Abstract
The etiology of autism spectrum disorders (ASD) is for the majority of cases unknown and more studies of risk factors are needed. Geographic variation in ASD occurrence has been observed, and urban residence has been suggested to serve as a proxy for etiologic and identification factors in ASD. We examined the association between urbanicity level and ASD at birth and during childhood. The study used a Danish register-based cohort of more than 800,000 children of which nearly 4,000 children were diagnosed with ASD. We found a dose–response association with greater level of urbanicity and risk of ASD. This association was found for residence at birth as well as residence during childhood. Further, we found an increased risk of ASD in children who moved to a higher level of urbanicity after birth. Also, earlier age of ASD diagnosis in urban areas was observed. While we could not directly examine the specific reasons behind these associations, our results demonstrating particularly strong associations between ASD diagnosis and post-birth migration suggest the influence of identification-related factors such as access to services might have a substantive role on the ASD differentials we observed.
Keywords: Autism, Risk, Urbanicity, Movement, Diagnosis
Introduction
Autism spectrum disorders (ASD) are pervasive developmental disorders characterized by deficits in social interaction and communication and by stereotypic repetitive behaviors (Lauritsen 2013). The occurrence of ASDs has increased dramatically over the last two decades. The current prevalence estimate in many developed countries is 0.6–1 % (Kawamura et al. 2008; Boyle et al. 2011; Fombonne 2009; Posserud et al. 2010; Manning et al. 2011; Centers for Disease Control and Prevention 2012; Elsabbagh et al. 2012), but a rate as high as 2 % has recently been found in South Korea (Kim et al. 2011). The explanation for the increasing ASD prevalence is not known, but diagnostic factors have been suggested to play a considerable role (Zaroff and Uhm 2012) and likely causes could be changing diagnostic criteria, greater awareness, improved case identification, and changes in age of diagnosis (Parner et al. 2008, 2011; Gal et al. 2012). Family and twin studies have previously suggested a strong genetic component in the etiology of ASD (Geschwind 2011; Miles 2011) but few specific genes have been identified. It has, however, been suggested based on a recent twin study where a smaller genetic component was found that environmental factors may have a larger role in ASD etiology than believed previously (Cook and Scherer 2008; Hallmayer et al. 2011) but only few environmental factors have been identified. Adverse perinatal outcomes and maternal birth complications have been shown to increase the risk of ASD (Kolevzon et al. 2007; Gardener et al. 2009, 2011). Other potential environmental risk factors include viral exposure or exposure to neurotoxicants such as inorganic mercury from environmental sources (Palmer et al. 2006; Lewandowski et al. 2009), and air pollutants like metals, particulates, and volatile organic compounds (Kalkbrenner et al. 2010), for some of which only weak reported associations were found. Most studies had methodological limitations, and for all of these risk factors, the timing and dose may most likely be important for their effect on neurodevelopment.
Geographic variation in the occurrence of ASD has been studied also, and geographical spatial clustering of ASD has recently been observed (Mazumdar et al. 2010, in press; Van Meter et al. 2010). An association between ASD and urbanicity (i.e. higher risk of autism in urban versus rural districts) has been documented (Lauritsen et al. 2005; Williams et al. 2006; Rosenberg et al. 2009; Lai et al. 2012). Urbanicity may act as a proxy for other factors, e.g. better access to diagnostic and medical services or higher exposure to as yet unknown environmental risk factors for ASD in urban vs. rural areas. Cultural influences related to family's place of residence have also been suggested to affect ASD prevalence, such as the hypothesis that in geographic areas with high ASD prevalence, the sharing of information on autism between parents increased community awareness about signs and symptoms of ASD. As a consequence children living in very close proximity to a child previously diagnosed with ASD may be more likely to be diagnosed with ASD than children not living close to a diagnosed child (Liu et al. 2010). In conclusion, the influence of etiological versus identification factors including cultural influences on the ongoing secular trend in ASDs is not well established. In addition, when studying the identification and etiological factors of ASD only limited attention has until now been put on the time from birth until a diagnosis of ASD is applied (Mazumdar et al., in press).
The aim of the present population-based cohort study was to study further our previous findings that the higher degree of urbanization at place of birth in Denmark is associated to a higher risk of autism (Lauritsen et al. 2005). The current study includes a more recent and uniformly diagnosed Danish cohort than the previous study (Lauritsen et al. 2005) and aim for a more rigorous confounder adjustment.
In Denmark, a specialised diagnostic assessment of children suspected to have ASD is generally necessary in order to be enrolled in special services. Psychiatric diagnoses are applied to children referred for evaluation at a child and adolescent psychiatric hospital which is free of charge. The multi-disciplinary psychiatric evaluation of the child is supervised by a child psychiatrist and the diagnoses are registered at the Danish Psychiatric Central Research Register (DPCRR) (Mors et al. 2011) which is mandatory and used primarily for clinical purpose and surveillance but also used for research. Here, we included data from DPCRR on all children born in Denmark and present data on both residence at time of birth and current residence during childhood and specifically analyzed changes in type of residence between birth and later childhood. The current “childhood residence” construct may incorporate factors related to social and family factors in autism identification and diagnosis. We hypothesized that a high risk of ASD at place of birth indicated etiological, and specifically environmental factors of importance for the risk of ASD. Identification factors were not considered to be involved at the time of birth of the child but generally urbanicity at birth correlates highly to current urbanicity in childhood which contains identification factors. The association between urbanicity at birth and the risk of autism may therefore be explained both by etiologic and identification factors. By including urbanicity in childhood residence we may attempt to explain if the well-established association between urbanicity at birth and autism spectrum is due to etiological factors related to urbanicity or differences in identification in different urban regions. If differences were seen between place of birth and current place of residence until a diagnosis of ASD, this may indicate identification factors to play a considerable role, but etiological factors can also be involved.
Methods
Study Population
The Danish Civil Registration System (CRS) (Pedersen 2011) was used to obtain a large set of data on all Danish residents aged 1 year or older. Our study population included all children born in Denmark between 1 January 1993 and 31 December 2005, who were alive at the 1st birthday, and who had information on maternal identity. The Danish CRS (Pedersen 2011) was established in 1968, and for all persons living in Denmark or Greenland, the CRS contains information on the current full address (municipality, road, and house number), and the date when they moved to that address. For immigrants, information on country of immigration, and for emigrants, information on the country of emigration is recorded along with dates of immigration and emigration. For persons whose residence is unknown to the Danish authorities, information is recorded concerning their date of disappearance. All residents in Denmark are obliged to inform the authorities about any change of permanent address within 5 days. It is very rare that this mandatory information is not reported as failure to supply this information will result in inability to receive supplementary benefits (e.g. unemployment, sickness or disablement benefits), to attend day nursery, nursery school, and primary and lower secondary school, and to avail of free national health care, etc.
Assessment of Degree of Urbanization in Denmark
Denmark is a small ethnically homogeneous country with a population of 5.3 million people and a total area of 43,000 km2. Municipalities in Denmark have been classified according to degree of urbanization by Statistics Denmark (1997) with population densities for the capital, capital suburbs, provincial cities, provincial towns, and rural areas respectively of 5,220, 845, 470, 180, and 55 people per km2 (Statistics Denmark 1997).
In line with previous studies on schizophrenia (Mortensen et al. 1999; Pedersen and Mortensen 2001; Pedersen 2006) and ASD (Lauritsen et al. 2005) performed by our research group, both a twelve and five level categorization scheme for degree of urbanization were used and we classified residence using both schemes. We assessed ASD rates using the twelve-level categorizations, but because no additional information was provided using this categorization compared to the five-level categorizations, we present the five-level categorizations for both residence at birth and during childhood.
Assessment of Psychiatric Disorder and Parental Age
Children within the study cohort and their parents were linked via their personal identifier to the DPCRR in which diagnoses applied during all admissions to psychiatric hospitals since 1969 and all outpatient contact data since 1995 are registered (Mors et al. 2011). Diagnoses are classified according to the International Classification of Diseases (ICD), Eighth Revision (ICD-8) and Tenth Revision (ICD-10). Cohort members were classified as having an ASD if they had been admitted to a psychiatric hospital or had received outpatient care for a diagnosis of ASD (ICD-10: F84.0, F84.1, F84.5, F84.8, F84.9).
Information about the parents of children born in Denmark was derived from the Danish Medical Birth Registry (DMBR) (Knudsen and Olsen 1998) which has been computerized since 1973 and information about parental age data was obtained from CRS (Pedersen 2011). Parental age was divided into the three age groups: <35, 35–39, and 40+ years.
Parents were classified as having a history of a mental health disorder if prior to the child's birth they had been admitted to a psychiatric hospital or had been in outpatient treatment for any mental health diagnosis according to ICD-8 and ICD-10. This variable was classified as yes, no, or missing due to missing information on the identity of the father.
Assessment of Obstetric Factors
We obtained information about birth weight and gestational age at birth from the DMBR (Knudsen and Olsen 1998). Gestational age was divided into four groups: <32, 32–36, 37–40, 41+ weeks, and birth weight was also divided into four groups: ≤2.500, 2.501–3.000, 3.001–4.000, >4.000 kg.
Statistical Analyses
Individuals within the cohort were followed from their 1st birthday during residence in Denmark and for each child, follow-up began after certain eligible criteria were met (e.g. alive and resident in Denmark at 1st birthday) and ended on 31 December 2006 or when an event occurred, such as diagnosis of ASD, death, or emigration from Denmark. The date 31 December 2006 was the end date of the previous municipality system in Denmark and was chosen because the new system could not be correlated to the old one. For purposes of calculating incidence of ASD diagnosis, the date of first diagnosis was defined as the first day of the first healthcare contact (inpatient or outpatient) in which an ASD diagnosis was given. Current place of residence during childhood was treated as a time-dependent variable where each cohort member contributed with person-years at risk corresponding to the time lived in each level of urbanization during follow-up. This means that each cohort member living in a certain area each year contributed 1 year of risk for an ASD diagnosis in this area, and if they moved to different areas prior to the end of follow-up then the cohort member contributed with person-years at risk for an ASD diagnosis in different levels of urbanization. Using competing risks survival analyses (Andersen et al. 1997; Rosthoj et al. 2004), we calculated the incidence of an ASD diagnosis at the 10th birthday, taking into account migration or death. These analyses were adjusted for sex and were also sub-divided according to degree of urbanization of birth residence.
The incidence rate ratios (IRRs) for each outcome were estimated by log linear Poisson regression (Breslow and Day 1987; SAS Institute Inc 2008). All IRRs were adjusted for calendar period, child's age, and sex, and these are referred to as crude estimates. In addition to calendar period, child's age, and sex, further adjustments were made for parental age at child's birth, gestational age, birth weight, and parental psychiatric history at birth and these are referred to as adjusted estimates. Child's age, calendar period, and current place of residence were treated as time-dependent variables (Clayton and Hill 1993), whereas all other variables were treated as independent of time. p values and 95 % confidence intervals (CIs) were based on the likelihood ratio method (Clayton and Hill 1993).
IRRs for ASD were presented for (1) a child's residence at birth according to different levels of urbanization and (2) a child's residence up to diagnosis/end of follow-up where cohort members contributed with person-years at risk corresponding to the time lived in each degree of urbanization.
We studied effect-modification on the risk of ASD between residence at birth and residence during follow-up by calculating IRRs for children who changed residence between birth and end of follow-up within strata that considered both birth and follow-up residences.
We showed the degree of movement by counting the number of moves between the 5 levels of urbanization through the whole follow-up for ASD children specifically and for children in the total study cohort, calculating the number (the percentages for each column) and estimated IRRs (95 % confidence intervals) of children not moving, moving one time, or two or more times in these two groups out of the ASD and study population, respectively. For the number of people the follow-up times were not necessarily of equal length while this was accounted for in the estimated incidence rate ratios. Movements from a certain urbanization level to a destination abroad or unknown was not counted as a move, while a move back from abroad was only counted for if the child returned to a different level of urbanization as the child originally came from.
This study was approved by the Danish Data Protection Agency.
Results
Overall, 857,499 individuals were born in Denmark between 1993 and 2005. Of these, 3,921 children were diagnosed with ASD before 31 December 2006. The male–female ratio in the ASD population was 5:1.
Table 1 shows the cumulative incidences of ASD diagnosis at the 10th birthday for covariates used in the study. The cumulative incidence proportion measures the probability of being diagnosed with ASD before the 10th birthday taken into account that persons may die or emigrate from Denmark; e.g. the estimated probability that a boy having a birth weight below 2,500 g was diagnosed with ASD before the 10th birthday was 14.7 (95 % CI: 12.7–17.1) per 1,000. In addition, a high cumulative incidence of ASD diagnosis was found for boys with gestational age below 32 weeks and boys with a parent with a psychiatric disorder. The cumulative incidence of ASD was greatest in the capital and capital suburb for both boys and girls and ASD incidence decreased with decreasing level of urbanization.
Table 1.
Cohort distribution in numbers (percentages) and sex-specific cumulative incidence of Autism Spectrum Disorders (ASDs) by study characteristics
| Covariate | Distribution ASDa | Distribution all births | Cumulative incidencea at age 10 years, boys | Cumulative incidencea at age 10 years, girls |
|---|---|---|---|---|
| Sex | ||||
| Girls | 652 (16.6) | 417,666 (48.7) | – | 2.1 (1.9–2.3) |
| Boys | 2,169 (83.4) | 439,833 (51.3) | 11.0 (10.6–11.4) | – |
| Birth weight (kg) | ||||
| ≤2.5 | 286 (7.3) | 41,380 (4.8) | 14.7 (12.7–17.1) | 4.2 (3.2–5.4) |
| 2.501–3.0 | 543 (13.8) | 99,648 (11.6) | 13.2 (12.0–14.7) | 2.5 (2.1–3.1) |
| 3.001–4.0 | 2,338 (59.6) | 508,603 (59.3) | 10.5 (10.0–11.0) | 1.9 (1.7–2.1) |
| >4.0 | 687 (17.5) | 133,658 (15.6) | 10.4 (9.5–11.4) | 1.7 (1.3–2.3) |
| Missing | 67 (1.7) | 74,210 (8.7) | 10.9 (8.2–14.5) | 3.7 (2.0–6.9) |
| Gestational age (weeks) | ||||
| <32 | 57 (1.5) | 6,702 (0.8) | 17.9 (13.3–24.3) | 2.3 (0.9–5.6) |
| 32–36 | 41,690 (6.0) | 41,690 (4.9) | 11.9 (10.2–13.9) | 2.7 (1.9–3.9) |
| 37–40 | 2,527 (64.4) | 527,838 (61.6) | 10.8 (10.3–11.3) | 2.1 (1.9–2.3) |
| 41+ | 1,049 (26.8) | 210,588 (24.6) | 11.1 (10.3–11.9) | 1.9 (1.6–2.3) |
| Missing | 54 (1.4) | 70,681 (8.2) | 9.2 (6.4–13.2) | 4.1 (2.3–7.2) |
| Maternal age (years) | ||||
| <35 | 3,322 (84.7) | 735,491 (85.8) | 10.7 (10.3–11.1) | 2.0 (1.8–2.2) |
| 35–39 | 508 (13.0) | 106,187 (12.4) | 12.6 (11.4–14.1) | 2.9 (2.3–3.7) |
| 40+ | 91 (2.3) | 15,821 (1.8) | 14.6 (11.3–18.9) | 3.7 (2.2–6.1) |
| Paternal age (years) | ||||
| <35 | 2,626 (67.0) | 603,891 (70.4) | 10.3 (9.8–10.7) | 1.8 (1.6–2.0) |
| 35–39 | 795 (20.3) | 169,009 (19.7) | 12.5 (11.5–13.6) | 2.4 (2.0–3.0) |
| 40+ | 462 (11.8) | 80,115 (9.3) | 13.3 (11.9–14.9) | 3.7 (3.0–4.5) |
| Missingb | 38 (1.0) | 4,484 (0.5) | 18.5 (12.2–27.8) | 3.3 (1.4–7.9) |
| Parental psychiatric history at birth | ||||
| No | 3,544 (90.4) | 799,668 (93.3) | 10.5 (10.0–10.9) | 2.1 (1.9–2.2) |
| Yes | 325 (8.3) | 50,490 (5.9) | 20.8 (18.2–23.7) | 3.2 (2.3–4.5) |
| Missingc | 52 (1.3) | 7,341 (0.9) | 17.1 (12.0–24.3) | 2.3 (0.9–5.5) |
| Residence at birth | ||||
| Capital | 929 (23.7) | 125,478 (14.6) | 17.7 (16.3–19.1) | 4.1 (3.5–4.8) |
| Capital suburb | 648 (16.5) | 114,571 (13.4) | 13.9 (12.6–15.2) | 2.7 (2.2–3.3) |
| Provincial city | 466 (11.9) | 108,948 (12.7) | 10.8 (9.8–12.1) | 1.9 (1.4–2.4) |
| Provincial town | 933 (23.8) | 233,460 (27.2) | 9.7 (9.0–10.5) | 1.7 (1.4–2.0) |
| Rural area | 945 (24.1) | 275,042 (32.1) | 8.1 (7.5–8.8) | 1.5 (1.2–1.8) |
Estimated cumulative incidence of ASD for 1,000 children (95 % confidence intervals) calculated in competing risk survival analyses
Age of father unknown
Father unknown
In Table 2, IRRs are presented for a diagnosis of ASD and 1) a child's residence at birth according to different levels of urbanization and 2) a child's residence up to diagnosis/end of follow-up. For both residence at birth and follow-up, diagnosed ASD incidence (both crude and adjusted) increased with increasing level of urbanization showing a dose–response relationship with increasing degree of urbanicity.
Table 2.
Incidence rate ratios (IRRs) (95 % confidence intervals) for Autism Spectrum Disorders according to level of urbanization
| Level | Residence at birth |
Current place of residence |
||
|---|---|---|---|---|
| Crudea IRR | Adjustedb IRR | Crudea IRR | Adjustedb IRR | |
| Capital | 2.35 (2.15–2.57) | 2.28 (2.09–2.50) | 2.96 (2.69–3.25) | 2.85 (2.59–3.13) |
| Capital suburb | 1.67 (1.51–1.85) | 1.64 (1.49–1.81) | 1.73 (1.57–1.91) | 1.71 (1.55–1.88) |
| Provincial city | 1.28 (1.14–1.43) | 1.28 (1.14–1.42) | 1.31 (1.16–1.47) | 1.30 (1.15–1.46) |
| Provincial town | 1.17 (1.07–1.28) | 1.16 (1.06–1.27) | 1.31 (1.20–1.43) | 1.30 (1.19–1.42) |
| Rural area | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
Adjustment has been made for age, sex, and calendar period
Adjustment has been made for age, sex, calendar period, maternal and paternal age, gestational age, birth weight, and parental psychiatric history at birth
When we evaluated the potential effect-modification on the risk of ASD between residence at birth and residence during follow-up with children who were both born in and resided in a rural area during childhood chosen as the reference category, a dose–response association in risk of ASD with level of urbanicity was observed among children with the same urbanicity level of residence at birth and follow-up, shown on the diagonal in Table 3 with bold numbers (Table 3).
Table 3.
Adjusted incidence rate ratios (95 % confidence intervals) for Autism Spectrum Disorders (ASDs) by residence at birth and current place of residence including percent of total cases of ASD and person year
| Residence at birth | Current place of residence |
||||
|---|---|---|---|---|---|
| Capital | Capital suburb | Provincial city | Provincial town | Rural area | |
| Capital | |||||
| % Cases | 18.57 | 3.19 | 0.08 | 1.12 | 0.74 |
| % Person year | 9.48 | 2.26 | 0.13 | 0.91 | 0.93 |
| IRR (95 % CI) | 3.04 (2.74–3.36) | 1.92 (1.58–2.31) | – | 1.64 (1.19–2.19) | 1.02 (0.69–1.45) |
| Capital suburb | |||||
| % Cases | 0.59 | 13.95 | 0.13 | 0.89 | 0.97 |
| % Person year | 0.29 | 11.78 | 0.07 | 0.53 | 0.77 |
| IRR (95 % CI) | 2.72 (1.74–4.02) | 1.80 (1.61–2.01) | – | 2.25 (1.57–3.10) | 1.63 (1.16–2.23) |
| Provincial city | |||||
| % Cases | 0.18 | 0.08 | 9.08 | 1.05 | 1.50 |
| % Person year | 0.11 | 0.10 | 10.28 | 0.66 | 1.45 |
| IRR (95 % CI) | – | – | 1.36 (1.20–1.55) | 2.21 (1.59–2.98) | 1.40 (1.06–1.80) |
| Provincial town | |||||
| % Cases | 0.23 | 0.41 | 0.46 | 19.89 | 2.81 |
| % Person year | 0.17 | 0.28 | 0.32 | 24.13 | 2.69 |
| IRR (95 % CI) | – | 1.97 (1.15–3.12) | 1.95 (1.18–3.02) | 1.26 (1.14–1.39) | 1.39 (1.13–1.69) |
| Rural area | |||||
| % Cases | 0.48 | 0.38 | 0.43 | 3.37 | 19.43 |
| % Person year | 0.10 | 0.23 | 0.44 | 2.21 | 29.66 |
| IRR (95 % CI) | 6.37 (3.90–9.75) | 2.20 (1.26–3.53) | 1.34 (0.79–2.09) | 2.09 (1.73–2.51) | 1.00 (Reference) |
Adjustment has been made for age, sex, calendar period, maternal and paternal age, gestational age, birth weight, and parental psychiatric history at birth
– indicates less than 10 observed cases of ASD in the category and hence the estimates are uncertain
In bold are shown the adjusted incidence rate ratios including 95 % confidence intervals for children with the same urbanicity level of residence at birth and follow-up
For example, children born in and residing in the capital suburb had an IRR of 1.80 indicating an 80 % increase in the rate as compared to children both born and residing in rural areas whereas children with residence at birth and residence at follow-up in provincial towns had an IRR of 1.26 relative to the rural group. We observed the highest IRRs of ASD for two groups of children, namely children who were both born and currently residing in the capital and children who were born in rural areas and were subsequently residing in the capital; IRRs for these two groups were 3.04 and 6.37, respectively. Any change of residence after birth for children born in the capital resulted in a marked decreased IRR for ASD. In addition, when comparing the diagonal IRRs to IRRs in the same row (which comprises children who change urbanicity level of residence from birth during follow-up) a tendency towards the diagonal IRRs most often being the lowest is seen. For example, for children born in provincial towns who did not change residence during follow-up, an IRR of 1.26 was found which was generally lower than the IRRs of children with residence at birth in provincial towns who moved to another urbanicity level. This tendency for lower diagonal IRRs compared to most of the other IRRs in the same row may be indicative of increased IRRs if the child not born in the capital changed level of urbanization after birth no matter whether it was to a higher or lower level of urbanicity.
Table 4 shows the same trend in a more simple way with place of residence categorized as lower, higher or the same level of urbanicity compared to residence at birth. Compared to the IRRs for children with same level of urbanicity for birth residence as place of residence during follow-up, we found higher IRRs for children who moved to either lower or higher level of urbanicity. For example, children residing in provincial towns at birth and residing there until ASD diagnosis or the end of follow-up had a lower IRR of 1.26 (as mentioned above) compared to children residing in provincial towns at birth and moving to a higher or lower level of urbanicity during upbringing which had IRRs of 1.95 and 1.39, respectively. Typically, however, the highest IRRs for children born in capital suburbs, provincial cities or provincial towns tended to be among children who moved to a higher level of urbanicity. The significant drop in IRR for children who were born in the capital and moved to a lower level of urbanicity and the significant increase in IRR for children who were born in the rural area and moved to a higher level of urbanicity is also apparent in Table 4.
Table 4.
Adjusted incidence rate ratios (95 % confidence interval) for Autism Spectrum Disorders for change of urbanization
| Residence at birth | Residence after birth |
||
|---|---|---|---|
| Lower | Same level of urbanicity as at the time of birth | Higher | |
| Capital | 1.62 (1.38–1.89) | 3.04 (2.74–3.36) | |
| Capital suburb | 1.91 (1.50–2.40) | 1.80 (1.61–2.01) | 2.72 (1.74–4.02) |
| Provincial city | 1.65 (1.33–2.02) | 1.36 (1.20–1.55) | 1.69 (0.84–2.97) |
| Provincial town | 1.39 (1.13–1.69) | 1.26 (1.14–1.39) | 1.95 (1.41–2.62) |
| Rural area | 1.00 (Reference) | 2.14 (1.81–2.51) | |
Adjustment has been made for age, sex, calendar period, maternal and paternal age, gestational age, birth weight, and parental psychiatric history at birth
Table 5 shows the number of moves for ASD children specifically and for all children in the total study cohort including IRRs, and the table supported the hypothesis of an increased risk associated with change of degree of urbanization. In fact, the more changes in degree of urbanization during childhood, the higher the risk of ASD (Table 5).
Table 5.
Number (percent of total cases) of moves between the 5 levels of urbanization and incidence rate ratios (IRRs) of Autism Spectrum Disorders (95 % confidence intervals for each child in the follow–up period)
| Number of moves | ASD (%) | Total (%) | Crude IRRa | Adjusted IRRb |
|---|---|---|---|---|
| 0 | 3,209 (81.84) | 718,799 (83.83) | 1 | 1 |
| 1 | 531 (13.54) | 105,865 (12.35) | 1.26 (1.15–1.38) | 1.26 (1.15–1.39) |
| 2+ | 181 (4.62) | 32,835 (3.83) | 1.61 (1.38–1.87) | 1.57 (1.34–1.82) |
Adjustment has been made for age, sex, and calendar period
Adjustment has been made for age, sex, calendar period, maternal and paternal age, gestational age, birth weight, and parental psychiatric history at birth
Figure 1 shows the effect of place of birth by child's age during follow-up and Fig. 2 shows the effect of residence of birth on ASD risk subdivided by birth year. In both figures, the highest IRRs were found in the capital and capital suburb. For all ages, the higher the degree of urbanization of place of birth, the higher the risk of ASD (Fig. 1). Similarly, for all birth years, the higher the degree of urbanization of place of birth, the higher the risk of ASD (Fig. 2). However, these differentials were greatest for children diagnosed at young age and in the most recent birth years. Figure 3 shows the effect of current place of residence by calendar year of diagnosis, and at all times the highest IRR was found in the capital and capital suburb. Also, the trends over calendar time in IRRs for the different degrees of urbanization of place of residence were very similar.
Fig. 1.
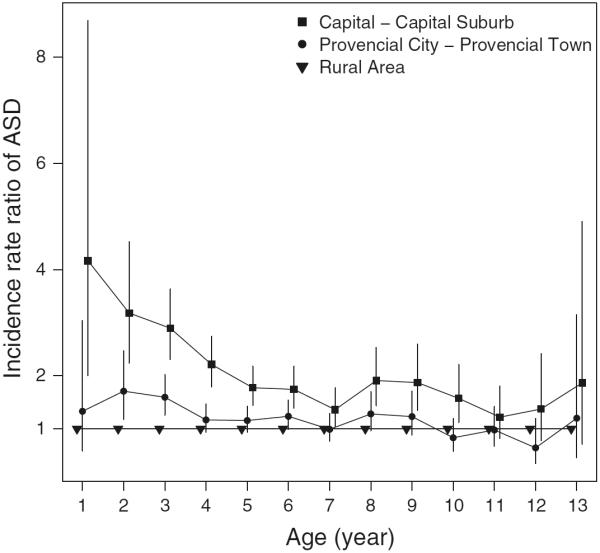
Incidence rate ratio of Autism Spectrum Disorders (ASDs) according to the degree of urbanicity of residence at birth and age during follow-up. Vertical lines indicate 95 % confidence intervals. Adjustment has been made for age, sex, calendar period, maternal and paternal age, gestational age, birth weight, and parental psychiatric history at birth
Fig. 2.
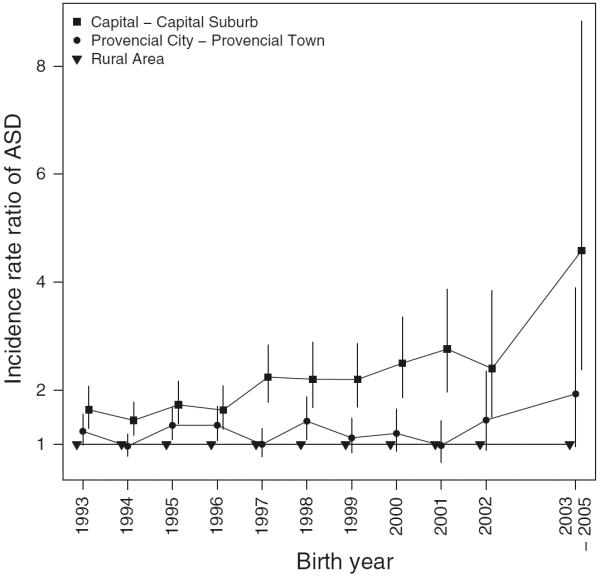
Incidence rate ratio of Autism Spectrum Disorders (ASDs) according to the degree of urbanicity of residence at birth and birth year. Due to a low number of observed cases of ASD in the rural area in birth year 2003, 2004, and 2005, they were grouped into one category. Vertical lines indicate 95 % confidence intervals. Adjustment has been made for age, sex, calendar period, maternal and paternal age, gestational age, birth weight, and parental psychiatric history at birth
Fig. 3.
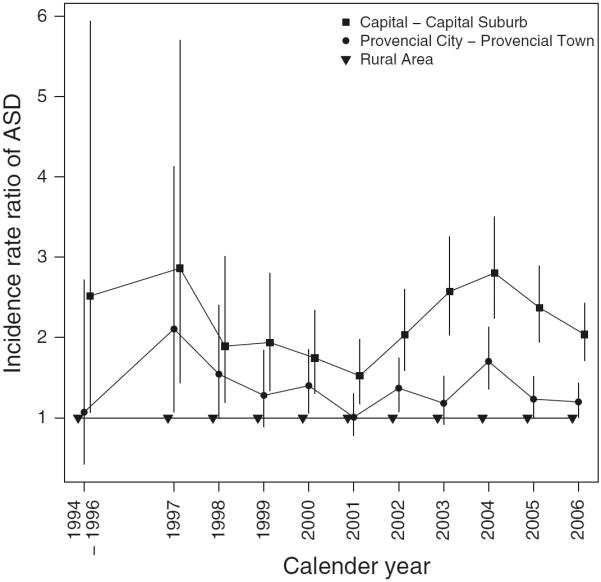
Incidence rate ratio of Autism Spectrum Disorders (ASDs) according to the degree of urbanicity of place of residence and calendar year of diagnosis. Due to a low number of observed cases of ASD in the rural area in birth year 1994, 1995, and 1996, they were grouped into one category. Vertical lines indicate 95 % confidence intervals. Adjustment has been made for age, sex, calendar period, maternal and paternal age, gestational age, birth weight, and parental psychiatric history at birth
Discussion
This study showed that the risk of ASD was positively associated with residence at birth as well as residence in childhood; the higher degree of urbanization of both residence at birth and during childhood, the higher the risk of ASD. This dose–response association was robust for all age periods and calendar year periods, especially in children diagnosed at the youngest ages and born in the most recent birth years. In addition, a trend towards a higher risk of ASD for children changing level of urbanicity between birth and the end of follow-up compared to children with the same urbanicity level of residence at birth as current residence was suggested. A notable exception to this pattern was children born in the highest level of urbanicity (capital) who had a marked decreased risk for ASD if they moved to a lower level of urbanicity after birth.
Our finding of very young children who were born in the capital having a higher risk of being diagnosed with ASD has been found previously by others (Mandell et al. 2005; Fountain et al. 2011) and it may indicate more awareness or attention to the disorder or that better or more readily accessible diagnostic and medical services are available in the urban areas. Kalkbrenner et al. (2011) addressed this issue in a population-based design and found that the median age at ASD diagnosis was lower for children living in areas with more psychologists and specialist physicians including neurologists and psychiatrists in contrast to areas with many primary care physicians.
Urbanicity at birth or in childhood may serve as a proxy for other factors influencing the risk of ASD. The high IRRs found in our study for children born in the capital and capital suburb could support the hypothesis of environmental agents acting during pregnancy or early in life of the child affecting the risk for ASD, such as infections or air pollutants containing neurotoxic or immunotoxic properties, a hypothesis that has been investigated by Kalkbrenner et al. (2010). To date, however, there is little evidence for the influence of such environmental pollutants including specific agents on the development of ASD (Kalkbrenner et al. 2010). Our finding of an increased risk of ASD in urban areas compared to rural areas is in accordance with findings from studies of geographically and ethnically diverse areas such as Japan (Hoshino et al. 1982), Taiwan (Chen et al. 2008), and the United States (Rosenberg et al. 2009). Furthermore, in a systematic review of prevalence studies of ASD, Williams et al. (2006) found an association between urban residence of the child and ASD. In the study from Taiwan (Chen et al. 2008) the urban/rural differences in risk of ASD have been suggested to be due to insufficient access to and availability of health care services. Another research group also found higher rates of ASD in urban areas compared to rural areas (Palmer et al. 2006), and they suggested that some unidentified socio-cultural factors related to ethnicity were involved in differences in rates of ASD.
The finding of the lowest risk of ASD in rural areas may be in concordance with the “hygiene hypothesis” first proposed by Strachan (1989) in the field of allergic diseases. The hypothesis had its origin in studies showing decreased incidence or risk of allergic diseases in rural, farming communities. For ASD, the explanatory mechanism could be a protective effect of living in rural areas because of exposure to allergens like e.g. farm animal contact stimulating the immune system.
While we did not have data to directly assess the specific factors contributing to increased risk of ASD among children born or residing in urban areas compared to rural areas, our data indirectly point to identification rather than etiologic explanations. If the primary explanation for the increased risk of ASD among children born in the most urbanized areas were etiologic, we would not expect post-birth migrations to have much influence on ASD prevalence and this is not the case in our study. We found the highest IRRs among children who moved to a higher level of urbanicity compared to children not moving when we look at children born in capital suburbs, provincial cities, or provincial towns, and particularly we found a high IRR of 6.37 for children born in rural areas who subsequently moved to urban areas (Table 2). With respect to etiologic factors involved in ASD, evidence from numerous past studies, however, points to the perinatal period (or before) as the likely key exposure time frame for environmental etiologic risk factors for ASD (Kolevzon et al. 2007; Gardener et al. 2009, 2011).
The finding of higher IRRs for children moving to a higher level of urbanicity compared in particular to children moving to a lower level of urbanicity (Table 3) could further support a hypothesis of identification factors involved with different access to or availability of health services corresponding to the different levels of urbanicity, a hypothesis that has been further studied by Mazumdar et al. (in press). The movement patterns for some families might also be related to the need for diagnostic and treatment services for a child who appears developmentally delayed where the child and/or the family with ASD may not be optimal functioning and as a consequence may move to another area with better services. Such services are more available in urbanized than rural areas. Socio-cultural factors affecting the risk of being diagnosed with ASD, such as differences in awareness corresponding with different level of urbanicity may also explain some of our observed findings. In summary, the fact that post-birth migrations were in some cases more strongly associated with ASD than urbanicity level at birth, plus the drop in risk associated with movement out of the urban area after birth, is suggestive of an identification effect.
Strengths of our study include the population-based design, the large sample size, and the ability to analyze child's residence at different points in time by use of the Danish registers. However, although the analyses have been corrected for calendar year, the results need to be interpreted with caution as the data analyzed are from a period during which the incidence of autism was increasing.
An urban–rural geographic gradient has been detected for other psychiatric disorders than ASD, e.g. schizophrenia, bipolar affective disorder, and depression (Sundquist et al. 2004; Pedersen and Mortensen 2006; Vassos et al. 2012). Explanations for these findings include some of the same hypotheses as has been suggested for ASD. The research in the field of urbanicity will benefit from comparing the associations found for different psychiatric disorders in order to detect specific causal or non-etiologic mechanism increasing the risk of the different disorders.
We were not able to investigate the influence of socioeconomic factors on the associations presented here. However, past studies have shown that in Denmark, ASD prevalence is not as variable across SES groups as has been observed in more heterogeneous countries such as the United States (Larsson et al. 2005; Durkin et al. 2010; Thomas et al. 2012). In Denmark, due to the organization of the health care system there is not a large differential in access to health services between different socioeconomic groups. Nonetheless, we cannot rule out potential SES effects entirely as care-seeking behavior and residential mobility might differ between different social groups in Denmark.
In contrast to other studies investigating geographic variation in the occurrence of ASD, we used a classification of geographic variation that only allowed us to study conditions that could be described from the density of population size throughout Denmark. We were, therefore, unable to pinpoint certain geographical areas where the risk of ASD was greater which could facilitate the identification of environmental factors acting in that specific area.
In conclusion, the incidence of ASD varies across an urban–rural gradient in Denmark. Both residence at birth and residence later in childhood are associated with diagnosis of ASD, and with the exception of children residing in the most urbanized area at birth (the capital), post-birth migration was even more strongly associated with a subsequent ASD diagnosis. Thus, associations with residence may reflect the impact of identification factors on geographic variation in ASD incidence although we cannot rule out the influence of etiologic factors as well. Our findings also support the notion that children with ASD residing in less populated areas might be at risk for a later diagnosis compared to children living in more densely populated areas. Further studies are needed to more fully understand the possible influences of both identification and environmental factors underlying geographic variation in ASD.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease and Control and Prevention.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- Andersen PK, Borgan Ø, Gill RD, Keiding N. Statistical models based on counting processes. Springer; New York: 1997. [Google Scholar]
- Boyle CA, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, et al. Trends in the prevalence of developmental disabilities in US Children, 1997–2008. Pediatrics. 2011;127:1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- Breslow NE, Day NE. Statistical methods in cancer research volume II—The design and analysis of cohort studies. 82nd ed. IARC Scientific Publications; France: 1987. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Prevalence of autism spectrum disorders—Autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveillance Summaries. 2012;61:1–19. [PubMed] [Google Scholar]
- Chen CY, Liu CY, Su WC, Huang SL, Lin KM. Urbanicity-related variation in help-seeking and services utilization among preschool-age children with autism in Taiwan. Journal of Autism and Developmental Disorders. 2008;38:489–497. doi: 10.1007/s10803-007-0416-y. [DOI] [PubMed] [Google Scholar]
- Clayton D, Hill M. Statistical model of epidemiology. Oxford University Press; Oxford, New York, Tokyo: 1993. [Google Scholar]
- Cook EH, Jr, Scherer SW. Copy-number variations associated with neuropsychiatric conditions. Nature. 2008;455:919–923. doi: 10.1038/nature07458. [DOI] [PubMed] [Google Scholar]
- Durkin MS, Maenner MJ, Meaney FJ, Levy SE, DiGuiseppi C, Nicholas JS, et al. Socioeconomic inequality in the prevalence of autism spectrum disorder: Evidence from a U.S. cross-sectional study. PLoS ONE. 2010;5:e11551. doi: 10.1371/journal.pone.0011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcin C, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Research. 2012;5:160–179. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E. Epidemiology of pervasive developmental disorders. Pediatric Research. 2009;65:591–598. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- Fountain C, King MD, Bearman PS. Age of diagnosis for autism: Individual and community factors across 10 birth cohorts. Journal of Epidemiology and Community Health. 2011;65:503–510. doi: 10.1136/jech.2009.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal G, Abiri L, Reichenberg A, Gabis L, Gross R. Time trends in reported autism spectrum disorders in Israel, 1986–2005. Journal of Autism and Developmental Disorders. 2012;42:428–431. doi: 10.1007/s10803-011-1252-7. [DOI] [PubMed] [Google Scholar]
- Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: Comprehensive meta-analysis. British Journal of Psychiatry. 2009;195:7–14. doi: 10.1192/bjp.bp.108.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: A comprehensive meta-analysis. Pediatrics. 2011;128:344–355. doi: 10.1542/peds.2010-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH. Genetics of autism spectrum disorders. Trends in Cognitive Sciences. 2011;15:409–416. doi: 10.1016/j.tics.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y, Kumashiro H, Yashima Y, Tachibana R, Watanabe M. The epidemiological study of autism in Fukushima-ken. Folia Psychiatrica et Neurologica Japonica. 1982;36:115–124. doi: 10.1111/j.1440-1819.1982.tb00262.x. [DOI] [PubMed] [Google Scholar]
- Kalkbrenner AE, Daniels JL, Chen JC, Poole C, Emch M, Morrissey J. Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8. Epidemiology. 2010;21:631–641. doi: 10.1097/EDE.0b013e3181e65d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE, Daniels JL, Emch M, Morrissey J, Poole C, Chen JC. Geographic access to health services and diagnosis with an autism spectrum disorder. Annals of Epidemiology. 2011;21:304–310. doi: 10.1016/j.annepidem.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Takahashi O, Ishii T. Reevaluating the incidence of pervasive developmental disorders: Impact of elevated rates of detection through implementation of an integrated system of screening in Toyota, Japan. Psychiatry and Clinical Neurosciences. 2008;62:152–159. doi: 10.1111/j.1440-1819.2008.01748.x. [DOI] [PubMed] [Google Scholar]
- Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, et al. Prevalence of autism spectrum disorders in a total population sample. American Journal of Psychiatry. 2011;168:904–912. doi: 10.1176/appi.ajp.2011.10101532. [DOI] [PubMed] [Google Scholar]
- Knudsen LB, Olsen J. The Danish medical birth registry. Danish Medical Bulletin. 1998;45:320–323. [PubMed] [Google Scholar]
- Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: A review and integration of findings. Archives of Pediatrics and Adolescent Medicine. 2007;161:326–333. doi: 10.1001/archpedi.161.4.326. [DOI] [PubMed] [Google Scholar]
- Lai DC, Tseng YC, Hou YM, Guo HR. Gender and geographic differences in the prevalence of autism spectrum disorders in children: Analysis of data from the national disability registry of Taiwan. Research in Developmental Disabilities. 2012;33:909–915. doi: 10.1016/j.ridd.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Larsson HJ, Eaton WW, Madsen KM, Vestergaard M, Olesen AV, Agerbo E, et al. Risk factors for autism: Perinatal factors, parental psychiatric history, and socioeconomic status. American Journal of Epidemiology. 2005;161:916–925. doi: 10.1093/aje/kwi123. [DOI] [PubMed] [Google Scholar]
- Lauritsen MB. Autism spectrum disorders. European Child and Adolescent Psychiatry. 2013;22(Suppl 1):37–42. doi: 10.1007/s00787-012-0359-5. [DOI] [PubMed] [Google Scholar]
- Lauritsen MB, Pedersen CB, Mortensen PB. Effects of familial risk factors and place of birth on the risk of autism: A nationwide register-based study. Journal of Child Psychology and Psychiatry. 2005;46:963–971. doi: 10.1111/j.1469-7610.2004.00391.x. [DOI] [PubMed] [Google Scholar]
- Lewandowski TA, Bartell SM, Yager JW, Levin L. An evaluation of surrogate chemical exposure measures and autism prevalence in Texas. Journal of Toxicology and Environmental Health. Part A. 2009;72:1592–1603. doi: 10.1080/15287390903232483. [DOI] [PubMed] [Google Scholar]
- Liu KY, King M, Bearman PS. Social influence and the autism epidemic. AJS. 2010;115:1387–1434. doi: 10.1086/651448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell DS, Novak MM, Zubritsky CD. Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics. 2005;116:1480–1486. doi: 10.1542/peds.2005-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning SE, Davin CA, Barfield WD, Kotelchuck M, Clements K, Diop H, et al. Early diagnoses of autism spectrum disorders in Massachusetts birth cohorts, 2001–2005. Pediatrics. 2011;127:1043–1051. doi: 10.1542/peds.2010-2943. [DOI] [PubMed] [Google Scholar]
- Mazumdar S, King M, Liu KY, Zerubavel N, Bearman P. The spatial structure of autism in California, 1993–2001. Health Place. 2010;16:539–546. doi: 10.1016/j.healthplace.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar S, Winter A, Liu KY, Bearman P. Spatial clusters of autism births and diagnoses point to contextual drivers of increased prevalence. Social Science & Medicine. doi: 10.1016/j.socscimed.2012.11.032. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles JH. Autism spectrum disorders—a genetics review. Genetic Medicine. 2011;13:278–294. doi: 10.1097/GIM.0b013e3181ff67ba. [DOI] [PubMed] [Google Scholar]
- Mors O, Perto GP, Mortensen PB. The Danish psychiatric central research register. Scandinavian Journal of Public Health. 2011;39:54–57. doi: 10.1177/1403494810395825. [DOI] [PubMed] [Google Scholar]
- Mortensen PB, Pedersen CB, Westergaard T, Wohlfahrt J, Ewald H, Mors O, et al. Effects of family history and place and season of birth on the risk of schizophrenia. New England Journal of Medicine. 1999;340:603–608. doi: 10.1056/NEJM199902253400803. [DOI] [PubMed] [Google Scholar]
- Palmer RF, Blanchard S, Stein Z, Mandell D, Miller C. Environmental mercury release, special education rates, and autism disorder: An ecological study of Texas. Health Place. 2006;12:203–209. doi: 10.1016/j.healthplace.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Parner ET, Schendel DE, Thorsen P. Autism prevalence trends over time in Denmark: Changes in prevalence and age at diagnosis. Archives of Pediatrics and Adolescent Medicine. 2008;162:1150–1156. doi: 10.1001/archpedi.162.12.1150. [DOI] [PubMed] [Google Scholar]
- Parner ET, Thorsen P, Dixon G, de Klerk N, Leonard H, Nassar N, et al. A comparison of autism prevalence trends in Denmark and Western Australia. Journal of Autism and Developmental Disorders. 2011;41:1601–1608. doi: 10.1007/s10803-011-1186-0. [DOI] [PubMed] [Google Scholar]
- Pedersen CB. No evidence of time trends in the urban-rural differences in schizophrenia risk among five million people born in Denmark from 1910 to 1986. Psychological Medicine. 2006;36:211–219. doi: 10.1017/S003329170500663X. [DOI] [PubMed] [Google Scholar]
- Pedersen CB. The Danish civil registration system. Scandinavian Journal of Public Health. 2011;39:22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- Pedersen CB, Mortensen PB. Family history, place and season of birth as risk factors for schizophrenia in Denmark: A replication and reanalysis. British Journal of Psychiatry. 2001;179:46–52. doi: 10.1192/bjp.179.1.46. [DOI] [PubMed] [Google Scholar]
- Pedersen CB, Mortensen PB. Urbanicity during upbringing and bipolar affective disorders in Denmark. Bipolar Disorders. 2006;8:242–247. doi: 10.1111/j.1399-5618.2006.00307.x. [DOI] [PubMed] [Google Scholar]
- Posserud M, Lundervold AJ, Lie SA, Gillberg C. The prevalence of autism spectrum disorders: Impact of diagnostic instrument and non-response bias. Social Psychiatry and Psychiatric Epidemiology. 2010;45:319–327. doi: 10.1007/s00127-009-0087-4. [DOI] [PubMed] [Google Scholar]
- Rosenberg RE, Daniels AM, Law JK, Law PA, Kaufmann WE. Trends in autism spectrum disorder diagnoses: 1994–2007. Journal of Autism and Developmental Disorders. 2009;39:1099–1111. doi: 10.1007/s10803-009-0723-6. [DOI] [PubMed] [Google Scholar]
- Rosthoj S, Andersen PK, Abildstrom SZ. SAS macros for estimation of the cumulative incidence functions based on a Cox regression model for competing risks survival data. Computer Methods and Programs in Biomedicine. 2004;74:69–75. doi: 10.1016/S0169-2607(03)00069-5. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc . SAS/STAT 9.2 user's guide. SAS Institute Inc; Cary, NC: 2008. The GENMOD Procedure; pp. 1609–1730. [Google Scholar]
- Statistics Denmark . Befolkningen i kommunerne 1. Januar 1997 [Population in municipalities 1 January 1997] Statistics Denmark; Copenhagen: 1997. [Google Scholar]
- Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist K, Frank G, Sundquist J. Urbanisation and incidence of psychosis and depression: Follow-up study of 4.4 million women and men in Sweden. British Journal of Psychiatry. 2004;184:293–298. doi: 10.1192/bjp.184.4.293. [DOI] [PubMed] [Google Scholar]
- Thomas P, Zahorodny W, Peng B, Kim S, Jani N, Halperin W, et al. The association of autism diagnosis with socioeconomic status. Autism. 2012;16:201–213. doi: 10.1177/1362361311413397. [DOI] [PubMed] [Google Scholar]
- Van Meter KC, Christiansen LE, Delwiche LD, Azari R, Carpenter TE, Hertz-Picciotto I. Geographic distribution of autism in California: A retrospective birth cohort analysis. Autism Research. 2010;3:19–29. doi: 10.1002/aur.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassos E, Pedersen CB, Murray RM, Collier DA, Lewis CM. Meta-analysis of the association of urbanicity with schizophrenia. Schizophrenia Bulletin. 2012;38:1118–1123. doi: 10.1093/schbul/sbs096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JG, Higgins JP, Brayne CE. Systematic review of prevalence studies of autism spectrum disorders. Archives of Disease in Childhood. 2006;91:8–15. doi: 10.1136/adc.2004.062083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaroff CM, Uhm SY. Prevalence of autism spectrum disorders and influence of country of measurement and ethnicity. Social Psychiatry and Psychiatric Epidemiology. 2012;47:395–398. doi: 10.1007/s00127-011-0350-3. [DOI] [PubMed] [Google Scholar]


