Abstract
Women with Multiple sclerosis (MS) often experience clinical improvement during pregnancy, indicating that sex hormones might have therapeutic effects in MS. Our previous studies have demonstrated that B cells and PD-L1 are crucial for E2 (17β-estradiol) - mediated protection against experimental autoimmune encephalomyelitis (EAE). We here demonstrate that transfer of IL-10+ B cells into E2-treated PD-L1−/− mice after EAE induction could partially restore E2-mediated protection and decrease the frequency of pro-inflammatory cells in the CNS compared to E2/saline treated PD-L1−/− mice. Hence, co-administration of IL-10+ B cells and E2 might have a powerful therapeutic potential for treatment of EAE.
Keywords: IL-10, regulatory B cell, PD-L1, PD-L2, estrogen, EAE
1. Introduction
Multiple sclerosis is a debilitating neurological and inflammatory autoimmune disease of central nervous system, which is characterized by demyelination and chronic neurodegeneration (Frohman et al., 2006, Lassmann et al., 2012). Although the incidence of MS is higher in women, clinical improvement may occur during pregnancy, which is followed by temporary post-partum exacerbations (Confavreux et al., 1998, Vukusic et al., 2004). It is now well accepted that sex hormones have immunoregulatory activity and may prevent exacerbations in MS during pregnancy. Our previous work demonstrated that treatment with pregnancy levels of estrogen (E2, 17β-estradiol) reduces CNS lesions in mice with experimental autoimmune encephalomyelitis (EAE) (Offner and Polanczyk, 2006, Polanczyk et al., 2003).
B cells were shown to have a dual role in EAE. Effector B cells contribute to the pathogenesis of EAE though the production of anti-myelin antibodies and as antigen presenting cells (APC) (Molnarfi et al., 2013, Owens et al., 2009). Conversely, regulatory B cells (Bregs) have been shown to have a protective role in EAE. Distinct subsets of IL-10 producing Bregs were identified as having immunosuppressing activity that inhibits the secretion of pro-inflammatory cytokines and migration of activated cells to the CNS (Bodhankar et al., 2013a, 2014).
We previously demonstrated that 17β-estradiol (E2) could induce complete protection for EAE in WT C57BL/6 mice and increase the frequency of CD1dhiCD5+ Bregs (Bodhankar et al., 2011). In addition, we demonstrated that this protection is mediated by the interaction of programmed death receptor 1 (PD-1) with PD-L1 but not PD-L2 on B cells (Bodhankar et al., 2013b). Recently, we demonstrated that co-administration of E2 and IL-10 secreting CD1dhiCD5+ Bregs ameliorates EAE severity in B cell deficient mice (Zhang et al., 2015). However it was not yet established whether this protective effect of Bregs in combination with E2 is dependent on PD-L1 expression on Bregs or whether co-adminstration of E2 and Bregs would be sufficient to treat EAE in PD-L1 deficient mice.
Herein, we demonstrate that IL-10 producing B cells significantly restored E2-mediated protection from EAE in PD-L1−/− mice. Moreover, this treatment decreased the frequencies of CD11b+CD45hi activated microglia/macrophages, dendritic cells and CD4+ T cells in the CNS and reduced the expression levels of pro-inflammatory cytokines in spinal cord compared to E2/saline treated PD-L1−/− mice.
2. Materials and methods
2.1 Animals
Female PD-L1−/− mice were a gift from Dr. Indira Guleria, Ph.D. (Transplantation Research Center, Brigham and Women’s Hospital and Children’s Hospital Boston, Harvard Medical School, Boston, MA, USA) (Keir et al., 2006). Female PD-L2−/− mice were a gift from Arlene Sharpe, Ph.D. (Department of Pathology, Harvard Medical School, Boston, MA, USA) (Latchman et al., 2004).
IL-10 transcriptional reporter mice were obtained from Dr. Christopher Karp, Division of Molecular Immunology, University of Cincinnati College of Medicine, Cincinnati, Ohio. The generation and characterization of these mice has been described (Madan et al., 2009). Briefly, the IL10-GFP reporter mice have a floxed neomycin-IRES eGFP cassette (Mohrs et al., 2001) inserted between the endogenous stop site and the poly (A) site of Il10 to allow identification of IL-10 producing cells in vivo. The mice (designated as Vert-X) are homozygous, develop normally and are viable and fertile without any obvious abnormal phenotype.
All animals were housed in the Animal Resource Facility at the VAPHCS in accordance with institutional guidelines. This study was conducted in accordance with National Institutes of Health guidelines for the use of experimental animals and the VAPHCS Animal Care and Use Committee approved all protocols.
2.2 Isolation of leukocytes from spleen, spinal cord and brain
Spleens were removed from euthanized animals under sterile conditions and single cell suspensions of leukocytes were prepared by disaggregation of the tissue through a 100µm nylon mesh (BD Falcon, Bedford, MA). Cells were washed once with RPMI 1640 supplemented with 10% heat-inactivated FBS (hiFBS; Thermo Scientific, Waltham, MA), then incubated with RBC Lysis Buffer (eBioscience, Inc., San Diego, CA) for 1 min to remove red cells, then washed in RPMI 1640+hiFBS. Cells were enumerated in a 1:1 dilution with 0.2% trypan blue stain (Life Technologies, Grand Island, NY) using a Cellometer Auto T4 Cell Viability Counter (Nexcelom, Lawrence, MA), washed, and resuspended at 107 cells/mL in stimulation medium (RPMI 1640 containing 10% FBS (Thermo Scientific, Waltham, MA), 1% sodium pyruvate (Life Technologies, Grand Island, NY), 1% L-glutamine [Thermo Scientific, Waltham, MA], and 0.4% β ME (Sigma-Aldrich, St. Louis, MO).
Brains and spinal cords were passed through 100µm mesh screens and washed as above. Cells were resuspended in 80 % Percoll (GE Healthcare, Pittsburgh, PA) then overlaid with 40 % Percoll to establish a density gradient and centrifuged at 1,600 rpm for 30min following a method previously described (Campanella et al., 2002). Leukocytes were collected from the resultant interface, counted, and resuspended in stimulation media for assay.
2.3 Hormone treatment and induction of EAE
Female PD-L1−/− and PD-L2−/− mice were implanted subcutaneously with 2.5mg/60-day release 17β-estradiol pellets (Innovative Research of America, Sarasota, FL) or sham-treated (control) one week prior to EAE induction. EAE was induced by immunization with 200µg mouse (m) MOG-35-55 peptide (PolyPeptide Laboratories, San Diego, CA) in 200µg Complete Freund’s adjuvant (Incomplete Freund’s adjuvant (IFA, Sigma-Adrich, St. Louis, MO) completed with heat-killed Mycobacterium tuberculosis (Mtb, Difco, Detroit, MI). Mice received pertussis toxin (Ptx, List Biologicals, Campbell, CA) on the day of immunization (75 ng) and 2 days later (200 ng).
All mice were monitored daily for clinical signs of disease and scored using the following scale: 0=normal; 1=limp tail or mild hind limb weakness; 2=moderate hind limb weakness or mild ataxia; 3=moderately severe hind limb weakness; 4=severe hind limb weakness or mild forelimb weakness or moderate ataxia; 5=paraplegia with no more than moderate forelimb weakness; and 6=paraplegia with severe forelimb weakness or severe ataxia or moribund condition. Mice were scored daily and were evaluated for incidence, day of onset, day of maximal clinical signs (peak) and total disease score over the course of the experiment (Cumulative Disease Index, CDI). Mean ± SEM were calculated for these parameters for each experimental group.
2.4 Flow cytometry
Cell isolates were prepared as above, then washed and resuspended in staining medium (1X PBS, 0.5% BSA, 0.02% sodium azide). Leukocytes were labeled with a combination of the following antibodies obtained from BD Bioscience (San Jose, CA): PE-Cy7 CD4 (L3T4), APC CD8 (53-6.7), APC CD19 (1D3), PE CD1d (1B1), PerCP CD5 (53–7.3), PerCP-Cy5.5 CD11b (M1/70), PE CD45 (30-F11). Data were acquired using a BD Accuri C6 (BD Biosciences) and BD’s proprietary software.
2.5 Cell sorting and adoptive transfer of B cells
Donor B cells were acquired from female IL-10 GFP reporter mice that were age matched to recipients. Splenic CD19+ B cells were purified using paramagnetic bead-conjugated antibodies (Abs) from the CD19 cell isolation kit and subsequently separated by AutoMACS (both from Miltenyi Biotec, Auburn, CA). The positive fraction of the cells thus separated were CD19+ B cells with a purity of ≥95 %. CD19+ B cells were suspended in stimulation medium as described above and cultured in the presence of 1 µg/mL of lipopolysaccharide (LPS from E. coli serotype 055:B5) (Sigma-Adrich, St. Louis, MO) for 48hrs at 37°C, 5% CO2 and 95% humidity. After 48hrs of culture, B cells were harvested from culture plates, washed free of LPS and viable cells were counted. The cells were then resuspended at 5×107 purified IL-10-GFP+ B cells/mL in 0.9% sodium chloride irrigation solution (saline, Baxter, Deerfield, IL) and 100µL of either cell suspension (5×106 cells) or saline were transferred intravenously (i.v.) via tail vein into PD-L1−/− and PD-L2−/− recipient mice.
2.6 RNA Isolation and Reverse transcription-Polymerase Chain Reaction
Total RNA was isolated using the RNeasy Mini Kit according to the manufacturer's instructions (Qiagen, Valencia, CA). cDNA was synthesized using the SuperScript II Reverse Transcriptase cDNA synthesis kit (Life Technologies, Grand Island, NY). Quantitative real time PCR was performed using the StepOnePlus Real-Time PCR System with TaqMan Gene Expression probes. Individual samples were analyzed in triplicate for IL-1β, IL-6, IL-17A, TNF-α and IFN-γ (Life Technologies, Grand Island, NY). GAPDH housekeeping gene was used as an endogenous control. Results were analyzed using ExpressionSuite Software (Life Technologies, Grand Island, NY).
2.7 Statistical analysis
Data were reported using GraphPad Prism (v5.0, San Diego, CA) and expressed as the mean ± SEM. Statistical significance for the disease course was calculated using the Mann-Whitney U test. The Student’s t-test and the Kruskal–Wallis Test (non-parametric analysis of variance) with Dunn’s multiple comparison of means post-test were used for analysis of the cumulative disease index. Statistical significance for flow cytometry data was calculated using One-way ANOVA (one way analysis of variance) and Newman-Kuels Multiple Comparison post-test. P-values ≤0.05 were considered to be significant (* p≤0.05; ** p≤0.01; *** p≤0.001).
3. Results
3.1 IL-10 producing B cells partially restore E2-mediated protection against EAE in PD-L1−/− mice
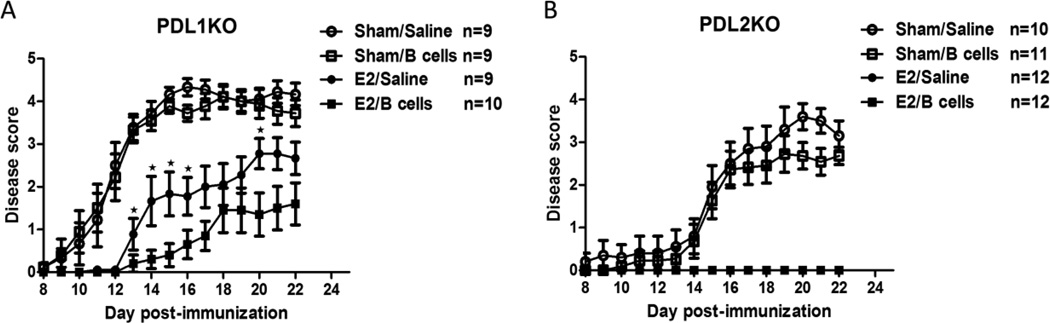
We have reported previously that E2 treatment requires B cells to ameliorate clinical signs of EAE (Bodhankar, Wang, 2011). We further have shown that E2 protection is dependent on PD-L1 expression on B cells. In order to determine the role of PD-L1 or PD-L2 expression on IL-10 secreting regulatory B cells in E2-mediated protection against EAE, recipient female PD-L1−/− and PD-L2−/− mice were either sham treated or implanted with E2 pellets 7 days prior to immunization with mMOG-35-55 peptide with CFA/Ptx. Three days post-immunization, IL-10 producing B cells from WT donor IL-10 GFP+ reporter female mice that were stimulated with LPS for 48 hr (Supplementary Figure 1), were transferred into recipient female PD-L1−/− and PD-L2−/− mice. As shown in Figure 1 and consistent with our previous report, there were marked differences in the course of EAE in PD-L1−/− vs. PD-L2−/− mice, with the PD-L1−/− mice exhibiting significantly earlier onset and more severe clinical signs (Peak and CDI scores) of EAE than PD-L2−/− mice that were similar to WT control mice (Bodhankar, Galipeau, 2013b). E2 implants induced complete protection against EAE in the PD-L2−/− mice in the absence or presence of the transferred IL-10 producing B cells, indicating that E2 protection could still be mediated through resident PD-L1 sufficient B cells. In contrast, E2 implants in PD-L1−/− mice in the absence of transferred B cells did not protect, but did delay EAE onset by ~4 days to that observed in the PD-L2−/− mice. However, co-administration of E2 with IL-10 producing PD-L1+ B cells in PD-L1 deficient recipient mice not only reduced the incidence of EAE from 100% to 50%, but also significantly reduced disease severity (both Peak and CDI scores) compared to all of the other PD-L1−/− mouse treatment groups (p<0.05, Table 1). Interestingly, mice that were given IL-10 producing B cells without E2 (Sham/B cells) displayed no difference in clinical disease course compared to the control group (Sham/Saline). These findings demonstrate that PD-L2 is not required for E2 mediated protection against EAE, whereas E2 treatment of PD-L1−/− mice delayed but not inhibit EAE. We further demonstrate that co-treatment with IL-10 producing regulatory B cells could significantly restore E2-mediated protection against EAE in the PD-L1−/− mice.
Figure 1. IL-10 producing B cells and E2 ameliorate clinical signs of EAE in PD-L1−/− mice.
Seven-8 week-old PD-L1−/− (KO) (Fig. 1A) and PD-L2−/− (KO)(Fig. 1B) mice were implanted with E2 pellets one week prior to immunization with mouse (m) MOG-35–55 peptide in CFA/Ptx. IL-10 GFP+ B cells were transferred intravenously via tail vein into recipients on day 3 post MOG-35-55/CFA/Ptx immunization and mice were monitored for clinical signs of EAE. Figures show the mean daily disease scores from 3 independent experiments with 3–4 mice/group/experiment. *significant difference (*p≤0.05) as compared to E2/B cell treated mice (Mann-Whitney U test).
Table 1.
Clinical course of EAE in PD-L1−/− and PD-L2−/− mice.
| Mice | Incidence | Onset | Peak | CDI |
|---|---|---|---|---|
| PD-L1−/− Sham/Saline | 9/9 | 11.8 ± 0.5a,c | 4.6 ± 0.2a | 45.3 ± 2.8a,c |
| PD-L1−/− Sham/B cells | 9/9 | 11.3 ± 0.6b,d | 4.7 ± 0.2b,d | 43.1 ± 2.5b,d |
| PD-L1−/− E2/Saline | 9/9 | 16.0 ± 1.2 | 3.1 ± 0.4 | 20.8 ± 3.5 |
| PD-L1−/− E2/B cells | 5/10 | 16.4 ± 1.4 | 1.7 ± 0.5a | 9.8 ± 3.3a |
| PD-L2−/− Sham/Saline | 10/10 | 15.5 ± 1.2 | 3.8 ± 0.3 | 26.8 ± 4.5 |
| PD-L2−/− Sham/B cells | 11/11 | 15.6 ± 1.0 | 3.3 ± 0.3 | 21.0 ± 3.0 |
| PD-L2−/− E2/Saline | 0/12 | N/A | 0.0 ± 0.0 | 0.0 ± 0.0 |
| PD-L2−/− E2/B cells | 0/12 | N/A | 0.0 ± 0.0 | 0.0 ± 0.0 |
: significant (P<0.05) as Compared to PD-L1−/− E2/Saline group
: significant (P<0.05) as Compared to PD-L1−/− E2/B cell group
: significant (P<0.05) as Compared to PD-L2−/− Sham/Saline group
: significant (P<0.05) as Compared to PD-L2−/− Sham/B cell group
Statistical evaluation of EAE disease course for Control and E2-implanted recipient PD-L1−/− and PD-L2−/− mice with or without B cell transfer, including the Cumulative Disease Index (CDI) through day 22 post-immunization for the 3 experiments (n = 3–4 mice/group/experiment). The Incidence, Day of Onset and Peak Daily Disease scores from pooled data and corresponding CDI data from each of the independent experiments (mean ± SEM) are presented. Additional information for the experiments is shown in Fig. 1.
3.2 IL-10 producing B cells in combination with E2 treatment inhibit the infiltration of CD4+ T lymphocytes into spinal cords of PD-L1−/− mice
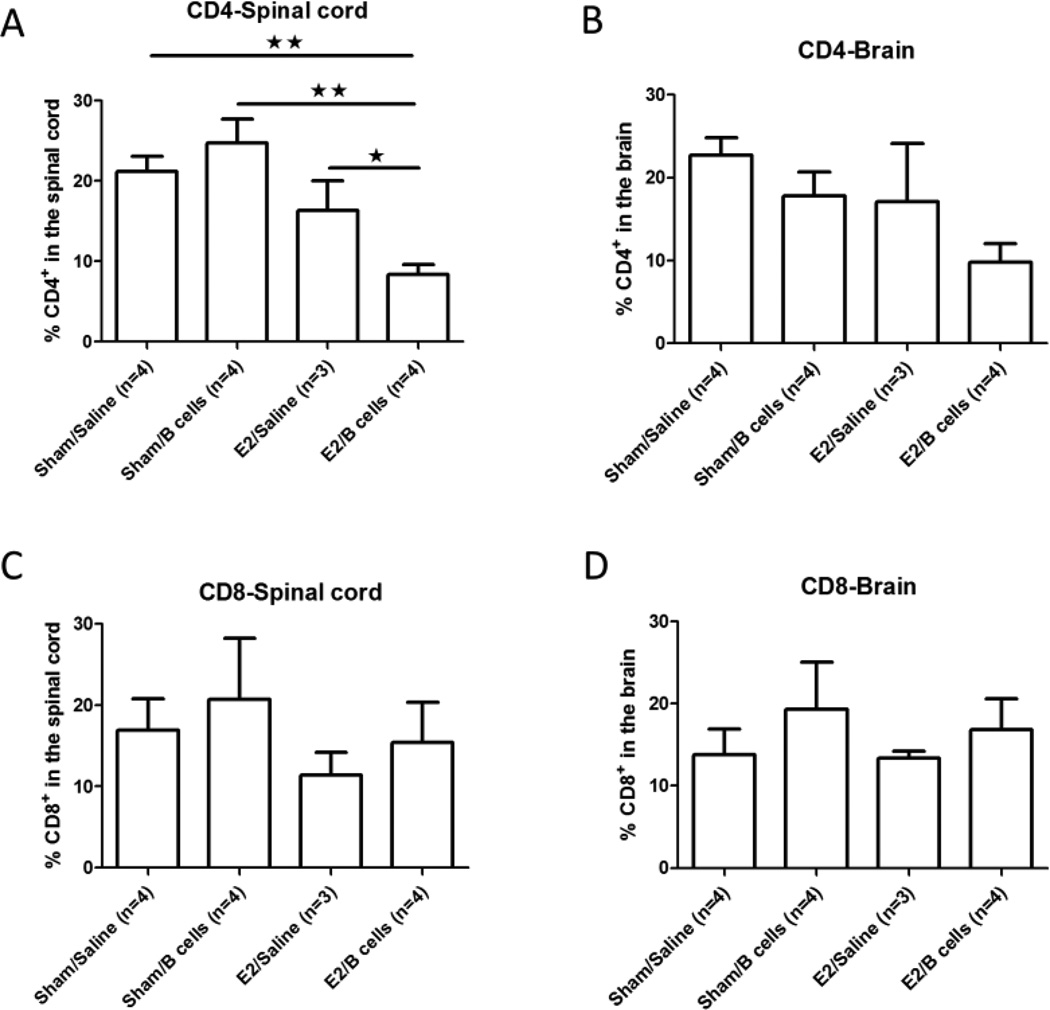
In order to assess the combined effect of E2 and IL-10 producing B cells on T cell migration into the CNS during EAE, infiltrating leukocytes were isolated from the spinal cords and brains of treated mice on day 22 post-immunization. Since E2 treated PD-L2 deficient mice were protected from EAE in the presence or absence of IL-10 producing regulatory B cells (Fig. 1), we focused on the contribution of regulatory B cells to the E2 mediated protection against EAE in PD-L1−/− mice. We observed that the frequency of infiltrating CD4+ T cells was significantly lower in spinal cords but not brains of PD-L1−/− mice that received both E2 and IL-10 producing B cells compared to other groups that received Sham/Saline, Sham/B cells or E2/Saline (p<0.01, p<0.01 and p<0.05, respectively, Figs. 2A and 2B). In contrast, there were no significant differences in the frequency of CD8+ T cells in the spinal cord or brain of E2/B cell treated PD-L1−/− mice (Figs. 2C and 2D).
Figure 2. IL-10 producing B cells in combination with E2 treatment inhibit the infiltration of CD4+ T lymphocytes into spinal cords of PD-L1−/− mice.
Frequencies of infiltrating CD4+ T cells (Fig 2A, 2B) and CD8+ T cells (Fig 2C, 2D) were determined in individual spinal cords and brains of mice with EAE. Data are presented as mean ± SEM. Statistical analysis was performed with ANOVA followed by Newman-Kuels post hoc test. Significant differences between sample means are indicated (*p≤0.05; **p≤0.01).
3.3 Treatment of EAE with E2 in combination with IL-10 producing B cells decreases the frequency of activated microglia/macrophages and DC in spinal cords of PD-L1−/− mice
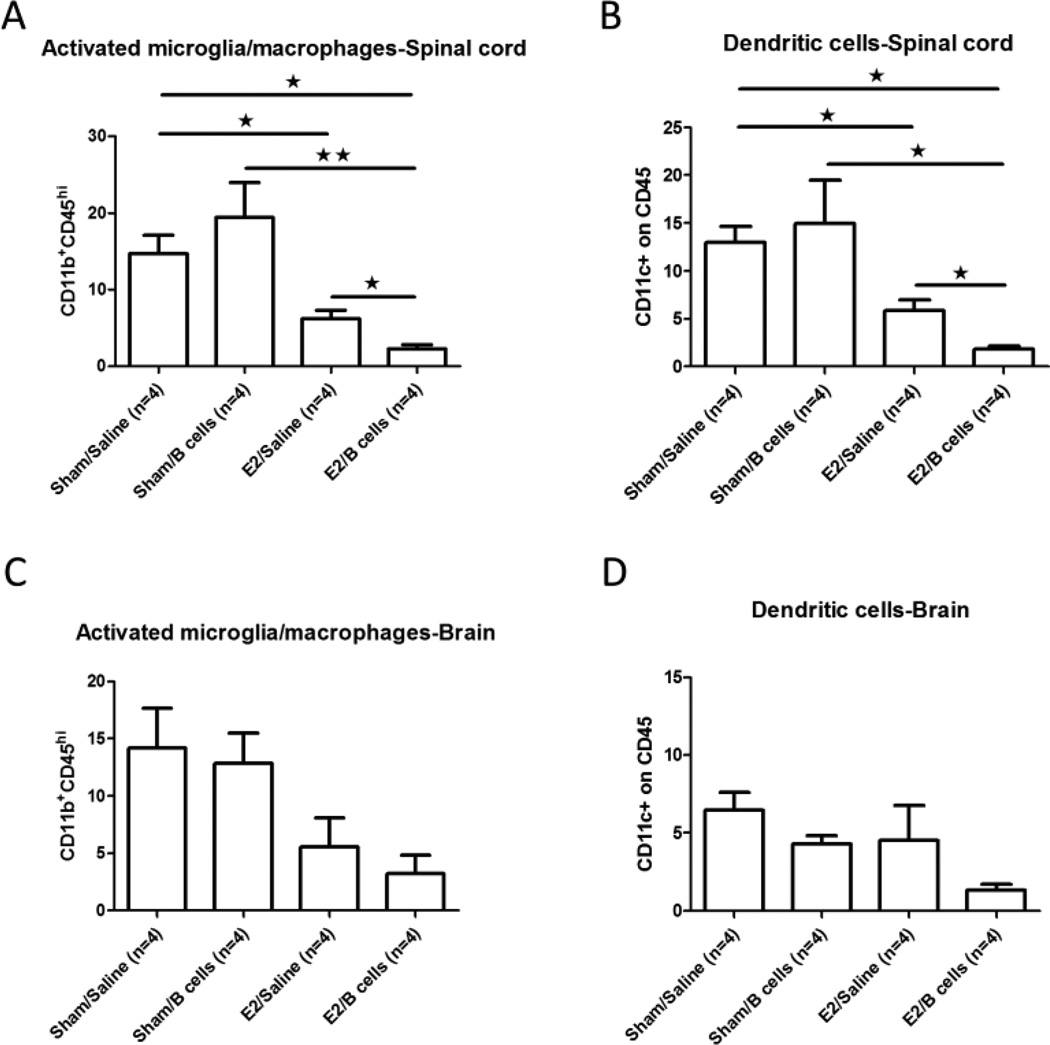
Monocytes and macrophages play a pathogenic role in multiple sclerosis and EAE, both of which are characterized by extensive lymphocytic infiltration into the CNS. Several reports have demonstrated that monocytes are involved in the exacerbation of EAE, with monocyte depletion resulting in a marked suppression of clinical disease (Ajami et al., 2011, Dijkstra et al., 2008). In the current study, we found a significant reduction in the frequency of CD11b+CD45hi activated microglia/macrophages in the spinal cords of E2/Saline vs. Sham/Saline treated PD-L1−/− mice with EAE, with an even greater reduction observed in the E2/B cell treated group (p<0.05, Fig 3A). Moreover, a nearly identical pattern of reduced frequencies of infiltrating CD11c+CD45+ dendritic cells (DC) was observed (p<0.05, Fig. 3B). In brain, however, no significant differences in the frequencies of CD11b+CD45hi activated microglia/macrophages or CD11c+CD45+ dendritic cells were noted among the different treatment groups, although a similar trend was observed (Figs. 3C and 3D). Moreover, there were no differences in the frequencies of CD4+ T cells or CD11b+ cells in the CNS between E2 treated PD-L2−/− mice with or without IL-10 producing B cells vs. Sham/Saline or Sham/B cell controls (data not shown). Taken together, our results demonstrate that IL-10 producing regulatory B cells which also express PD-L1 could enhance the E2 protection effect in PD-L1−/− mice with EAE by inhibiting the frequencies of pro-inflammatory cells found in spinal cord.
Figure 3. Treatment of EAE with E2 in combination with IL-10 producing B cells decreases the frequency of activated microglia/macrophages and DC in spinal cords of PD-L1−/− mice.
CD11b+CD45hi activated microglia/macrophages (Fig. 3A, 3C) and CD11c+CD45+ dendritic cells (Fig. 3B, 3D) were determined in individual spinal cords and brains of mice with EAE. Data are presented as mean ± SEM. Statistical analysis was performed with ANOVA followed by Newman-Kuels post hoc test. Significant differences between sample means are indicated (*p≤0.05; **p≤0.01).
3.4 Treatment of EAE with E2 in combination with IL-10 producing B cells reduces the overall pro-inflammatory milieu in the CNS
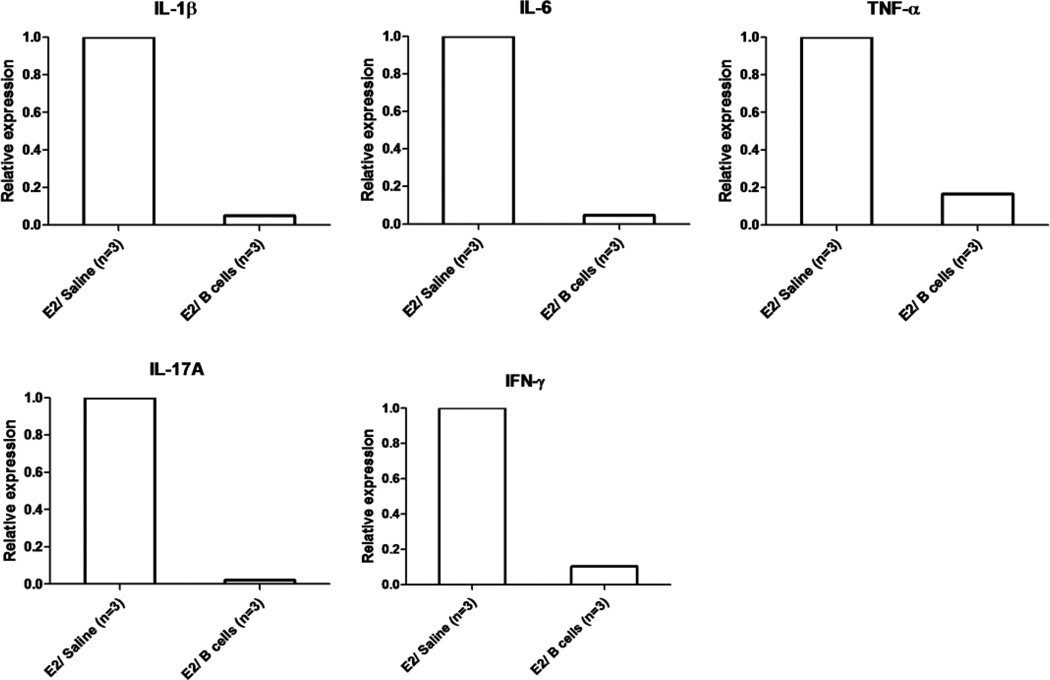
To evaluate the effects of IL-10 producing B cells on CNS inflammation during EAE in E2 treated PD-L1−/− mice, we performed real-time PCR analysis for expression of pro-inflammatory cytokines on day 22 post-immunization. As shown in Figure 4, the expression levels of IL-1β, IL-6, TNF-α, IL-17A and IFN-γ were profoundly inhibited in spinal cords of E2/IL-10 producing B cell vs. E2/Saline treated PD-L1−/− mice with EAE, although these differences did not reach significance due to a large variation in the E2/saline group. These data, in total, suggest that IL-10 producing B cells can reduce CNS inflammation in E2 treated PD-L1−/− mice with EAE.
Figure 4. Treatment of EAE with E2 in combination with IL-10 producing B cells reduces the overall pro-inflammatory milieu in the CNS.
Spinal cords were individually collected from E2/saline and E2/Breg groups of PD-L1−/− mice with EAE. Relative expression of pro-inflammatory cytokines was determined by Real-Time PCR. Data are presented as mean ± SEM. Statistical analysis was performed with ANOVA followed by Newman-Kuels post hoc test. Significant differences between sample means are indicated (*p≤0.05).
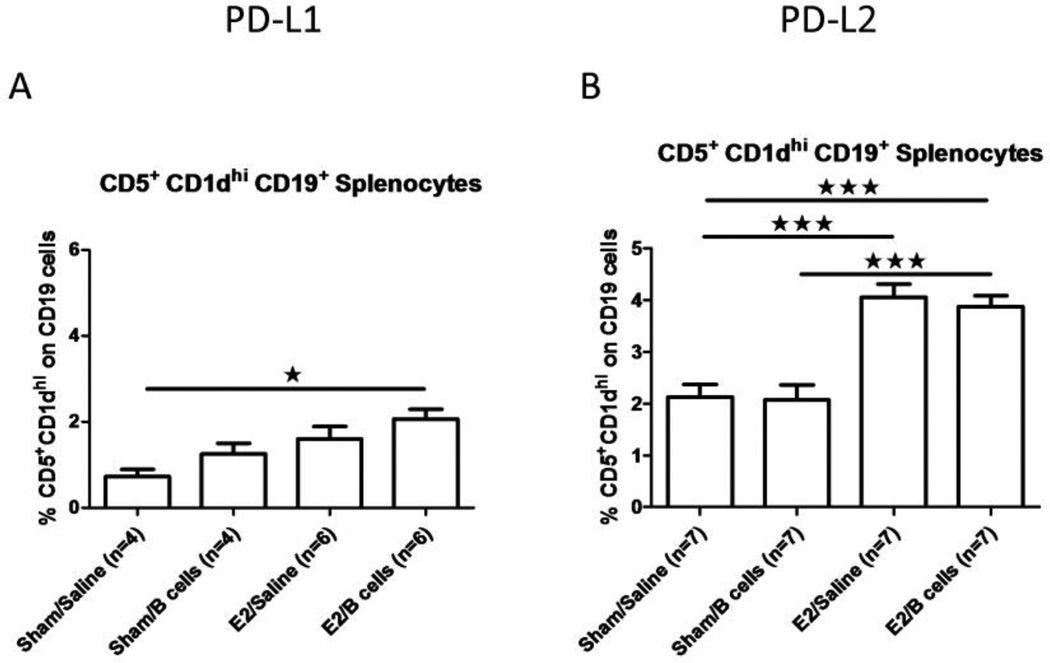
3.5 Treatment of EAE in PD-L2−/− mice with E2 in combination with resident PD-L1+ B cells induces optimal increases in the peripheral frequency of CD5+CD1dhi regulatory B cells
Our previous study demonstrated that E2 treatment induced a significant increase in the frequency of CD5+CD1dhi B cells in the spleens of mice with EAE in a PD-L1, but not a PD-L2 dependent manner (Bodhankar, Wang, 2011). We thus investigated whether the increased frequency of CD5+CD1dhi B cells was dependent upon the administration of PD-L1+ IL-10 secreting regulatory B cells. As shown in Fig. 5A, CD5+CD1dhi splenic B cells from PD-L1 deficient mice were modestly increased to <2% of total splenocytes after E2 treatment with or without co-administration of IL-10 secreting B cells, whereas a more vigorous and highly significant increase to ~4% CD5+CD1dhi splenic B cells occurred in E2-treated PD-L2−/− mice that retained intact resident PD-L1+ B cells, again with or without co-administered IL-10 secreting B regulatory cells (Fig. 5B). Thus, it would appear that E2 treatment in the presence of resident PD-L1+ regulatory B cells was critical for optimal increases in the regulatory CD5+CD1dhi splenic B cell subpopulation, but was not dependent upon the transfer of PD-L1+ IL-10 secreting regulatory B cells in mice completely protected against EAE (as in Fig. 1B).
Figure 5. Treatment of EAE in PD-L2−/− mice with E2 in combination with resident PD-L1+ B cells induces optimal increases in the peripheral frequency of CD5+CD1dhi regulatory B cells.
CD5+CD1dhi B cells were determined in individual spleens of PD-L1−/− and PD-L2−/− mice with EAE (Figs. 5A and 5B). Data are presented as mean ± SEM. Statistical analysis was performed with ANOVA followed by Newman-Kuels post hoc test. Significant differences between sample means are indicated (*p≤0.05; ***p≤0.001).
4. Discussion
Like many autoimmune diseases, MS predominantly affects females. However, women with MS often show clinical improvement during pregnancy, followed by temporary post-partum exacerbations, suggesting immunoregulatory and neuroprotective roles for pregnancy induced sex hormones, especially estriol and E2 (Bebo et al., 2001, Polanczyk et al., 2006, Wang et al., 2009). We previously demonstrated that relatively low doses of E2 confer potent protection against clinical and histological signs of EAE (Polanczyk et al., 2004). Recently, we demonstrated that E2 mediated protection against EAE is dependent on the presence of PD-L1 expressing B cells (Bodhankar, Galipeau, 2013b). We further showed that E2 treatment could increase the frequency of CD5+CD1dhi regulatory B cells and increase the cell surface expression of PD-L1 and IL-10 secretion from these cells (Bodhankar, Wang, 2011). Herein, we demonstrate that transferred PD-L1+ IL-10 producing B cells could partially restore E2 mediated protection against EAE in PD-L1 deficient mice.
Programmed death-1 (PD-1), a new member of the B7 superfamily of costimulatory molecules, plays a critical role in regulating autoimmune diseases. There are two known ligands known that bind PD-1: PD-L1 which was identified through homology searches with known B7 molecules (Dong et al., 1999, Freeman et al., 2000); and PDL2 which was identified among a library of genes differentially expressed between DCs and activated macrophages (Tseng et al., 2001). The engagement of PD-1 with PDL1/L2 plays a critical role in the maintenance of tolerance by negatively regulating autoreactive pathogenic CD4+ T cells (Dai et al., 2014, Latchman et al., 2001, Latchman, Liang, 2004). Recent studies indicated that PD1−/− and PD-L1−/− mice develop more severe EAE after immunization with MOG-35-55 peptide while PD-L2−/− develop EAE similar to wild type control mice. Consistent with this, PD-1−/− and PD-L1−/− cells produced elevated levels of the pro-inflammatory cytokines (Carter et al., 2007, Polanczyk et al., 2007). Our previous study also have demonstrated PD-1 interaction with PD-L1 but not PD-L2 on B cells is crucial for E2-mediated protection in EAE (Bodhankar, Galipeau, 2013b). When we evaluated and compared the immune status between PD-L1−/− mice and PD-L2−/− mice in E2-mediated protection against EAE, we found that PD-L2−/− mice exhibited decreased infiltating CD4+ and CD8+ T cells, a lower frequency of activated microglia/macrophages and a higher frequency of CD5+CD1dhi regulatory B cells compared to PD-L1−/− mice. In addition, we found that E2 treatment was able to induce IL-10 producing regulatory B cells, which have a beneficial role in protection against EAE in WT mice (Bodhankar, Wang, 2011). Moreover, Dong, et al. demonstrated that PD1/PD-L1 interaction could increase IL-10 production (Dong, Zhu, 1999). Our current study clearly demonstrates that transfer of IL-10 producing B cells, which also express PD-L1 on their cell surface, could partially resotre E2 mediated protection against EAE, mainly by inhibiting the infiltration of pro-inflammatory cells from the periphery, which in turn leads to a less inflammatory CNS. This finding is in concert with our previous report that IL-10 producing regulatory B cells in conjunction with E2 ameliorates EAE severity and decreases CNS inflammation in B cell-deficient mice (Zhang, Lapato, 2015)
Contradictory roles for B cells in EAE pathogenesis have been reported (Matsushita et al., 2008, Pollinger et al., 2009). Although not essential for EAE induction or progression, MOG-specific autoantibodies can enhance demyelination and inflammation (Linington et al., 1988, Lyons et al., 1999). On the other hand, additional studies demonstrated that B-cell deficient mice develop a severe non-remitting form of EAE (Fillatreau et al., 2002, Matsushita et al., 2006, Wolf et al., 1996) and B-cell depletion with anti-CD20 antibody before EAE induction resulted in exacerbation of disease signs and increased encephalitogenic T cell influx into the CNS (Matsushita, Yanaba, 2008). These studies led to the identification of regulatory B-cells, which when depleted resulted in increased clinical severity. Indeed, phenotypically distinct subsets of IL-10 producing regulatory B cells have now been identified, including CD19+CD1dhiCD5+, CD19+CD21hiIgMhiCD23hi, CD19+Tim-1+ and B220+CD138+CD44hi (Evans et al., 2007, Matsumoto et al., 2014, Matsushita, Yanaba, 2008, Mauri and Bosma, 2012). It is well known that these Bregs act mainly, but not exclusively, via the release of IL-10, which can induce the differentiation and activation of Tregs and inhibit the activation of microglia and production of pro-inflammatory cytokines.
Our current results demonstrate that IL-10 producing B cells could significantly restore E2-mediated protection against EAE induced in PD-L1−/− mice, with full protection (no clinical signs) observed in 50% (5/10) of the mice and overall delayed onset of disease and lower disease peak and CDI scores for the group. The mouse to mouse variation in response to E2/B cell treatment suggests that in addition to the IL-10 producing B cells other regulatory factors may be involved in the E2-mediated protection against EAE. Moreover, we found that administration of IL-10 producing B cells without E2 treatment, 3 days post-immunization, was not sufficient to protect against EAE in either PD-L1−/− or PD-L2−/− mice (Fig. 1). It is possible that different conditions for B cell administration (time point or cell number) might have a more beneficial effect in protecting against EAE in PD-L1−/− mice that are treated with or without E2. Lastly, it is apparent that optimal production of the regulatory CD5+CD1dhi splenic B cell subpopulation occurred in fully protected E2 treated PD-L2−/− mice that retain resident PD-L1+ regulatory B cells. In contrast, only minor changes were observed in PD-L1−/− mice after transfer of PD-L1+ IL-10 secreting regulatory B cells, suggesting a predominant involvement of the resident PD-L1+ B cells as the source of the regulatory CD5+CD1dhi B cells.
It is not clear whether the protective effect of the IL-10 producing B cells in the E2 treated PD-L1−/− mice is caused by the IL-10 secretion, the expression of PD-L1 on the cell surface of these cells or an additive effect of both of these factors. In this regard, it is important to note that when B cells form PD-L1−/− mice, which were not sorted for IL-10 production, were transferred into recipient E2 treated µMT−/− mice (that lack B cells); the recipient mice had only a slightly lower CDI score compared to the no cell recipient E2 treated µMT−/− mice. However, E2 mediated protection was sustained in recipient E2 treated µMT−/− mice that were administered B cells form PD-L2−/− mice (Bodhankar, Galipeau, 2013b). These experiments underline the importance of PD1/PD-L1 interaction in mediating E2 protection effects. Future studies using B cells transferred from IL-10 deficient donor mice to E2 treated PD-L1−/− mice with EAE could further delineate the effects of IL-10 in this model.
In summary, our present study demonstrates that IL-10 producing B cells partially restore E2-mediated protection in EAE-induced PD-L1−/− mice. IL-10 producing B cells decreased the frequency of CD11b+CD45hi activated microglia/macrophages, dendritic cells and infiltrating CD4 T cells in spinal cords of E2-treated PD-L1−/− mice compared to E2-treated PD-L1−/− mice that did not receive these regulatory cells. Our present data also demonstrate a lower level of pro-inflammatory cytokines in the spinal cord of PDL1−/− mice treated with E2 and IL-10 producing B cells relative to PD-L1−/− mice treated with E2/saline treatment. Taken together, these findings suggest optimal protection against EAE using treatment with E2 in combination with IL-10 producing B cells.
Supplementary Material
Highlights.
IL-10+ B cells partially restore E2 protection against EAE in PD-L1−/− mice.
IL-10+ B cells and E2 inhibit cell infiltration into the CNS of EAE PD-L1−/− mice.
IL-10+ B cells and E2 reduce the pro-inflammatory milieu in the CNS of PD-L1−/− mice.
Acknowledgements
This work was supported by NIH/NINDS grant RO1 NS080890. The authors wish to thank Gail Kent for assistance with manuscript preparation and OHSU research cores for histopathology. This material is the result of work supported with resources and the use of facilities at the VA Portland Health Care System, Portland, OR. The contents do not represent the views of the Department of Veterans Affairs or the US government.
Abbreviations
- MS
multiple sclerosis
- EAE
experimental autoimmune encephalomyelitis
- Breg
regulatory B cell
- Treg
regulatory T cell
- CNS
central nervous system
- CDI
cumulative disease index
- PD1
programmed cell death protein 1
- PD-L
programmed death-ligand
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Jun Zhang, Email: zhanju@ohsu.edu.
Gil Benedek, Email: benedek@ohsu.edu.
Sheetal Bodhankar, Email: bodhanka@ohsu.edu.
Andrew Lapato, Email: lapato@ohsu.edu.
Arthur A. Vandenbark, Email: vandenba@ohsu.edu.
Halina Offner, Email: offnerva@ohsu.edu.
References
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nature neuroscience. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- Bebo BF, Jr, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. Journal of immunology. 2001;166:2080–2089. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. IL-10-producing B-cells limit CNS inflammation and infarct volume in experimental stroke. Metabolic brain disease. 2013a28:375–386. doi: 10.1007/s11011-013-9413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. Treatment of experimental stroke with IL-10-producing B-cells reduces infarct size and peripheral and CNS inflammation in wild-type B-cell-sufficient mice. Metabolic brain disease. 2014;29:59–73. doi: 10.1007/s11011-013-9474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Galipeau D, Vandenbark AA, Offner H. PD-1 Interaction with PD-L1 but not PD-L2 on B-cells Mediates Protective Effects of Estrogen against EAE. Journal of clinical & cellular immunology. 2013b;4:143. doi: 10.4172/2155-9899.1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Wang C, Vandenbark AA, Offner H. Estrogen-induced protection against experimental autoimmune encephalomyelitis is abrogated in the absence of B cells. European journal of immunology. 2011;41:1165–1175. doi: 10.1002/eji.201040992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke; a journal of cerebral circulation. 2002;33:586–592. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- Carter LL, Leach MW, Azoitei ML, Cui J, Pelker JW, Jussif J, et al. PD-1/PD-L1, but not PD-1/PD-L2, interactions regulate the severity of experimental autoimmune encephalomyelitis. Journal of neuroimmunology. 2007;182:124–134. doi: 10.1016/j.jneuroim.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. The New England journal of medicine. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- Dai S, Jia R, Zhang X, Fang Q, Huang L. The PD-1/PD-Ls pathway and autoimmune diseases. Cellular immunology. 2014;290:72–79. doi: 10.1016/j.cellimm.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Dijkstra S, Kooij G, Verbeek R, van der Pol SM, Amor S, Geisert EE, Jr, et al. Targeting the tetraspanin CD81 blocks monocyte transmigration and ameliorates EAE. Neurobiology of disease. 2008;31:413–421. doi: 10.1016/j.nbd.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nature medicine. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. Journal of immunology. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nature immunology. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. The Journal of experimental medicine. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. The New England journal of medicine. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. The Journal of experimental medicine. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nature reviews Neurology. 2012;8:647–656. doi: 10.1038/nrneurol.2012.168. [DOI] [PubMed] [Google Scholar]
- Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nature immunology. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. The American journal of pathology. 1988;130:443–454. [PMC free article] [PubMed] [Google Scholar]
- Lyons JA, San M, Happ MP, Cross AH. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. European journal of immunology. 1999;29:3432–3439. doi: 10.1002/(SICI)1521-4141(199911)29:11<3432::AID-IMMU3432>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. Journal of immunology. 2009;183:2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Baba A, Yokota T, Nishikawa H, Ohkawa Y, Kayama H, et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity. 2014;41:1040–1051. doi: 10.1016/j.immuni.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Fujimoto M, Hasegawa M, Komura K, Takehara K, Tedder TF, et al. Inhibitory role of CD19 in the progression of experimental autoimmune encephalomyelitis by regulating cytokine response. The American journal of pathology. 2006;168:812–821. doi: 10.2353/ajpath.2006.050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. The Journal of clinical investigation. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri C, Bosma A. Immune regulatory function of B cells. Annual review of immunology. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- Molnarfi N, Schulze-Topphoff U, Weber MS, Patarroyo JC, Prod'homme T, Varrin-Doyer M, et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. The Journal of experimental medicine. 2013;210:2921–2937. doi: 10.1084/jem.20130699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Polanczyk M. A potential role for estrogen in experimental autoimmune encephalomyelitis and multiple sclerosis. Annals of the New York Academy of Sciences. 2006;1089:343–372. doi: 10.1196/annals.1386.021. [DOI] [PubMed] [Google Scholar]
- Owens GP, Bennett JL, Lassmann H, O'Connor KC, Ritchie AM, Shearer A, et al. Antibodies produced by clonally expanded plasma cells in multiple sclerosis cerebrospinal fluid. Annals of neurology. 2009;65:639–649. doi: 10.1002/ana.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk M, Zamora A, Subramanian S, Matejuk A, Hess DL, Blankenhorn EP, et al. The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha. The American journal of pathology. 2003;163:1599–1605. doi: 10.1016/s0002-9440(10)63516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. Journal of neuroscience research. 2006;84:370–378. doi: 10.1002/jnr.20881. [DOI] [PubMed] [Google Scholar]
- Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (PD-1) International immunology. 2007;19:337–343. doi: 10.1093/intimm/dxl151. [DOI] [PubMed] [Google Scholar]
- Polanczyk MJ, Jones RE, Subramanian S, Afentoulis M, Rich C, Zakroczymski M, et al. T lymphocytes do not directly mediate the protective effect of estrogen on experimental autoimmune encephalomyelitis. The American journal of pathology. 2004;165:2069–2077. doi: 10.1016/S0002-9440(10)63257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollinger B, Krishnamoorthy G, Berer K, Lassmann H, Bosl MR, Dunn R, et al. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. The Journal of experimental medicine. 2009;206:1303–1316. doi: 10.1084/jem.20090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. The Journal of experimental medicine. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukusic S, Hutchinson M, Hours M, Moreau T, Cortinovis-Tourniaire P, Adeleine P, et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain : a journal of neurology. 2004;127:1353–1360. doi: 10.1093/brain/awh152. [DOI] [PubMed] [Google Scholar]
- Wang C, Dehghani B, Li Y, Kaler LJ, Vandenbark AA, Offner H. Oestrogen modulates experimental autoimmune encephalomyelitis and interleukin-17 production via programmed death 1. Immunology. 2009;126:329–335. doi: 10.1111/j.1365-2567.2008.03051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. The Journal of experimental medicine. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lapato A, Bodhankar S, Vandenbark AA, Offner H. Treatment with IL-10 producing B cells in combination with E2 ameliorates EAE severity and decreases CNS inflammation in B cell-deficient mice. Metabolic brain disease. 2015 doi: 10.1007/s11011-015-9661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.