Abstract
Introduction
The objective of this study was to assess the bone repair process of crystallized Biosilicate in surgically created defects on rats’ calvaria. This biomaterial was recently developed for odontological use.
Materials and methods
We used fifteen rats (rattus norvegicus albinus, Wistar), and two 5 mm surgical defects were performed on each of them; the defects were made with trephine drill on the calvarium region prior to the biomaterial placement. Groups were divided as follows: Group 1—defect filled with clot; Group 2—defect filled with crystallized Biosilicate. After 7, 14 and 28 days the animals were killed, the parts were retrieved and slides were prepared for histological studies.
Results
Bone formation was satisfactory in all groups, with direct contact between biomaterial surface and bone and absence of infection signs. The 28 days periods showed better results, and statistically significant difference between Clot Group (90.2 %) and Biosilicate (58 %; p = 0.002) was seen, regarding presence of bone tissue on the surgical defects.
Conclusion
Our study revealed that defects filled with clot present better results on bone formation compared to crystallized Biosilicate, which is considered a biocompatible material with favorable osteoconductive properties.
Keywords: Biomaterial, Biosilicate, Bioglass
Introduction
Biomaterials such as silicate, ceramics and calcium silicate glasses have been used to improve, repair and promote hard tissue replacement, being able to interact with bone tissue and stimulate osteogenesis. The use of such materials has been applied in medical and dental field for more than 30 years [1, 2] and tested in different animal studies to evaluate the potential of new bone formation and its strength, with excellent responses and considered a quite promising material to replace bone [3].
Techniques of bone reconstructions with the use of autogenous bone grafts are considered the first choice due to the many advantages these techniques present, highlighting among others the possibility of transporting live cells from the donor site, the impossibility of unfavorable body’s immune reaction, reduced risk of infection and hence facilitating regeneration process. However, this technique requires donor area, which in certain situations requires extensive exposure increasing the risk of morbidity and the need for hospitalization, when general anesthesia is required [4].
Synthetic biomaterials presenting calcium and phosphate like hydroxyapatite, bioactive bioceramics and glasses are widely used when autogenous bone grafts are not indicated or in association with other techniques, demonstrating the best results among others with particular emphasis to bioglasses [5]. Previous studies demonstrated the excellent biocompatibility of this material and favorable osteoconduction proprieties leading to a significant increase in bone matrix [6, 7], promoting the deposition of a layer of calcium phosphate in contact with the implant (osseointegration).
In contrast to the excellent osteoconductive properties, bioactive glass has limited use due to its low mechanical strength [8]. Studies using bioglass cylinders implanted in rabbits’ femurs showed that these biomaterials are viable for odontological use due to its high biocompatibility rate, which also shows that microfractures occurred throughout the implant’s length, as seen in histological findings [7]. The industry has sought to develop bioglasses that present similar characteristics to Bioglass®45S5 and better mechanical strength during application. Recently a 100 % crystallized glass ceramic based on formula SiO2P2O5Na2OCaO was developed and classified as Biosilicate®. The material is made of silica, oxygen, sodium, calcium and phosphorus, and it releases small quantities of Si(OH)4, Na+, Ca2+ e PO42− during its dissolution, forming Hydroxycarbonate Apatite (HCA) on the surface, and consequent union with bone tissue (Patent EP1601623B1, Federal University of São Carlos, São Paulo, Brazil). Hench and Polak [9] confirm such results, demonstrating that bioglasses, in contact with viable bone tissue and body fluids, have the ability to form a layer of HCA, promoting a fairly strong chemical bond between biomaterial and tissue. This layer resembles chemically and structurally the mineral phase of bone tissue, with high osteogenic activity.
In vitro studies with saline solution, similar to body fluids, show that Biosilicate® is highly bioactive with greater bone matrix formation when compared to Bioglass®45S5. After 24 h the formation of an apatite layer was observed on bioglass surface, and after 17 days the presence of osteogenic cells were also observed [10]. Granito et al. [11] compared the behavior of Bioglass®45S5 and Biosilicate® on defects created on tibias of rats and found that Biosilicate® had similar mechanical strength to Bioglass®45S5 and osteogenic activity of Biosilicate® was better. Roriz et al. [2] analyzed alveolar defects filled Biosilicate® and found that this biomaterial presented great quantity of mineralized tissue in close contact with the surface, with excellent biocompatibility and satisfactory mechanical properties. In vivo studies are relevant to the quantitative and qualitative assessment of Biosilicate® behavior in close contact with tissues. The present study aims to evaluate the bone repair process of defects created on rats’ calvarias filled with a new biomaterial called crystallized Biosilicate®, through histological and histometric analysis in different periods, along with the investigation of the bioglass’s influence on bone formation and viability for use in clinical dentistry.
Materials and Methods
Sample Preparation
Biosilicate and Bioglass®45S5 materials based on the general formula SiO2P2O5Na2OCaO were obtained after weighing and mixing in rubber bottles, high pure silica, calcium carbonate, sodium carbonate, and sodium phosphate for 30 min. Materials fusion took place between 1,250 and 1,380 °C after 3 to 4 h in an electric furnace (Rapid Temp 1710BL; CM Furnaces Inc., Bloomfield, NJ, USA) at the Vitreous Material Laboratory of the Federal University of São Carlos, São Carlos, SP, Brazil and the Vitrovita—Instituto de Inovação em Vitrocerâmicos Ltda, São Carlos, SP, Brazil. The melt was poured into graphite molds for making cylinders, which were then crushed to obtain particulate material with dimensions between 180 and 220 micrometers. Biosilicate composition and the thermal treatment protocol are described in detail at EP1601623B1 patent (Brazil).
Experimental Surgery
This study was approved by the Ethics Committee for Animal Research of Ribeirão Preto Dental School, University of São Paulo, Brazil (Protocol 11.1.989.53.6). Fifteen 3-month-old rats (Rattus norvegicu, Albinus, Wistar) weighing between 250 and 300 g were used in this study. The rats were kept in individual cages with standard diet, solid food and water “ad libitum” in climatic conditions. Each animal was submitted to calvarial surgery exposure and two 5 mm defects were done using a trephine drill. Right calvarial defects were filled with bioglass and left defects with blood clot. Sutures were placed and antibiotic (0.2 ml; Penicilin) and analgesic (0.1 ml; Tramadol) were administered immediately after surgery. The animals were maintained in cages with free access to food and water and the wounds were inspected daily for clinical signs of complications or adverse reactions. After 7, 14 and 28 days of implantation the animals were euthanized with a lethal dose of sodium thiopental and calvarial bone harvested and processed for morphological and histomorphometrical analyses.
Histological Processing and Histometric Analysis
Calvarial bones were ground sectioned for light microscopy the protocol of which has been previously described (7). After that Leica DM LB2 optical microscope (Leica Microsystems Wetzlar GmbH, Wetzlar, Germany) equipped with a digital video camera Leica DFC 280 (Leica Microsystems AG Imaging, Cambridge, England) was used for image capture. The images were processed using Qwin Leica software (Leica Imaging Systems Ltd, Cambridge, England) to obtain the bone volume fraction (%). Five fields were analyzed in each defect in order to estimated the bone percentage volume through a method of differential count of 500 points per defect.
Statistical Analysis
Differences between groups were analyzed by ANOVA and Mann–Whitney (p = 0.05) using Assistat 7.6 Beta software updated in 01/01/2013 (Patent #0004051-2). The results were expressed in graphs and tables.
Results
Clinical Analysis
All animals were clinically observed throughout the study period and there was no evidence of infection among the animals used for obtaining the results. The macroscopically analyzed parts presented no clinical signs of inflammation or infection, necrosis or other lesions affecting soft or bone tissues; the animals remained healthy throughout the whole experimental period.
Histological Analysis
Group 1: Blood Clot
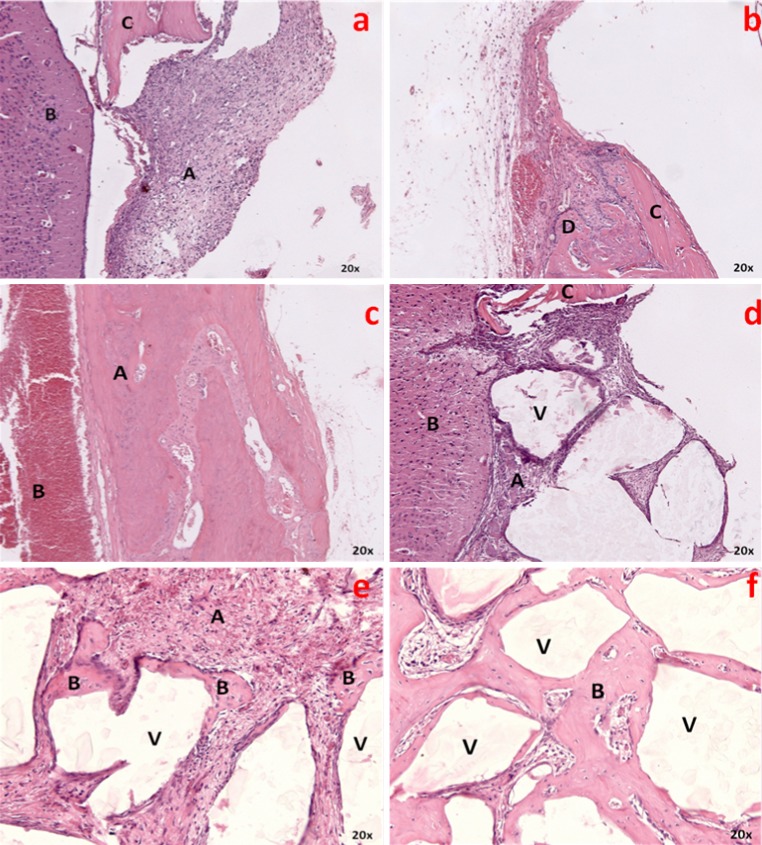
7 days—After 7 days, there is formation of granulation tissue and presence of disorganized Connective Tissue (CT) on a large extent of the surgical defect. The bone defect is clear, with complete disruption of inner and outer cortical plates and presence of erythrocytes and fibroblasts. There is no bone tissue at margins, central region or inner and outer cortical plates; only the skull bone tissue is observed. There are no signs of infection (Fig. 1a).
Fig. 1.
a, b, c Blood clot group after 7, 14 and 28 days respectively. e, f, g Biosilicate group after 7, 14 and 28 days respectively
14 days—After 14 days, there is disorganized CT with chronic inflammatory infiltration. Bone tissue formation starts next to defects margins; the osteoblastic activity is rather intense, with irregular cellular matrix deposition within the vascular conjunctive tissue. There are no episodes of infection or necrotic tissue (Fig. 1b).
28 days—Extensive area is filled with newly formed bone tissue both in center and edge areas of surgical defects, with mature bone; sharp decrease of CT and absence of infection signs (Fig. 1c).
Group 2: Crystallized Biosilicate
7 days—After 7 days, the defect is clear, as implant material occupies an extensive area; irregular granules are seen in contact with granulation tissue, which projects among fragments. There is great quantity of CT with no signs of infection and presence of giant multinuclear cells next to the material, with chronic inflammatory infiltrate. There is also a moderate quantity of fibroblasts and clots in some areas. There is no sign of newly formed bone tissue, only small fragments near the defects borders and bone tissues of the remaining skull bone (Fig. 1d).
14 days—After 14 days, as the particles of the implant material occupy a large area of the surgical defect, with decreased CT in contact with the bioglass’ surface, there is newly formed bone tissue at the margins of the defect, with no presence of bone in the central portion. There are no giant multinuclear cells, and some particles of the biomaterial are discreet and randomly surrounded by immature bone. The clot areas and signs of infection are severely reduced (Fig. 1e).
28 days—After 28 days, the bioglass presence could be observed within the surgical defect, with an increase of newly formed bone tissue along the entire area and in close contact with Biosilicate surface, mainly on smaller fragments. There is a marked decrease of CT amount, as great part of the defect is filled by bone tissue, with no signs of infection (Fig. 1f).
Histometric and Statistical Analysis
Conjunctive Tissue
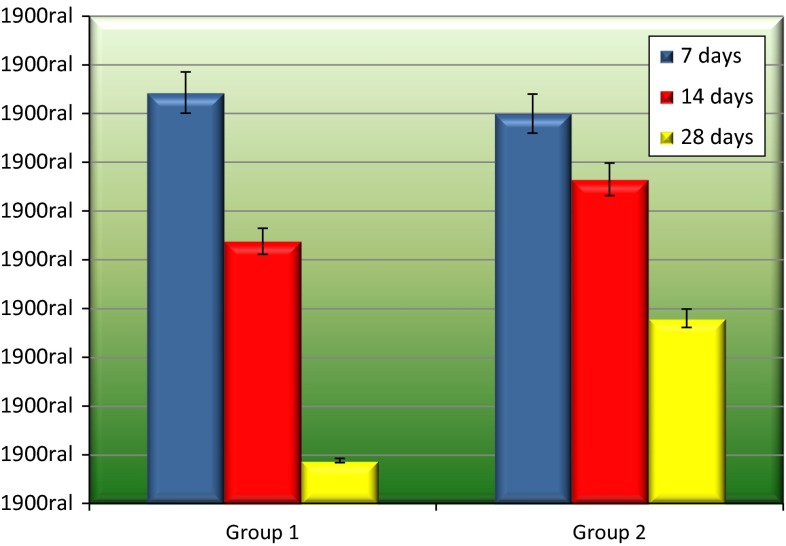
The 28 days period of Group 1 presented the best results (8.8 %). A statistically significant difference was only observed on comparing this period to the periods of Group 2 (p = 0.004, p < 0.05 and p = 0.002, respectively 7, 14 and 28 days). The periods of 7 days (84.3 %) and 14 days (53.8 %) of Group 1 showed no significant difference (p > 0.05) compared to Group 2 (Table 1 and Fig. 2).
Table 1.
Connective tissue amount (%) after 7, 14 and 28 days
| Groups | Periods | ||
|---|---|---|---|
| 7 (%) | 14 (%) | 28 (%) | |
| Blood clot | 84.3 | 53.8 | 8.8 |
| Biosilicate | 80 | 66.5 | 38 |
Fig. 2.
Connective tissue amount (%) and standard deviation after 7, 14 and 28 days
Bone Tissue
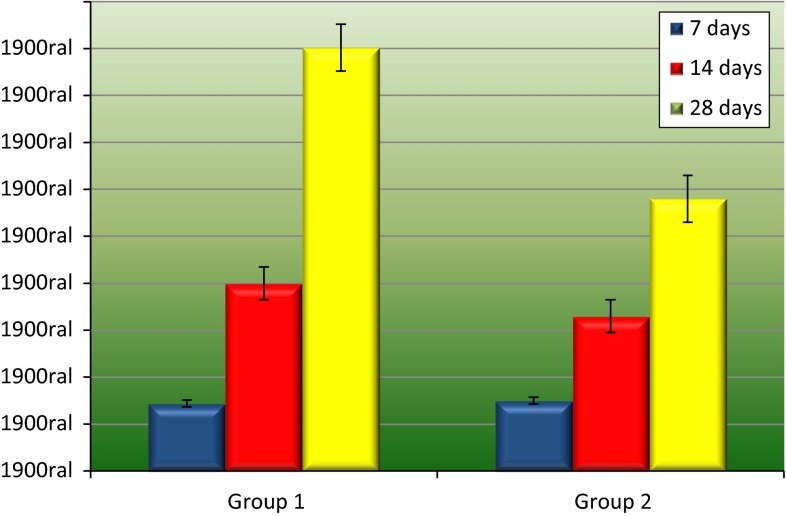
No statistically significant differences were found between both groups when the amount of bone tissue on the surgical defect was assessed in the 7 and 14 days periods (p = 0.5 and p = 0.35, respectively). In the 7 days period, Group 2 presented slightly better results than Group 1 (15 and 14.4 %); the results were inverted after 14 days, as Group 1 showed higher formation of bone tissue (40 % against 33 %). After analyzing 28 days periods, Group 1 presented the best results (90.2 %)—statistically higher than Group 2 (58 %; p = 0.002; Table 2 and Fig. 3).
Table 2.
Bone tissue amount (%) after 7, 14 and 28 days
| Groups | Períods | ||
|---|---|---|---|
| 7 (%) | 14 (%) | 28 (%) | |
| Blood clot | 14.4 | 40 | 90.2 |
| Biosilicate | 15 | 33 | 58 |
Fig. 3.
Bone tissue amount (%) and standard deviation after 7, 14 and 28 days
Discussion
The results presented in this research show excellent biological behavior of crystallized Biosilicate. The influence of a bioglass on the process of repairing calvarial defects considered to be of critical size was studied. According to previous studies [12, 13], the small and disorganized bone formation in the early stages confirmed the critical nature of defects of 5 mm diameter which were surgically made to rat calvaria. The mentioned results are similar to the ones found in this research, which, during the periods of 7 and 14 days, showed a greater connective tissue amount in the defect areas of both study groups when compared to the bone neoformation. Defects considered to be critical are ideal and recommended when the objective of the study is to evaluate the bone tissue behavior concerning grafting with different biomaterials, since spontaneous osteogenesis does not take place throughout the animal’s life, except in situations when the implanted material enables osteoconduction and osteoinduction, causing the formation of new tissue. Choosing to use rat calvaria is due to the fact that there are two cortical bones with medullar tissue between them in this area, which is similar to the mandible bone. Another advantage is that the chances of infection caused by the animal itself (through its mouth and paws) are smaller, allowing the material to remain in the cavity with minimal movement [14].
Seeking for a synthetic absorbable material with biomechanical characteristics similar to those of the cortical bone and enough elasticity to support the local loads and forces has become the greatest challenge for innumerous research works on tissue engineering. Variables, such as the preparing method of the material and particles composition and size, have huge influence on the results. Therefore, bioglasses have been studied and improved, aiming at finding a synthetic bone substitute with the right characteristics. Initially developed by Hench and Wilson [3], the bioactive glass has been exhaustively researched and modified. The Bioglass®45S5 has been considered to be the bioactive glass which presents the best characteristics to suit the health field, being extensively tested in both in vitro and in vivo situations as the control for other biomaterials. When in contact with tissue fluids, the bioglass particles trigger a series of chemical reactions, leading to the formation of HCA layers on the particles surfaces, attracting the osteoblasts and organic constituents and, as a consequence, forming a substrate whose chemical composition is similar to the bone tissue [15]. The development of novel bone substitute biomaterials such as Bioglass or Biosilicate is extreme important when considering the oral and maxillofacial region. Bone fractures of difficult reduction, bone defects resulting from trauma or pathology sequel, rehabilitation of edentulous ridges, periodontal defects, apicoectomies, and maxillary sinus augmentation are some of the indications for the use of Bioglass that has been recently used to reconstruct orbital wall fractures with similar results to autogenous grafts [16].
When the use of biomaterials is indicated, their easy handling and insertion in the recipient bed become important characteristics for a successful procedure besides representing low costs. The used material in this experiment proved to be very favorable to handling, being the mixing of bioglass fragments to physiological solution prior to its insertion, as indicated in the procedure, as it makes the granules stay together and it is easier for it to be transported to the surgical defect. Shapoff et al. [17] affirm that the preparation before insertion can also be performed with sterilized water or blood from the patient and that bioactive glass possesses the ability to promote hemostasis by incorporating the granules into the host site without fibrous encapsulation, and remaining where placed. Schepers and Ducheyne [18] reported that bioglass is a material which is easy to handle and fast to prepare; it is easily mixed to other grafts and tends to remain in the insertion spot. These results were confirmed by this study, since whenever the crystallized Biosilicate® was inserted in the surgical defects, it was possible to observe that it remained where it was inserted during the histological analysis.
Recently, the complete crystallization of the Bioglass (crystallized Biosilicate) by researchers from the Universidade Federal de São Carlos (São Paulo, Brazil) and the use of this new biomaterial in experiments have proved that this synthetic material shows similar characteristics or even better behavior than Bioglass®45S5. Moura et al. [9] showed that the complete crystallization of the bioactive glass with SiO2P2O5Na2OCaO tends to favor the bone tissue formation in vitro studies using the cell culture system. Granito et al. [11] suggest that the Biosilicate® shows higher osteogenic activity when compared to Bioglass®45S5 in histological analyses and no differences were observed concerning the load absorption ability, energy absorption and structural rigidity. Roriz et al. [2] found similar histological results when comparing the Biosilicate and Biogran® for reconstructing alveolar ridges and installing implants in dogs. Both materials showed favorable biological characteristics and were appropriate for maintaining the alveolar crest bone, thus allowing the placement of osseointegrated implants in 8 and 12 weeks after the graft, suggesting that this new biomaterial can be indicated for this type of surgery.
Another very important and desired characteristic of Biosilicate is its antimicrobial activity, considering that such biomaterial can be used in maxillary reconstruction surgeries, where the oral microbiota favors the development of infections when synthetic materials are used. After different laboratorial analyses, it could be observed that the Biosilicate shows resistance even to anaerobic bacteria [19].
The bone differentiation regarding critical defects filled with bioglasses tends to be from the defect edges towards the center, or in a centripetal way. This research, as well as previous studies, showed that in the early stages a higher amount of bone could be observed in the edge of the defects, increasing in the central area as time passed, and in contact with the particles of the biomaterial which was away from the edges, confirming the osteoconductive characteristic of the material [15, 16, 20]. Schepers and Ducheyne [18] evaluated the bone growth around the particles of bioactive glass implanted in dogs. After different analyses periods, it was concluded that after biomaterial implantation some fragments of the bioglass may lead to the formation of protective pouches, that may lead to formation of new bone without this bone being connected to the bone tissue outside the particles and that these islands of newly formed bone tissue function as nuclei for further bone growth and enhance the repair of the defect. We agree with these authors because we found similar histological findings as presented by them. In our study it was possible to observe the presence of new bone tissue surrounding the surface of the Biosilicate® particles and isolated in a few areas inside the particles.
Previous studies showed that the complete crystallization of the bioglass tends to decrease its biocompatibility, suggesting that the crystallization converts bioactive glass into an inert material [21]. The results presented in this study go against the mentioned findings. Neither inflammatory reaction nor infection was observed and the bone formation next to or in contact with the bioglass after grafting with crystallized Biosilicate increased gradually when compared to initial and final periods, which shows excellent biocompatibility regarding the used material. Xynos et al. [22] and Loty et al. [23] affirm that total Biosilicate crystallization changes the material’s important characteristics with pH changes as a consequence: it becomes alkaline, favoring osteoblastic differentiation. Azenha et al. [7] confirmed these results when they observed excellent biological behavior and strong bone formation in close contact with the surface of different bioglasses and without multinucleus giant cells. Through histological and histometric analyses, it was discovered that crystallized Biosilicate showed similar results to Bioglass®45S5, proving it to be a safe and promising biomaterial to be used in dentistry. In a study using hydroxyapatite from cattle and B-tricalcium phosphate, it was possible to observe close contact of the used biomaterials with bone, as well as foreign body reaction with the presence of multinucleus giant cells [24], which leads to a decrease in the bone tissue formation in contact with the biomaterial and consequent decrease in the biocompatibility [17].
Matsumoto et al. [25] in a study using rabbit calvaria observed that Biosilicate associated with autogenous bone showed histometric and immunohistochemical results similar to the defects which were filled with nothing but autogenous bone. The microscopic analyses showed very similar neoformed tissue in both groups, suggesting that the degradation level of the biomaterial tends to be compatible to the bone formation. This can be interpreted as an advantage of using this material as scaffold in the early stages of the repairing process, maintaining the local anatomy and favoring tissue formation. Researchers do not agree when complete resorption of bioglass particles are done, with long term research being necessary to elucidate this issue and take advantage of the best degradation characteristics of bioglass, once the bone neoformation happens at the same time as the material reabsorption process, which provides a scaffold for the cell proliferation and synthesis of organic matrix [13, 19]. Also in agreement with what was found by Matsumoto et al. the immunohistochemical analyses showed intense osteoblastic and osteogenic activity both in the defects filled with autogenous bone and in the ones filled with autogenous bone and Biosilicate®, leading to the conclusion that the glass ceramic had a similar biological behavior when compared to the autogenous bone.
Based on the findings, it is possible to conclude that the crystallized Biosilicate has positive response in many in vitro and in vivo studies and that it can be used in dentistry in different ways and situations. In this research, this biomaterial proved to be biocompatible despite the fact that it showed worst results compared to defects filled by coagulum. Its role regarding the defects in Wistar rats calvaria are apparently related to osteoconduction, not being absorbed throughout all the time necessary to conduct this experiment.
References
- 1.Bosetti M, Cannas M. The effect of bioactive glasses on bone marrow stromal cells differentiation. Biomaterials. 2005;26:3873–3879. doi: 10.1016/j.biomaterials.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 2.Roriz VM, Rosa AL, Peitl O, Zanotto ED, Panzeri H, de Oliveira PT. Efficacy of a bioactive glass-ceramic (Biosilicates) in the maintenance of alveolar ridges and in osseointegration of titanium implants. Clin Oral Implants Res. 2010;21:148–155. doi: 10.1111/j.1600-0501.2009.01812.x. [DOI] [PubMed] [Google Scholar]
- 3.Wilson J, Clark AE, Hall M, Hench LL (1993) Tissue response to bioglass endosseous ridge maintenance implants. J Oral Implantol 19:295–302 [PubMed]
- 4.Herford AS, Dean JS. Complications in bone grafting. Oral Maxillofac Surg Clin North Am. 2011;23:433–442. doi: 10.1016/j.coms.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Araújo MG, Liljenberg B, Lindhe J. Beta-tricalcium phosphate in the early phase of socket healing: an experimental study in the dog. Clin Oral Implants Res. 2010;21:445–454. doi: 10.1111/j.1600-0501.2009.01876.x. [DOI] [PubMed] [Google Scholar]
- 6.Rocha Barros VM, Liporaci JLJ, Jr, Rosa AL, Junqueira MC, Oliveira PT, Johnson A, et al. Bone response to three different chemical compositions of fluorcanasite glass-ceramic. J Biomed Mater Res A. 2007;83:480–483. doi: 10.1002/jbm.a.31219. [DOI] [PubMed] [Google Scholar]
- 7.Azenha MR, Peitl O, Barros VMR. Bone response to biosilicates® with different crystal phases. Braz Dent J. 2010;21:383–389. doi: 10.1590/s0103-64402010000500001. [DOI] [PubMed] [Google Scholar]
- 8.James PF. Glass ceramics: new compositions and uses. J Non-Cryst Solids. 1995;181:1–15. doi: 10.1016/0022-3093(94)00515-X. [DOI] [Google Scholar]
- 9.Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;8:1014–1017. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 10.Moura J, Teixeira LN, Ravagnani C, Peitl O, Zanotto ED, Beloti MM, Panzeri H, Rosa AL, de Oliveira PT. In vitro osteogenesis on a highly bioactive glass-ceramic (Biosilicate) J Biomed Mater Res A. 2007;82:545–547. doi: 10.1002/jbm.a.31165. [DOI] [PubMed] [Google Scholar]
- 11.Granito RM, Ribeiro DA, Rennó ACM, Ravagnani C, Bossini OS, Peitl-Filho O. Effects of biosilicate and bioglass 45S5 on tibial bone consolidation on rats: a biomechanical and a histological study. J Mater Sci Mater Med. 2009;20:2521–2526. doi: 10.1007/s10856-009-3824-z. [DOI] [PubMed] [Google Scholar]
- 12.Bosch C, Melsen B, Vargevic K. Importance of the critical size bone defect in testing bone-regeneration materials. J Craniofac Surg. 1998;9:310–316. doi: 10.1097/00001665-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Furlaneto FAC, Nagata MJH, Fucini SE, Deliberador TM, Okamoto T, Messora MR. Bone healing in critical-size defects treated with bioactive glass/calcium sulfate: a histologic and histometric study in rat calvaria. Clin Oral Implants Res. 2007;18:311–318. doi: 10.1111/j.1600-0501.2006.01331.x. [DOI] [PubMed] [Google Scholar]
- 14.Schimitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986;205:299–308. [PubMed] [Google Scholar]
- 15.Hench LL. Bioceramics: material characteristics versus in vivo behaviour. Ann N Y Acad Sci. 2006;523:54–71. doi: 10.1111/j.1749-6632.1988.tb38500.x. [DOI] [PubMed] [Google Scholar]
- 16.Gunarajah DR, Samman N. Biomaterials for repair of orbital floor blowout fractures: a systematic review. J Oral Maxillofac Surg. 2013;71:550–570. doi: 10.1016/j.joms.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Shapoff CA, Alexander DC, Clarck AE. Clinical use of a bioactive glass particulate in the treatment of human osseous defects. Compend Contin Educ Dent. 1997;18:352–363. [PubMed] [Google Scholar]
- 18.Schepers EJG, Ducheyne P. Bioactive glass particles of narrow size range for the treatment of oral bone defects: a 1–24 month experiment with several materials and particle sizes and size ranges. J Oral Rehabil. 1997;24:171–181. doi: 10.1111/j.1365-2842.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 19.Martins CHG, Carvalho TC, Souza MGM, Ravagnani C, Peitl O, Zanotto ED, Panzeri H, Casemiro LA. Assessment of antimicrobial effect of Biosilicate against anaerobic, microaerophilic and facultative anaerobic microorganisms. J Mater Sci Mater Med. 2011;22:1439–1446. doi: 10.1007/s10856-011-4330-7. [DOI] [PubMed] [Google Scholar]
- 20.Vogel M, Voigt C, Gross UM, Muller-Mai CM. In vivo comparison of bioactive glass particles in rabbits. Biomaterials. 2001;22:357–362. doi: 10.1016/S0142-9612(00)00191-5. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Yang Q, Zhang F, Kokubo T. The effect of residual glassy phase in a bioactive glass-ceramic on the formation of its surfasse apatite layer in vitro. J Biomed Mater Res. 1992;3:452–456. [Google Scholar]
- 22.Xynos ID, Edgar AJ, Buttery LD, Hench LL, Polak JM. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem Biophys Res Commun. 2000;276:461–465. doi: 10.1006/bbrc.2000.3503. [DOI] [PubMed] [Google Scholar]
- 23.Loty C, Sautier JM, Tan MT, Oboeuf M, Jallot E, Boulekbache H. Bioactive glass stimulates in vitro osteoblast differentiation and creates a favorable template for bone tissue formation. J Bone Miner Res. 2001;16:231–239. doi: 10.1359/jbmr.2001.16.2.231. [DOI] [PubMed] [Google Scholar]
- 24.De Souza-Nunes LS, De Oliveira RV, Holgado LA, Nary Filho H, Ribeiro DA, Matsumoto MA. Immunoexpression of Cbfa-1/Runx2 and VEGF in sinus lift procedures using bone substitutes in rabbits. Clin Oral Implants Res. 2010;21:584–590. doi: 10.1111/j.1600-0501.2009.01858.x. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto MA, Caviquioli G, Biguetti CC, Holgado LA, Saraiva PP, Renno ACM, Kawakami RY. A novel bioactive vitroceramic presents similar biological responses as autogenous bone grafts. J Mater Sci Mater Med. 2012;23:1447–1456. doi: 10.1007/s10856-012-4612-8. [DOI] [PubMed] [Google Scholar]