Abstract
The mutualism between fungus-growing animals and fungi is a classic example of a complex interspecies association. A handful of insects, notably the well-recognized fungus-farming ants, termites and beetles, have developed advanced agriculture, which includes seeding new gardens with crop propagules, improving growth conditions and protecting and harvesting the fungal crop. More examples, which could be called ‘proto-fungiculture', involve fewer adaptations, as exemplified by marine snails that farm intertidal fungi on marsh grass. Recent work has indicated that the solitary leaf-rolling weevil Euops chinensis (family Attelabidae) has a protofarming symbiosis with the mycangial fungus Penicillium herquei (family Trichocomaceae). In this study, we investigated how the weevils create cradles (leaf-rolls) for their offspring and protect the fungal garden. We describe new specialized structures and behaviors that E. chinensis females use for leaf-rolling and fungus inoculation. The fungus P. herquei produces the antibiotic (+)-scleroderolide in laboratory culture and in leaf-rolls, which can serve to inhibit microbial ‘weeds' and pests, thus protecting the fungal garden against potential infection. The fungiculture of E. chinensis differs from other advanced insect fungiculture systems because female weevils do not continuously tend the inoculated microbe and do not depend nutritionally on the fungus. The defensive role of the cultivated fungus makes the attelabid weevils exceptional in ‘proto-fungiculture' animals.
Introduction
Insects developed the capacity to cultivate fungi as a food source approximately 40–60 million years ago, and agricultural life ultimately enabled all of these insect farmers to rise to major ecological importance (Mueller and Gerardo, 2002; Mueller et al., 2005). The capacity to grow fungus has not been restricted to insect lineages; also snails have been discovered to be nutritionally dependent on external fungal symbionts (Silliman and Newell, 2003).
Insect fungiculture shares some defining features of agriculture, including habitual planting (‘inoculation'), cultivation, harvesting and nutritional dependency (Mueller et al., 2005). Instances of advanced fungiculture have been extensively documented and studied, including fungus-farming ants, termites and ambrosia beetles (Farrell et al., 2001; Aanen et al., 2002; Currie et al., 2003a). The farming strategies of these insect farmers include evolved mechanisms for substrate preparation, inoculation with crop propagules, optimization of fungal growth through regular activities, protection of the crop against parasites or diseases and harvest and consumption of fungi (Schultz et al., 2004; Mueller et al., 2005). The insect farmers have developed task-partitioned societies cooperating in their fungiculture biology (Biedermann and Taborsky, 2011; Nobre et al., 2011). In ant and termite farmers, agriculture tasks are partitioned between different castes, each specialized on one main task (Hart et al., 2002; Nobre et al., 2011). In ambrosia beetle (Xyleborinus saxesenii), division of labor is shown between larval and adult colony members (Biedermann and Taborsky, 2011).
Proto-fungiculture mutualism cases have been reported from non-social organisms, and the insects show no division of labor. Outside the class Insecta, marine snails farming intertidal fungi provide an example in the marine environment. Littoria snails (Littoraria irrorata) ‘protofarm' fungi by creating plant wounds that become infected with fungal mycelia that are part of the snails' diet, but the snails do not actively inoculate plant wounds or otherwise garden the fungi (Silliman and Newell, 2003). The fungus-growing lizard beetle Doubledaya bucculenta transmits symbiotic yeast into bamboo internode cavities and disperses the yeast in the cavities to make gardens (Toki et al., 2013). These proto-farming phenomena have also been reported in some species of gall midges and wood wasps (Kukor and Martin, 1983; Heath and Stireman, 2010; Pazoutova et al., 2010).
A seldom-studied example of proto-fungiculture is exhibited by attelabid weevils. Among the subfamily Attelabinae, species in the genus Euops have a symbiotic association with fungi: the female weevils roll leaf pieces and inoculate them with a fungal symbiont stored in their mycangium. They lay one or a few eggs in the leaf cradles (leaf-rolls), and then the larvae develop by feeding on the inside layers of the leaf cradles (Sakurai, 1985; Wang et al., 2010). Inoculation with the fungal symbiont decreases the cellulose content of the host plant's leaves. Experimental removal of the cultivated fungus results in increased larval mortality (Li et al., 2012). The mycangial fungi have been identified as species of the genus Penicillium (Kobayashi et al., 2008; Li et al., 2012).
Morphological and behavioral adaptations for a ‘fungiculture lifestyle' have been observed in attelabid weevils. These adaptations include the female's mycangium, the comb plates on her abdomen, and the characteristic posture she assumes when depositing fungal spores (Sakurai, 1985). Recent studies suggested that the fungal cultivar tissues have only a minor nutritional value (Kobayashi et al., 2008; Li et al., 2012).
Our study of the attelabid weevil, E. chinensis, and its symbiotic fungus Penicillium herquei have indicated that important aspects of this symbiosis have not been previously described. Based on both video recordings of living specimens in the field and detailed laboratory investigations, we describe new specialized structures and behaviors that E. chinensis females use for leaf-rolling and fungus inoculation. We also report that P. herquei produces an antibiotic, (+)-scleroderolide, that protects the fungal garden from ‘weed microorganisms'. The characteristics of E. chinensis–P. herquei mutualism include the leaf-roll habitat, non-social nature and the highly evolved morphological and behavioral adaptations of the insect. The defensive role of the cultivar is to make the attelabid weevils' fungiculture exceptional in the proto-farming systems known today.
Material and methods
Collection of weevils, leaf-rolls and non-rolled host plant leaves
E. chinensis adults and 70 weevil-rolled and 103 non-rolled leaves of Fallopia japonica were collected in four locations in Jiangxi and Hubei provinces, China. The collected host plant leaves and weevils were placed in separate containers. The leaf-rolls were maintained individually on sterilized moist soil to permit the weevils to undergo complete metamorphosis.
Scanning electron microscopy (SEM)
Dissected mycangia and leaf-rolls were used for SEM screening. The mycangia of 20 adult female weevils were dissected. After the eggs and larvae were removed, 1.5 cm2 pieces were cut from 20 leaf-rolls. These specimens (20 each of mycangia and leaf-rolls without insects) were fixed in glutaraldehyde and processed according to previously published methods (Yuceer et al., 2011). The specimens were subsequently examined using a Quanta 200 scanning electron microscope (FEI, Czech).
Isolation of microorganisms from mycangia
A total of 89 E. chinensis females were collected from the host shrub F. japonica in Jiangxi and Hubei provinces, China. Spores stored in the mycangia of these females were removed with a sterile needle and placed on 1/4-strength potato dextrose agar (PDA) plates at room temperature. After the spores germinated, the colonies were transferred to full-strength PDA plates. Sporulating colonies were used to identify the fungi. Nutrient agar (NA; per liter: 5 g peptone, 3 g beef extract, and 15 g agar) and chitin medium (Hsu and Lockwood, 1975) were used to isolate bacteria (including Actinobacteria) from female weevils' mycangia. The plates were incubated in the dark at 25 °C for fungal isolation (including Actinobacteria) and at 30 °C for bacterial isolation.
Isolation of microorganisms from non-rolled host plant leaves and leaf-rolls
The un-cut parts of the leaves left by weevils were used as the non-rolled leaf samples. Leaf-rolls were unrolled, and the eggs or larvae inside were removed. The non-rolled leaf parts and the leaf-rolls were processed separately. The leaf tissues were washed twice with sterile 0.1% Tween 20. The samples were then homogenized in 10 ml of sterile deionized water, and the homogenate was serially diluted from 10−1 to 10−4. Each dilution was divided into three parts and used for isolation of fungi and bacteria, with three plates for each dilution and each type of microorganism. Fungal isolation was performed on 1/4-strength PDA amended with 0.1 mg ml−1 each of streptomycin and chloromycetin. NA and chitin agar were used to isolate bacteria (including Actinobacteria).
Identification of microorganisms
Colony morphology and microscopic characteristics were used to identify isolates (Klaus et al., 2007; Kirk et al., 2008). Isolated microorganisms were further identified based on DNA sequence information (White et al., 1990; Altschul et al., 1997; Hall, 1999; Stach et al., 2003; Sharma et al., 2008). The mycangia from 65 female weevils were dissected and distributed in three Eppendorf tubes. The tubes were submerged in liquid nitrogen, and the mycangia were ground with a sterile pestle. The DNA was extracted using the Promega Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA) according to the manufacturer's instructions.
For analysis of the microbial community in mycangia, fungal and bacterial universal primer pairs were used for PCR. The ITS1 and ITS4 regions were used for fungi, the yeast internal transcribed region was used for yeast, and 27F and 1495R primers of the 16S rDNA were used for bacteria, using previously published PCR procedure (Bianciotto et al., 1996). The specific primer pairs S-C-Act-0235-a-S-20 and S-C-Act-0878-a-A-19 were also used to detect Actinobacteria, as previously described (Stach et al., 2003). The positive (the DNA of Escherichia coli and Streptomycetes sp.) and negative (ddH2O) controls were used in PCR amplification. The amplicons were ligated into the TA vector pMD 18-T (TaKaRa Biotechonology, Dalian, China) and were cloned into E. coli DH5α following the manufacturer's protocol. In total, six clone libraries (three each for fungal and bacterial libraries) were established for the DNA pool obtained from the 65 mycangia. A total of 200–250 positive clones from each library were finally sequenced using the universal primer M13-47.
Preparation of the crude extract from leaf-rolls
A total of 70 leaf-rolls were dissected, and the larvae within were removed. The leaf-rolls were thawed, and adherent sand particles were removed using small forceps. The leaf-rolls were extracted three times (12 h each time) with 500 ml of ethyl acetate (EtOAc), and the organic solvent was evaporated to dryness under vacuum to obtain the crude extract. A total of 25 mg crude extract from 70 leaf rolls was prepared. A portion of the extract was dissolved in dimethyl sulfoxide (DMSO; 10 mg ml−1) and used for the inhibition assays described below. The remaining portion (from 13 leaf-rolls) was dissolved in methanol (MeOH) and subjected to high-performance liquid chromatography (HPLC) fractionation and HPLC-MS (mass spectrometry) analysis to verify the presence of the isolated antibiotics in the leaf-rolls.
Large-scale fermentation and extraction of P. herquei
The fungal strain P. herquei (CGMCC 3.15277) was cultured on slants of PDA at 25 °C for 10 days. Agar plugs were cut into small pieces (approximately 0.5 × 0.5 × 0.5 cm3) under aseptic conditions, and 15 pieces were used to inoculate three Erlenmeyer flasks (250 ml) (Shengbang Laboratory equipment, Haimen, China), each containing 50 ml media (0.4% glucose, 1% malt extract and 0.4% yeast extract; final pH adjusted to 6.5, sterilized by autoclave). Three flasks of the inoculated media were incubated at 25 °C on a rotary shaker at 170 r.p.m. for 5 days to prepare the seed culture. Fermentation was performed in 36 Fernbach flasks (500 ml) (Shengbang Laboratory equipment), each containing 80 g of rice. Distilled H2O (120 ml) was added to each flask, and the contents were soaked overnight before autoclaving at 15 psi for 30 min. After cooling to room temperature, each flask was inoculated with 5.0 ml of the spore inoculum and incubated at 25 °C for 40 days.
Isolation and structure determination of (+)-scleroderolide
The fermented material was extracted repeatedly with EtOAc (4 × 3.0 liters), and the organic solvent was evaporated to yield a crude extract (25.0 g), which was fractionated by silica gel vacuum liquid chromatography using a CH2Cl2–MeOH gradient elution (specific glass vessel; 12 × 40 cm2). Among the fractions, the 1% MeOH-eluted fraction (750 mg) demonstrated the greatest inhibition of microorganisms isolated from the non-rolled leaves and leaf-rolls. This fraction was further separated using Sephadex LH-20 column chromatography with 1:1 CH2Cl2–MeOH as the eluent, and the resulting subfractions were purified by repeated reversed-phase HPLC (Agilent Zorbax SB-C18 column (Agilent Technology, Santa Clara, CA, USA); 5 μm; 9.4 × 250 mm2; 60–80% CH3CN in H2O over 30 min; 2 ml min−1) to afford a yellow compound (29.8 mg; tR 28.38 min) over a total of three runs. Based on its nuclear magnetic resonance (NMR) and MS data, the compound was identified as (+)-scleroderolide (Supplementary Figures S2–S4 and S10).
Inhibitory effects of the mycangial fungus
Bioassays were conducted to determine whether the fungus isolated from mycangia (P. herquei) inhibited the 16 main microorganisms isolated from the non-rolled and rolled F. japonica leaves. Inhibition of fungi and bacteria was measured on PDA and NA, respectively, following the published methods (Poulsen et al., 2007). Bioassays were run for 6 weeks or until the growth of microorganisms ceased.
Inhibition of other microorganisms by (+)-scleroderolide or the leaf extracts
Agar diffusion assays were conducted to determine whether (+)-scleroderolide or the crude extracts of the leaf-rolls inhibited the 16 microbial species isolated from the non-rolled and rolled F. japonica leaves. For each test microorganism, 200 μl mycelia or spore suspension was spread onto 9.0-cm-diameter plates containing PDA (for fungi) or NA (for bacteria). A 6-mm-diameter well was cut in the middle of the plate. Serial dilutions of (+)-scleroderolide (5, 7.5, 10, 15, 30, 45, 60 and 75 nmol) was used to test the inhibitory effects. The compound was dissolved in 50 μl DMSO and added to the wells. Inhibitory effects of the crude extracts from the leaf-rolls were also tested, and 50 μl of DMSO solution of the crude extract (10 mg ml−1) was added to the well; the control plates received 50 μl of DMSO alone. All assays were performed at least in triplicate. The plates were kept at 25 °C, and the inhibition zones were measured daily. Inhibition was expressed relative to the growth on the control plates.
Quantification of (+)-scleroderolide in the leaf-rolls
Thirteen leaf-rolls were extracted repeatedly with EtOAc (4 × 0.15 L), and the organic solvent was evaporated to dryness under a vacuum to yield a crude extract of 4.4 mg, which was dissolved in methanol (MeOH; 1.6 ml). The sample (10.0 μl) was separated by HPLC (Zorbax Eclipse plus-C18 1.8 μm 2.1 × 50 mm2; 40–60% CH3CN in H2O over 30 min; 1 ml min−1) to afford the base-line separation for (+)-scleroderolide with a retention of (tR) 16.78 min. Using the same method, the standard curve of (+)-scleroderolide was prepared using the standard sample (10.0 μl) with the concentrations of 0.390, 0.780, 1.56, 3.12, 6.25, 12.5 and 25.0 μg ml−1. Based on the above results, the concentration of (+)-scleroderolide in the MeOH extract from 13 leaf-rolls was determined to be 1.79 μg ml−1, corresponding to an average of 0.22 μg of (+)-scleroderolide in each leaf-roll.
Results
Morphological and behavioral adaptations
Female weevil (Figure 1a) uses one of her forelegs, which have jagged teeth (Figure 1b), to partially cut a strip from the whole leaf (Supplementary Movie S1) of the host plant F. japonica. The cut piece remains connected to the rest of the leaf by a narrow band of tissue (Figure 2a). The specialized maxillary palps (Figure 1c) puncture multiple pairs of holes on each side of the leaf strip (Figure 1d). The female then adopts the characteristic posture for fungal inoculation (Supplementary Movie S1); she holds up her front and middle legs towards the leaf piece, bends her rear legs, presses her abdomen onto the leaf and forces the spores of the fungal symbiont out of the mycangium (Figure 1e). Then the six rows (in three pairs) of comb-like setae on the female weevil's abdomen (Figure 1f) brush the spores towards the punctured holes. The holes appear to facilitate spore germination and fungal colonization of the leaf (Figure 1g). Males lack mycangia and comb-like plates, as observed in this and previous studies (Sakurai, 1985). After inoculation of the spores, the female mates with a male and then uses her legs to fold the leaf piece longitudinally, leaving the apex of the leaf piece unfolded. When the leaf piece is partially rolled, one egg is deposited (70 leaf-rolls examined), and the remaining leaf is continuously folded and rolled (Supplementary Movie S1). The female then uses her legs to tighten the leaf-roll and prevents it from unrolling by puncturing its overlapping edges (Supplementary Movie S1). Finally, the female bites the narrow band of tissue that connects the leaf-roll to the main leaf, causing the leaf-roll to fall to the soil (Supplementary Movie S1). The moist ground environment is favorable for fungal growth and weevil development. A weevil will develop through the stages of egg, larva, pupa and adult in the leaf-roll cradle (Figure 2).
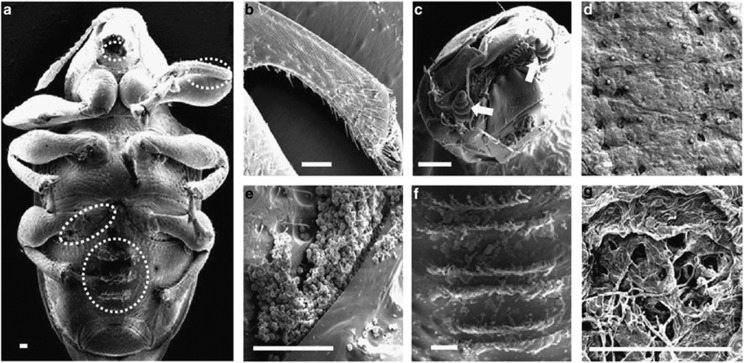
Figure 1.
Specialized structures of E. chinensis females for leaf-roll formation and inoculation of fungal spores. (a) Ventral side of the female. (b) Protibia with teeth. (c) A pair of maxillary palps (arrows). (d) Pairwise holes in the leaf that serve as inoculation sites. (e) Fungal spores are squeezed out of the mycangium. (f) Comb-like plate. (g) Fungal symbiont growing in a leaf-roll. Scale bars: 100 μm.
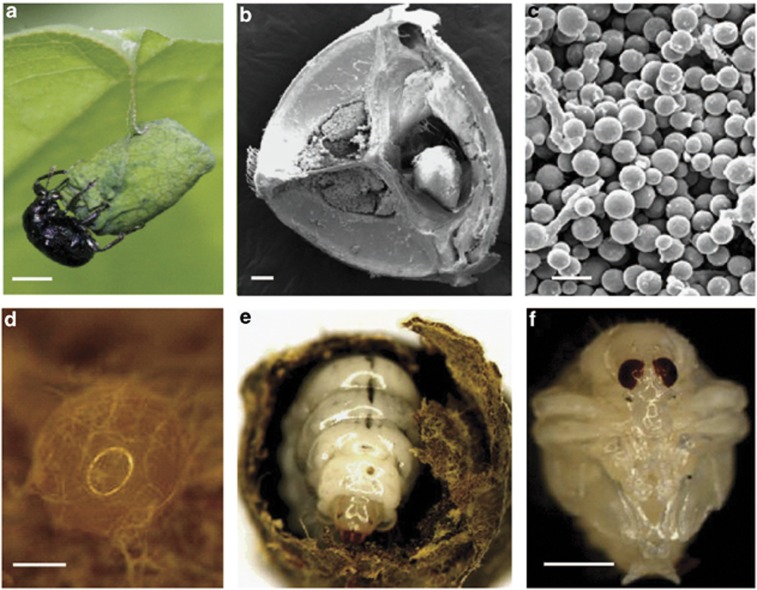
Figure 2.
Fungiculture by E. chinensis females. (a) A female has constructed a ‘cradle' by rolling a leaf of the host plant (F. japonica). (b) The female's mycangium is filled with fungal spores (SEM). (c) The fungal spores in the mycangium (SEM). (d) The female has deposited a fertilized egg in the leaf-roll inoculated with fungus, and the fungus rapidly envelops the egg within 5 days after oviposition. (e) The larva feeds on both the leaf-roll and the fungus. (f) The pupa. Scale bars: (a, f) 1000 μm; (b, d) 100 μm; (c) 10 μm.
Microbial community in the mycangium
Only a single fungal species, P. herquei (Genbank Accession NO. KC203056), was isolated from 89 E. chinensis female mycangia (Figure 2b). The spores from the mycangia of 65 females were pooled, and the DNA was extracted and PCR-amplified using fungal-specific primers (ITS1 and ITS4). The PCR product was purified and inserted into the pMD18-T vector to construct a clone library. The sequencing of 1536 clones confirmed the results obtained by culturing, that is, P. herquei (Genbank Accession No. KM236586) was the only fungus detected in the mycangia of E. chinensis females. SEM further indicated that spores of only one phenotype were present in mycangia (Figure 2c).
Plating the dissected mycangia on nutrient agar medium did not reveal the presence of any bacteria. This result was also confirmed via PCR amplification using bacteria-specific primers (27F and 1495R) on the 16S rRNA fragments from the mycangial DNA pool. Similarly, no Actinobacteria were detected either by plating the mycangia on the chitin agar plates or by PCR with Actinobacteria-specific primers (S-C-Act-0235-a-S-20, S-C-Act-0878-a-A-19).
The development of E. chinensis in the cradle was observed by carefully opening the leaf-rolls and immediately rewrapping them at 2-day intervals. Eggs, larvae, pupae and adults were examined; all of the larvae examined in this manner successfully developed into adults. The ochre-yellow mycelia of P. herquei were also observed to cover both the inside and outside of the leaf-roll (Figures 3a–c).
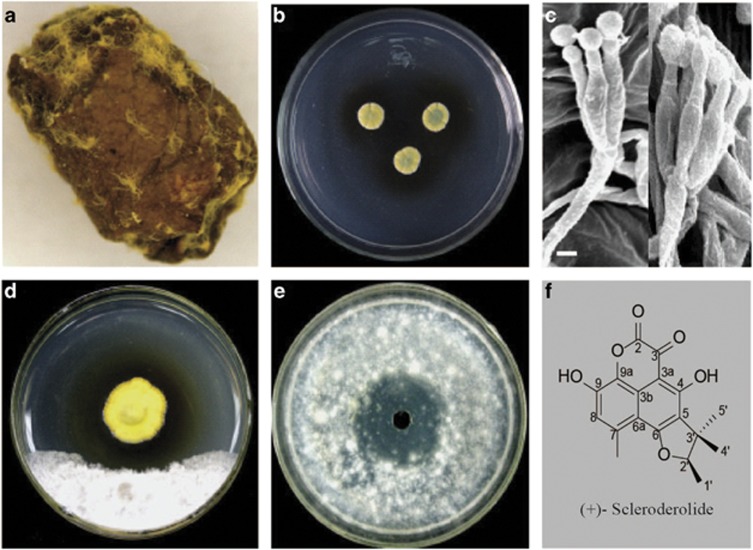
Figure 3.
The inhibitory effect of leaf-rolls and the fungal symbiont. (a) Leaf cradle covered by mycelium. (b) P. herquei colonies on CYA medium. (c) Conidiophores of P. herquei on leaf cradles. (d) In vitro inhibition of other fungi by P. herquei (the yellow colony in the center). (e) In vitro inhibition of other microorganisms by the crude extract from leaf-rolls. (f) The structure of (+)-scleroderolide, an antibiotic produced by P. herquei. Scale bar: 5 μm.
Microorganisms on host plant leaves and leaf-rolls
In the symbiosis between the weevil and the fungus, rolls are made of leaves that naturally harbor many other microorganisms. A total of 13 filamentous fungi, 2 yeasts and 1 bacterium were isolated from the non-rolled host plant leaves (Supplementary Table S1), but only 7 microbial species were isolated from the leaf-rolls that were collected over a 3-year period. The collected leaves that were not rolled harbored many different microorganisms, but they did not harbor P. herquei. It is possible that the microorganisms naturally occurring on the host leaves inhibit the weevil–fungus symbiosis by competing with P. herquei for nutrients, and they may potentially function as opportunistic pathogens of either the fungal garden or the weevil.
Inhibitory effects of the mycangial fungus
The in vitro inhibition of microorganisms isolated from the non-rolled host leaves by P. herquei (Figure 3d) suggested that P. herquei produced an antibiotic that inhibits potential competitors or parasites. To determine whether the leaf-rolls contain inhibitory substances, we collected 13 leaf-rolls from the natural environment and removed the weevil larvae. The leaf-rolls were then extracted with EtOAc. The crude extract (10 mg ml−1) from the leaf-rolls inhibited the growth of the tested microorganisms (Figure 3e). The crude extract from the non-rolled leaves showed little or no inhibitory effects (Supplementary Figures S1 and S9).
Structure identification and inhibitory effect of (+)-scleroderolide
The 16 microorganisms isolated from the non-rolled and rolled leaves were used as the test organisms to guide the fractionation of the active extract prepared from the large-scale fermentation culture of P. herquei. Using the results of this bioassay, a yellow compound was obtained from the bioactive fractions. The compound was assigned the molecular formula C18H16O6 (11 degrees of unsaturation) on the basis of the high-resolution electrospray ionization MS results. An analysis of its Proton (1H) and carbon-13 (13C) NMR data (Supplementary Figure S2) revealed the presence of 1 exchangeable proton (δH 13.74), 4 methyl groups, 1 oxygenated methine, 10 aromatic carbons (one of which was protonated), 1 sp3 quaternary carbon and 2 additional downfield sp2 carbons (δC 155.9 and 170.2, respectively). These data accounted for all of the resonances observed in the NMR spectra with the exception of the one exchangeable proton. In the Heteronuclear multiple bonds coherence (HMBC) spectrum (Supplementary Figures S3 and S4), correlations from the methyl protons at 2.75 p.p.m. to C-6a, C-7 and C-8 and from H-8 to C-6a, C-9 and C-9a indicated a penta-substituted aryl ring. HMBC correlations from OH-4 to C-3a, C-4 and C-5 revealed the presence of a naphthalene skeleton. HMBC cross-peaks from H3-4′ and H3-5′ to C-5, C- 2′ and C-3′ suggested that both C-2′ and C-5′ were connected to C-3′, and an HMBC correlation from H3-1′ to C-2′ demonstrated that the 2,3-dihydrofuran ring was fused to the naphthalene at C-5/C-6. Considering the existence of the intramolecular hydrogen-bond and the chemical shift of the sp2 carbon C-3 at 170.2 p.p.m., C-3 was attached to both C-3a and C-2. Finally, to agree with the degrees of unsaturation and the chemical shifts of C-2 (δC155.9), C-9 (δC144.7) and C-9a (δC130.0), the remaining hydroxy group was determined to be connected to C-9 and both the C-9a and C-2 carbons were attached to the same oxygen atom to form a dihydro-2H-pyran-2,3(4H)-dione partial structure, despite the fact that no HMBC data were observed to support this linkage. A compound called sclerodin has the same molecular formula as (+)-scleroderolide. Sclerodin has been reported as a metabolite of P. herquei. The structures of (+)-scleroderolide and sclerodin resemble each other closely, but that the NMR data are a far better match with those of (+)-scleroderolide. On the basis of these data, and by analogy to (+)-scleroderolide (Ayer et al., 1987), the compound was determined to have the same planar structure.
The absolute configuration of the compound was established based on its specific rotation value ([α]D). The (−)-form of scleroderolide ([α]D −116.0) was initially isolated from the extract of Gremmeniella abietina, although it was isolated in racemic form in one report. The levorotary enantiomer was assigned the 2′S absolute configuration (Ayer et al., 1987). However, in our experiment, the positive specific rotation value ([α]25D +107.5) of the compound suggested the 2′R absolute configuration.
In agar diffusion assay, (+)-scleroderolide showed a visible (Supplementary Figure S5) and the largest (Figure 4 and Supplementary Figure S6) inhibitory zones at 0.049 mg ml−1 (7.5 nmol in 50 μl DMSO) and 0.39 mg ml−1 (60 nmol in 50 μl DMSO), respectively. HPLC-MS analysis also detected the presence of (+)-scleroderolide in the EtOAc crude extract of the leaf-rolls. Based on the standard curve, an average of 0.22 μg of (+)-scleroderolide was detected in each leaf-roll (Supplementary Figures S7 and S8).
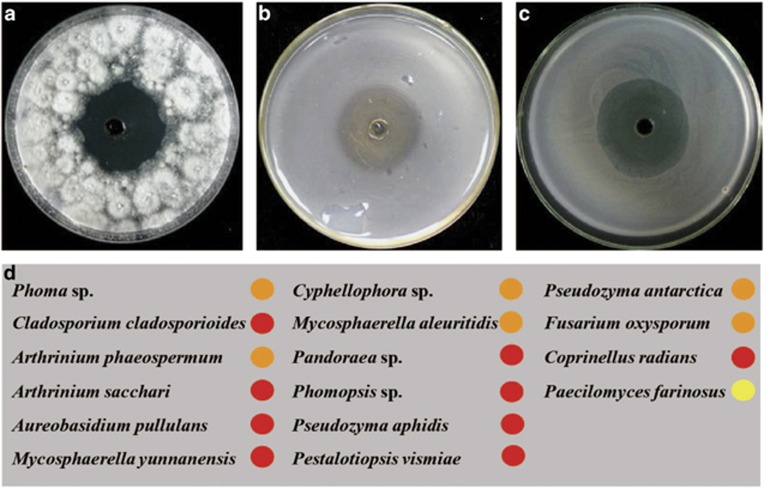
Figure 4.
In vitro inhibition of filamentous fungi, bacteria and yeast by (+)-scleroderolide. When added to the central well of an agar plate, (+)-scleroderolide inhibited a wide range of (a) filamentous fungi, (b) bacteria and (c) yeast. (d) The microorganisms were isolated from non-rolled and rolled host leaves, and the diameters of the inhibition zones are indicated by different colors: red=2.99–3.99 cm; orange=1.99–2.98 cm; yellow=0.99–1.98 cm.
Discussion
Examples of proto-farming systems have been previously reported. The marine snails Littoraria irrorata farm intertidal fungi on marsh grass (Silliman and Newell, 2003), the fungus-growing lizard beetle Doubledaya bucculenta make a fungal garden inside bamboo (Toki et al., 2012), and the attelabid weevils inoculate symbiotic fungus on leaf-rolls (Sakurai, 1985). In these systems, the elaborate structural and behavioral adaptations of the insects have been involved for fungal cultivation. In the fungiculture of attlebid weevils, we demonstrated that, in addition to the reported mycangia and comb plates, the female's serrated front legs and maxillary palps are used for cutting the leaf and depositing the fungal spores. These highly evolved structural and behavioral characteristics of the weevil enable the fungal symbiont to colonize the leaf-roll successfully. Compared with other proto-fungiculture examples, the relationship of the weevil E. chinensis and the fungus P. herquei is distinguished by the defensive role of the fungal cultivar.
In our study, the SEM screening, culture-dependent and -independent methods indicated that P. heruqei is the unique microorganism in the weevils' mycangia, with no other microorganisms found. This may relate to the antibiotic-producing characteristic of P. herquei. Some studies show that when teneral females with soft cuticle newly emerge from leaf-rolls, spores could not be found in their mycangia (Sakurai, 1985); we also observed this phenomenon in our study. Till now, it is still unclear how the fungal cultivar is transmitted in generations.
With the identification of the (+)-scleroderolide, we characterized an antimicrobial agent produced by the fungal cultivar of attelabid weevils. (+)-scleroderolide was proved to be efficient agents against the microorganism colonized on non-rolled host plant leaves and leaf-rolls. Other P. herquei strains have been reported to be isolated from diverse plant materials and soil and produce diverse antibiotics (Ayer et al., 1987). That suggests the E. chinensis–P. herquei mutualism appears to originate from the insects using the fungi as a source of antibiotics in the evolutionary transition to agriculture.
The chemical protection is well known in the fungal gardens of the advanced fungiculture systems of attine ants. The ants' metapleural glands have been reported to produce antibiotics in order to increase the competitive advantage of the mutualistic fungus (Poulsen et al., 2002). The ant Apterostigma dentigerum cultivate Pseudonocardia bacteria on their cuticles, and the bacteria produce the bioactive compound dentigerumycin, which specifically inhibits the growth of Escovopsis but not the cultivar fungus (Currie et al., 2003b; Oh et al., 2009). The fungal cultivar of the fungus-growing ant Cyphomyrmex minutus can produce several antifungal diketopiperazines that may suppress or eliminate fungal competitors in the garden (Wang et al., 1999). Similar to the last case, the mycangial fungus produces the antibiotic (+)-scleroderolide. To date, (+)-scleroderolide is the first discovered antibiotic molecule in a proto-fungiculture system.
Division of labor among the workers of insect societies is a conspicuous feature of the advanced fungiculture of attine ants, termites and ambrosia beetles. After their fungal gardens are established, both the fungus-growing ants and termites divide labor among castes, while in ambrosia beetles tasks are divided between adults and larvae instead of castes. Cooperation and task specialization have not been reported in proto-fungiculture systems. The attelabid weevil is solitary; after establishing her garden and ovipositing, the female leaf-rolling weevil moves on to form another leaf-roll and does nothing more to further the growth of the fungus or her offspring. The fungal gardens of Euops weevils last for only 15–17 days; they are planted when the female weevils finish forming the leaf-rolls and inoculate the fungal spores and decay once the newly developed adults emerge.
The farming strategis of proto- and advanced-fungiculture systems are different. In advanced fungiculture systems of ants, termites and ambrosia beetles, the insects put great efforts into the maintenance of their fungal gardens after the gardens are established (Little et al., 2006). Insect farmers prepare substrates to improve the growth of fungal cultivar, fertilize the crop with fecal material or oral exudates and control pathogens early in disease outbreaks by physical weeding and antibiotics application (Currie and Stuart, 2001). In proto-fungiculture stystems, the farming strategies are relatively simpler. The Littoraria snails promote fungal growth on live Spartina plants through grazing activities and direct application of fecal pellets but do not utilize intricate pest management or inoculating techniques (Silliman and Newell, 2003). The attelabid weevils put great effort into planting the fungal garden (by forming leaf-rolls and inoculating them with spores) but do nothing more to maintain the gardens. It is obvious that ‘cultivation' of fungal gardens is a major component of the fungicultures of ants, beetles and termites, whereas ‘habitual planting' is the major component of the fungiculture of attelabid weevils.
In conclusion, the mutualism of E. chinensis and P. herquei has distinct characteristics. The Euops weevil's fungal farming strategy shows that fungal farmers can achieve evolutionary success through simple inoculating behavior without continuous tending of the inoculated microbe. Furthermore, the identification of (+)-scleroderolide as active antifungal compounds indicates that the chemical protection may be common in proto-farming systems that needs to be further discovered.
Acknowledgments
We thank Zhiqiang An at the University of Texas and Chengshu Wang at the Shanghai Institute for Life Science, Chinese Academy of Sciences for their valuable suggestions and comments on this study and Joan W Bennett at Rutgers University and John Taylor and Bruce Jaffee at the University of California for serving as presubmission reviewers. We gratefully acknowledge the financial support from the National Natural Science foundation of China (31270070) and the National Program of Drug Research and Development (2012ZX09301-003).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Aanen DK, Eggleton P, Rouland-Lefevre C, Guldberg-Froslev T, Rosendahl S, Boomsma JJ. The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc Natl Acad Sci USA. 2002;99:14887–14892. doi: 10.1073/pnas.222313099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer WA, Hoyano Y, Pedras MS, Clardy J, Arnold E. Metabolites produced by the scleroderris canker fungus, Gremmeniella-Abietina. 2. The structure of scleroderolide. Can J Chem. 1987;65:748–753. [Google Scholar]
- Bianciotto V, Bandi C, Minerdi D, Sironi M, Tichy HV, Bonfante P. An obligately endosymbiotic mycorrhizal fungus itself harbors obligately intracellular bacteria. Appl Environ Microbiol. 1996;62:3005–3010. doi: 10.1128/aem.62.8.3005-3010.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann PH, Taborsky M. Larval helpers and age polyethism in ambrosia beetles. Proc Natl Acad Sci USA. 2011;108:17064–17069. doi: 10.1073/pnas.1107758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie CR, Stuart AE. Weeding and grooming of pathogens in agriculture by ants. Proc Biol Sci. 2001;268:1033–1039. doi: 10.1098/rspb.2001.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie CR, Wong B, Stuart AE, Schultz TR, Rehner SA, Mueller UG, et al. Ancient tripartite coevolution in the attine ant-microbe symbiosis. Science. 2003;299:386–388. doi: 10.1126/science.1078155. [DOI] [PubMed] [Google Scholar]
- Currie CR, Scott JA, Summerbell RC, Malloch D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature. 2003;423:461. [Google Scholar]
- Farrell BD, Sequeira AS, O' Meara BC, Normark BB, Chung JH, Jordal BH. The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae) Evolution. 2001;55:2011–2027. doi: 10.1111/j.0014-3820.2001.tb01318.x. [DOI] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Hart AG, Anderson C, Ratnieks FL. Task partitioning in leafcutting ants. Acta Ethol. 2002;5:1–11. [Google Scholar]
- Heath JJ, Stireman JO. Dissecting the association between a gall midge, Asteromyia carbonifera, and its symbiotic fungus, Botryosphaeria dothidea. Entomol Exp Appl. 2010;137:36–49. [Google Scholar]
- Hsu SC, Lockwood JL. Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. Appl Microbiol. 1975;29:422–426. doi: 10.1128/am.29.3.422-426.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA.2008Dictionary of the Fungi10th ednCromwell Press: Trowbridge, UK [Google Scholar]
- Klaus HD, Walter G, Anderson TH.2007Compendium of Soil Fungi2nd ednAcademic Press [Google Scholar]
- Kobayashi C, Fukasawa Y, Hirose D, Kato M. Contribution of symbiotic mycangial fungi to larval nutrition of a leaf-rolling weevil. Evol Ecol. 2008;22:711–722. [Google Scholar]
- Kukor JJ, Martin MM. Acquisition of digestive enzymes by sicrid woodwasps from their fungal symbiont. Science. 1983;220:1161–1163. doi: 10.1126/science.220.4602.1161. [DOI] [PubMed] [Google Scholar]
- Little AE, Murakami T, Mueller UG, Currie CR. Defending against parasites: fungus-growing ants combine specialized behaviors and microbial symbionts to protect their fungus gardens. Biol Lett. 2006;2:12–16. doi: 10.1098/rsbl.2005.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XQ, Guo WF, Ding JQ. Mycangial fungus benefits the development of a leaf-rolling weevil Euops chinensis. J Insect Physiol. 2012;58:867–873. doi: 10.1016/j.jinsphys.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Mueller UG, Gerardo NM, Aanen DK, Six DL, Schultz TR. The evolution of agriculture in insects. Annu Rev Ecol Evol Syst. 2005;36:563–595. [Google Scholar]
- Mueller UG, Gerardo N. Fungus-farming insects: multiple origins and diverse evolutionary histories. Proc Natl Acad Sci USA. 2002;99:15247–15249. doi: 10.1073/pnas.242594799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre T, Rouland-Lefevre C, Aanen DK.2011Comparative biology of fungus cultivation in termites and antsIn: Bignell DE, Roisin Y, Lo N (eds). Biology of Termites: A Modern Synthesis Springer: New York, NY, USA; 193–201. [Google Scholar]
- Oh DC, Poulsen M, Currie CR, Clardy J. Dentigerumycin: a bacterial mediator of an ant-fungus symbiosis. Nat Chem Biol. 2009;5:391–393. doi: 10.1038/nchembio.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazoutova S, Srutka P, Holusa J, Chudickova M, Kolarik M. Diversity of xylariaceous symbionts in Xiphydria wood wasps: role of vector and a host tree. Fungal Ecol. 2010;3:392–401. [Google Scholar]
- Poulsen M, Bot ANM, Nielsen MG, Boomsma JJ. Experimental evidence for the costs and hygienic significance of the antibiotic metapleural gland secretion in leaf-cutting ants. Behav Ecol Sociobiol. 2002;52:151–157. [Google Scholar]
- Poulsen M, Erhardt DP, Molinaro DJ, Lin TL, Currie CR. Antagonistic bacterial interactions help shape host-symbiont dynamics within the fungus-growing ant-microbe mutualism. PLoS One. 2007;2:e960. doi: 10.1371/journal.pone.0000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K. An attelabid weevil (Euops splendida) cultivates fungi. J Ethol. 1985;3:151–156. [Google Scholar]
- Schultz TR, Mueller UG, Currie CR, Rehner SA.2004A comparison of agriculture in humans and in fungus-growing antsIn: Fernando EV, Meredith B (eds). Insect-Fungal Associations: Ecology and Evolution Oxford University Press: Oxford, USA; 149–190. [Google Scholar]
- Sharma M, Schmid M, Rothballer M, Hause G, Zuccaro A, Imani J, et al. Detection and identification of bacteria intimately associated with fungi of the order Sebacinales. Cell Microbiol. 2008;10:2235–2246. doi: 10.1111/j.1462-5822.2008.01202.x. [DOI] [PubMed] [Google Scholar]
- Silliman BR, Newell SY. Fungal farming in a snail. Proc Natl Acad Sci USA. 2003;100:15643–15648. doi: 10.1073/pnas.2535227100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stach JEM, Maldonado LA, Ward AC, Goodfellow M, Bull AT. New primers for the class Actinobacteria: application to marine and terrestrial environments. Environ Microbiol. 2003;5:828–841. doi: 10.1046/j.1462-2920.2003.00483.x. [DOI] [PubMed] [Google Scholar]
- Toki W, Tanahashi M, Togashi K, Fukatsu T. Fungal farming in a non-social beetle. PLoS One. 2012;7:e41893. doi: 10.1371/journal.pone.0041893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki W, Takahashi Y, Togashi K. Fungal garden making inside bamboos by a non-social fungus-growing beetle. PloS One. 2013;8:e79515. doi: 10.1371/journal.pone.0079515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Mueller UG, Clardy J. Antifungal diketopiperazines from symbiotic fungus of fungus-growing ant Cyphomyrmex minutus. J Chem Ecol. 1999;25:935–941. [Google Scholar]
- Wang YZ, Wu K, Ding JQ. Host specificity of Euops chinensis, a potential biological control agent of Fallopia japonica, an invasive plant in Europe and North America. Biocontrol. 2010;55:551–559. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor JW. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. Academic Press: New York, NY, USA; 1990. [Google Scholar]
- Yuceer C, Hsu CY, Erbilgin N, Klepzig KD. Ultrastructure of the mycangium of the southern pine beetle, Dendroctonus frontalis (Coleoptera:Curculionidae, Scotylinae): complex morphology for complex interactions. Acta Zool. 2011;92:216–224. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.