Abstract
In this study we examined Th1 and Th17 immune responses to rat myelin basic protein (MBP), bovine MBP, human MBP, MBP 68-86, MBP 63-81 and ovalbumin in Lewis rats to determine which MBP antigen is recognized following ischemic brain injury. Responses were compared to animals immunized to rat MBP. Data show that immune responses following immunization with rat MBP are promiscuous with cross reaction to MBP from other species. After stroke, few animals develop Th1 or Th17 responses to MBP, but when those responses occur, especially Th1 responses to rat MBP in brain, they are predictive of worse stroke outcome.
Keywords: stroke, Th1, Th17, MBP, immune response
Th1 type responses to myelin basic protein (MBP) after experimental stroke are associated with, and seem to mediate, worse functional outcome (Becker et al., 2005; Zierath et al., 2013; Zierath et al., 2010b). The exact mechanisms by which Th1 immune responses to MBP worsen stroke outcome are unclear, but there is evidence of enhanced CD8+ infiltration and increased brain atrophy at one month after middle cerebral artery occlusion (MCAO) in animals with Th1(+) responses (Becker et al, 2005). And while animals with Th1(+) responses to MBP perform worse on behavioral tests after stroke, they do not manifest overt signs of experimental allergic encephalomyelitis (EAE) (Becker et al., 2005; Gee et al., 2008; Gee et al., 2009; Zierath et al., 2010b). It is also true that the phenotype and pathology of EAE differs depending upon the antigen used to initiate EAE, the species from which the antigen is derived, the portion of antigen to which the immune response is directed, and the type of cells mediating the immune response (Domingues et al., 2010; Jager et al., 2009; Mannie et al., 2009; Stromnes et al., 2008).
Following stroke there is necrosis of brain tissue and antigens from dying cells are presented to the immune system, either within the brain or in the periphery (Becker et al., 2005; Planas et al., 2012; van Zwam et al., 2009). Given the degree of necrosis that accompanies severe brain injury, these neural antigens have undoubtedly been transformed from their native state. It is thus unclear which antigens drive the post-ischemic immune response and whether the outcomes differ depending upon the target epitope. In prior studies we assessed the immune response to MBP after stroke using commercially available bovine MBP and human MBP and found that the immune responses to both of these heterologous proteins were predictive of functional outcome (Becker et al., 2005; Zierath et al., 2013). In this study we aimed to determine whether the immune response to MBP after stroke was more robust to whole MBP or one its encephalitogenic peptides and whether there were differences in the responses to autologous rat MBP and heterologous (bovine, human) MBP. Further, the immune responses were assessed in spleen, lymph node and brain to evaluate whether the responses differed based on the compartment (spleen, lymph node, brain) tested. Finally, we aimed to determine if the responses to a particular antigen were more predictive of outcome than responses to other antigens in animals subjected to stroke. As an immunologic control, a subset of animals was immunized to rat MBP and immune responses to the same set of antigens assessed.
Methods
Animals
Male Lewis rats (275–325 grams) were purchased from Taconic Farms. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC).
Immunization to MBP
Male Lewis rats were immunized with either recombinant rat MBP (prepared by Neo BioScience™) or OVA (Sigma). Antigens were mixed in complete Freund’s adjuvant and injected into the hind foot pad. Two doses of MBP were used, 50μg (n=16) and 100 μg (N=14). Control animals were immunized with OVA 50μg (N=18). Twelve days after immunization, animals were sacrificed and lymphocytes isolated from the brain, spleen, and cervical lymph nodes for ELISPOT assay.
Middle Cerebral Artery Occlusion (MCAO)
Anesthesia was induced with 5% and maintained with 1.5% isoflurane. After midline neck incision, the right common carotid, internal carotid and pterygopalantine arteries were ligated. A silicone rubber-coated monofilament suture (4-0, Doccol, Sharon MA) was inserted into the common carotid artery and advanced into the internal carotid artery (Longa et al., 1989). Animals were maintained at normothermia during surgery and reperfused 2 hours after MCAO. In sham-operated animals, the suture was inserted into the carotid artery but not advanced. Rectal temperature and body weight were assessed at set time intervals. Animals were sacrificed one month after surgery.
ELISPOT Assays
At the time of sacrifice, lymphocytes were isolated from the spleen, cervical lymph nodes and brain as previously described (Becker et al., 2005). (Note: for a subset of animals [N=15], brains were frozen and lymphocytes from this organ were not available for analysis.) ELISPOT assays were done to detect the MBP specific secretion of IFN-γ, IL-17 and TGF-β1 (R&D Systems) by lymphocytes. Rat MBP (rMBP) was manufactured by NeoBioSci™. Bovine MBP (bMBP), human MBP (hMBP), ovalbumin (OVA) and lipopolysaccharide (LPS) were purchased from Sigma. Encephalitogenic peptides of MBP (MBP 68-86 and MBP 63-81) were purchased from AnaSpec. All antigens/mitogens were used at a concentration of 50 μg/mL; responses were assessed in triplicate.
Briefly, cells were cultured in media alone or in media supplemented with MBP (or an MBP peptide), OVA or LPS for 48 hours in 96 well plates (Multiscreen®-IP, Millipore). Plates were developed using standard protocols (R & D Systems). After plate development, spots were counted with the aid of a semi-automated system (AID iSPOT®) and expressed as the ratio of the relative increase in the number of MBP specific IFN-γ secreting cells to the relative increase in the number of MBP specific TGF-β1 secreting cells (Th1 response) or as the ratio of the relative increase in the number of MBP specific IL-17 secreting cells to the relative increase in the number of MBP specific TGF-β1 secreting cells (Th17 response).
The ratios of the number of MBP specific IFN-γ and MBP specific IL-17 secreting cells to that of MBP specific TGF-β1 secreting cells (Th1 and Th17 responses, respectively) were used to reflect the overall immunologic phenotype of the response to MBP and OVA. For the purposes of this study, animals were considered to be Th1(+) or Th17(+) if the Th1 or Th17 response to MBP was greater than the median value for that antigen in the organ of interest when compared to animals immunized with rat MBP.
Behavioral Outcomes
The neurological score of animals undergoing MCAO was determined at routine intervals up to 1 month after MCAO (Bederson et al., 1986). Animals were trained on the rotarod prior to MCAO. After MCAO, rotarod performance was assessed weekly until one month after stroke. Performance of the foot fault test was assessed at these time points and the results expressed as a percentage of foot faults per total number of steps taken (Lubics et al., 2005).
Statistics
Non-parametric data are displayed as median (interquartile range) and compared using the Mann-Whitney U test or Kruskal-Wallis H test, as appropriate. Categorical data are evaluated using the χ2-test statistic. Significance was set at P≤0.05.
Results
Immunization with MBP
Immunization with rat MBP, using either the 50 μg or 100 μg dose, did not lead to overt signs of EAE. The percentage of animals with weight loss at 12 days did not differ between MBP or OVA immunized animals. Given the lack of clinical difference between the 2 doses of rat MBP, all rat MBP immunized animals were considered together for immunologic analyses. The Th1 and Th17 responses to whole MBP, MBP peptides and OVA are displayed in Table 1. The responses to whole MBP and MBP peptides were not as robust in spleen as in other organs. For instance, among MBP immunized animals, Th1 responses to hMBP were most robust in the lymph node (P=0.04), while Th17 responses to rMBP, (P<0.001), bMBP (P=0.008) and hMBP (P=0.002) were most robust in the brain. Nonetheless, animals immunized with rat MBP had increased Th17 responses to rat and bovine MBP in spleen, as well as to MBP 68-86, when compared to OVA immunized animals. In the cervical lymph nodes, both Th1 and Th17 responses to rat and human MBP were more robust in rat MBP immunized animals than OVA immunized animals. And in brain, Th1 and Th17 responses to rat, bovine and human MBP were greater in rat MBP immunized animals. The data for animals subjected to MCAO are also presented in Table 1. Overall, the immune responses to MBP and MBP peptides among animals subjected to cerebral ischemia did not differ from that of OVA immunized (control) animals with the exception of more robust Th17 responses to rat MBP and bovine MBP in the spleen. In animals subjected to MCAO, the numbers of cells secreting cytokines (IFN-γ, IL-17, TGF-β1) to LPS (ie. in an antigen independent fashion) were greater among splenocytes (Table 2).
Table 1.
Comparison of Th1 and Th17 responses to whole MBP, MBP peptides and OVA in animals immunized with OVA or rat MBP and in animals subjected to MCAO. Data are displayed as median (interquartile range). Statistics are by Mann-Whitney U test.
| spleen | lymph node | brain | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Th1 | OV A N=14 |
MBP N=22 |
MCA O N=37 |
OV A N=10 |
MBP N=19 |
MCA O N=36 |
OV A N=11 |
MBP N=23 |
MCAO (ischemic hemisphere ) N=22 |
MCAO (non- ischemic hemisphere ) N=22 |
| rMBP | 0.80 (0.66, 1.06) | 0.98 (0.72, 1.34) | 0.82 (0.46, 1.75) | 0.67 (0.40, 1.05) | 1.35* (0.78, 2.00) | 1.26 (0.61, 2.13) | 0.74 (0.40, 1.04) | 1.26** (0.88, 1.43) | 0.88 (0.49, 1.68) | 0.89 (0.63, 1.51) |
| MBP 68-86 | 0.72 (0.54, 1.18) | 1.00 (0.55, 1.36) | 0.91 (0.48, 1.73) | 1.00 (0.55, 1.34) | 1.37 (0.80, 2.27) | 1.06 (0.56, 1.80) | 1.00 (0.66, 1.90) | 0.95 (0.86, 1.33) | 1.10 (0.56, 1.78) | 1.34 (0.71, 1.97) |
| MBP 63-81 | 0.70 (0.61, 1.36) | 1.01 (0.53, 1.45) | 0.84 (0.54, 1.86) | 0.75 (0.64, 1.83) | 1.00 (0.82, 1.34) | 1.05 (0.49, 1.86) | 0.91 (0.57, 1.08) | 1.16 (0.81, 1.45) | 0.91 (0.48, 1.22) | 1.41 (0.59, 2.35) |
| bMBP | 0.77 (0.64, 1.15) | 1.00 (0.66, 1.27) | 0.92 (0.56, 1.64) | 0.98 (0.70, 1.08) | 1.34 (0.77, 2.53) | 1.02 (0.61, 1.87) | 0.73 (0.31, 1.00) | 1.16** (0.92, 1.54) | 0.93 (0.55, 1.48) | 1.27 (0.57, 1.84) |
| hMBP | 1.02 (0.72, 1.24) | 0.88 (0.43, 1.97) | 1.02 (0.60, 1.58) | 0.75 (0.50, 1.34) | 2.15** (1.36, 3.33) | 0.89† (0.52, 2.02) | 0.64 (0.33, 1.17) | 1.44* (0.74, 2.68) | 0.95† (0.66, 1.33) | 1.18 (0.53, 2.32) |
| OVA | 0.88 (0.72, 1.27) | 1.03 (0.64, 1.64) | 0.93 (0.49, 1.58) | 1.00 (0.75, 2.91) | 1.35 (0.86, 3.79) | 0.98 (0.48, 1.49) | 0.62 (0.26, 0.92) | 0.92 (0.72, 1.96) | 0.90 (0.39, 1.56) | 0.97* (0.67, 1.54) |
| Th17 |
OVA N=14 |
MBP N=22 |
MCAO N=37 |
OVA N=10 |
MBP N=19 |
MCAO N=36 |
OVA N=11 |
MBP N=23 |
MCAO (ischemic hemisphere) N=22 |
MCAO (non-ischemic hemisphere) N=22 |
| rMBP | 0.60 (0.44, 0.76) | 0.84** (0.70, 1.18) | 0.85* (0.45, 1.47) | 0.89 (0.62, 1.00) | 1.69** (1.00, 2.39) | 0.76† (0.40, 2.00) | 0.79 (0.56, 0.99) | 1.47** (1.20, 1.96) | 1.11† (0.41, 1.78) | 0.80‡ (0.45, 1.46) |
| MBP 68-86 | 0.66 (0.27, 1.18) | 1.26* (0.76, 2.40) | 0.84 (0.53, 1.40) | 1.00 (0.70, 1.74) | 1.50 (0.89, 2.30) | 0.67† (0.34, 1.46) | 0.98 (0.58, 1.38) | 1.42 (0.88, 2.02) | 1.10 (0.40, 1.85) | 1.05 (0.54, 2.16) |
| MBP 63-81 | 0.61 (0.35, 1.26) | 0.98 (0.63, 2.10) | 0.88 (0.56, 1.24) | 1.00 (0.68, 1.94) | 0.95 (0.43, 1.69) | 0.89 (0.37, 1.61) | 1.04 (0.46, 1.55) | 1.67 (1.10, 2.15) | 0.82 (0.44, 1.86) | 0.97† (0.47, 1.37) |
| bMBP | 0.57 (0.30, 0.77) | 0.93* (0.62, 1.18) | 0.92* (0.56, 1.64) | 1.00 (0.34, 1.08) | 1.00 (0.71, 1.48) | 0.69 (0.44, 1.50) | 1.00 (0.65, 1.31) | 1.41* (1.23, 2.01) | 0.83 (0.34, 2.13) | 1.36 (0.62, 2.29) |
| hMBP | 0.72 (0.39, 0.85) | 0.85 (0.69, 1.48) | 0.92 (0.58, 1.55) | 0.94 (0.55, 1.00) | 1.35* (0.80, 2.89) | 0.95 (0.52, 1.80) | 1.00 (0.44, 1.31) | 1.74** (1.20, 3.83) | 0.83‡ (0.38, 2.19) | 1.03† (0.64, 2.05) |
| OVA | 0.80 (0.68, 0.98) | 1.01 (0.80, 1.35) | 0.71 (0.55, 1.16) | 1.00 (0.75, 1.74) | 1.31 (0.56, 1.59) | 0.87 (0.46, 1.52) | 0.94 (0.30, 1.90) | 1.24 (0.58, 1.90) | 1.13 (0.45, 2.06) | 1.24* (0.62, 2.43) |
Note: In a subset of animals undergoing MCAO (N=15), brains were removed and frozen. Lymphocytes are thus not available from these brains.
MBP=myelin basic protein, rMBP=rat MBP, bMBP=bovine MBP, hMBP=human MBP, ova=ovalbumin, MCAO=middle cerebral artery occlusion.
signifies P≤0.05 compared to OVA immunized animals;
signifies P≤0.001 compared to OVA immunized animals,
signifies P≤0.05 compared to rMBP immunized animals,
signifies P≤0.001 compared to rMBP immunized animals.
Table 2.
Relative increase in the number of cells secreting the given cytokine after stimulation with LPS.
Data are presented as median (IQR) and statistics are by Kruskal-Wallis H test.
| spleen | lymph node | ischemic brain | non-ischemic brain | P | |
|---|---|---|---|---|---|
| IFN-γ | 12.6 (7.4, 22.6) | 3.1 (1.6, 6.3) | 1.4 (1.0, 2.0) | 1.3 (0.9, 1.7) | <0.001 |
| IL-17 | 13.2 (5.3, 18.5) | 4.0 (2.1, 6.6) | 1.5 (0.8, 2.5) | 1.6 (1.2, 4.5) | <0.001 |
| TGF-β1 | 22.8 (10.9, 33.8) | 4.4 (3.4, 7.3) | 2.1 (1.1, 2.6) | 1.6 (1.4, 2.1) | <0.001 |
LPS=lipopolysaccharide, IFN=interferon, IL=interleukin, TGF=transforming growth factor
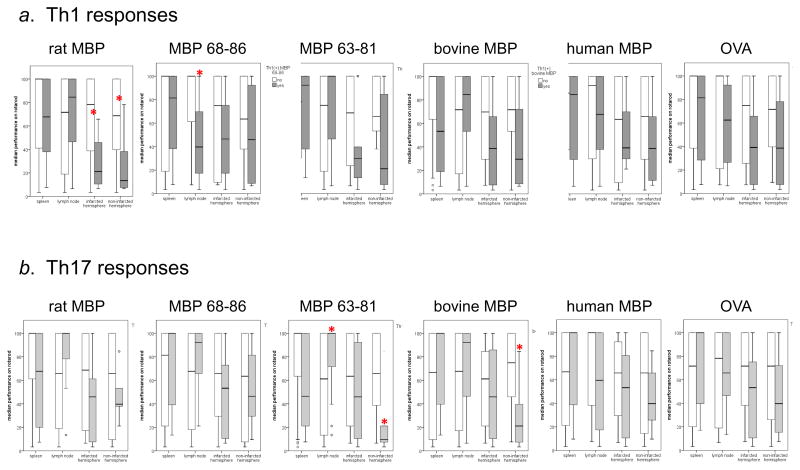
Table 3 shows the percentage of animals that were deemed to be Th1(+) or Th17(+) based on the immune responses in each organ compared to animals immunized with rat MBP (Th1+ or Th17+ defined as a value greater than the median for that organ in rMBP immunized animals). There were no differences in the neurological scores or the percentage of foot faults at one month in animals that were Th1(+) or Th17(+) and those that were Th1(−) or Th17(−), respectively. The difference in rotarod performance at 1 month among animals with a Th1(+) or Th1(−) response to the tested antigens is displayed in Figure 1. Worse outcome was seen with Th1(+) responses to rat MBP in both the ischemic and non-ischemic hemisphere of brain and Th1(+) responses to MBP 68-86 in the cervical lymph nodes. Th17(+) responses to MBP 63-81 and bovine MBP in the non-infarcted hemisphere were also associated with worse outcome. Surprisingly, Th17(+) responses to MBP 63-81 in the lymph node were associated with better rotarod performance.
Table 3.
Percentage of Th1(+) and Th17(+) animals 1 month after MCAO.
| Th1 response in: | spleen | lymph node | ischemic hemisphere | non-ischemic hemisphere |
|---|---|---|---|---|
| rMBP | 17/37 (46%) | 15/36 (42%) | 7/22 (32%) | 7/21 (33%) |
| MBP 68-86 | 16/37 (43%) | 11/37 (30%) | 12/22 (54%) | 12/22 (54%) |
| MBP 63-81 | 14/37 (38%) | 19/37 (51%) | 6/22 (27%) | 13/22 (59%) |
| bMBP | 17/36 (47%) | 13/34 (38%) | 9/21 (43%) | 12/21 (57%) |
| hMBP | 19/37 (51%) | 9/37 (24%) | 4/22 (18%) | 7/22 (32%) |
| OVA | 16/37 (43%) | 8/30 (27%) | 10/22 (45%) | 13/22 (59%) |
| Th17 response in: | spleen | lymph node | ischemic hemisphere | non-ischemic hemisphere |
| rMBP | 19/37 (51%) | 10/35 (29%) | 6/22 (27%) | 5/22 (23%) |
| MBP 68-86 | 11/37 (30%) | 8/35 (23%) | 7/22 (32%) | 8/22 (36%) |
| MBP 63-81 | 14/37 (38%) | 17/36 (47%) | 8/22 (36%) | 5/22 (23%) |
| bMBP | 18/36 (50%) | 12/33 (36%) | 8/21 (38%) | 9/21 (43%) |
| hMBP | 19/37 (51%) | 12/35 (34%) | 7/22 (32%) | 7/22 (32%) |
| OVA | 10/37 (27%) | 7/28 (25%) | 11/22 (50%) | 11/22 (50%) |
Note: In a subset of animals undergoing MCAO (N=15), brains were removed and frozen. Lymphocytes were thus not available from these brains. rMBP=rat MBP, bMBP=bovine MBP, hMBP=human MBP, OVA=ovalbumin, MCAO=middle cerebral artery occlusion
Figure 1.
Rotarod performance (as a function of baseline performance) 1 month after MCAO. Panel a shows the Th1(+) responses and panel b the Th17(+) responses. Shaded bars depict animals with a Th1(+) or Th17(+) response; open bars depict animals without such responses. Statistics are by Mann-Whitney U test. *P≤0.05.
Among the 37 rats in the study, 17 (46%) demonstrated worse performance on the rotarod at one month when compared to earlier time points after stroke. The decline in performance was highly associated with Th1(+) responses to rat MBP in brain, both in the ischemic and non-ischemic hemisphere (Table 4). In addition, Th1(+) responses to bovine MBP in brain were associated with a decline in rotarod performance, as were Th1(+) responses to MBP 68-86 in the lymph node. Th17(+) responses to human MBP in the non-ischemic hemisphere of the brain were also associated with worsening rotarod performance at one month after MCAO.
Table 4.
Percentage of animals that experienced a decline in rotarod performance at 1 month in Th1(+) and Th17(+) animals. Statistics are by likelihood ratio.
| spleen | lymph node | ischemic hemisphere | non-ischemic hemisphere | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Th1(−) | Th1(+) | P | Th1(−) | Th1(+) | P | Th1(−) | Th1(+) | P | Th1(−) | Th1(+) | P | |
| rMBP | 7/20 (35%) | 10/17 (59%) | NS | 10/21 (48%) | 7/15 (47%) | NS | 7/15 (47%) | 7/7 (100%) | 0.004 | 0/7 | 7/14 (50%) | 0.007 |
| MBP 68-86 | 9/21 (43%) | 8/16 (50%) | NS | 8/26 (31%) | 9/11 (82%) | 0.004 | 6/10 (60%) | 8/12 (67%) | NS | 4/8 (50%) | 8/14 (57%) | NS |
| MBP 63-81 | 11/23 (48%) | 6/14 (43%) | NS | 8/18 (44%) | 9/19 (47%) | NS | 9/16 (56%) | 5/6 (83%) | NS | 4/8 (50%) | 9/14 (64%) | NS |
| bMBP | 8/19 (42%) | 9/17 (53%) | NS | 10/21 (48%) | 6/13 (46%) | NS | 6/12 (50%) | 8/9 (89%) | 0.05 | 2/7 (29%) | 10/14 (71%) | 0.06 |
| hMBP | 9/18 (50%) | 8/19 (42%) | NS | 12/28 (43%) | 5/9 (56%) | NS | 11/18 (61%) | 3/4 (75%) | NS | 1/8 (12%) | 6/14 (43%) | NS |
| OVA | 9/21 (43%) | 8/16 (50%) | NS | 9/22 (41%) | 5/8 (62%) | NS | 6/12 (50%) | 8/10 (80%) | NS | 3/8 (38%) | 10/14 (71%) | NS |
| Spleen | lymph node | ischemic hemisphere | non-ischemic hemisphere | |||||||||
| Th17(−) | Th17(+) | P | Th17(−) | Th17(+) | P | Th17(−) | Th17(+) | P | Th17(−) | Th17(+) | P | |
| rMBP | 6/18 (33%) | 11/19 (58%) | NS | 14/25 (56%) | 3/10 (30%) | NS | 10/16 (62%) | 4/6 (67%) | NS | 1/8 (12%) | 4/14 (29%) | NS |
| MBP 68-86 | 12/26 (46%) | 5/11 (45%) | NS | 14/27 (52%) | 3/8 (38%) | NS | 10/15 (67%) | 4/7 (57%) | NS | 2/8 (25%) | 6/14 (43%) | NS |
| MBP 63-81 | 9/23 (39%) | 8/14 (57%) | NS | 11/19 (58%) | 6/17 (35%) | NS | 9/14 (64%) | 5/8 (62%) | NS | 1/8 (12%) | 4/14 (29%) | NS |
| bMBP | 11/18 (61%) | 6/18 (33%) | 0.09 | 11/21 (52%) | 5/12 (42%) | NS | 8/13 (62%) | 6/8 (75%) | NS | 1/7 (14%) | 8/14 (57%) | 0.05 |
| hMBP | 10/18 (56%) | 7/19 (37%) | NS | 10/23 (43%) | 7/12 (58%) | NS | 10/15 (67%) | 4/7 (57%) | NS | 1/8 (12%) | 8/14 (57%) | NS |
| OVA | 13/27 (48%) | 4/10 (40%) | NS | 10/21 (48%) | 4/7 (57%) | NS | 7/11 (64%) | 7/11 (64%) | NS | 2/8 (25%) | 9/14 (64%) | 0.07 |
Note: In a subset of animals undergoing MCAO (N=15), brains were removed and frozen. Lymphocytes were thus not available from these brains.
rMBP=rat MBP, bMBP=bovine MBP, hMBP=human MBP, NS signifies P>0.10
Discussion
Not surprisingly, immunization of rats with autologous rat MBP leads to Th1 and Th17 immune responses to rat MBP that can be detected reliably within the brain and cervical lymph nodes, and less readily so in the spleen. Despite immunization with rMBP, the immune response shows abundant cross reaction to bovine and human MBP in the brain and lymph nodes of immunized animals. Of note, detection of an immune response to rMBP, bMBP or hMBP in the brains and lymph nodes of rat MBP immunized animals, these animals did not show classical signs of EAE.
If one considers the antigens to which an immune response was detected following immunization with rat MBP (rat MBP, human MBP and bovine MBP), very few animals would be considered to be Th1(+) or Th17(+) after stroke; on average, only 1/4 to 1/3 of animals had immune responses greater than the median of that seen in rat MBP immunized animals. These proportions are similar to what we have seen previously among animals subjected to MCAO (although the likelihood of developing a Th1 response can be increased by exposing animals to an inflammatory stimulus at the time of MCAO) (Becker et al., 2005; Gee et al., 2009; Zierath et al., 2010a; Zierath et al., 2013). In the previous studies, we also showed that Th1 (and Th17) immune responses to either bovine or human MBP were associated with worse outcome. Given that bovine and human proteins are heterologous for rat, we wanted to determine if there would also be a response to rat MBP after stroke and whether such a response would be associated with worse outcome. Indeed, our data show that Th1(+) responses to rat MBP in brain (both in the ischemic and non-ischemic hemispheres) were associated with a higher rate of neurological decline and worse outcome, based on rotarod performance, at one month after MCAO. In keeping with the lack of specificity of the immune response to rat MBP in rat MBP immunized animals, Th1(+) and Th17(+) responses to bovine MBP in brain were also associated with neurologic decline after stroke. Of note, Th17(+) responses to bovine MBP in the non-ischemic hemisphere were more predictive of worse outcome/neurological decline than those in the ischemic hemisphere.
MBP 68-86 is an encephalitogenic epitope of MBP and induces EAE in Lewis rats while MBP 63-81 does not (Stepaniak et al., 1997). Among the cohort of animals in our study, immune responses to the encephalitogenic portions of MBP were not reliably seen in animals immunized with rat MBP. Further, the associations between the immune responses to the encephalitogenic portions of MBP and outcome after stroke were quite variable. For instance, a Th1(+) response to MBP 68-86 in the lymph node was associated with worse outcome while a Th17(+) response to MBP 63-81 in the lymph node was associated with better outcome. And a Th17(+) response to MBP 63-81 in the non-ischemic brain was associated with neurological decline.
We assessed the immune response to MBP and MBP peptides in the blood, lymph nodes and spleen as there is a growing literature that shows the immune response is highly regulated and the nature of the response differs from compartment to compartment. For instance, in actively induced EAE, the detected immune response is highly dependent upon the stage of the disease; MBP specific responses can vary dramatically among the draining lymph nodes, peripheral blood, spleen and central nervous system (Hofstetter et al., 2005). And while both Th1 and Th17 cells can induce EAE, the phenotype and pathology of this EAE differs (Domingues et al., 2010; Jager et al., 2009; Stromnes et al., 2008). Following stroke, few animals had Th1(+) or Th17(+) responses to MBP in brain, but for those that did, these responses tended to be associated with neurological decline and worse outcome. As might be expected, we found that Th1 responses to rat MBP in brain correlated best to outcome after stroke. And perhaps unexpectedly for the Th17 response to bovine MBP, it was the response in the non-ischemic hemisphere that was most predictive of a poor outcome after stroke. It is important to stress that for the data presented here, we analyzed the immune response at 1 month after MCAO. It is unclear whether these responses would persiste indefinitely, resolve, or transform into something completely different. It has been recognized that the Treg and Th17 phenotypes are inherently unstable, with Th17 cells becoming Treg cells and Treg cells becoming Th17 cells over the course of time (Gagliani et al., 2015, Koenen et al., 2008). Future studies will have to evaluate the time course of the immune response in each organ following stroke.
It is important to appreciate that the immune responses to MBP are likely just a reflection of the overall immune response to brain antigens. That an immune response to MBP mediates worse outcome may be true, but it is quite likely that the animals have immune responses to a multiplicity of other antigens, and the one(s) that are most responsible for mediating worse outcome are unclear. For instance, in patients with stroke, humoral responses to neurofilaments, portions of the N-methyl-D-aspartate (NMDA) receptor and neuronal potassium channels have been detected, as have cellular responses to MBP and brain homogenate (Bornstein et al., 2001; Dambinova et al., 2003; Kalev-Zylinska et al., 2013; Kallen et al., 1977; Rocklin et al., 1971; Simal et al., 2012; Wang et al., 1992; Youngchaiyud et al., 1974). It is likely that these responses are just the tip of the iceberg, and that the immune response to neural antigens after brain injury are as individual as the patients who experience the injury. And the data from our study suggest that the pathologic significance of these responses likely depends on the robustness of the response and the immunological compartment in which it was detected.
Also notable in this study is the fact that the the responses to LPS were markedly different in spleen, lymph node and brain. Here we show that even when removed from the brain and washed in phosphate buffered saline, the lymphocytes isolated from the brain are still limited in their ability to respond to LPS relative to the lymphocytes removed from the spleen and lymph nodes. This finding highlights the fact that the brain’s microenvironment is generally thought to limit the immune response, and despite this relative inhibition of the immune response in brain, robust responses to MBP were seen. Undoubtedly this modulation of lymphocyte function in the brain is important in limiting the post-ischemic immune response.
The findings outlined in this study are important as they show the immune responses to whole MBP are not species specific, whether they induced by immunization with rat MBP or by stroke. Additionally, immunization with whole rat MBP does not elicit EAE or immune responses to the encephalitogenic portions of MBP. And despite not causing EAE, immune responses to MBP, especially Th1 responses to autologous rat MBP, are associated with neurological decline and worse outcome after stroke, particularly when these self-reactive cells are found in brain, as opposed to peripheral lymphoid organs.
Highlights.
Rats immunized to rat MBP develop responses to autologous and heterologous MBP.
The responses to MBP in immunized animals are most robust in brain.
Few animals develop Th1(+) or Th17(+) immune response to MBP after stroke.
Th1(+) responses to rat MBP in brain are associated with worse stroke outcome.
Th17(+) responses to MBP in non-infarcted (vs. infarcted) brain predict outcome.
Acknowledgments
This work was funded by NINDS 5RO1NS056457.
Abbreviations
- MCAO
middle cerebral artery occlusion
- MBP
myelin basic protein
- IFN
interferon
- IL
interleukin
- TGF
transforming growth factor
- LPS
lipopolysaccharide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25:1634–44. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–6. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Bornstein NM, Aronovich B, Korczyn AD, Shavit S, Michaelson DM, Chapman J. Antibodies to brain antigens following stroke. Neurology. 2001;56:529–30. doi: 10.1212/wnl.56.4.529. [DOI] [PubMed] [Google Scholar]
- Dambinova SA, Khounteev GA, Izykenova GA, Zavolokov IG, Ilyukhina AY, Skoromets AA. Blood test detecting autoantibodies to N-methyl-D-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clin Chem. 2003;49:1752–62. doi: 10.1373/49.10.1752. [DOI] [PubMed] [Google Scholar]
- Domingues HS, Mues M, Lassmann H, Wekerle H, Krishnamoorthy G. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS ONE. 2010;5:e15531. doi: 10.1371/journal.pone.0015531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N, Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015 doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee JM, Kalil A, Thullbery M, Becker KJ. Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke. 2008;39:1575–82. doi: 10.1161/STROKEAHA.107.501486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee JM, Zierath D, Hadwin J, Savos A, Kalil A, Thullbery M, et al. Long term immunologic consequences of experimental stroke and mucosal tolerance. Exp Transl Stroke Med. 2009;1:3. doi: 10.1186/2040-7378-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter HH, Targoni OS, Karulin AY, Forsthuber TG, Tary-Lehmann M, Lehmann PV. Does the frequency and avidity spectrum of the neuroantigen-specific T cells in the blood mirror the autoimmune process in the central nervous system of mice undergoing experimental allergic encephalomyelitis? J Immunol. 2005;174:4598–605. doi: 10.4049/jimmunol.174.8.4598. [DOI] [PubMed] [Google Scholar]
- Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–77. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalev-Zylinska ML, Symes W, Little KC, Sun P, Wen D, Qiao L, et al. Stroke patients develop antibodies that react with components of N-methyl-D-aspartate receptor subunit 1 in proportion to lesion size. Stroke. 2013;44:2212–9. doi: 10.1161/STROKEAHA.113.001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen B, Nilsson O, Thelin C. Effect of encephalitogenic protein on migration in agarose of leukoytes from patients with multiple sclerosis. A longitudinal study of patients with relapsing multiple sclerosis or with cerebral infarction. Acta Neurol Scand. 1977;55:47–56. [PubMed] [Google Scholar]
- Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–52. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Lubics A, Reglodi D, Tamas A, Kiss P, Szalai M, Szalontay L, et al. Neurological reflexes and early motor behavior in rats subjected to neonatal hypoxic-ischemic injury. Behav Brain Res. 2005;157:157–65. doi: 10.1016/j.bbr.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Mannie M, Swanborg RH, Stepaniak JA. Experimental autoimmune encephalomyelitis in the rat. Curr Protoc Immunol. 2009;Chapter 15(Unit 15):2. doi: 10.1002/0471142735.im1502s85. [DOI] [PubMed] [Google Scholar]
- Planas AM, Gomez-Choco M, Urra X, Gorina R, Caballero M, Chamorro A. Brain-derived antigens in lymphoid tissue of patients with acute stroke. J Immunol. 2012;188:2156–63. doi: 10.4049/jimmunol.1102289. [DOI] [PubMed] [Google Scholar]
- Rocklin RE, Sheremata WA, Feldman RG, Kies MW, David JR. The Guillain-Barre syndrome and multiple sclerosis. In vitro cellular responses to nervous-tissue antigens. N Engl J Med. 1971;284:803–8. doi: 10.1056/NEJM197104152841501. [DOI] [PubMed] [Google Scholar]
- Simal P, Garcia-Garcia AM, Serna-Candel C, Egido JA. Stroke preceding autoimmune encephalitis with neuronal potassium channel antibody. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr.08.2011.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepaniak JA, Wolf NA, Sun D, Swanborg RH. Interstrain variability of autoimmune encephalomyelitis in rats: multiple encephalitogenic myelin basic protein epitopes for DA rats. J Neuroimmunol. 1997;78:79–85. doi: 10.1016/s0165-5728(97)00084-2. [DOI] [PubMed] [Google Scholar]
- Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–42. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zwam M, Huizinga R, Melief MJ, Wierenga-Wolf AF, van Meurs M, Voerman JS, et al. Brain antigens in functionally distinct antigen-presenting cell populations in cervical lymph nodes in MS and EAE. J Mol Med. 2009;87:273–86. doi: 10.1007/s00109-008-0421-4. [DOI] [PubMed] [Google Scholar]
- Wang WZ, Olsson T, Kostulas V, Hojeberg B, Ekre HP, Link H. Myelin antigen reactive T cells in cerebrovascular diseases. Clin Exp Immunol. 1992;88:157–62. doi: 10.1111/j.1365-2249.1992.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngchaiyud U, Coates AS, Whittingham S, Mackay IR. Cellular-immune response to myelin protein: absence in multiple sclerosis and presence in cerebrovascular accidents. Aust N Z J Med. 1974;4:535–8. doi: 10.1111/j.1445-5994.1974.tb03233.x. [DOI] [PubMed] [Google Scholar]
- Zierath D, Hadwin J, Savos A, Carter KT, Kunze A, Becker KJ. Anamnestic recall of stroke-related deficits: an animal model. Stroke. 2010a;41:2653–60. doi: 10.1161/STROKEAHA.110.592865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierath D, Schulze J, Kunze A, Drogomiretskiy O, Nhan D, Jaspers B, et al. The immunologic profile of adoptively transferred lymphocytes influences stroke outcome of recipients. Journal of Neuroimmunology. 2013;263:28–34. doi: 10.1016/j.jneuroim.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierath D, Thullbery M, Hadwin J, Gee JM, Savos A, Kalil A, et al. CNS immune responses following experimental stroke. Neurocrit Care. 2010b;12:274–84. doi: 10.1007/s12028-009-9270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]