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D. The study also explored the intervention-induced changes in VDR and CYP24 expression in a subset of participants with qualifying benign melanocytic nevi. The University of Arizona Institutional Review Board approved the study and informed consent was obtained from all study participants.
Study Drug
Study drug capsules were supplied by the National Cancer Institute, Division of Cancer Prevention. Each capsule contains 50,000 IU of cholecalciferol and inactive ingredients microcrystalline cellulose, silica, and gelatin. The study capsules were stored at room temperature and protected from environmental extreme.
Study populations
We recruited healthy men and women who had serum 25-hydroxyvitamin D levels <30 ng/mL, had moderate to severe sun damage on the forearm, were at least 40 years of age, had normal liver, renal, and marrow function, had normal serum calcium and parathyroid hormone (PTH) levels, and had skin phototype II or III. Participants were excluded if they had acute or chronic hypervitaminosis D or hypercalcemia, had a history of increased arterial calcification or atherosclerosis, sarcoidosis, histoplasmosis, hyperparathyroidism, lymphoma, or kidney disease, were taking digoxin, cholestyramine, colestipol, oral steroids, and antacids that contain magnesium, were taking other investigational agents, had a history of allergic reactions to cholecalciferol, lidocaine or xylocaine, had uncontrolled intercurrent illness, were pregnant or breastfeeding. Additional exclusion criteria were based on medical and drug history including diagnosis of invasive cancer or cancer treatment within the past five years, except non-melanoma skin cancer, were immunosuppressed by virtue of medication or disease, were unwilling or unable to refrain from taking herbal medicines or above-standard vitamin or mineral supplements during the study, had used tanning beds or other methods to promote sun-tanning within 6 months of study entry, were unwilling to minimize their exposure to sunlight, were treated with topical retinoids, steroids, 5-fluorouracil, Levulan, eflornithine, diclofenac, or imiquimod within 30 days of study entry, had received treatment for basal cell carcinoma or squamous cell carcinoma at study sites within six months of study entry.
Study Procedures
Pre-screening visit
Participants received a skin exam and had a blood sample collected for serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D analysis. Participants were asked to return if their 25-hydroxyvitamin D serum levels were <30 ng/mL and they had evidence of at least moderate skin photodamage on the forearms. A blood sample was collected for complete blood count, comprehensive metabolic panel and intact PTH. A urine pregnancy test was done for women with childbearing potential. Photodamage of the right dorsal forearm was measured using a clinical assessment scale. Selection of Benign Melanocytic Nevi: Subjects were then evaluated for the presence of two benign melanocytic nevi, ≥ 4 mm, located on photoprotected areas of the body where biopsies would be appropriate. Epiluminescent-microscopy (ELM) (25) was used by the study physician aided by the pattern analysis (26) and Modified ABCD (27) rule algorithms to exclude atypical/dysplastic nevi and exclusively select either intradermal or compound nevi to maximize the population of melanocytes in the study samples. Baseline Specimen Collection: Participants who met all eligibility criteria returned for baseline skin specimen collection. A urine pregnancy test was repeated at baseline, follow-up, and end of study if indicated. The clinician selected two areas on the mid-upper right dorsal forearm (photodamaged area) plus two areas on the left buttock (photoprotected area), for biopsy. If available, BN were photographed (standard and ELM photos) and a baseline lesion was biopsied. One set of biopsies were immediately fixed in 10% neutral buffered formalin for 24 hours then transferred to 70% ethanol prior to routine processing and paraffin embedding. Another set of biopsies were immediately separated from surrounding connective and fat tissue, placed in RNAlater overnight at 4°C, and stored at −80°C. Agent Intervention: Following baseline specimen collection, participants were instructed to take one cholecalciferol capsule (50,000 IU per capsule) twice each week for a period of 8–9 weeks. Participants returned to the clinic after 3–5 weeks of agent intervention for adherence and safety evaluation. A blood sample was drawn for assessment of serum calcium levels. Post-intervention Evaluation: Participants then returned after 8–9 weeks of study agent intervention for post-intervention evaluation. Photodamage of the right forearm was measured using a clinical assessment scale. Two skin biopsy samples were collected adjacent to the baseline biopsy sites on the right dorsal forearm and two skin samples collected from the left buttock. When applicable, the other BN identified at screening was re-evaluated by ELM using the pattern analysis and Modified ABCD rules algorithms, photographed, and then collected in an excisional manner. The post-intervention biopsies were handled as described above for the baseline biopsies. A blood sample was collected for laboratory evaluation (CBC-diff, CMP, intact PTH, 25-hydroxyvitamin D, and 1,25-dihydroxyvitamin D serum level analysis). Participants were followed for approximately 10–14 days after the post-intervention evaluation and returned for a final visit for suture removal and examination of the biopsy sites.
Measurement of CYP24 and VDR expression
CYP24 and VDR expression was evaluated using real time-PCR. Total RNA was isolated from the frozen skin tissue (with RNAlater) using an RNeasy Fibrous Tissue Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. The RNA obtained was then quantified using A260/A280 spectrophotometry. DNase treated RNA (2 μg) was reverse transcribed using the High Capacity RNA-to-cDNA kit (Life Technologies, Grand Island, NY). The obtained cDNA was used in 20 μL PCR reactions containing 10 μL Maxima SYBR Green qPCR Master Mixes (Thermo Scientific, Pittsburg, PA) 0.4 μL primers, 0.86 μL of cDNA template sample, and 8.74 μL of molecular grade water. Reactions were performed in 284-well PCR plates and read on a ABI Prism 7900HT Sequence Detection System. Data were analyzed using the comparative Ct method as a means of relative quantitation, normalized to an endogenous reference (TBP cDNA) and relative to a calibrator (normalized Ct value obtained from controls) and expressed as 2−DDCt according to Applied Biosystems User Bulletin 2: Rev B, “Relative Quantitation of Gene Expression.”
Measurement of Skin Layers Thickness
The thickness of skin layers was measured on 4 micron sections of formalin-fixed paraffin-embedded (FFPE) biopsies stained with H&E. The measurement was accomplished using an imaging system composed of a Leica microscope equipped with a Sony DXC 9000 3CCD color video camera, a full complement of Leica plan app objectives and the Image-Pro Plus version 6.3 imaging capture and analysis software (Media Cybernetics, Rockville, MD). Three areas from each skin biopsy were imaged using 40× magnification and the Image-Pro Plus line draw tool, which provides measurements in microns. The stratum corneum was measured from the outer edge of the layer to the base of the lucidum. Epidermis was measured from the base of the lucidum to the basal layer-dermis interface. The three measurements were averaged for analysis.
Measurement of Differentiation Markers
Loricrin and caspase-14 protein expression was determined by immunohistochemistry. Four micron FFPE tissue sections were deparaffinized and rehydrated. All tissue sections were subjected to antigen retrieval using a citrate buffer for 30 seconds. Immunohistochemical staining was performed using a Vectastain Elite Standard ABC kit immunoperoxidase detection system, a Vector Nova Red substrate for peroxidase (Vector Laboratories, Burlingame, CA), and a hematoxylin counterstain (Surgipath, Buffalo Grove, IL). Antibodies included caspase-14 (mouse monoclonal, 1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) and loricrin (rabbit polyclonal, 1:1000 dilution; Covance, Princeton, NJ), which were incubated 1 hour at room temperature. Skin from routine surgical procedures was used as a positive control. Image-Pro Plus image analysis software was used to capture and analyze 3 representative areas at 40X magnification. A standardized intensity score (mean intensity divided by the standard deviation) for each participant was derived at both baseline and post-intervention and used to calculate the change from baseline to post-intervention. For loricin, the entire epidermis was imaged and an intensity score generated. In contrast, for caspase 14, the basal and suprabasal were measured separately and each was given an intensity score.
Measurement of circulating concentrations of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D
The analysis of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D was performed by validated radioimmunoassays (RIA) at Heartland Assays (Ames, IA) (28, 29).
Statistical Analysis
Descriptive statistics, e.g. mean and standard deviation, were calculated on each of the endpoints. A two-sided paired t test was performed to test if the percent change from baseline to post-intervention in each of the endpoints is significantly different from zero. Each analysis was performed comparing results within each individual from baseline to post-intervention values. A two-sided paired t test was also performed to compare the baseline tissue biomarkers between photoprotected and photodamaged skin except a signed rank test was used to compare the baseline CYP24 expression between photoprotected and photodamaged skin because the data were not normally distributed. Pearson correlation coefficients between changes in serum levels of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and changes in each tissue biomarker were calculated to evaluate whether the changes are significantly correlated.
Results
The study pre-screened 50 and accrued 25 participants between October 2011 and July 2012. Characteristics of study participants are summarized in Table 1. The mean age of the study participants was 57.8 years with a male:female distribution of 8:17. The mean BMI was 29.7 kg/m2.
Table 1.
Study Participants Characteristics
| Variables | |
|---|---|
| Age, yr (mean ± SD) | 57.8 ± 7.2 |
| Weight, pounds (mean ± SD) | 185.2 ± 40.8 |
| Height, inches (mean ± SD) | 66.2 ± 3.9 |
| BMI, kg/m2 (mean ± SD) | 29.7 ± 5.9 |
| Sex, male/female | 8/17 |
| Race, White/Other | 23/2 |
High dose cholecalciferol (50,000 IU) taken twice a week for 8–9 weeks was well tolerated in our study participants. None of the reported adverse events was related to the drug. The two most frequently reported AEs were headache (4 Grade 1 and 2 Grade 2 reports) and flu-like symptoms (3 Grade 1 and 1 Grade 2).
The intervention also did not result in clinically significant changes in hematology, blood chemistry, and PTH. As shown in Table 2, the study intervention significantly increased serum levels of 25-hydroxyvitamin D from 21.6 ± 5.2 to 70.5 ± 18.2 ng/mL (p<0.0001) and 1,25-dihydroxyvitamin D from 31.1 ± 12.4 to 51.4 ± 13.5 ρg/mL (p<0.0001). However, the systemic increase in these 2 metabolites did not correlate with individual modulations in VDR and CYP expression.
Table 2.
Serum levels of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D before and after agent intervention
| Analyte | Baseline mean ± SD (range) | Post-intervention mean ± SD (range) | Change mean ± SD (range) | % change mean ± SD (range) | pa |
|---|---|---|---|---|---|
| 25-hydroxyvitamin D, ng/mL | 21.6 ± 5.2 (12.7 – 29.6) | 70.5 ± 18.2 (41.5 – 105.1) | 48.9 ± 16.9 (22.2 – 87.7) | 239.4 ± 106.2 (77.6 – 596.6) | < 0.0001 (<0.0001) |
| 1,25-dihydroxyvitamin D, pg/mL | 31.1 ± 12.4 (8.4 – 56.6) | 51.4 ± 13.5 (24.4 – 86.5) | 20.3 ± 13.9 (−11.0 – 48.3) | 86.3 ± 76.0 (−19.5 – 358.2) | < 0.0001 (<0.0001) |
Note: after adjusting for baseline level by linear regression, p=0.01 (<0.0001) for change (% change) in 25-hydroxyvitamin D and <0.0001 (<0.0001) for 1,25-dihydroxyvitamin D
Table 3 summarizes the changes in VDR and CYP24 expression. The baseline VDR expression was similar between photoprotected and photodamaged skin. The intervention induced minimal changes in VDR expression in both photoprotected and photodamaged skin. Baseline CYP24 expression was higher in photodamaged skin compared to photoprotected skin (37,698 ± 10,801 vs. 10,859 ± 21,767, p<0.0001). The intervention induced a consistent upregulation trend of CYP24 expression (% change: 134.43 ± 347.55; p=0.07 in photoprotected skin and 185.92 ± 502.19; p=0.08 in photodamaged skin). VDR and CYP24 expression was also evaluated in 11 subjects with eligible benign melanocytic nevi. This number of nevi was consistent with our anticipated recruitment rate of approximately 40% of subjects who would have eligible nevi for inclusion in this exploratory endpoint. An upregulation trend of VDR (% change: 19.86 ± 33.61; p=0.08) and CYP24 (% change: 544.02 ± 955.27; p=0.09) was observed in nevi following supplementation.
Table 3.
Measurements of VDR and CYP24 expression
| Baseline Mean ± SD |
Post-Intervention Mean ± SD |
Change Mean ± SD |
% change Mean ± SD |
pb | |
|---|---|---|---|---|---|
| VDR (2-DDCt) | |||||
| Photoprotected skin (n = 25) | 4993 ± 2488 | 5073 ± 3525 | 81 ± 3546 (0.91a) | 35.3 ± 159.2 (0.28) | 0.10 (0.01) |
| Photodamaged skin (n = 25) | 5171 ± 2785 | 5009 ±2678 | −163 ± 1207 (0.51) | −0.99 ± 28.00 (0.86) | 0.31 (0.58) |
| Benign Nevi (n = 11) | 2607 ± 1180 | 2993 ±1396 | 386 ± 712 (0.10) | 19.86 ± 33.61 (0.08) | 0.56 (0.09) |
| CYP24 (2-DDCt) | |||||
| Photoprotected skin (n = 25) | 10859 ± 21767 | 19403 ±41494 | 8544 ± 21533 (0.06) | 134.43 ± 347.55 (0.07) | 0.87 (0.07) |
| Photodamaged skin (n = 24) | 37698 ± 10801 | 48502 ±96677 | 10804 ± 102729 (0.61) | 185.92 ± 502.19 (0.08) | 0.10 (0.08) |
| Benign Nevi (n = 11) | 9247 ± 17218 | 19311 ±36018 | 10063 ± 39247 (0.42) | 544.02 ± 955.27 (0.09) | 0.19 (0.07) |
p-value derived from paired t test
p-value derived from linear regression after adjusting for baseline value for change (% changes) from baseline
Table 4 summarizes the measurements of differentiation markers. Baseline loricrin and caspase 14 staining intensity was similar between photoprotected and photodamaged skin in different skin layers. The staining intensity of these markers did not change significantly after oral cholecalciferol supplementation with the exception of a significant increase (p<0.0001) in caspase 14 staining intensity in the basal layer of the photodamaged skin (Figure 1). In addition, VD supplementation did not result in significant changes in skin layer thickness (data not shown).
Table 4.
Measurements of differentiation markers
| Baseline | Post-intervention | % change | pa | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Photoprotected skin | ||||
| Loricrin (n = 25) | 5.67 ± 1.60 | 6.03 ± 1.95 | 13.97 ± 47.05 | 0.15 |
| Caspase 14 | ||||
| Basal (n = 23) | 2.57 ± 0.54 | 2.52 ± 0.39 | 0.19 ± 17.87 | 0.96 |
| Suprabasal (n = 25) | 3.46 ± 1.18 | 3.68 ± 1.02 | 15.58 ± 43.71 | 0.10 |
| Photodamaged skin | ||||
| Loricrin (n = 25) | 5.65 ± 1.69 | 5.18 ±1.14 | −4.99 ± 22.95 | 0.29 |
| Caspase 14 | ||||
| Basal (n = 25) | 2.58 ± 0.31 | 3.80 ± 1.03 | 48.63 ± 38.63 | <0.0001 |
| Suprabasal (n = 25) | 3.84 ± 1.58 | 3.76 ± 0.80 | 7.41 ± 39.13 | 0.35 |
derived from paired t-test for % change from baseline
Figure 1. Effect of oral vitamin D supplementation on epidermal differentiation.
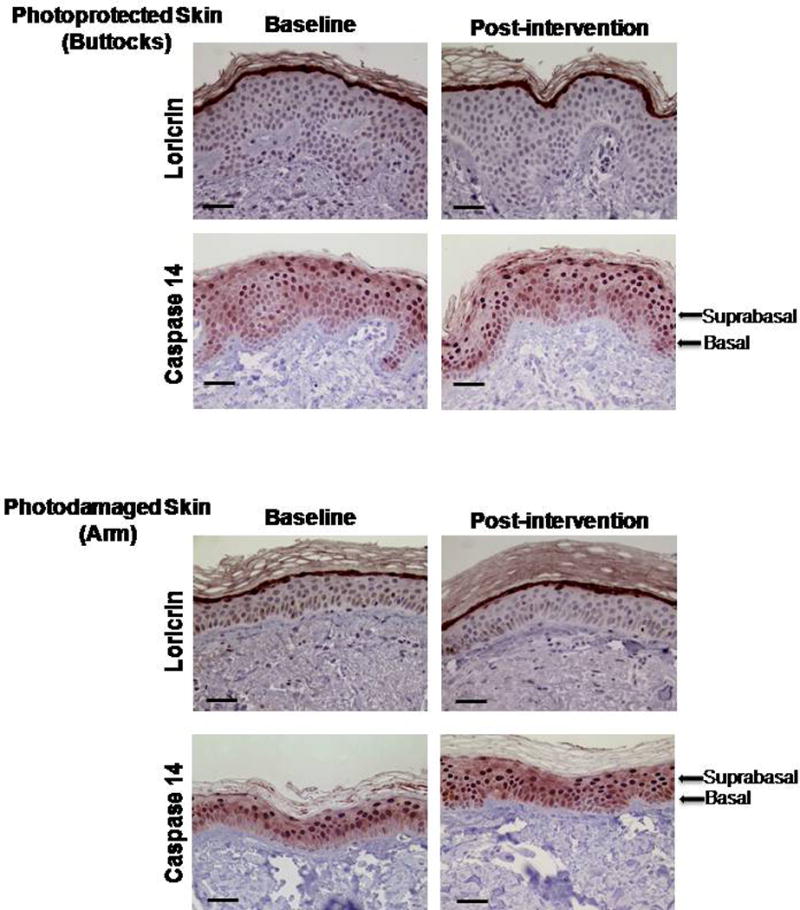
Both loricrin and caspase 14 staining were used to analyze epidermal differentiation. Images are at a magnification of 40× and the scale bar represents 50 μm. Loricrin stain is localized to the stratum granulosum while caspase 14 expression is observed throughout the entire thickness of the epidermis (cytoplasmic and nuclear). The images depict examples of similar Loricrin expression in photoprotected and photodamaged skin at baseline and post-intervention time points. In the case of caspase 14, an increased expression was observed in the basal layer of photodamaged skin after intervention when compared to baseline and photoprotected skin.
Discussion
Our study is one of the first to assess the levels of putative markers of vitamin D bioactivity in human skin and to determine the changes in these markers after oral supplementation. We showed that supplementation with 50,000 IU of cholecalciferol biweekly for eight weeks improved the D serum levels status of 25(OH)D and 1,25(OH)2D in individuals who were considered vitamin D insufficient. Armas et al (30) showed that a single dose of 50,000 IU of cholecalciferol resulted in persistent elevation of serum 25-hydroxyvitamin D levels over a 4-week period, which supported the significant increase in 25-hydroxyvitamin D with the dosing regimen used in our study. In addition, we showed that high dose vitamin D supplementation also resulted in a significant elevation in serum levels of 1,25-dihydroxyvitamin D, the bioactive form of vitamin D that is generally considered to be tightly regulated.
We selected measurements of VDR mRNA expression as the primary measure of the bioactivity of vitamin D in the skin because vitamin D supplementation to vitamin D deficient rats has been shown to increase VDR mRNA expression in skin keratinocytes (31). Despite showing a significant elevation in circulating levels of 25(OH)D and 1,25(OH)2D, we did not find a change in the expression of VDR mRNA after high dose oral cholecalciferol supplementation in either photodamaged and photoprotected human skin. One explanation may be that the expression of VDR in skin is not suppressed in individuals with this level of vitamin D insufficiency. Thus, Vitamin D supplementation may not be able to exert a significant modulatory effect in cells with normal VDR expression. Interestingly, the baseline VDR expression was similar between photodamaged and photoprotected skin, suggesting that VDR expression may not be sensitive to solar exposure and/or photodamage. Given the important role of VDR in cell function, it is plausible that the expression level is preserved despite broad changes in 25 hydroxyvitamin D and 1,25 dihydroxyvitamin D. Studies evaluating the effect of oral supplementation in more profoundly depleted patients might provide additional information as to the possibility of modulating VDR expression in this clinical setting. However, VDR modulatory findings in such extreme circumstances and beneficial effect of supplementation might not be applicable to the vast majority of patients. An additional explanation to the significant variability in VDR expression observed across study participants and lack of reaching clinical significance in this pilot study include the range of VDR polymorphisms that might result in different binding capabilities to VD and further activation of downstream signaling a different serum levels (32, 33). Additional studies involving VDR and CYP24 genotyping might provide further information on the role of this potential confounder and possibility to identify those individuals that will be more likely to respond to oral supplementation. Also changes in a larger array of gene expression profiles or proteomics analysis in samples collected from our study may help identify more suitable biomarkers for future clinical research.
We also assessed the CYP24 mRNA expression as a measure of the vitamin D bioactivity in the skin because multiple in vitro studies have shown an induction of CYP24 with vitamin D treatment (34). We observed a consistent upregulation of CYP24 mRNA expression after high dose cholecalciferol supplementation, although the increase did not reach statistical significance. We also observed a significantly higher baseline CYP24 mRNA expression in photodamaged than that in photoprotected skin, possibly due to differential solar exposure and/or photodamage in different anatomical areas. This suggests that CYP24 may be a more sensitive biomarker to changes in solar exposure, photodamage, and vitamin D status, thus it could be more easily modulated by vitamin D supplementation. Overall, a significant variability in the range of VDR and CYP24 expression was identified across study samples. We consider this a relevant consideration when carrying out future studies evaluating the in vivo modulation at targeted tissue following systemic VD supplementation.
In 11 individuals with qualifying nevi, we observed a trend of higher melanocytic nevus expression of both VDR and CYP24 in the post-intervention samples. The upregulation of CYP24 in melanocytic nevi following cholecalciferol supplementation is consistent with that observed in keratinocytes. The observed upregulation of VDR in melanocytic nevi suggests that VDR expression may be more susceptible to modulation in VD status in human melanocytes when compared to keratinocytes.
We incorporated measures of epidermal differentiation in our study because preclinical studies suggested that an increase in differentiation is one of the potential mechanisms responsible for the vitamin D effects on cutaneous carcinogenesis (35, 36). However, high dose cholecalciferol supplementation had minimal effects on the protein markers of differentiation and skin layer thickness, although we did observe a significant increase in caspase 14 expression in the basal layer of the photodamaged skin after 8 weeks of treatment. The supplementation effects on VDR and CYP24 mRNA expression did not translate to a similar change in protein markers of differentiation or in measures of skin layer thickness. This lack of correlation is not surprising as previous studies have shown that 1,25-dihydroxyvitamin D alters keratinocyte differentiation through multiple complex mechanisms and such regulations may be affected by calcium homeostasis making the identification of terminal differentiation markers more challenging based on isolated endpoints (17, 37).
Our study has several limitations. First, we have observed a large inter-individual variation in VDR and CYP24 mRNA expression. A larger sample size may be needed to observe a significant effect from oral supplementation on these measurements. Second, an intervention of 8 weeks might have been sufficient to correct the serum levels but not long enough to observe changes in epidermal differentiation markers. Third, we have selected markers of bioactivity based primarily on studies in cell line and small animal models. These markers may not be the optimal endpoints to reflect clinical bioactivity. Because of the complexity of the vitamin D signaling pathways, additional studies on changes in gene expression profiles or proteomic analysis in samples collected from our study may help identify more suitable biomarkers for future clinical research. Lastly, we designed this study as a pilot project to assess changes in study endpoints after the supplementation and the feasibility of biomarker assessments. Future clinical investigation should include a controlled arm to minimize potential confounders in single arm studies.
We conclude that high dose cholecalciferol supplementation resulted in significant elevation of circulating levels of both 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D in individuals who are considered vitamin D insufficient and variable changes in selected markers of VD bioactivity in the skin. Our data suggest that CYP24 expression in the skin may be a sensitive marker for solar exposure, photodamage, and vitamin D status and supplementation. Our study highlights the importance of evaluating the bioactivity of vitamin D supplementation in the target tissue in order to optimize the development of vitamin D supplementation for cancer prevention.
Acknowledgments
Our appreciation to Christine Brooks, Clinic Coordinator, for her tireless effort and professional execution of the study. To Dr. Beth Jacobs for her expert guidance on the assessment of biomarker determination at the time of study design. Lastly, to the study subjects for their willingness to participate in the study.
Funding source: Supported by a contract (NO1CN35158 – All authors received grant) from the National Cancer Institute (NCI), Division of Cancer Prevention, University of Arizona Cancer Center Support Grant (CA023074 – University of Arizona Cancer Center members – C Curiel-Lewandrowski, JG Einspahr, Y Bermudez, C-H Hsieh, DS Alberts, H-H. S. Chow), and Janice and Alan Levine Endowed Chair in Cancer Research (C Curiel-Lewandrowski), University of Arizona Cancer Center and NCI P01CA027502 (C Curiel-Lewandrowski, JG Einspahr, Y Bermudez, DS Alberts).
Abbreviations
- VD
Vitamin D
- VD3
Cholecalciferol
- VDR
Vitamin D receptor
- CYP24
Cytochrome P450
- CM
Cutaneous melanoma
- NMSC
Non-melanoma skin cancer
- BCC
Basal cell carcinoma
- SCC
Squamous cell carcinoma
- BN
Benign nevi
- ELM
Epiluminescence microscopy
Footnotes
Disclosures:
Clara Curiel-Lewandrowski: MelaSciences, Inc-Consulting; Medical Directions, Inc-Consulting; DermSpectra LLC-Founder/Member
References
- 1.Tang JY, Parimi N, Wu A, Boscardin WJ, Shikany JM, Chren MM, et al. Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men. Cancer Causes Control. 2010;21:387–91. doi: 10.1007/s10552-009-9470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asgari MM, Tang J, Warton ME, Chren MM, Quesenberry CP, Jr, Bikle D, et al. Association of prediagnostic serum vitamin D levels with the development of basal cell carcinoma. J Invest Dermatol. 2010;130:1438–43. doi: 10.1038/jid.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandini S, Raimondi S, Gnagnarella P, Dore JF, Maisonneuve P, Testori A. Vitamin D and skin cancer: a meta-analysis. Eur J Cancer. 2009;45:634–41. doi: 10.1016/j.ejca.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Tang JY, Fu T, Leblanc E, Manson JE, Feldman D, Linos E, et al. Calcium plus vitamin D supplementation and the risk of nonmelanoma and melanoma skin cancer: post hoc analyses of the women’s health initiative randomized controlled trial. J Clin Oncol. 2011;29:3078–84. doi: 10.1200/JCO.2011.34.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linos E, Keiser E, Kanzler M, Sainani KL, Lee W, Vittinghoff E, et al. Sun protective behaviors and vitamin D levels in the US population: NHANES 2003–2006. Cancer Causes Control. 2012;23:133–40. doi: 10.1007/s10552-011-9862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Pols JC, Russell A, Bauer U, Neale RE, Kimlin MG, Green AC. Vitamin D status and skin cancer risk independent of time outdoors: 11-year prospective study in an Australian community. J Invest Dermatol. 2013;133:637–41. doi: 10.1038/jid.2012.346. [DOI] [PubMed] [Google Scholar]
- 7.Schafer A, Emmert S, Kruppa J, Schubert S, Tzvetkov M, Mossner R, et al. No association of vitamin D metabolism-related polymorphisms and melanoma risk as well as melanoma prognosis: a case-control study. Arch Dermatol Res. 2012;304:353–61. doi: 10.1007/s00403-012-1243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy KK. Vitamin D level and basal cell carcinoma, squamous cell carcinoma, and melanoma risk. J Invest Dermatol. 2013;133:589–92. doi: 10.1038/jid.2012.427. [DOI] [PubMed] [Google Scholar]
- 9.Afzal S, Nordestgaard BG, Bojesen SE. Plasma 25-hydroxyvitamin D and risk of non-melanoma and melanoma skin cancer: a prospective cohort study. J Invest Dermatol. 2013;133:629–36. doi: 10.1038/jid.2012.395. [DOI] [PubMed] [Google Scholar]
- 10.Tang JY, Fu T, Lau C, Oh DH, Bikle DD, Asgari MM. Vitamin D in cutaneous carcinogenesis: part I. J Am Acad Dermatol. 2012;67:803e1–12. doi: 10.1016/j.jaad.2012.05.044. quiz 15–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berwick M, Erdei EO. Vitamin D and melanoma incidence and mortality. Pigment Cell Melanoma Res. 2013;26:9–15. doi: 10.1111/pcmr.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Major JM, Kiruthu C, Weinstein SJ, Horst RL, Snyder K, Virtamo J, et al. Pre-diagnostic circulating vitamin D and risk of melanoma in men. PLoS One. 2012;7:e35112. doi: 10.1371/journal.pone.0035112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowd DR, MacDonald PN. The 1,25-dihydroxyvitamin D3-independent actions of the vitamin D receptor in skin. J Steroid Biochem Mol Biol. 2010;121:317–21. doi: 10.1016/j.jsbmb.2010.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reichrath J, Rafi L, Rech M, Mitschele T, Meineke V, Gartner BC, et al. Analysis of the vitamin D system in cutaneous squamous cell carcinomas. J Cutan Pathol. 2004;31:224–31. doi: 10.1111/j.0303-6987.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 15.Sertznig P, Seifert M, Tilgen W, Reichrath J. Activation of vitamin D receptor (VDR)- and peroxisome proliferator-activated receptor (PPAR)-signaling pathways through 1,25(OH)(2)D(3) in melanoma cell lines and other skin-derived cell lines. Dermatoendocrinol. 2009;1:232–8. doi: 10.4161/derm.1.4.9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bikle DD, Gee E, Pillai S. Regulation of keratinocyte growth, differentiation, and vitamin D metabolism by analogs of 1,25-dihydroxyvitamin D. J Invest Dermatol. 1993;101:713–8. doi: 10.1111/1523-1747.ep12371681. [DOI] [PubMed] [Google Scholar]
- 17.Popadic S, Ramic Z, Medenica L, Mostarica Stojkovic M, Trajkovic V, Popadic D. Antiproliferative effect of vitamin A and D analogues on adult human keratinocytes in vitro. Skin Pharmacol Physiol. 2008;21:227–34. doi: 10.1159/000135639. [DOI] [PubMed] [Google Scholar]
- 18.Zinser GM, Suckow M, Welsh J. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J Steroid Biochem Mol Biol. 2005;97:153–64. doi: 10.1016/j.jsbmb.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Tremezaygues L, Seifert M, Tilgen W, Reichrath J. 1,25-dihydroxyvitamin D(3) protects human keratinocytes against UV-B-induced damage: In vitro analysis of cell viability/proliferation, DNA-damage and -repair. Dermatoendocrinol. 2009;1:239–45. doi: 10.4161/derm.1.4.9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seifert M, Rech M, Meineke V, Tilgen W, Reichrath J. Differential biological effects of 1,25-dihydroxyVitamin D3 on melanoma cell lines in vitro. J Steroid Biochem Mol Biol. 2004;89–90:375–9. doi: 10.1016/j.jsbmb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Egan KM. Vitamin D and melanoma. Ann Epidemiol. 2009;19:455–61. doi: 10.1016/j.annepidem.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Watabe H, Soma Y, Kawa Y, Ito M, Ooka S, Ohsumi K, et al. Differentiation of murine melanocyte precursors induced by 1,25-dihydroxyvitamin D3 is associated with the stimulation of endothelin B receptor expression. J Invest Dermatol. 2002;119:583–9. doi: 10.1046/j.1523-1747.2002.00116.x. [DOI] [PubMed] [Google Scholar]
- 23.Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593–8. doi: 10.1111/1523-1747.ep12477126. [DOI] [PubMed] [Google Scholar]
- 24.Sauer B, Ruwisch L, Kleuser B. Antiapoptotic action of 1alpha,25-dihydroxyvitamin D3 in primary human melanocytes. Melanoma Res. 2003;13:339–47. doi: 10.1097/00008390-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 25.English DR, MacLennan R, Rivers JK, Kelly J, Armstrong BK. Epidemiological studies of melanocytic naevi: protocol for identifying and recording naevi. Lyon, France: International Agency for Research on Cancer; 1990. (IARC Internal Report No. 90/002). [Google Scholar]
- 26.Annessi G, Bono R, Sampogna F, Faraggiana T, Abeni D. Sensitivity, specificity, and diagnostic accuracy of three dermoscopic algorithmic methods in the diagnosis of doubtful melanocytic lesions: the importance of light brown structureless areas in differentiating atypical melanocytic nevi from thin melanomas. J Am Acad Dermatol. 2007;56:759–67. doi: 10.1016/j.jaad.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Blum A, Rassner G, Garbe C. Modified ABC-point list of dermoscopy: A simplified and highly accurate dermoscopic algorithm for the diagnosis of cutaneous melanocytic lesions. J Am Acad Dermatol. 2003;48:672–8. doi: 10.1067/mjd.2003.282. [DOI] [PubMed] [Google Scholar]
- 28.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39:529–33. [PubMed] [Google Scholar]
- 29.Hollis BW, Kamerud JQ, Kurkowski A, Beaulieu J, Napoli JL. Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with 125I-labeled tracer. Clin Chem. 1996;42:586–92. [PubMed] [Google Scholar]
- 30.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–91. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 31.Zineb R, Zhor B, Odile W, Marthe RR. Distinct, tissue-specific regulation of vitamin D receptor in the intestine, kidney, and skin by dietary calcium and vitamin D. Endocrinology. 1998;139:1844–52. doi: 10.1210/endo.139.4.5903. [DOI] [PubMed] [Google Scholar]
- 32.Raimondi S, Johansson H, Maisonneuve P, Gandini S. Review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Carcinogenesis. 2009;30:1170–80. doi: 10.1093/carcin/bgp103. [DOI] [PubMed] [Google Scholar]
- 33.Lucas RM, Ponsonby AL, Dear K, Valery PC, Taylor B, van der Mei I, et al. Vitamin D status: multifactorial contribution of environment, genes and other factors in healthy Australian adults across a latitude gradient. J Steroid Biochem Mol Biol. 2013;136:300–8. doi: 10.1016/j.jsbmb.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Tuckey RC, Janjetovic Z, Li W, Nguyen MN, Zmijewski MA, Zjawiony J, et al. Metabolism of 1alpha-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1alpha,20-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 2008;112:213–9. doi: 10.1016/j.jsbmb.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lippens S, Kockx M, Denecker G, Knaapen M, Verheyen A, Christiaen R, et al. Vitamin D3 induces caspase-14 expression in psoriatic lesions and enhances caspase-14 processing in organotypic skin cultures. Am J Pathol. 2004;165:833–41. doi: 10.1016/S0002-9440(10)63346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, et al. 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol. 2004;122:984–92. doi: 10.1111/j.0022-202X.2004.22424.x. [DOI] [PubMed] [Google Scholar]
- 37.Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Dardenne O, et al. Mice lacking 25OHD 1alpha-hydroxylase demonstrate decreased epidermal differentiation and barrier function. J Steroid Biochem Mol Biol. 2004;89–90:347–53. doi: 10.1016/j.jsbmb.2004.03.113. [DOI] [PubMed] [Google Scholar]


