Abstract
Purpose
Investigate the risk of ear-associated diseases after zygomaticomaxillary complex (ZMC) fracture in a population-based retrospective cohort study.
Materials and Methods
This is a retrospective cohort study using Taiwan’s National Health Insurance Research Database of reimbursement claims. A total of 1,330 ZMC fracture patients and 5,320 non-ZMC fracture participants were included and newly developed ear-associated disease data were collected. A Poisson regression and multivariate Cox proportion hazard regression were used for data analysis.
Results
The ZMC fracture cohort had a higher incidence of tinnitus than non-ZMC fracture cohort (IRR 1.64, 95 % CI 1.37–1.96), particularly in younger patients (≤34 years of age; IRR 4.05, 95 % CI 3.18–5.15) and male patients (IRR 2.08, 95 % CI 1.12–3.73). ZMC fracture patients also showed a significantly increased risk of having trigeminal neuralgia [IRR 4.06, 95 % CI 3.34–4.94, adjusted HR 4.07 (1.02–16.3)]. For sudden hearing loss and peripheral vertigo, the incidence densities were higher in the ZMC fracture cohort, but these relationships were not significant in the multivariate Cox proportional hazard regression analyses (HR 2.69, 95 % CI 0.76–9.53 for risk of sudden hearing loss; HR 1.36, 95 % CI 0.77–2.40 for risk of peripheral vertigo).
Conclusions
The findings of the study suggest an increased risk of ear-associated diseases among individuals with ZMC fractures, particularly within 2-years follow-ups after injury. We suggest performing detailed examinations for ear-associated diseases in patients with ZMC fractures for early diagnosis and adequate treatment.
Keywords: Ear-associated diseases, Zygomaticomaxillary complex fracture, Cohort study
Introduction
The zygoma and maxilla form the main structure of the middle third facial skeleton called the zygomaticomaxillary complex (ZMC). The ZMC consists of alternating thick and thin sections of bone that are capable of resisting substantial force. This structurally strong bone provides protection for the globes and brain, projection of the midface, and support for occlusion. However, the prominent convex shape of the ZMC makes it particularly vulnerable to injury [1].
The frequency of ZMC fractures is second only to nasal fractures, which are the most common type of facial fracture [2]. Assault is assumed to be the main etiology other than motor vehicle crashes in developed countries, whereas road traffic accidents are the most frequent cause in developing regions [3, 4].
ZMC fractures refer to the osseous disruption of the malar eminence at 4 buttresses: the zygomaticomaxillary, frontozygomatic, zygomaticosphenoid, and zygomaticotemporal buttresses. Because of its unique anatomical structure, even minimally displaced fractures in this region can result in both functional and aesthetic deformities. ZMC-fractured patients may have various presentations, such as soft tissue swelling, edema, ecchymosis, cheek numbness, trismus, diplopia, and epistaxis, depending on the associated injuries. In addition to these, ear-associated problems such as sudden hearing loss [5–7], tinnitus [8, 9], trigeminal neuralgia [10, 11], and vertigo [12, 13] have also been reported.
The objective of this study was to investigate whether ZMC fracture is associated with an increased risk and incidence of ear-associated disease by using the National Health Insurance Research Database (NHIRD) in Taiwan.
Methods
Data Sources
The data were retrieved from Taiwan’s NHIRD, which is managed by Taiwan’s National Health Research Institutes (NHRI). In 1995, the Taiwanese government commenced a state-run National Health Insurance (NHI) program, which registers all medical claims and provides affordable health care for all residents. The NHI program covers more than 99 % of the Taiwanese population and has contracts with 97 % of the island’s hospitals and clinics [14].
The data sets available from the NHIRD encompass all medical services availed by each insurant from 1996 to 2010, as well as the characteristics of the patients, hospitals, and physicians. In this study, we analyzed the claims of 1 million beneficiaries randomly selected from all the enrollees in 2000, with age and sex distributions nearly identical to that of the entire insured population. The database contained patient information such as sex, date of birth, admission and discharge dates, diagnoses, operation procedures performed, discharge status, and expenditures. The diagnoses were coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).
Ethical Considerations
The NHRID encrypts the patients’ personal information for privacy protection and provides researchers with anonymous identification numbers associated with the relevant claim information, which includes the patient’s sex, date of birth, registry of medical services, and medication prescriptions. Patient consent is not required for accessing the NHIRD. This study was approved by the Institutional Review Board of China Medical University (CMU-REC-101-012). Our IRB specifically waived the requirement for consent.
Study Population
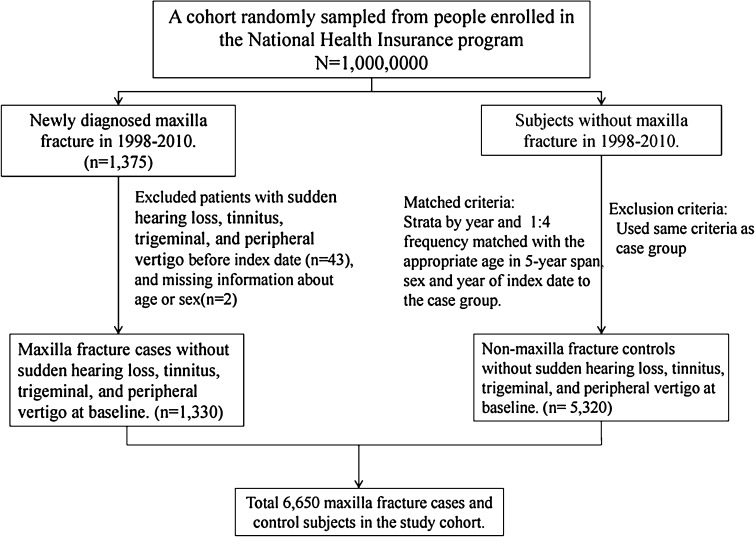
Patients with newly diagnosed ZMC fractures (ICD-9-CM 802.4 and 802.5) from 1998 to 2010 were identified in the database. The first diagnosis date for ZMC fracture was used as the index date. Patients were excluded if they had a history of sudden hearing loss (ICD-9-CM 388.2), tinnitus (ICD-9-CM 388.3), trigeminal neuralgia (ICD-9-CM 350.1), or peripheral vertigo (ICD-9-CM 350.1) before the index date. A total of 1,330 patients were extracted for the study, defined as the case cohort. Excluding the case cohort in the entire insured population, we randomly selected the non-ZMC fracture cohort who were frequency matched by sex, age (5 years), and index-year to the case cohort (n = 5,320) at a ratio of 1:4 (Fig. 1). The same exclusion criteria for case cohort were carried out in the non-ZMC fracture cohort.
Fig. 1.
The process for identifying cases relevant to the cohort study
Outcome Measurement and Covariates
We identified the first diagnosis of sudden hearing loss, tinnitus, trigeminal neuralgia, or peripheral vertigo from the database as the study endpoint. Each patient was followed from the index date to either the end point, withdrawal from the database, or December 31, 2010, whichever came first.
At baseline, major comorbidities affecting the risk of outcomes, such as otitis media (ICD-9-CM 382) or otalgia (ICD-9-CM 388.7), were coded. Selected comorbidities were determined based on diagnostic codes in ambulatory visits or hospitalization databases before the index date.
Statistical Analysis
The distributions of baseline characteristics (sex, age, and comorbidities) were compared between both cohorts by using the Chi squared test or Student’s t test. The incidence rates and incidence rate ratios (IRR) stratified by baseline characteristics were determined under Poisson assumption. The multivariate Cox proportion hazard regression model was used to examine the effect of ZMC fractures on the risk of outcomes, as shown using hazard ratios (HRs) with 95 % confidence intervals (CIs). A 2-tailed p value of <.05 was considered statistically significant. All statistical analyses were performed using the SAS statistical software. (version 9.2 for Windows; SAS Institute, Inc., Cary, NC, USA).
Results
The distributions of the baseline demographic and comorbidity data of the patients in the 2 cohorts are presented in Table 1. The mean (standard deviation; SD) age of the patients in the ZMC fracture and non-ZMC fracture cohorts was 37.9 (17.3) years. In the ZMC fracture cohort, 50.5 % were younger than 34 years of age and 67.5 % of them were male patients. No significant difference in age (p = .99) and sex (p = .99) was determined. The ZMC fracture cohort was more likely to have otitis media (2.86 %, p = .003).
Table 1.
Demographic characteristics and comorbidity in patients with and without ZMC fracture
| ZMC fracture | p value | ||
|---|---|---|---|
| No (n = 5,320) | Yes (n = 1,330) | ||
| Age [mean (SD)] | 37.9 (17.3) | 37.9 (17.3) | 0.96# |
| Stratify age | |||
| ≤34 | 2,684 (50.5) | 671 (50.5) | 0.99 |
| 35–49 | 1,344 (25.3) | 336 (25.3) | |
| 50–64 | 856 (16.1) | 214 (16.1) | |
| 65+ | 436 (8.20) | 109 (8.20) | |
| Gender | |||
| Women | 1,728 (32.5) | 432 (32.5) | 0.99 |
| Men | 3,592 (67.5) | 898 (67.5) | |
| Comorbidity | |||
| Otitis media | 86 (1.62) | 38 (2.86) | 0.003 |
| Otalgia | 4 (0.08) | 0 (0.00) | 0.59‡ |
Chi square test; # t test; ‡ Fisher’s exact test
In estimating tinnitus risk, a higher incidence rate was found in the ZMC fracture cohort (33.0 per 10,000 person-years) than in the non-ZMC fracture cohort (20.14 per 10,000 person-years; IRR 1.64, 95 % CI 1.37–1.96), and the rate was much higher in younger patients (≤34 years; IRR 4.05, 95 % CI 3.18–5.15) and in male patients (IRR 2.08, 95 % CI 1.12–3.73) (Table 2). The multivariate Cox proportional hazard regression analysis revealed that the risk of developing tinnitus among ZMC fracture patients younger than 34 years of age was significantly higher than that of the patients in the non-ZMC fracture cohort (HR 4.01, 95 % CI 1.67–9.64).
Table 2.
Incidence, incidence rate ratio and hazard ratio of outcome compared between with and without ZMC fracture by demographic characteristics
| Outcome | ZMC fracture | Compared to non-ZMC fracture | ||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| Event | PY | Rate# | Event | PY | Rate# | IRR† (95 % CI) | Adjusted HR‡ (95 % CI) | |
| Sudden hearing loss | 6 | 27,491 | 2.18 | 4 | 6,762 | 5.92 | 2.71 (2.23, 3.28)*** | 2.69 (0.76, 9.53) |
| Stratify age | ||||||||
| ≤34 | 2 | 14,622 | 1.37 | 1 | 3,657 | 2.73 | 2.00 (1.51, 2.65)*** | 1.98 (0.18, 21.8) |
| >34 | 4 | 12,869 | 3.11 | 3 | 3,106 | 9.66 | 3.11 (2.38, 4.05)*** | 3.10 (0.69, 13.9) |
| Gender | ||||||||
| Women | 1 | 9,184 | 1.09 | 2 | 2,303 | 8.68 | 7.97 (5.69, 11.2)*** | 8.02 (0.73, 88.5) |
| Men | 5 | 18,308 | 2.73 | 2 | 4,459 | 4.49 | 1.64 (1.27, 2.12)*** | 1.64 (0.32, 8.44) |
| Tinnitus | 55 | 27,313 | 20.14 | 22 | 6,660 | 33.0 | 1.64 (1.37, 1.96)*** | 1.62 (0.99, 2.65) |
| Stratify age | ||||||||
| ≤34 | 10 | 14,587 | 6.86 | 10 | 3,062 | 32.7 | 4.05 (3.18, 5.15)*** | 4.01 (1.67, 9.64)** |
| >34 | 45 | 12,726 | 35.4 | 12 | 3,058 | 39.2 | 1.11 (0.85, 1.45) | 1.10 (0.58, 2.07) |
| Gender | ||||||||
| Women | 23 | 9,094 | 25.3 | 6 | 2,274 | 26.4 | 1.04 (0.75, 1.46) | 1.04 (0.42, 2.55) |
| Men | 32 | 18,219 | 17.6 | 16 | 4,386 | 36.5 | 2.08 (1.68, 2.56)*** | 2.05 (1.12, 3.73)* |
| Trigeminal neuralgia | 4 | 27,496 | 1.45 | 4 | 6,769 | 5.91 | 4.06 (3.34, 4.94)*** | 4.07 (1.02, 16.3)* |
| Stratify age | ||||||||
| ≤34 | 0 | 14,623 | 0.00 | 1 | 3,661 | 2.73 | – | – |
| >34 | 4 | 12,874 | 3.11 | 3 | 3,108 | 9.65 | 3.11 (2.38, 4.05)*** | 3.08 (0.69, 13.8) |
| Gender | ||||||||
| Women | 3 | 9,172 | 3.27 | 2 | 2,306 | 8.67 | 2.65 (1.90, 3.71)*** | 2.66 (0.44, 15.9) |
| Men | 1 | 18,324 | 0.55 | 2 | 4,463 | 4.48 | 8.21 (6.34, 10.6)*** | 8.19 (0.74, 90.3) |
| Peripheral vertigo | 47 | 27,287 | 17.2 | 16 | 6,719 | 23.8 | 1.38 (1.15, 1.67)*** | 1.36 (0.77, 2.40) |
| Stratify age | ||||||||
| ≤34 | 10 | 14,570 | 6.86 | 3 | 3,646 | 8.23 | 1.20 (0.90, 1.60) | 1.21 (0.33, 4.40) |
| >34 | 37 | 12,717 | 29.1 | 13 | 3,074 | 42.3 | 1.45 (1.13, 1.87)** | 1.41 (0.75, 2.66) |
| Gender | ||||||||
| Women | 28 | 9,037 | 31.0 | 12 | 2,260 | 53.1 | 1.71 (1.28, 2.30)*** | 1.70 (0.86, 3.34) |
| Men | 19 | 18,250 | 10.4 | 4 | 4,459 | 8.97 | 0.86 (0.65, 1.14) | 0.86 (0.29, 2.52) |
* p < 0.05; ** p < 0.01; *** p < 0.001
#Rate, incidence rate, per 10,000 person-years
†IRR, incidence rate ratio, per 10,000 person-years
‡Adjusted HR, model adjusted for age, sex and otitis media
In estimating trigeminal neuralgia risk, the overall incidence rate for the ZMC fracture cohort was 5.91 per 10,000 person-years. Compared with the patients in the non-ZMC fracture cohort, the ZMC fracture patients showed a significantly increased risk of trigeminal neuralgia with an IRR of 4.06 (95 % CI 3.34–4.94) and an adjusted HR of 4.07 (1.02–16.3). However, no significant associations were observed between ZMC fracture and the risk of trigeminal neuralgia in the age- and sex-stratified analysis (Table 2).
In estimating risks for sudden hearing loss and peripheral vertigo, the incidence densities were higher in the ZMC fracture cohort than in the non-ZMC fracture cohort (5.92 vs. 2.18 per 10,000 person-years, IRR 2.71 for the risk of sudden hearing loss; 23.8 vs. 17.2 per 10,000 person-years, IRR 1.38 for the risk of peripheral vertigo). However, these relationships were not significant when the covariates in the multivariate Cox proportional hazard regression analyses were adjusted (HR 2.69, 95 % CI 0.76–9.53 for the risk of sudden hearing loss; HR 1.36, 95 % CI 0.77–2.40 for risk of peripheral vertigo) (Table 2).
Furthermore, the incidence densities and HRs of each outcome between the 2 cohorts were compared by studying the follow-up duration. We observed that when the follow-up duration was <2 years, the risks of sudden hearing loss (HR 6.09, 95 % CI 1.02–36.4), tinnitus (HR 3.40, 95 % CI 1.62–7.16), and trigeminal neuralgia (HR 6.03, 95 % CI 1.01–36.1) were significantly higher in the ZMC fracture cohort than in the non-ZMC fracture cohort. By contrast, these relationships were not associated with a significantly increased or decreased outcome when the follow-up duration was more than 2 years (Table 3).
Table 3.
Hazard ratio for outcome compared between with and without ZMC fracture by follow-up duration
| Follow time | ZMC fracture | Compared to non-ZMC fracture | ||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| Event | PY | Rate# | Event | PY | Rate# | IRR† (95 % CI) | Adjusted HR‡ (95 % CI) | |
| Sudden hearing loss | ||||||||
| ≤2 | 2 | 9,245 | 2.16 | 3 | 2,305 | 13.0 | 6.02 (4.95, 7.31)*** | 6.09 (1.02, 36.4)* |
| >2 | 4 | 18,246 | 2.19 | 1 | 4,457 | 2.24 | 1.02 (0.78, 1.34) | 1.02 (0.11, 9.11) |
| Tinnitus | ||||||||
| ≤2 | 15 | 9,231 | 16.3 | 13 | 2,290 | 56.8 | 3.49 (2.92, 4.18)*** | 3.40 (1.62, 7.16)** |
| >2 | 40 | 18,082 | 22.1 | 9 | 4,370 | 20.6 | 0.93 (0.73, 1.18) | 0.92 (0.45, 1.90) |
| Trigeminal neuralgia | ||||||||
| ≤2 | 2 | 9,246 | 2.16 | 3 | 2,302 | 13.0 | 6.02 (1.92, 7.38)*** | 6.03 (1.01, 36.1)* |
| >2 | 2 | 18,250 | 1.10 | 1 | 4,467 | 2.24 | 2.04 (1.62, 2.57)*** | 2.09 (0.19, 23.0) |
| Peripheral vertigo | ||||||||
| ≤2 | 25 | 9,225 | 27.1 | 6 | 2,301 | 26.1 | 0.96 (0.77, 1.20) | 0.95 (0.39, 2.33) |
| >2 | 22 | 18,062 | 12.2 | 10 | 4,419 | 22.6 | 1.86 (1.51, 2.28)*** | 1.84 (0.87, 3.88) |
* p < 0.05; ** p < 0.01; *** p < 0.001
#Rate, incidence rate, per 10,000 person-years
†IRR, incidence rate ratio, per 10,000 person-years
‡Adjusted HR, model adjusted for age, sex and otitis media
Discussion
Tinnitus
Tinnitus is the perception of sound in the absence of external acoustic stimulation and is a common disorder with a prevalence ranging from 10 to 15 %. Trauma to the head and neck region, ototoxic drugs, and even psychological stress have been reported to cause tinnitus [15, 16]. Vernon and Press reported that 8 % of patients with head injuries developed tinnitus [16]. Folmer and Greist considered tinnitus a critical symptom, and if it results from head or neck injury, it tends to be more severe than tinnitus resulting from other causes [8]. The pathophysiology might damage the auditory pathway or nonauditory pathway, resulting in an increased spontaneous firing rate or increased neuronal activity [17–20].
ZMC fractures typically result from a strong impact to the midface area and associated injuries to nearby structures, such as the skull base, external ear canal, eardrum, cochlea, and even the cranial nerve or the brain. This might explain why there is an increased tinnitus incidence in patients after a ZMC fracture. However, cases of ZMC fracture must be examined individually because every trauma mechanism is unique.
Trigeminal Neuralgia
Trigeminal neuralgia, also called tic douloureux, causes extreme, sporadic, sudden burning, or shock-like face pain that lasts from a few seconds to several minutes; it can be physically and mentally incapacitating. The onset is typically in middle or old age, but young adults and children can also be affected [21, 22].
Trigeminal neuralgia is typically caused by the demyelination of trigeminal sensory fibers either in an intracranial part, such as the brainstem, root, ganglion, and divisions, or in the extracranial part, such as the bone canal level or terminal branches. The causes of demyelination could be trauma force or external compression caused by either vessels or various types of tumor. Systemic diseases, such as multiple sclerosis [23–25], Charcot-Marie-Tooth disease [26], and amyloidosis [27, 28] could lead to trigeminal neuralgia. Other cases without exact pathophysiologies are referred to as idiopathic.
In this study, ZMC fracture increased the incidence of trigeminal neuralgia. Direct trauma to nerves, bone fragment compression, hematoma, or even posttraumatic tissue swelling and edema could have been the cause of trigeminal neuralgia. Meticulous physical examination, thorough history taking, and advanced image study could help physicians make accurate diagnoses and recommend timely treatment to patients.
Sudden Hearing Loss
Hearing loss after head trauma is a well-known phenomenon, particularly in patients with temporal bone fractures [29]. Even after minor head traumas, patients have a relatively high risk for hearing impairment immediately after sustaining the injury and during the early recovery phases. Both conductive and sensorineural hearing loss have been demonstrated in cases of closed head injuries with or without bone fracture [30]. Ipsilateral sensorineural hearing loss is commonly associated with transverse type temporal bone fractures, and labyrinthine concussions are believed to be the mechanism of contralateral profound hearing loss [5, 31]. Research regarding the association between ZMC fractures and sudden hearing loss is limited; therefore, the etiology could still be attributed to trauma, sensorineural injury, or both. Otologic and neuro-otologic examination, audiologic testing, and image studies should be performed on patients with ZMC fractures if hearing loss is present in the early posttraumatic period. In our study, the incidence density was higher in the ZMC fracture cohort than in the non-ZMC fracture cohort, but the relationship was not significant when the covariates in the multivariate Cox proportional hazard regression analyses were adjusted. Thus, the relationship between ZMC fracture and hearing loss requires further investigation.
Vertigo
Vertigo is classified as either peripheral or central depending on the location of the dysfunction of the vestibular pathway; it can also be caused by psychological factors [32]. Posttraumatic vertigo was reported as a major symptom in head injury patients, and its incidence ranged from 34 to 50 % [13].
Trauma of the head, neck, and craniocervical junction can have a major impact on the brainstem, the cerebellum or the vestibular system at various sites, such as the semicircular canals, the vestibule, or the vestibular nerve, and thereby induce vertigo [33]. If vertigo is induced by posttraumatic perilymphatic fistula, surgery can be performed to correct the symptoms [34, 35].
In ZMC fracture patients, the impact force is typically large and associated injuries are common. In this cohort study, the HRs for peripheral vertigo between the ZMC and non-ZMC fracture patients were not significantly increased. However, the IRRs in patients older than 34 years and in female patients are increased. Patients should be examined carefully and repeated physical examinations are necessary. High resolution CT imaging or other imaging tools such as MRI can be used to obtain detailed information concerning associated injuries. Electronystagmography, the Dix-Hallpike maneuver, rotation tests, the head-thrust test, the caloric reflex test, and computerized dynamic posturography could be used to help physicians make accurate diagnoses.
Study Limitations
The strengths of this study included its use of population-based data that are highly representative of the general population. However, certain limitations of our findings should be considered. First, the NHIRD does not contain detailed information regarding smoking habits and alcohol consumption, which may be potential comorbidities or risk factors for ZMC fractures or ear disorders. Second, the evidence derived from a retrospective cohort study is generally lower in statistical quality than that from randomized trials because of potential biases related to adjustments for confounding variables. Despite our meticulous study design and the control measures taken for confounding factors, bias resulting from unknown confounders might have affected the results. Third, all data in the NHIRD are anonymous. Thus, relevant clinical variables such as imaging results, pathology findings, and serum laboratory data were unavailable. However, the data regarding the diagnoses for ZMC fractures or ear disorders were reliable.
Conclusions
This cohort study suggests an increased risk of ear-associated disease (such as sudden hearing loss, tinnitus, trigeminal neuralgia, and peripheral vertigo) among individuals with ZMC fractures, particularly within the first 2 years after injury. Because the ZMC has a unique structure and location, impacts resulting in ZMC fractures could also induce associated injuries to surrounding structures. These could possibly be the cause of ear-associated diseases. We suggest performing detailed examinations on every patient with ZMC fractures for early diagnosis and adequate treatment.
Acknowledgments
This study was supported in part by the study projects (DMR-103-012 and DMR-103-018) in China Medical University Hospital, Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW103-TDU-B-212-113002), and health and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TD-B-111-03, Taiwan) and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
All authors declare that they have no conflicts of interest. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
Footnotes
Chao-Chih Yang and Chih-Jaan Tai have contributed equally to this work.
References
- 1.Strong EB, Sykes JM. Zygoma complex fractures. Facial Plast Surg. 1998;14:105–115. doi: 10.1055/s-0028-1085306. [DOI] [PubMed] [Google Scholar]
- 2.Donald PJ (1976) Zygomatic fractures. In: English GM (ed) Otolaryngology: a text book. Medical Department, Harper & Row, Hagerstown, MD
- 3.Bouguila J, Zairi I, Khonsari RH, Hellali M, Mehri I, Landolsi A, et al. Fractured zygoma: a review of 356 cases. Ann Chir Plast Esthet. 2008;53:495–503. doi: 10.1016/j.anplas.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Obuekwe O, Owotade F, Osaiyuwu O. Etiology and pattern of zygomatic complex fractures: a retrospective study. J Natl Med Assoc. 2005;97:992–996. [PMC free article] [PubMed] [Google Scholar]
- 5.Sakallıoğlu Ö, Polat C, Soylu E, Orhan İ, Altınsoy HB. Contralateral profound hearing loss after head trauma: a case report. Turk Arch Otolaryngol. 2011;49:58–60. doi: 10.5152/tao.2011.16. [DOI] [Google Scholar]
- 6.Bergemalm PO. Progressive hearing loss after closed head injury: a predictable outcome. Acta Otolaryngol. 2003;123:836–845. doi: 10.1080/00016480310002474. [DOI] [PubMed] [Google Scholar]
- 7.Chau JK, Lin JR, Atashband S, Irvine RA, Westerberg BD. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope. 2010;120:1011–1021. doi: 10.1002/lary.20784. [DOI] [PubMed] [Google Scholar]
- 8.Folmer RL, Griest SE. Chronic tinnitus resulting from head or neck injuries. Laryngoscope. 2003;113:821–827. doi: 10.1097/00005537-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Ceranic BJ, Prasher DK, Raglan E, Luxon LM. Tinnitus after head injury: evidence from otoacoustic emissions. J Neurol Neurosurg Psychiatry. 1998;65:523–529. doi: 10.1136/jnnp.65.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fried K, Bongenhielm U, Boissonade FM, Robinson PP. Nerve injury-induced pain in the trigeminal system. Neuroscientist. 2001;7:155–165. doi: 10.1177/107385840100700210. [DOI] [PubMed] [Google Scholar]
- 11.Becker M, Kohler R, Vargas MI, Viallon M, Delavelle J. Pathology of the trigeminal nerve. Neuroimaging Clin N Am. 2008;18:283–307. doi: 10.1016/j.nic.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Schuknecht HF, Davison RC. Deafness and vertigo from head injury. AMA Arch Otolaryngol. 1956;63:513–528. doi: 10.1001/archotol.1956.03830110055006. [DOI] [PubMed] [Google Scholar]
- 13.Berman JM, Fredrickson JM. Vertigo after head injury: a five year follow-up. J Otolaryngol. 1978;7:237–245. [PubMed] [Google Scholar]
- 14.Cheng TM. Taiwan’s National Health Insurance system: high value for the dollar. In: Okma KGH, Crivelli L, editors. Six countries, six reform models: the health reform experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland and Taiwan. New Jersey: World Scientific; 2009. pp. 71–204. [Google Scholar]
- 15.Kreuzer PM, Landgrebe M, Vielsmeier V, Kleinjung T, De Ridder D, Langguth B (2014) Trauma-associated tinnitus. J Head Trauma Rehabil 29:432–442 [DOI] [PubMed]
- 16.Vernon JA, Press LS. Characteristics of tinnitus induced by head injury. Arch Otolaryngol Head Neck Surg. 1994;120:547–551. doi: 10.1001/archotol.1994.01880290057010. [DOI] [PubMed] [Google Scholar]
- 17.Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Kaltenbach JA. Tinnitus: models and mechanisms. Hear Res. 2011;276:52–60. doi: 10.1016/j.heares.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010;30:14972–14979. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shore SE. Plasticity of somatosensory inputs to the cochlear nucleus: implications for tinnitus. Hear Res. 2011;281:38–46. doi: 10.1016/j.heares.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manson WE, Kollros P, Jannetta PJ. Trigeminal neuralgia and its treatment in a 13-month-old child: a review and case report. J Craniomandib Disord. 1991;5:213–216. [PubMed] [Google Scholar]
- 22.Resnick DK, Levy EI, Jannetta PJ. Microvascular decompression for pediatric onset trigeminal neuralgia. Neurosurgery. 1998;43:804–807. doi: 10.1097/00006123-199810000-00047. [DOI] [PubMed] [Google Scholar]
- 23.Jensen TS, Rasmussen P, Reske-Nielsen E. Association of trigeminal neuralgia with multiple sclerosis: clinical and pathological features. Acta Neurol Scand. 1982;65:182–189. doi: 10.1111/j.1600-0404.1982.tb03076.x. [DOI] [PubMed] [Google Scholar]
- 24.Moulin DE, Foley KM, Ebers GC. Pain syndromes in multiple sclerosis. Neurology. 1988;38:1830–1834. doi: 10.1212/WNL.38.12.1830. [DOI] [PubMed] [Google Scholar]
- 25.Soustiel JF, Hafner H, Chistyakov AV, Yamitzky D, Sharf B, Guilburd JN, et al. Brain-stem trigeminal and auditory evoked potentials in multiple sclerosis: physiological insights. Electroencephalogr Clin Neurophysiol. 1996;100:152–157. doi: 10.1016/0013-4694(95)00172-7. [DOI] [PubMed] [Google Scholar]
- 26.Coffey RJ, Fromm GH. Familial trigeminal neuralgia and Charcot-Marie-Tooth neuropathy. Report of two families and review (Review) Surg Neurol. 1991;35:49–53. doi: 10.1016/0090-3019(91)90202-K. [DOI] [PubMed] [Google Scholar]
- 27.Bornemann A, Bohl J, Hey O, Storkel S, Gamm H, Muller-Forell W, et al. Amyloidoma of the gasserian ganglion as a cause of symptomatic neuralgia of the trigeminal nerve: report of three cases (Review) J Neurol. 1993;241:10–14. doi: 10.1007/BF00870665. [DOI] [PubMed] [Google Scholar]
- 28.Love S, Batemann DE, Hirschowitz L. Bilateral lambda light chain amyloidomas of the trigeminal ganglion, nerve and roots. Neuropathol Appl Neurobiol. 1997;23:512–515. doi: 10.1111/j.1365-2990.1997.tb01329.x. [DOI] [PubMed] [Google Scholar]
- 29.Lyos AT, Marsh MA, Jenkins HA, Coker NJ. Progressive hearing loss after transverse temporal bone fracture. Arch Otolaryngol Head Neck Surg. 1995;121:795–799. doi: 10.1001/archotol.1995.01890070081017. [DOI] [PubMed] [Google Scholar]
- 30.Bergemalm PO, Borg E. Long-term objective and subjective audiologic consequences of closed head injury. Acta Otolaryngol. 2001;121:724–734. doi: 10.1080/00016480152583674. [DOI] [PubMed] [Google Scholar]
- 31.Khairi M, Irfan M, Rosdan S. Traumatic head injury with contralateral sensorineural hearing loss. Ann Acad Med Singapore. 2009;38:1017–1018. [PubMed] [Google Scholar]
- 32.Reeves AG, Swenson RS (eds) (2008) Chapter 14: evaluation of the dizzy patient. In: Disorders of the nervous system: a primer. Dartmouth Medical School. http://www.dartmouth.edu/~dons/index.html
- 33.Ernst A, Basta D, Seidl RO, Todt I, Scherer H, Clarke A. Management of posttraumatic vertigo. Otolaryngol Head Neck Surg. 2005;132:554–558. doi: 10.1016/j.otohns.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 34.Lehrer JF, Rubin RC, Poole DC, Hubbard JH, Wille R, Jacobs GB. Perilymphatic fistula: a definitive and curable cause of vertigo following head trauma. West J Med. 1984;141:57–60. [PMC free article] [PubMed] [Google Scholar]
- 35.Maitland CG (2001) Perilymphatic fistula. Curr Neurol Neurosci Rep 1:486–491 [DOI] [PubMed]