Abstract
In collectively foraging groups, communication about food resources can play an important role in the organization of the group’s activity. For example, the honeybee dance communication system allows colonies to selectively allocate foragers among different floral resources according to their quality. Because larger groups can potentially collect more information than smaller groups, they might benefit more from communication because it allows them to integrate and use that information to coordinate forager activity. Larger groups might also benefit more from communication because it allows them to dominate high-value resources by recruiting large numbers of foragers. By manipulating both colony size and the ability to communicate location information in the dance, we show that larger colonies of honeybees benefit more from communication than do smaller colonies. In fact, colony size and dance communication worked together to improve foraging performance; the estimated net gain per foraging trip was highest in larger colonies with unimpaired communication. These colonies also had the earliest peaks in foraging activity, but not the highest ones. This suggests they may find and recruit to resources more quickly, but not more heavily. The benefits of communication we observed in larger colonies are thus likely a result of more effective informationgathering due to massive parallel search rather than increased competitive ability due to heavy recruitment.
Keywords: Apis mellifera, collective behaviour, colony size, communication, foraging, honeybee, information, resource distribution, social insect
In self-organized societies like social insect colonies, the flow of information among individuals shapes group organization. Some species have evolved elaborate communication mechanisms, allowing specific information collected by a few individuals to be shared with other colony members. One well-known example is the honeybee waggle dance, which is used to communicate the location of resources like food, water and nest sites (von Frisch 1967). Because successful foragers choose whether to dance and how long to dance based on resource quality (among other features), a forager choosing a random dance to follow is likely to be directed to a high-quality resource (Seeley & Visscher 1988). The dance communication system thus serves not only to recruit naive foragers to specific locations, but also, at the colony level, to integrate that information, resulting in a dynamic allocation of foragers among resources that tracks changes in resource quality (Seeley 1986; Schmickl & Crailsheim 2004).
If the effective distribution of information within a group is key to group organization, we might expect communication to become more valuable with larger group size. First of all, in large groups, coordination may be more important because otherwise individuals interfere with each other’s task performance (Pacala et al. 1996). For example, if there are many independently searching central-place foragers, they are likely to encounter the same nearby food sources, which will quickly be stripped of their reward. At the same time, effective coordination may become more difficult in large groups, because information must be distributed more widely (Naug 2009). However, as group size increases, more individuals mean that more information can be gathered and integrated, and the potential value of that information may be greater. In collective foraging, it has been suggested that larger groups may be able to find resources more quickly (Naug & Wenzel 2006) and/or recruit more group members to help collect them (Johnson & Hubbell 1987), both of which could provide an advantage in competition. Larger groups might also search their surroundings more effectively, identifying more potential resources, and then use communication to select the most profitable ones to concentrate on (Beekman et al. 2004).
Although there are many reasons to think that communication could be of particular benefit to large groups, there is surprisingly little evidence that this is so (Dornhaus et al. 2012). One theoretical study showed that large colony size and recruitment are complementary features of a collective foraging strategy that performs well when resources are rich and patchily distributed (Johnson & Hubbell 1987). In line with this general prediction, a comparative study across 98 ant species found that more complex communication systems seemed to be associated with larger mature colony size (Beckers et al. 1989). A similar relationship has been suggested among eusocial bees (Michener 1974). A particular contrast can be drawn between the ‘cottage-industry’ foraging strategy of bumblebees, and the ‘big markets’ foraging strategy of honeybees (Heinrich 2004). The bumblebees’ strategy, which involves relatively few foragers, searching independently, may work particularly well for foraging on small, scattered resources in northern temperate regions. In contrast, the enormous colonies of honeybees, coupled with their unique dance communication system, may give them the opportunity to exploit rich, ephemeral resources in their ancestral habitat: tropical forests. Although it has been argued that dance communication may indeed be more valuable in tropical forests than in other environments (Dornhaus & Chittka 2004), there is no evidence so far that colony size plays a role. In fact, an agent-based model of honeybee foraging concluded just the opposite: that the benefits of communication are independent of colony size, regardless of environment (Dornhaus et al. 2006).
In this article, we empirically evaluate the benefits of dance communication for honeybee colonies of different sizes foraging on natural floral resources. We find that larger colonies benefit more from communicating location information than smaller colonies do. We then explore the evidence for several hypotheses about why this might be so. First, because larger colonies must forage over greater distances in order to feed the entire colony, communication about resource location might enable them to overcome that disadvantage (hypothesis H1). Second, larger colonies have more individual foragers searching in parallel, so they may be able to locate resources more quickly. Communicating about the location of those resources could allow them to take advantage of early discovery by mobilizing foragers to exploit the resource before others do (hypothesis H2). Third, larger colonies have more individuals available to be recruited, so they may be able to recruit more foragers to particularly rewarding resources, giving them an edge in the competition for highquality, ephemeral resources (hypothesis H3).
We assessed support for these three nonmutually exclusive hypotheses in two stages. In the first stage, we divided the hypotheses into two categories according to the predictions they make about the foraging performance of larger colonies compared to smaller ones, each with dance communication impaired and intact. If H1 is true, we predicted that larger colonies with impaired communication would perform worse than both smaller colonies and larger colonies with intact communication. If either H2 or H3 is true, we predicted that larger colonies with intact communication would perform better than both smaller colonies and larger colonies with impaired communication. In the second stage, we used patterns of foraging activity to distinguish between H2 and H3; in particular, we looked for peaks in activity that could be evidence of strong recruitment events. If H2 is true, we predicted that larger colonies with intact communication would have earlier peaks in foraging activity than any of the other three groups. If H3 is true, we predicted that larger colonies with intact communication would have higher peaks in foraging activity than any of the other three groups.
METHODS
Experiment
We measured the benefits of dance communication in honeybee colonies of different sizes by manipulating both colony size and communication and characterizing colony-level foraging success. The experiment was performed at the Santa Rita Experimental Range Headquarters in Arizona during the summer monsoon season (27 August–19 September 2011). This location and season was chosen because, in an earlier series of experiments across different environments, it was found to be the one where dance communication was of greatest benefit (Donaldson-Matasci & Dornhaus 2012). We assessed the diversity and abundance of floral resources twice during that period (2–4 and 16–18 September) using censored t-square sampling (Diggle 1983; Zimmerman 1991; Donaldson-Matasci & Dornhaus 2012).
The experiment was performed on eight colonies of domestic Italian honeybees, Apis mellifera ligustica. To control for genetic differences between colonies that could be linked to natural differences in colony size, we created four matched pairs of colonies. For each pair, we mixed stock from two original colonies to create one larger colony of about 6000 bees and one smaller colony of about 3000 bees. Although both these sizes would be considered small for managed honeybee colonies, 6000 workers is probably within the range observed for wild colonies in their natural habitat (Schneider 1990). All colonies were housed in 10-frame Langstroth hives, but the smaller colonies were given only five frames and were confined to one half of the box. Each larger colony received approximately twice as much stored honey, brood and empty honeycomb as the smaller colony in its pair. We estimated the number of bees in each colony by placing a 4 × 8 grid over each side of each frame and counting the number of squares covered by bees, and estimating the number of bees per square. We then took the average of two counts, one before and one after the experiment, as our estimate of the number of bees in each colony.
We used an established technique to manipulate the bees’ ability to communicate the location of food resources (von Frisch 1967; Sherman & Visscher 2002; Dornhaus & Chittka 2004; Donaldson-Matasci & Dornhaus 2012). The waggle dance usually takes place on vertical honeycomb; the angle of the dance relative to straight up indicates the angle of the resource being advertised relative to the azimuth of the sun. When the hive is turned on its side so that honeycomb is horizontal, the usual reference point for the dance is removed. However, if a directional light source is available, it can be used as an alternative reference point for the dance (von Frisch 1967). This allows us to compare colonies that can communicate location information via the waggle dance (horizontal comb with a directional light source) with those that cannot (horizontal comb with a nondirectional light source). Each colony was housed in a modified hive box with frames held horizontally. Entering bees were constrained to walk across the top frame, so that the majority of dances would take place there (for example, Seeley & Towne 1992 found that 94% of dances take place within one frame-width of the hive entrance). The top frame was illuminated by a window, which was cut into the hive body. In the diffuse-light treatment, the window was covered with translucent white Plexiglas. In the oriented-light treatment, a cool fibre-optic light source was placed directly over the window. It has been shown previously that colonies in the diffuse-light treatment perform disoriented dances, while those in the oriented-light treatment perform directionally oriented dances that are as precise as those performed by colonies in the normal vertical position (Sherman & Visscher 2002). Furthermore, colonies in the diffuse-light treatment dance just as much, but recruit to artificial feeders less effectively than colonies in the oriented-light treatment (Sherman & Visscher 2002; Granovskiy et al. 2012).
The experiment consisted of eight 3-day treatment blocks, for a total of 24 days. During each treatment block, two pairs of colonies were given the diffuse-light treatment and two pairs were given the oriented-light treatment; in the following treatment block the treatments were switched. To measure foraging success, we weighed each hive every evening after sunset, when most foragers had returned. The daily weight change is a standard measure of the net nectar gain for that day (Seeley 1995). Because honeybee colonies require large amounts of stored honey to survive the winter, the amount of nectar a colony can collect and store has important implications for its fitness (Seeley & Visscher 1985). We also collected and weighed the pollen from front-mounted pollen traps. Pollen traps were mounted on all colonies only for a subset of the experiment (8 days total, 2 days each in four different treatment blocks) to minimize the impact on overall foraging activity.
Traffic in and out of the hive was measured with custom-built automated counters mounted on the hive entrances. The counters were built using photointerrupter gates (Sparkfun Electronics, Boulder, CO, U.S.A.), which emit a beam of infrared light on one side and detect it on the other. When a bee passes through the gate, the beam is blocked. These gates were mounted over four short, narrow, transparent plastic tubes (approximately 5 cm long, with an 8 mm inner diameter). Each tube allowed only one bee to pass at a time; having four parallel tubes greatly reduced traffic bottlenecks because some tubes tended to be used for entry while others were used for exit. The photo-interrupter gates were wired to an Arduino microcontroller, which logged data continuously and recorded the number of bees that had passed once per minute. Again, to minimize any impact on overall foraging activity, we mounted the counters on half of the colonies for only a subset of the experiment (8 days total, 2 days each in four different treatment blocks).
We estimated the number of foraging trips for each colony on each day as one half of the total traffic counter tally for that day, since each bee presumably triggered the counter twice: once on exit and once on return. This is an overestimate of foraging activity because some bees will leave the hive to defecate, perform orientation flights, and so on. In unmanipulated colonies, naturally occurring differences in colony size could affect the proportion of orientation flights in particular by changing the relative numbers of new foragers compared to experienced foragers. However, because we created matched pairs of larger and smaller colonies by joining and then splitting two original colonies, we would expect no consistent demographic differences between larger and smaller colonies. We therefore believe the overestimate to be consistent across treatments.
Foraging Performance
We assessed the effects of communication treatment and colony size on colony-level foraging performance in two ways. First, to look at the benefits of communicating location information separately in larger and smaller colonies, we compared the total daily weight change for each colony to the median weight change for other colonies of the same size class on that day. Second, to measure colony-level foraging performance in a way that is comparable across colony sizes, we divided each colony’s daily weight change by the total number of bees in that colony. We then compared the per-bee weight change of each colony to the median for that day across all colonies, including both larger and smaller colonies. By comparing only to other colonies on the same day, we accounted for strong day-to-day differences in nectar and pollen collection that may have been due to changing weather or floral availability (Donaldson-Matasci & Dornhaus 2012).
We also assessed individual-level foraging performance across treatments by estimating the net weight gain per foraging trip. We expected the total weight change for a colony, ΔW, to depend on the number of foraging trips f, the net gain per foraging trip gf, the total number of bees in the colony n, and the nectar weight used by a nonforager cn: ΔW = gf f − cn n. To estimate the net gain per foraging trip, we constructed a linear mixed-effects model of daily weight change based on the equation above: the total number of bees and number of foraging trips were included as fixed effects, while date and colony were included as crossed random effects. We constrained the coefficient for n (the cost per nonforager, cn) to be the same for all four combinations of colony size and communication treatment, while allowing the coefficient for f (the net gain per foraging trip, gf) to differ. This allowed us to estimate the net gain per foraging trip for each of the four categories separately: larger and smaller colonies in the diffuse-light treatment and the oriented-light treatment. We used the R programming language with the package lme4 for mixed-effects models (R Development Core Team 2009; Bates et al. 2011).
Patterns of Foraging Activity
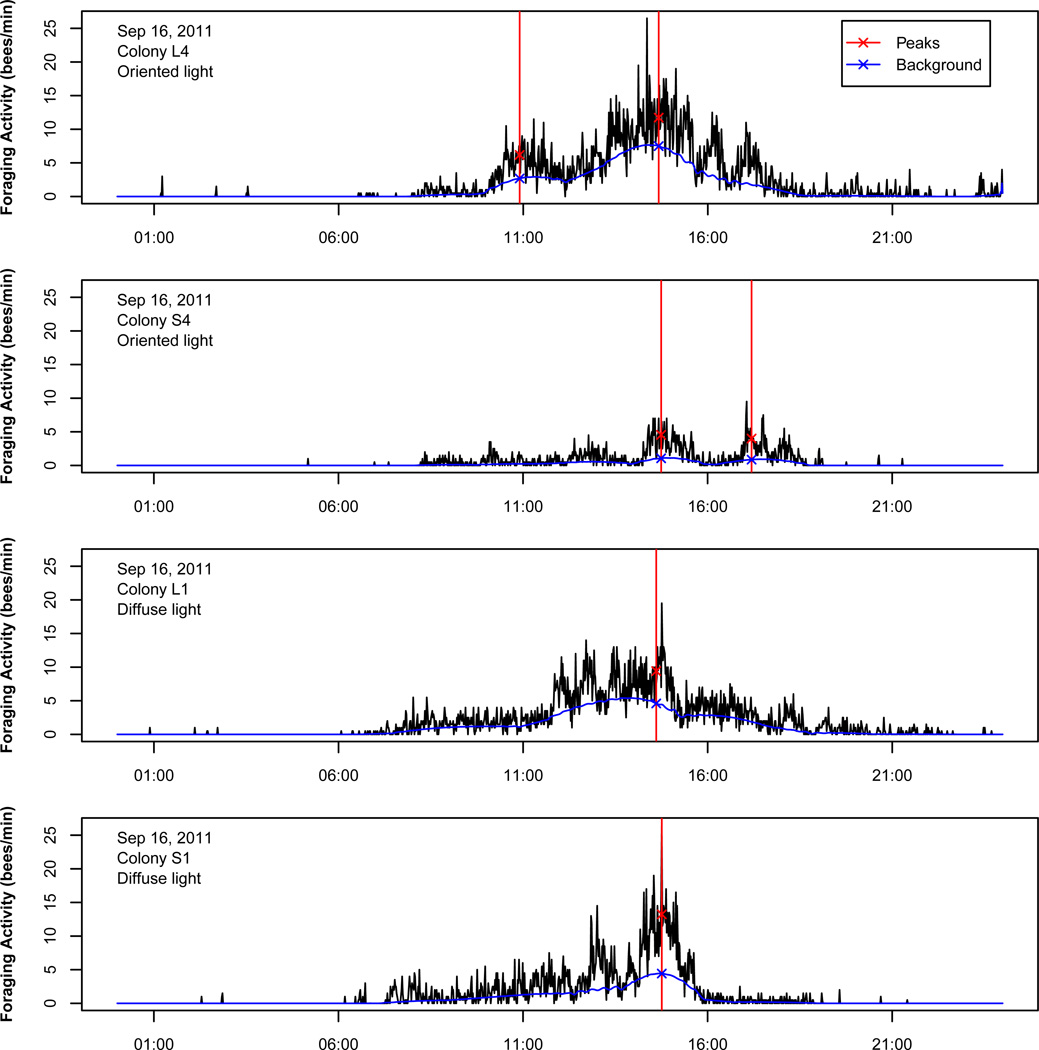
To quantify patterns of foraging activity, we sought to identify peaks in activity that could be a result of dance recruitment (for an example, see Fig. 1). General foraging activity levels may change over the course of the day in response to many other factors, such as changing weather, resource availability, competition and the nutritional needs of the hive. How can we pick out the effects of dance recruitment against the background of so many other factors? To estimate the background foraging activity level and identify peaks in foraging activity that surpass it, we borrowed a technique known as deconvolution from the field of signal processing. Deconvolution is the process of separating an observed signal into two component parts. We used a deconvolution algorithm developed to simultaneously estimate both peak location and background signal level in the presence of noise, as implemented in the R package Peaks (Morhač et al. 2000; Morhač 2008). Sudden rises in activity that are sustained tend to be classified as peaks, while gentler rises tend to be classified as background. This method tells us the time of day when foraging peaks occurred, and gives an estimate of how nonpeak activity changes over the course of the day.
Figure 1.
Measuring peaks in foraging activity of honeybees. Foraging activity, as measured by custom-built automated counters, is shown over the course of 1 day for four colonies. For each colony on each day, the background level of foraging activity is estimated and peaks relative to that background are identified by signal deconvolution (see text). From the peaks and background, we can identify the timing of the earliest peak, the height of the highest peak (the difference between peak activity and background activity at that time) and the proportion of all activity that is peak activity rather than background activity.
We measured three aspects of foraging activity for each colony on each day. First, we determined the time of day of the earliest foraging peak. Second, we determined the height of the highest foraging peak. The height of a foraging peak is the difference between the observed activity level and the estimated background activity level (both recorded as number of foraging trips per minute) at the time the peak occurs. The peak height was log transformed so that it would fit a normal distribution. Third, we summed the background activity levels over the entire day to get a total measure of background activity, and subtracted that from the total activity to find the total amount of peak foraging activity. We then divided by the total activity to determine the proportion of all activity that was due to foraging peaks.
RESULTS
Weather throughout the experiment was generally warm and dry, excellent for foraging. Precipitation came in the form of short, intense monsoon thunderstorms in the late afternoon. During the first three treatment blocks (27 August–4 September), precipitation was minimal (average 0.6 mm/day) and potential bee forage, as measured at the end of that period, was quite sparse (estimated blooming plant density 0.01 plant/m2). However, beginning in the fourth treatment block, precipitation increased (average 4.2 mm/day) and many more plants came into bloom (estimated blooming plant density 0.51 plants/m2). Observed species richness also increased from 29 to 37 species in bloom. Bees probably foraged mainly on several species of composites (e.g. Viguiera dentata, Bidens leptocephala and Trixis californica) as well as a prolific leguminous shrub, Mimosa dysocarpa, and two conspicuous succulents, Agave palmeri and Dasylirion wheeleri.
All colonies lost weight on most days, with occasional gains (see Fig. 2). This indicates that colonies generally used up more honey stores than they were able to collect, a situation that is not unusual for much of the year (Seeley 1995; Sherman & Visscher 2002). Gains were more frequent after 4 September, once precipitation had increased floral availability (Fisher’s exact test: P = 0.047). Over the course of the experiment, the larger colonies lost slightly more weight than the smaller ones (weight change after 24 days: larger colonies, −1.40 ± 0.29 kg, N = 4; smaller colonies, −0.97 ± 0.36 kg, N = 4).
Figure 2.
Daily weight change in (a) smaller and (b) larger honeybee colonies (N = 4 colonies each, indicated by red, yellow, green and blue bars, respectively). Pairs of colonies created from the same stock are shown in the same colour. Vertical grey bars indicate colonies in the diffuse-light treatment; open bars indicate colonies in the oriented-light treatment. Dashed lines divide 3-day treatment blocks, when colonies changed communication treatments. (c) Daily precipitation (mm) during the experimental period.
Effects on Foraging Performance
We first assessed the effect of communication treatment in larger and smaller colonies separately. To do this, we compared the daily weight change of each colony to the median weight change for all colonies of the same size class on the same day. We asked whether colonies that could communicate location information were likely to perform better than those that could not. We found that while communication treatment had a significant effect in the larger colonies (Fisher’s exact test: P = 0.03), there was no such effect in the smaller colonies (P = 1; Fig. 3a). We performed an analogous test for the weight of pollen collected and found no significant effect of communication treatment in either larger or smaller colonies.
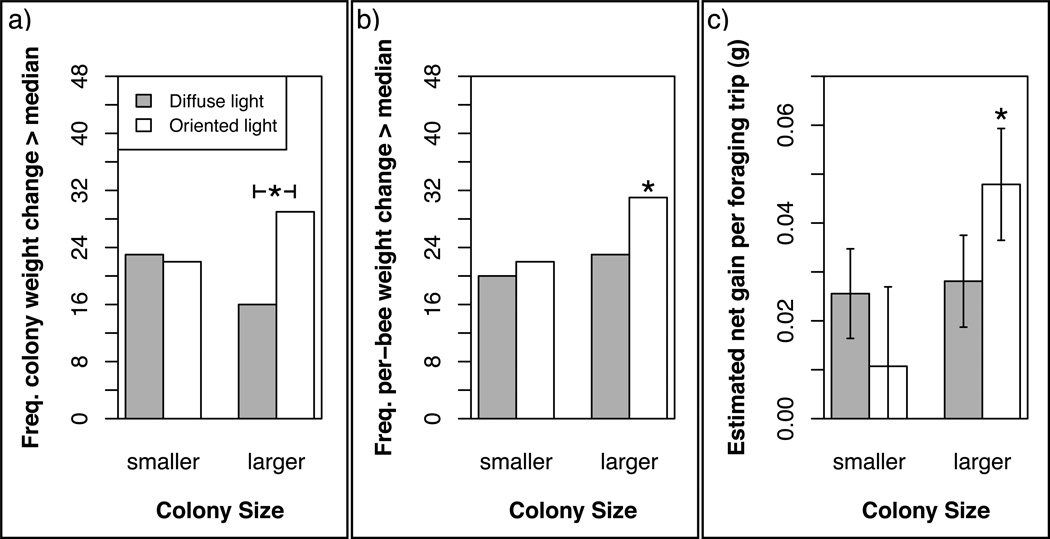
Figure 3.
Effects of communication (diffuse light = impaired; oriented light = unimpaired) on foraging efficiency of smaller and larger honeybee colonies. (a) Change in daily weight gain per colony relative to the median for that day and size class. *Denotes a significant difference between treatments within size class (Fisher’s exact test: P = 0.03). (b) Change in daily weight gain per bee relative to the median for that day across colonies and size classes. *Denotes a significant difference from the other three groups combined (P = 0.03). (c) Estimated average net gain per foraging trip per treatment group. *Denotes a significant difference from the other three groups combined (P = 0.01). Bar heights are estimated coefficients from a linear model (see text); error bars are standard errors.
Why did larger colonies benefit more from dance communication? Was it because they did particularly well when they could communicate about resource location (e.g. H2 or H3), and/or particularly poorly when they could not (e.g. H1)? To standardize our measure of foraging performance across larger and smaller colonies, we compared the daily per-bee weight change for each colony to the median for all colonies on that day. According to this measure, we found that large colonies in the oriented-light treatment performed well significantly more often than colonies in the other three categories combined (Fisher’s exact test: P = 0.03; Fig. 3b). There was no evidence that large colonies in the diffuse-light treatment performed well any less often than those in the other three categories combined (Fisher’s exact test: P = 0.87).
We saw that larger colonies in the oriented-light treatment performed better per bee than both smaller colonies and larger colonies in the diffuse-light treatment, suggesting that larger colonies with communication intact do the best job of balancing their energy budget. How do they achieve this? Is it because individual foragers are able to collect more nectar on average? To answer this question, we used our measurements of foraging activity to estimate the average net gain per foraging trip for each of the four treatment categories (see Methods). We found that foragers in larger colonies in the oriented-light treatment gathered significantly more nectar per trip than foragers in the other three categories combined (LR test: D = 6.48, P = 0.01; Fig. 3c).
Effects on Patterns of Foraging Activity
To explore why larger colonies in the oriented-light treatment had the highest net gain per foraging trip, we compared daily patterns of foraging activity for smaller and larger colonies across communication treatments. First, we predicted that if larger colonies find resources particularly quickly, and using a directional dance allows them to recruit to those resources (H2), then larger colonies in the oriented-light treatment should have foraging peaks earlier in the day. We found that the first peak in foraging activity did indeed occur earlier in the day for larger colonies in the oriented-light treatment than for any of the other three categories (one-sided Wald test: t13 = −2.12, P = 0.03; Fig. 4a). Second, we predicted that if larger colonies use directional dances to recruit more heavily to high-quality resources (H3), then larger colonies in the oriented-light treatment should show either higher foraging peaks or a greater proportion of activity during peaks than colonies in the other three categories. However, there was no evidence that larger colonies in the oriented-light treatment had higher foraging peaks (t13 = −1.05, P = 0.84; Fig. 4b). There was also no evidence that foraging peaks in larger colonies in the oriented-light treatment constituted a greater proportion of their total foraging activity (t13 = −1.88, P = 0.96; Fig. 4c).
Figure 4.
Effects of communication (diffuse light = impaired; oriented light = unimpaired) on patterns of foraging activity in smaller and larger honeybee colonies. (a) Time of earliest peak foraging activity. *Denotes a significant difference from the other three groups combined (P = 0.03). (b) Height of maximum peak in foraging activity. (c) Proportion of foraging activity occurring in peaks. Box plots show medians as well as upper and lower quartiles; whiskers show maximum and minimum values.
Because the automated counters restricted the size of the hive entrance, it is possible that they limited the amount of traffic to some extent, particularly in the larger colonies. This could explain why there seemed to be no difference between larger and smaller colonies in the height of the maximum foraging peak (see Fig. 4b). Still, we did see that larger colonies in the oriented-light treatment performed better than any other treatment combination (see Fig. 3b). Without the potential constraint of the automated counter, could they have performed even better? To assess this possibility, we asked whether large colonies in the oriented-light treatment were less likely to perform well when they had the automated counter installed. We found that their per-bee weight change was no less likely to be above the median when the counter was present than when it was not, suggesting that foraging performance was not strongly affected by the counter (Fisher’s exact test: P = 0.40).
DISCUSSION
It has often been proposed that the honeybees’ large colony size is an important feature of a cooperative foraging strategy that allows them to exploit highly rewarding but ephemeral resources (Michener 1974; Roubik 1980; Anderson 2001; Heinrich 2004). Here, we demonstrate that larger colonies of honeybees benefit more from the location information in their dance communication system than smaller colonies do. This observation fits well with general models of collective foraging that suggest that large colony size and communication are complementary strategies (Johnson & Hubbell 1987). The empirical evidence for such a connection has so far been limited, resting mainly on one comparative study across ant species (Beckers et al. 1989). A few studies within species have manipulated colony size and looked at changes in foraging strategy (Beekman & Sumpter 2001; Mailleux et al. 2003; Beekman et al. 2004). However, to understand how colony size and communication interact to affect foraging success, we need to manipulate both colony size and the ability to communicate, and then measure the resulting foraging performance, as done here. This allows us not only to demonstrate a clear connection between colony size and communication, but also to explore the reasons why such a connection might exist.
One reason that communicating about resource location might be more valuable to large colonies than to small ones is because it helps them overcome the disadvantage of foraging over greater distances (H1; see e.g. Goulson 2003; Jun et al. 2003). Larger honeybee colonies forage on more distant resources, particularly when resources are scarce (Beekman et al. 2004); furthermore, dance communication is of greater benefit when used for more distant resources (Kirchner & Grasser 1998; Dornhaus 2002). However, if this were the only factor in play, then we would have expected individuals in larger colonies to forage less effectively than those in smaller colonies when communication was impaired, because they would have to expend more energy in searching and travelling. This was not the case. We did find, however, that the estimated net gain per foraging trip was significantly higher for larger colonies with intact communication than it was for any other group. This is consistent with the results of previous work that explicitly measured individual foraging success, but without manipulating communication: nectar loads were found to be higher for individuals in larger colonies than for those in smaller ones (Eckert et al. 1994). The greater benefits of communication to larger colonies thus seem to be due to some particular advantage their foragers have, both compared to smaller colonies and to colonies with impaired communication.
One advantage that large colony size may offer is in the gathering of information. If more individuals are available to search the environment, larger colonies should be able to find resources more quickly; this, coupled with the ability to recruit to those resources could give them a competitive advantage over smaller colonies (H2; see e.g. Roubik 1980; Naug & Wenzel 2006). We found that larger colonies that were able to communicate location information tended to have foraging peaks earlier in the day than both smaller colonies and larger colonies whose communication was impaired. This suggests that with directional dances, larger colonies are indeed able to locate and recruit to rewarding resources more quickly than smaller colonies. Improved search ability could help larger colonies succeed in scramble competition. Even without competition, it might allow them to take advantage of short-lived flowers that bloom at night and wilt the next morning.
The greater information-gathering capacity of larger colonies compared to smaller ones may also enable them to search the environment more thoroughly. Previously, we found that dance communication increased nectar foraging performance particularly in environments with many species of flowers in bloom (Donaldson-Matasci & Dornhaus 2012). This could indicate that the value of the dance communication system is highest when variation in resource quality is high, because that is when the ability to selectively exploit only the most profitable resources is most important. In such environments, we might expect large colonies of honeybees to do particularly well because their large size allows them to discover more resources, and the ability to communicate resource location allows them to concentrate on the most rewarding ones. The study site and season for this experiment were chosen from those used in Donaldson-Matasci & Dornhaus (2012) to be just such an environment, and observed species richness in this experiment was similarly high. Consistent with this, we found that the net nectar gain per trip was higher in larger colonies with intact communication than it was in smaller colonies and larger colonies where communication was impaired. Further research will be required to assess whether this advantage accrues because, in environments where resource quality is highly variable, larger colonies find more resources and then use the dance communication system to select the best ones.
Another advantage that large colonies may have over small colonies is in the ability to recruit massive numbers of otherwise inactive foragers to windfall resources (H3; see e.g. Johnson & Hubbell 1987; Anderson 2001; Heinrich 2004). This ability could help large colonies dominate in contest competition, if individuals aggressively defend resources. It could also help in scramble competition or in the exploitation of ephemeral resources, by allowing large colonies to take advantage of resources before they disappear. However, it has been argued that recruitment rate in honeybees may be relatively insensitive to colony size, because, unlike trail-laying ants, one dancer can recruit only a small number of bees at a time (Dornhaus et al. 2006). We found that larger colonies with intact communication did not show higher peaks in foraging activity than smaller colonies and larger colonies where communication was impaired. We also found that foraging activity in larger colonies with intact communication was not any more concentrated in foraging peaks than in the other groups. Larger colonies, if anything, actually showed a smaller proportion of their total activity concentrated in foraging peaks, which could mean that less of their total foraging activity occurred in response to recruitment. Both of these observations suggest that, in our experiment, larger colonies did not use dance communication to quickly mobilize more foragers than smaller colonies did. Thus, it is unlikely that higher recruitment rate explains the greater benefits of communication we observed in larger colonies.
Although we found no evidence that heavy recruitment provides a key advantage to large colonies, that result could be context dependent. In our experiment, candidates for resource bonanzas included Mimosa dysocarpa, a leguminous shrub that flowers copiously after rain, and Dasylirion wheeleri, a succulent that bears a single tall inflorescence consisting of hundreds of flowers. Both of these plants offer large numbers of flowers with nectar and are highly attractive to honeybees (M. C. Donaldson-Matasci, personal observation); however, neither can compare to a mass-flowering dipterocarp in the Asian tropics (Roubik 1992). Further research across different types of environments will be required to determine whether in other environments, communication benefits large colonies by enabling mass recruitment to short-lived, prolific resources.
In conclusion, we found that honeybees in larger colonies were more effective foragers than those in smaller colonies, and this is mediated by their ability to communicate resource location via the waggle dance. Their advantage seems to be a result of being in colonies with greater information-gathering capacity: larger colonies may be able to locate rich resources more quickly, and perhaps also to select among them more effectively. However, larger colonies do not seem to recruit more foragers to particularly rich resources, at least not in our study environment. Together, these observations provide empirical evidence for the idea that large colony size and the ability to communicate resource location work together to make social insect colonies into highly efficient distributed information-gathering systems.
-
➢
Larger colonies of honeybees benefit more from communication than smaller colonies.
-
➢
Larger colony size and communication together improve per-trip foraging performance.
-
➢
Earlier peaks in foraging activity suggest larger colonies find resources faster.
-
➢
Larger colonies may benefit more because they gather information more effectively.
Acknowledgments
M.C.D. was funded by the University of Arizona’s Center for Insect Science through a National Institutes of Health (NIH) Training Grant (1K12GM000708). A.D. was funded by National Science Foundation grants (IOS-0921280 and IOS-0841756). We thank M. Heitlinger at the Santa Rita Experimental Range Headquarters for logistical support and P. Jenkins at the University of Arizona Herbarium for help with plant species identification. We gratefully acknowledge the fieldwork assistance of N. Matasci, J. Brown, S. Boleyn and J. Henkel. We also thank two anonymous referees for their helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson C. The adaptive value of inactive foragers and the scout-recruit system in honey bee (Apis mellifera) colonies. Behavioral Ecology. 2001;12:111–119. [Google Scholar]
- Bates DM, Maechler M, Bolker BM. lme4: Linear Mixed-effects Models Using S4 Classes. 2011 http://cran.r-project.org/package=lme4. [Google Scholar]
- Beckers R, Goss S, Deneubourg JL, Pasteels JM. Colony size, communication and ant foraging strategy. Psyche: A Journal of Entomology. 1989;96:239–256. [Google Scholar]
- Beekman M, Sumpter DJT. Phase transition between disordered and ordered foraging in Pharaoh’s ants. Proceedings of the National Academy of Sciences U.S.A. 2001;98:9703–9706. doi: 10.1073/pnas.161285298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman M, Sumpter DJT, Seraphides N. Comparing foraging behaviour of small and large honey-bee colonies by decoding waggle dances made by foragers. Functional Ecology. 2004;18:829–835. [Google Scholar]
- Diggle P. Statistical Analysis of Spatial Point Patterns. London: Academic Press; 1983. [Google Scholar]
- Donaldson-Matasci MC, Dornhaus A. How habitat affects the benefits of communication in collectively foraging honey bees. Behavioral Ecology and Sociobiology. 2012;66:583–592. doi: 10.1007/s00265-011-1306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornhaus A. Significance of honeybee recruitment strategies depending on foraging distance (Hymenoptera : Apidae : Apis mellifera) Entomologia Generalis. 2002;26:93–100. [Google Scholar]
- Dornhaus A, Chittka L. Why do honey bees dance? Behavioral Ecology and Sociobiology. 2004;55:395–401. [Google Scholar]
- Dornhaus A, Klügl F, Oechslein C, Puppe F, Chittka L. Benefits of recruitment in honey bees: effects of ecology and colony size in an individual-based model. Behavioral Ecology. 2006;17:336–344. [Google Scholar]
- Dornhaus A, Powell S, Bengston S. Group size and its effects on collective organization. Annual Review of Entomology. 2012;57:123–141. doi: 10.1146/annurev-ento-120710-100604. [DOI] [PubMed] [Google Scholar]
- Eckert CD, Winston ML, Ydenberg RC. The relationship between population size, amount of brood, and individual foraging behaviour in the honey bee, Apis mellifera L. Oecologia. 1994;97:248–255. doi: 10.1007/BF00323157. [DOI] [PubMed] [Google Scholar]
- von Frisch K. The Dance Language and Orientation of Bees. Cambridge, Massachusetts: Belknap Press of Harvard University Press; 1967. [Google Scholar]
- Goulson D. Bumblebees: Their Behaviour and Ecology. Oxford: Oxford University Press; 2003. [Google Scholar]
- Granovskiy B, Latty T, Duncan M, Sumpter DJT, Beekman M. How dancing honey bees keep track of changes: the role of inspector bees. Behavioral Ecology. 2012;23:588–596. [Google Scholar]
- Heinrich B. Bumblebee Economics. Cambridge, Massachusetts: Harvard University Press; 2004. [Google Scholar]
- Johnson L, Hubbell S. Defense of food supply by eusocial colonies. American Zoologist. 1987;27:347–358. [Google Scholar]
- Jun J, Pepper JW, Savage VM, Gillooly JF, Brown JH. Allometric scaling of ant foraging trail networks. Evolutionary Ecology Research. 2003;5:297–303. [Google Scholar]
- Kirchner WH, Grasser A. The significance of odor cues and dance language information for the food search behavior of honeybees (Hymenoptera: Apidae) Journal of Insect Behavior. 1998;11:169–178. [Google Scholar]
- Mailleux A-C, Deneubourg J-L, Detrain C. How does colony growth influence communication in ants? Insectes Sociaux. 2003;50:24–31. [Google Scholar]
- Michener CD. The Social Behavior of the Bees. Cambridge, Massachusetts: Harvard University Press; 1974. [Google Scholar]
- Morháč M. Peaks: Peaks. R package Version 0.2. 2008 cran.r-project.org/web/packages/Peaks/. [Google Scholar]
- Morháč M, Kliman J, Matoušek V, Veselský M, Turzo I. Identification of peaks in multidimensional coincidence γ-ray spectra. Nuclear Instruments & Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2000;443:108–125. [Google Scholar]
- Naug D. Structure and resilience of the social network in an insect colony as a function of colony size. Behavioral Ecology and Sociobiology. 2009;63:1023–1028. [Google Scholar]
- Naug D, Wenzel J. Constraints on foraging success due to resource ecology limit colony productivity in social insects. Behavioral Ecology and Sociobiology. 2006;60:62–68. [Google Scholar]
- Pacala SW, Gordon DM, Godfray HCJ. Effects of social group size on information transfer and task allocation. Evolutionary Ecology. 1996;10:127–165. [Google Scholar]
- R Development Core Team. R: a Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- Roubik DW. Foraging behavior of competing Africanized honeybees and stingless bees. Ecology. 1980;61:836–845. [Google Scholar]
- Roubik DW. Ecology and Natural History of Tropical Bees. Cambridge: Cambridge University Press; 1992. [DOI] [PubMed] [Google Scholar]
- Schmickl T, Crailsheim K. Costs of environmental fluctuations and benefits of dynamic decentralized foraging decisions in honey bees. Adaptive Behavior. 2004;12:263–277. [Google Scholar]
- Schneider SS. Nest characteristics and recruitment behavior of absconding colonies of the African honey bee, Apis mellifera scutellata in Africa. Journal of Insect Behavior. 1990;3:225–240. [Google Scholar]
- Seeley TD. Social foraging by honeybees: how colonies allocate foragers among patches of flowers. Behavioral Ecology and Sociobiology. 1986;19:343–354. [Google Scholar]
- Seeley TD. The Wisdom of the Hive : the Social Physiology of Honey Bee Colonies. Cambridge, Massachusetts: Harvard University Press; 1995. [Google Scholar]
- Seeley TD, Towne W. Tactics of dance choice in honey bees: do foragers compare dances? Behavioral Ecology and Sociobiology. 1992;30:59–69. [Google Scholar]
- Seeley TD, Visscher PK. Survival of honeybees in cold climates: the critical timing of colony growth and reproduction. Ecological Entomology. 1985;10:81–88. [Google Scholar]
- Seeley TD, Visscher PK. Assessing the benefits of cooperation in honeybee foraging: search costs, forage quality, and competitive ability. Behavioral Ecology and Sociobiology. 1988;22:229–237. [Google Scholar]
- Sherman G, Visscher PK. Honeybee colonies achieve fitness through dancing. Nature. 2002;419:920–922. doi: 10.1038/nature01127. [DOI] [PubMed] [Google Scholar]
- Zimmerman D. Censored distance-based intensity estimation of spatial point processes. Biometrika. 1991;78:287. [Google Scholar]