Abstract
Background
A variety of materials have been used for bone augmentation, distraction osteotomy, and in post-cancer patients following tumor removal. However, a temporary metal implant that would resorb after successful treatment is a new concept. Magnesium was suggested as a suitable material for these purposes because it is biocompatible, has better mechanical properties than titanium, and stimulates new bone formation. This study evaluates histological appearance of magnesium-based implants and the surrounding bone.
Materials and Methods
Three magnesium-based biomaterials were tested in a rabbit bone defect model: magnesium–hydroxyapatite (Mg–HA), W4 (96 % magnesium, 4 % yttrium), and pure magnesium (pure Mg). Animals were sacrificed after 6 and 12 weeks and the samples were analyzed histologically and histomorphometrically.
Results
Mg–HA had the highest mean amount of tartrate-resistant acid phosphatase (TRAP) positive cells at the implantation site of all groups. It had shown the fastest degradation rate already at 6 weeks but the least amount of new bone formation. New bone was seen forming in direct contact with pure Mg and W4. The mean gas volume was highest in W4 compared to pure Mg and Mg–HA but this difference was not statistically significant. W4 had the lowest mean number of TRAP-positive cells of all materials.
Conclusion
Pure Mg and W4 were shown to be the most promising materials in this study in respect to the bone response to the implant material. They could be used for screws and plates in bone augmentation procedures.
Keywords: Biocompatibility, Biodegradability biomaterials, Magnesium
Introduction
Titanium screws are commonly used to provide rigid fixation for onlay grafting but have potential drawbacks including the need for a second surgery for removal before implant placement and screw fracture during removal [1]. These problems raised the need for temporary metal screws and plates that would resorb after the bone healing is complete. This would decrease the costs associated with repeated surgeries, minimize recovery times, decrease the risk of postoperative infections, and thus promote higher quality of life to each individual patient. The possible application areas of magnesium screws and plates could be among others fixation of traumatic orbital defects [2], treatment of zygomatic fractures [3], fixation of mandibular fractures [4], fixation in orthognathic and pediatric craniofacial surgeries [5], and fixation of calvarial bone grafts.
For metal alloy to be successfully used as a resorbable implant, several criteria must be met. It should provide enough strength to the healing tissues, it should resorb after a set time period, and it should be non-toxic and cause no harm to the organism.
Magnesium is considered a suitable material for biodegradable implants for a number of reasons. First of all, it is biocompatible [6]. By term biocompatible it is meant that material does not cause toxicological tissue reaction. Secondly, magnesium is natural for humans since our body contains about 25 g of this element and 50–60 % is found in bone [7]. Thirdly, magnesium seems to stimulate bone formation since magnesium ions enhance the cell attachment and proliferation [8]. New bone was seen forming in direct contact to magnesium’s degradation layer in previous studies [9, 10]. Last but not least, it has excellent mechanical properties, with values being closer to the healthy bone than titanium alloys [11].
However, an important problem of magnesium is a high corrosion rate with consistent hydrogen gas formation on contact with fluids [12]. In water, magnesium hydroxide accumulates on the surface of the magnesium implant to form a mildly protective corrosion layer, also known as a degradation layer. Although this film slows corrosion under aqueous conditions, it reacts with chlorine ions present in blood to produce a highly soluble MgCl2 and hydrogen gas [12]. Therefore, severe pitting corrosion can be observed on magnesium alloys where the chloride concentration of the body fluid is about 150 mM/L [9, 10]. Increase of the pH during this reaction further irritates tissues slowing the healing. This is a known challenge and several magnesium alloys were proposed and investigated, however, none of them was good enough for further development.
In general, adding extra elements to the alloy will strengthen material by forming inter-metallic phases. These inter-metallic phases act as obstacles for the dislocation movement [12]. Even ductility and corrosion properties might be improved [12, 13]. Alloying magnesium with yttrium could be an effective measure to improve the bio-corrosion properties of the magnesium alloy for biomedical application [14]. Yttrium belongs to the rare earth family and is known by its relatively low chemical toxicity and can be used in very small amounts without toxic effect on the organism [15].
Synthetic calcium phosphate materials like hydroxyapatite (HA) [Ca10(PO4)6(OH)2], are widely used today in orthopedics, dental and maxillofacial fields as bone substitutes and as coating material for metallic implants [16, 17]. This is because HAs have chemical composition close to bone mineral matrix which gives them excellent biocompatibility, osseointegration and osteoconductivity properties [16]. Coating the surface of the metallic implants with HA to enhance osseointegration is a common method. However, in case of degradable metals like magnesium, the quality of the HA coating is crucial since it determines the degradation rate of the underlying magnesium. If any defects in the coating are present, then magnesium is immediately exposed to the surrounding bone and starts degrading. This will result in uneven and unpredictable corrosion. Since the degradation of magnesium starts from the surface [17], it is more rational to combine magnesium with HA. This would make the degradation process more controllable.
For the above reasons, three types of biodegradable magnesium-based implants were chosen for this study: magnesium–hydroxyapatite (Mg–HA); an alloy containing 96 % magnesium and 4 % yttrium (W4), and 99.8 % pure magnesium (pure Mg).
The aim of the current observational study was to evaluate the histological appearance of magnesium-based implants and the bone response to these materials.
Materials and Methods
Production of Samples
Three types of magnesium-based implants were used in this study: (a) pure Mg (99.8 % of Mg by wt); (b) W4 alloy, VMWO 061 R116986; (c) Mg–HA (80 % W4, 20 % HA). The HA powder was produced by spray drying a HA slurry. Mg–HA samples were manufactured by high-energy milling of a mixture of HA and Mg granules and extruding the homogenate [18].
Pure Mg and W4 were cut from cast ingots and machined into rod samples with a dimension of 5.0 mm in length and 5.5 mm in diameter. Implants were ultrasonically cleaned and packed into airtight pouches. Gamma-sterilization was performed at the BBF Sterilisation service GmbH facility (Kernen, Germany) with a total dosage of 29 kGy.
Implantation
All animal experiments were conducted according to the European Commission Directive 86/609/EEC for animal experiments. Twenty-four female New Zealand white rabbits (Manfred Bauer, Neuenstein, Germany) were used. There were eight animals in each group. The pre-anesthetic procedure included an intramuscular administration of Ketamine (50 mg/kg) and Xylazine (1 mg/kg). General anesthesia was then achieved with intravenous administration of Ketamine (25 mg/kg) as well as Midazolam 0.5–1 mg/kg. Post-operative anesthesia was achieved with Bupreorphin 0.05 mg/kg.
An incision at the skin level was performed followed by a muscle layer and a periosteal incision. Next, a flap was reflected, and the bone exposed. The samples were implanted by lateral approach to the left distal femur condyle after predrilling with a 5.5 mm hand-operated diamond bone cutting system under constant irrigation with saline solution. Implantation was performed pressfit into the spongiosa. One magnesium-based implant was implanted per rabbit. The periosteum, muscle, and dermis layer were closed with 4–0 vicryl (Ethicon Johnson, Miami, FL) resorbable suture, using single interrupted nodes. The skin was sutured with 4–0 nylon (Ethicon Johnson, Miami, FL) sutures. After the surgical procedures, the animals were kept in their cages under controlled lighting and temperature.
After the operation, all the animals received 128 mg of Veracin as an antibiotic prophylaxis. Postoperatively, the rabbits were allowed to move freely in their cages without external support. Four rabbits per group were sacrificed randomly at 6 and 12 weeks post-operation, respectively.
Histological Preparations
The bone samples were embedded in paraffin and in methyl methacrylate based resin.
For the paraffin embedding, the specimens were first fixed in 4 % phosphate-buffered paraformaldehyde (Merck, Darmstadt, Germany), then decalcified with 10 % ethylenediaminetetraacetic acid (EDTA, pH 8.0; Sigma, Taufkirchen, Germany) in 3.5 M Tris buffer (pH 7.4; Sigma) for 21 days, dehydrated with graded ethanol concentrations, saturated in xylene, and finally embedded in paraffin. Sections of 3–5 μm thickness were cut with a rotator microtome (Leica, Bensheim, Germany), deparaffinized, and then stained with hematoxylin and eosin (HE) (Shandon Scientific Ltd, Cheshire, UK).
For the Technovit® 9100 new embedding, the samples were prepared according to the manufacturers protocol (Heraeus Kulzer, Hanau, Germany) and then grinded into 50 μm thick specimens using EXAKT-microgrinding system (400, CS). The grindings were stained with toluidine blue (TB) and tartrate-resistant acid phosphatase (TRAP). Briefly, the grindings were first declassified. For TB, they were etched in 20 % hydrogen peroxide for 40 min, stained with TB solution containing Sodium Tetraborate (Merck, Germany), Pyronin G (Merck, Germany) and TB (Chroma, Germany). The grindings were then let to dry for 24 h, washed in 100 % ethanol and xylene, and coverslipped with DePeX mounting medium (Serva Electrophoresis Life Science Products, Germany). For TRAP the sections were treated with 0.1 M Sodium Acetate buffer and incubated in Napthol-AS-TR phosphate (N6125-1G, Sigma, Germany) in N–N-Dimethyl formamide (Sigma Aldrich) and sodium tartrate (Merck, Germany) with Fast Red TR salt (Sigma Aldrich) at 37 °C for 60 min. Coverslipping was performed using DePeX.
Histomorphometric Analysis
Image capturing used Axioplan 2 Imaging system (Carl Zeiss, Germany) associated to a DC500 camera (Leica, Germany). Image evaluation was performed on Image-Pro® Plus (Weiss Imaging and Solutions GmbH, Germany) and Photoshop CS3 Extended® (Adobe, v.10.0.1, 2007, USA). The region of interest (ROI) was defined as 2 mm from the implant surface since we were mainly interested in implant stability in the surrounding bone and the reaction of the nearby tissues. Briefly, circles with diameter 7.5 mm were created in Photoshop CS3 Extended® and placed in the implant area. The implant was centered in the middle of the circle. All histomorphometric analysis was then limited to the ROI inside the circle. For measuring the amount of gas voids, corrosion layer and new bone, all areas containing these structures were first selected and then the total area calculated. For quantification of TRAP-positive cells, all cells were counted in the ROI and then the resultant number was divided by the circle area (mm2) to get the mean number of cells per mm2. In order to determine the implant-bone contact, the interface between material and trabeculae was measured in mm for each animal.
Transmission Electronic Microscope (TEM)
Examination under TEM was performed in order to study the cell morphology and bone response to magnesium materials. Sections were first fixed in 2 % phosphate-buffered paraformaldehyde with 2 % glutaraldehyde and 0.02 % picric acid, and later in 1 % osmium acid. After dehydration in ascending grades of ethanol, all samples were embedded in Epon (Serva, Heidelberg, Germany). Semithin sections of 0.5–1 µm were cut using an ultramicrotome (Reichert-Jung, Vienna, Austria), and then ultrathin sections of about 60–80 nm were prepared with the same machine. Examination was done with the transmission electron microscope LEO EM 912 (Zeiss, Oberkochen, Germany). Images were recorded with a 2 k × 2 k slow scan CCD camera (Troendle Restlichtverstärker-Systems, Moorenweis Germany).
Statistical Analysis
Data were analyzed using the Statistical Package for the Social Sciences, SPSS® (SPSS Inc. v18, Chicago, USA). The significance level was set at 5 %. ANOVA test was used to determine whether any significant differences in hydrogen gas production, bone contact, corrosion layer and implant resorption existed between the three groups. Bar graphs were plotted in Microsoft Excel® computer software (MS Excel 2003, Washington, USA).
Results
Bone Response
There was no significant difference between the pure Mg and W4 in the amount of new bone formation (p = 0.08), and these two materials showed similar appearance histologically (Fig. 1A–D). Both materials were often surrounded by host bone (Fig. 1B), with few areas with newly formed bone in direct contact to corrosion layer at 6 and 12 weeks (Fig. 1C, D). The least new bone was observed in Mg–HA group compared to all other materials both at 6 and 12 weeks (Fig. 1E, F). This difference was statistically significant (p = 0.03).
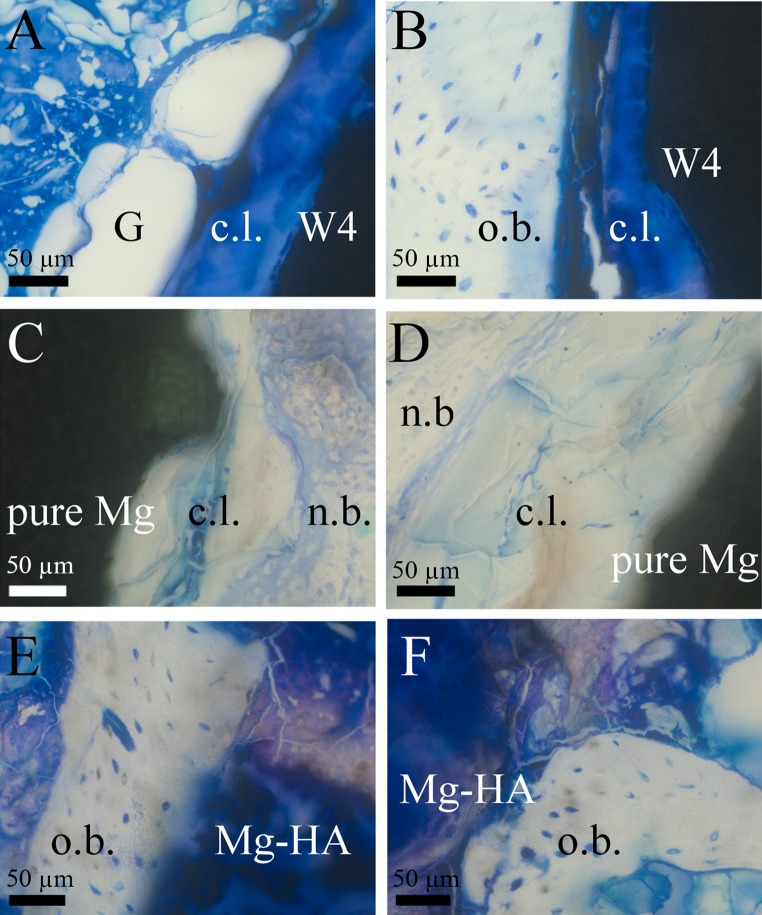
Fig. 1.
New bone formation patterns at implants’ surfaces. TB staining. A W4 at 6 weeks. Gas voids (G) and corrosion layer (c.l.) were observed on material’s surface. B W4 at 12 weeks. Bone-implant interface, corrosion layer (c.l.) formed next to host bone (o.b.). C Pure Mg at 6 weeks. New bone (n.b.) formed in direct contact to corrosion layer (c.l.). D Pure Mg at 12 weeks. New bone formed around corrosion layer (c.l.). Corrosion layer (c.l.) was thicker at 12 weeks than at 6 weeks. E Mg–HA at 6 weeks. The material was mostly surrounded by older bone (o.b.) and there were no signs of new bone formation. F Mg–HA at 12 weeks. Material had irregular shape and spread into the tissues. Still mostly old host bone (o.b.) was observed
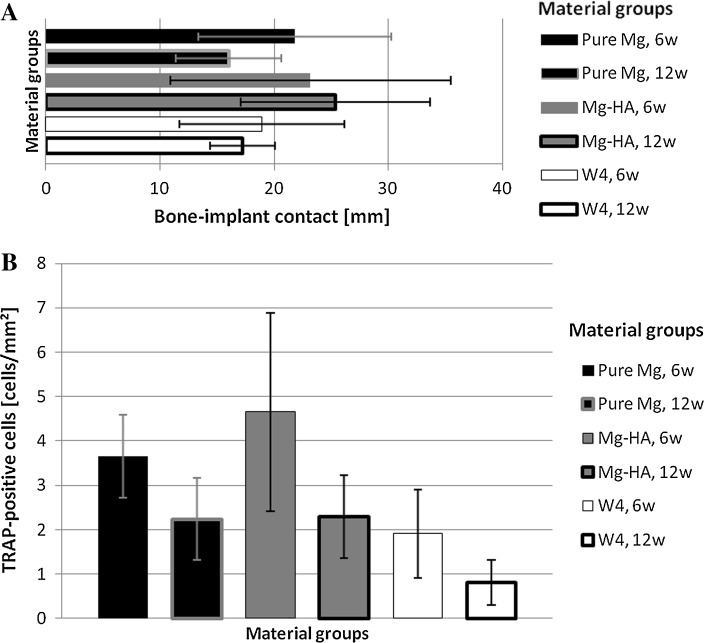
What was similar for all groups was that the clusters of new bone typically formed on the circumference of gas bubbles, encircling the gas (Fig. 2A, B). These newly formed bone clusters grew and appeared more matured and developed at 12 weeks (Fig. 2C, D). Mean bone contact to the implant surface was more extensive in Mg–HA group both at 6 and 12 weeks (Fig. 3A). Pure Mg and W4 had the lowest mean values for bone-implant contact (Fig. 3A).
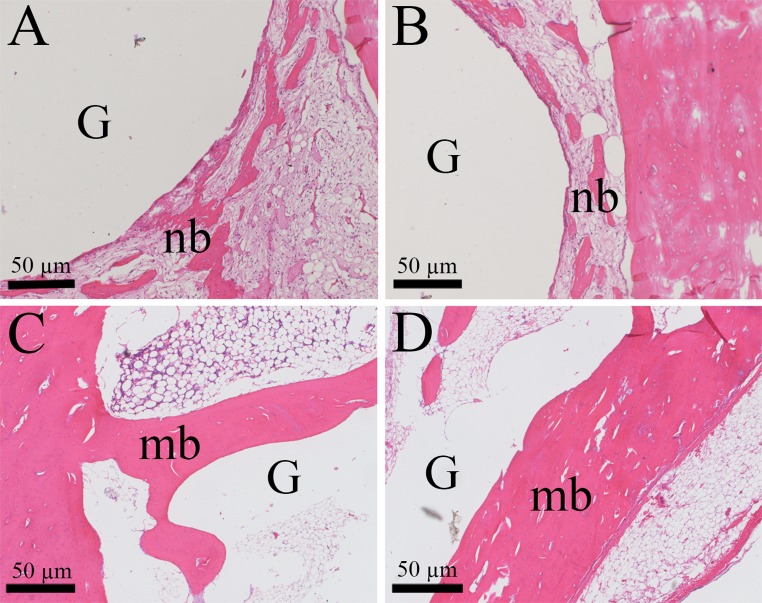
Fig. 2.
New bone formation patterns around gas voids. HE staining. A, B New bone (nb) clusters surrounding the gas void (G) at 6 weeks. C, D The bone grows stronger and matures (mb) at 12 weeks post-operation
Fig. 3.
Bar graphs showing quantitative data on bone response to tested materials at different time points. A Bone-to-implant contact (mm) at 6 and 12 weeks (6 w and 12 w respectively). B TRAP-positive cells (cells/mm2) around tested materials at 6w and 12 w
TRAP-positive multinucleated cells were found in all groups. The highest mean prevalence of TRAP-positive cells was seen in Mg–HA group at 6 weeks. There was no significant difference between the groups at 12 weeks post-operation, and the number of TRAP positive cells was similar in Mg–HA and pure Mg groups (Fig. 3B).
Gas Voids
In general, the gas voids’ diameter was biggest at some distance from the implants. The bubbles were smallest at material surface. Pure Mg and W4 were normally surrounded by huge bubbles up to 4 mm in diameter whereas Mg–HA implants were producing smaller gas volumes. The gas was spread in the bone marrow, not affecting trabecular or cortical bone. Least gas was seen in the bone surrounding Mg–HA biomaterial both at 6 and 12 weeks, whereas W4 produced the most gas of all groups (Fig. 4).
Fig. 4.
Graph showing the area of gas voids (mm2) around the tested materials at different time points
Implant Resorption Behaviour
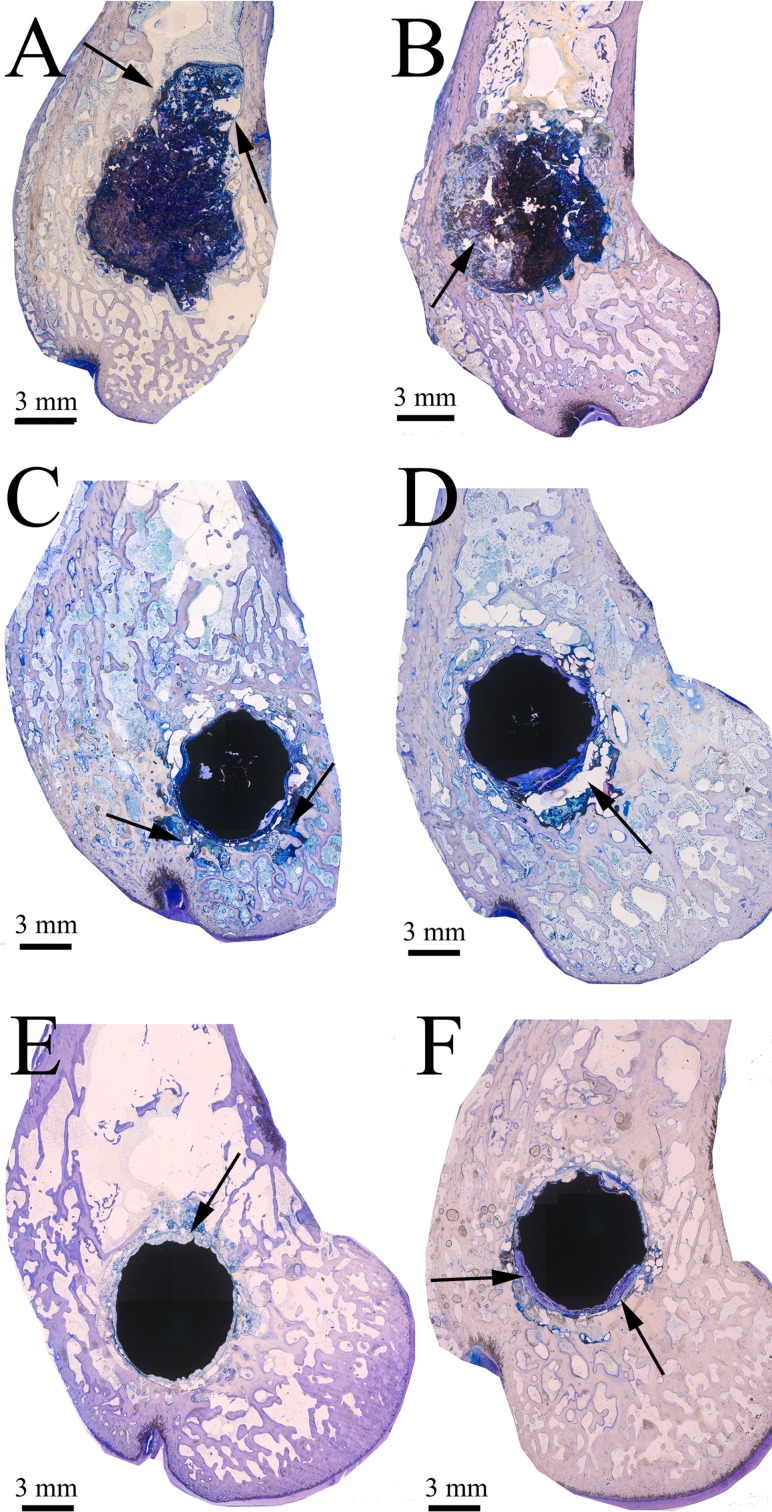
Mg–HA implants had the fastest corrosion rate of all evaluated materials (Fig. 5). Already at 6 weeks most implants lost their integrity, and degradation products together with the implant pieces were seen in the tissues. At 12 weeks the implants were even more degraded. Unlike Mg–HA, both pure Mg and W4 retained their round shape and density. Corrosion process was happening on their surface, whereas in Mg–HA corrosion was more uneven and uncontrollable.
Fig. 5.
Implants’ resorption behaviour at 6 and 12 weeks. TB staining. Arrows represent degradation products. A Mg–HA at 6 weeks. B Mg–HA at 12 weeks. C Pure Mg at 6 weeks. D Pure Mg at 12 weeks. E W4 at 6 weeks. F W4 at 12 weeks
TEM
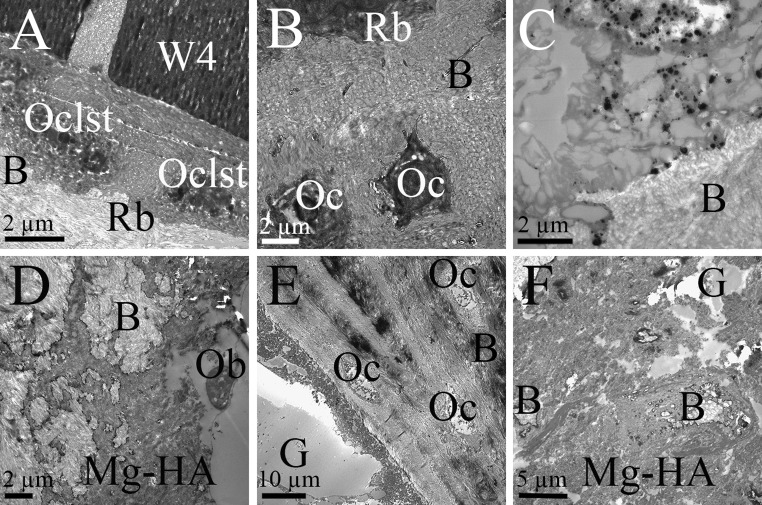
Bone remodeling was observed in direct proximity to implants’ surface. In W4 group osteoclasts were seen at implants interface (Fig. 6A). The signs of bone remodeling with typical rough borders at bone-implant interface were also seen at gas voids’ periphery in W4 (Fig. 6B). Osteocytes were evenly spread in the bone tissue (Fig. 6B). W4’s degradation particles which otherwise were not seen at light microscope magnifications were observed in the tissues at 12 weeks (Fig. 6C). Few osteoblasts were seen in Mg–HA group, and often these cells exhibited abnormal morphology (Fig. 6D). Unlike, other materials, bone remodeling around gas voids was not observed in Mg–HA (Fig. 6E). However, new bone-ingrowth into the material was found (Fig. 6F).
Fig. 6.
Images from TEM at 12 weeks. A Osteoclasts (Oclst) produced typical rough borders (Rb) on bone around W4. B Host bone rich in osteocytes (Oc) with typical appearance of rough borders (Rb) around gas voids (not shown in picture). C Degradation particles of W4 seen in the tissue. D Few osteoblasts (Ob) were seen in direct contact with Mg–HA, and their morphology was disturbed. Mg–HA’s degradation products covered host bone (B). E Gas (G) inside bone marrow in Mg–HA group. No active bone remodeling was observed. Numerous osteocytes (oc) in host bone. F View into Mg–HA material. Isles of newly formed bone (B) together with gas voids (G) were observed
Discussion
In this study three magnesium-based implants were compared qualitatively and quantitatively using such parameters as degradation behaviour, gas formation and bone response.
Formation of corrosion layer and implant resorption shows the rate of implant degradation. It is crucial that the implant starts resorbing after the bone has gained enough strength after the fracture. In this study, Mg–HA implants had the fastest corrosion rates. Already at 6 weeks these biomaterials lost their integrity, and degradation products together with the implant pieces spread in the tissue. At 12 weeks Mg–HA were even more disintegrated and spread outside the initial area of surgical placement. Hard-tissue repair typically requires implantation of the fixture for a minimum of 12 weeks [11]. Thus, Mg–HA tested in this study does not meet these criteria, most likely due to high concentration of HA reaching 20 % by wt. It was shown in one study that the corrosion rate of calcium-containing magnesium implants was highly dependent on the amount of calcium in the alloy: the higher the calcium concentration, the faster the degradation rate [19]. Based on these results it can be assumed that decreasing the amount of HA might improve resorption properties of Mg–HA. Unlike Mg–HA, pure Mg and W4 held their original round shape during the whole period of investigation. These two materials were covered by even corrosion layer, meaning that degradation was quite uniform. W4 had the most even degradation behaviour in this study. Material was covered by uniform corrosion layer and degradation products were rarely seen breaking off the surface unlike in the other implants. This means that the surface corrosion is more homogeneous and thus more predictable. This, of course, is more beneficial for the screws and plates that are to be used in bone augmentation procedures.
In this study we have observed an interesting pattern of new bone formation around magnesium-based implants. The bone formed not only around material itself, but also around the gas voids. Osteocytes, the principal sensors for mechanical loading of bone, regulate the onset of bone formation and resorption [20]. Moderate mechanical stress stimulates bone formation by osteoblasts, whereas high loads or no loads at all lead to bone resorption by osteoclasts [20]. It can be hypothesized that the gas produced by degradation process of magnesium acted as a moderate mechanical stress and stimulated osteocytic activation of bone remodeling process in this study. This could explain why the clusters of new bone were often seen forming around the gas voids.
When comparing the values for implant-bone contact (Fig. 3A) and the amount of gas (Fig. 4), a certain pattern between these two parameters could be observed: the less gas, the higher the implant-bone contact. Thus, for Mg–HA the highest mean bone-implant contact was most probably due to the lowest mean gas amounts compared to the other groups.
TRAP is an enzyme that is expressed in high amounts by osteoclasts, and even by inflammatory macrophages and dendritic cells [21]. TRAP-staining is widely used as a marker of osteoclasts. High amount of TRAP-positive cells, which in most cases are osteoclasts, is a first sign of bone remodeling since resorption by osteoclasts precedes bone formation by osteoblasts. Mg–HA group had the highest mean number of TRAP-positive cells at 6 weeks both surrounding the implant and in direct contact to material, compared to pure Mg and W4. This is probably because of fewer and smaller gas bubbles which, hypothetically, do not push out the cells from the implant resulting in higher cell counts in the ROI. Another explanation to such high numbers of the TRAP-positive cells could be the osteolysis caused by high degradation rate of Mg–HA and an increase in metal particulates in the bone. This immunological response can cause the loss of bone and lead to implant failure. Macrophages, fibroblasts, lymphocytes and osteoclasts are recruited to the implantation site and secrete proinflammatory and osteoclastogenic cytokines, exacerbating the inflammatory response. This chronic cell activation can break the balance between bone formation and bone resorption resulting in the osteolysis [22].
New bone formation in direct contact to the corrosion layer was shown in, among others, Mg–Mn–Zn and WE43 in earlier publications [9, 10]. Our results are hard to compare with previous research since slightly different alloy compositions and manufacture methods are used in various in vivo studies. However, also in this investigation it was observed that new bone can form directly on corrosion layer of pure Mg and W4. The least new bone formation was seen in Mg–HA. This material had the fastest degradation of all implants and thus its release of corrosion products was the highest. These products might have changed the internal environment and interfered with the normal tissue healing. This could also explain the abnormal appearance of osteoblasts coming in direct proximity to Mg–HA. As a result, new bone was not readily forming in Mg–HA group.
In previous research it was stated that magnesium might stimulate bone formation since magnesium ions enhance cell attachment and proliferation [8]. In our study, new bone was seen forming in direct contact to the materials, as well as around the gas voids. Thus, magnesium itself seems to be biocompatible. It is its product of degradation—gas—which causes mechanical barrier for the cells.
To conclude, pure Mg and W4 were shown to be the most promising materials in this study in respect to the bone response to the implant material. Mg–HA did not enhance the bone healing, had extremely fast degradation and high amount of TRAP-positive cells which might be the sign of the osteolysis. The limitation of this study is that it looks at histological appearance of magnesium materials and the surrounding bone; future research should look at mechanical performance of such materials in vivo. The possible applications of pure Mg and W4 could be the screws and plates for fixing minor bone fractures as well as for bone augmentation procedures in implant dentistry and maxillofacial fields in order to avoid the secondary surgery for screw removal.
Conclusion
Magnesium implants have huge potential for future medical applications in orthopedic, dental and maxillofacial fields. Pure Mg and W4 were shown to be the most promising materials in this histological study.
Acknowledgments
The authors would like to sincerely thank Ida Oberst, Iris Schütz and Rainer Braun from the Laboratory for Experimental Trauma Surgery, Justus-Liebig University Giessen, Germany, for their technical assistance.
Conflict of interests
The authors would like to declare no conflict of interests involved during conduction and preparation of this publication.
Footnotes
Olga Charyeva and Ulrich Thormann have contributed equally to the paper.
Contributor Information
Olga Charyeva, Email: olga.charyeva@gmail.com.
Katrin S. Lips, Phone: +49(0)6419433 117, Email: katrin.s.lips@chiru.med.uni-giessen.de
References
- 1.Quereshy FA, Dhaliwal HS, El SA, Horan MP, Dhaliwal SS. Resorbable screw fixation for cortical onlay bone grafting: a pilot study with preliminary results. J Oral Maxillofac Surg. 2010;68(10):2497–2502. doi: 10.1016/j.joms.2010.05.060. [DOI] [PubMed] [Google Scholar]
- 2.Iizuka T, Mikkonen P, Paukku P, Lindqvist C. Reconstruction of orbital floor with polydioxanone plate. Int J Oral Maxillofac Surg. 1991;20(2):83–87. doi: 10.1016/S0901-5027(05)80712-X. [DOI] [PubMed] [Google Scholar]
- 3.Bergsma EJ, Rozema FR, Bos RR, de Bruijn WC. Foreign body reactions to resorbable poly (l-lactide) bone plates and screws used for fixation of unstable zygomatic fractures. J Oral Maxillofac Surg. 1993;51(6):666–670. doi: 10.1016/S0278-2391(10)80267-8. [DOI] [PubMed] [Google Scholar]
- 4.Quereshy FA, Goldstein JA, Goldberg JS, Beg Z. The efficacy of bioresorbable fixation in the repair of mandibular fractures: an animal study. J Oral Maxillofac Surg. 2000;58(11):1263–1269. doi: 10.1053/joms.2000.16627. [DOI] [PubMed] [Google Scholar]
- 5.Edwards RC, Kiely KD, Eppley BL. Fixation of bimaxillary osteotomies with resorbable plates and screws: experience in 20 consecutive cases. J Oral Maxillofac Surg. 2001;59(3):271–276. doi: 10.1053/joms.2001.20988. [DOI] [PubMed] [Google Scholar]
- 6.Goyer RA, Clarkson TW. Toxicity of metals. In: Klaassen CD, editor. Casarett and Doull’s toxicology: the basic science of poisons. USA: McGraw-Hill Book Company Inc; 2001. pp. 811–868. [Google Scholar]
- 7.Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes (1997) Dietary reference intakes for calcium, phosphorous, magnesium, vitamin D, and fluoride. National Academy Press, Washington, DC, pp 20–30
- 8.Li Z, Gu X, Lou S, Zheng Y. The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials. 2008;29(10):1329–1344. doi: 10.1016/j.biomaterials.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Xu L, Yu G, Zhang E, Pan F, Yang K. In vivo corrosion behavior of Mg–Mn–Zn alloy for bone implant application. J Biomed Mater Res A. 2007;83(3):703–711. doi: 10.1002/jbm.a.31273. [DOI] [PubMed] [Google Scholar]
- 10.Witte F, Kaese V, Haferkamp H, Switzer E, Meyer-Lindenberg A, Wirth CJ, Windhagen H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials. 2005;26(17):3557–3563. doi: 10.1016/j.biomaterials.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 11.Staiger MP, Pietak AM, Huadmai J, Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27:1728–1734. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Witte F, Hort N, Vogt C, Cohen S, Kainer KU, Willumeit R, Feyerabend F. Degradable biomaterials based on magnesium corrosion. Curr Opin Solid State Mater Sci. 2008;12:63–72. doi: 10.1016/j.cossms.2009.04.001. [DOI] [Google Scholar]
- 13.Shaw BA. Corrosion resistance of magnesium alloys. In: Stephen D, editor. ASM handbook, vol 13a, Corrosion: fundamentals, testing and protection. UK: ASM International; 2003. pp. 692–696. [Google Scholar]
- 14.He W, Zhang E, Yang K. Effect of Y on the bio-corrosion behavior of extruded Mg–Zn–Mn alloy in Hank’s solution. Mater Sci Eng, C. 2010;3(1):167–174. doi: 10.1016/j.msec.2009.09.014. [DOI] [Google Scholar]
- 15.Reardon KA, McIntosh AF, Shilling AT, Hagspiel KD, Al-Osaimi A, Berg C, Caldwell SH, Northup PG, Angle F, Mulder R, Rich TA. Treatment of primary liver tumors with yttrium-90 microspheres (TheraSphere) in high risk patients: analysis of survival and toxicities. Technol Cancer Res Treat. 2009;8(1):71–77. doi: 10.1177/153303460900800109. [DOI] [PubMed] [Google Scholar]
- 16.Sartori M, Giavaresi G, Tschon M, Martini L, Dolcini L, Fiorini M, Pressato D, Fini M. Long-term in vivo experimental investigations on magnesium doped hydroxyapatite bone substitutes. J Mater Sci Mater Med. 2014;25(6):1495–1504. doi: 10.1007/s10856-014-5177-5. [DOI] [PubMed] [Google Scholar]
- 17.Ratna SB, Sampath KTS, Chakkingal U, Nandakumar V, Doble M. Nano-hydroxyapatite reinforced AZ31 magnesium alloy by friction stir processing: a solid state processing for biodegradable metal matrix composites. J Mater Sci Mater Med. 2014;25(4):975–988. doi: 10.1007/s10856-013-5127-7. [DOI] [PubMed] [Google Scholar]
- 18.Witte F, Feyerabend F, Maier P, Fischer J, Störmer M, Blawert C, Dietzel W, Hort N. Biodegradable magnesium–hydroxyapatite metal matrix composites. Biomaterials. 2007;28(13):2163–2174. doi: 10.1016/j.biomaterials.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Schaffler MB, Kennedy OD. Osteocyte signaling in bone. Curr Osteoporos Rep. 2012;10(2):118–125. doi: 10.1007/s11914-012-0105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frost HM. Wolff’s Law and bone’s structural adaptations to mechanical usage: an overview for clinicians. Angle Orthod. 1994;64(3):175–188. doi: 10.1043/0003-3219(1994)064<0175:WLABSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Halleen JM, Tiitinen SL, Ylipahkala H, Fagerlund KM, Väänänen HK. Tartrate-resistant acid phosphatase 5b (TRACP 5b) as a marker of bone resorption. Clin Lab. 2006;52(9–10):499–509. [PubMed] [Google Scholar]
- 22.Noordin Shahryar, Masri Bassam. Periprosthetic osteolysis: genetics, mechanisms and potential therapeutic interventions. Can J Surg. 2012;55(6):408–417. doi: 10.1503/cjs.003711. [DOI] [PMC free article] [PubMed] [Google Scholar]