Abstract
Importance
Valproate is used for the treatment of epilepsy and other neuropsychological disorders and may be the only treatment option for women of childbearing potential. However, prenatal exposure to valproate may increase the risk of autism.
Objective
To determine whether prenatal exposure to valproate is associated with an increased risk of autism in offspring.
Design, Setting, and Participants
Population-based study of all children born alive in Denmark from 1996 to 2006. National registers were used to identify children exposed to valproate during pregnancy and diagnosed with autism spectrum disorders (childhood autism [autistic disorder], Asperger syndrome, atypical autism, and other or unspecified pervasive developmental disorders). We analyzed the risks associated with all autism spectrum disorders as well as childhood autism. Data were analyzed by Cox regression adjusting for potential confounders (maternal age at conception, paternal age at conception, parental psychiatric history, gestational age, birth weight, sex, congenital malformations, and parity). Children were followed up from birth until the day of autism spectrum disorder diagnosis, death, emigration, or December 31, 2010, whichever came first.
Main Outcomes and Measures
Absolute risk (cumulative incidence) and the hazard ratio (HR) of autism spectrum disorder and childhood autism in children after exposure to valproate in pregnancy.
Results
Of 655 615 children born from 1996 through 2006, 5437 were identified with autism spectrum disorder, including 2067 with childhood autism. The mean age of the children at end of follow-up was 8.84 years (range, 4-14; median, 8.85). The estimated absolute risk after 14 years of follow-up was 1.53% (95% CI, 1.47%- 1.58%) for autism spectrum disorder and 0.48% (95% CI, 0.46%-0.51%) for childhood autism. Overall, the 508 children exposed to valproate had an absolute risk of 4.42% (95% CI, 2.59%-7.46%) for autism spectrum disorder (adjusted HR, 2.9 [95% CI, 1.7-4.9]) and an absolute risk of 2.50% (95% CI, 1.30%-4.81%) for childhood autism (adjusted HR, 5.2 [95% CI, 2.7-10.0]). When restricting the cohort to the 6584 children born to women with epilepsy, the absolute risk of autism spectrum disorder among 432 children exposed to valproate was 4.15% (95% CI, 2.20%-7.81%) (adjusted HR, 1.7 [95% CI, 0.9-3.2]), and the absolute risk of childhood autism was 2.95% (95% CI, 1.42%-6.11%) (adjusted HR, 2.9 [95% CI, 1.4-6.0]) vs 2.44% (95% CI, 1.88%-3.16%) for autism spectrum disorder and 1.02% (95% CI, 0.70%-1.49%) for childhood autism among 6152 children not exposed to valproate.
Conclusions and Relevance
Maternal use of valproate during pregnancy was associated with a significantly increased risk of autism spectrum disorder and childhood autism in the offspring, even after adjusting for maternal epilepsy. For women of childbearing potential who use antiepileptic medications, these findings must be balanced against the treatment benefits for women who require valproate for epilepsy control.
Antiepileptic drug exposure during pregnancy has been associated with an increased risk for congenital malformations and delayed cognitive development in the offspring,1-3 but little is known about the risk of other serious neuropsychiatric disorders. Autistic symptoms have been described in case series of children exposed in utero to valproic acid,4-7 and it has been suggested that valproate given to animals during pregnancy may constitute an experimental model of autism.8-10
Genes play a significant role in the risk of autism, and disease-related genes may be identified in up to 25% of children with autism.11 However, a variety of environmental factors may increase the risk of autism,12 and prenatal valproate exposure would be a modifiable environmental exposure.
Autism spectrum disorders are characterized by social and communication difficulties and by stereotyped or repetitive behaviors and interests.13 The autism spectrum disorders include childhood autism (autistic disorder), Asperger syndrome, atypical autism, and other or unspecified pervasive developmental disorders. We analyzed the risks associated with all autism spectrum disorders and with the most severe of the autism spectrum disorders, childhood autism. A diagnosis of childhood autism requires symptoms related to (1) stereotyped patterns of behavior, (2) impaired social interaction, and (3) impaired communication. Symptoms in at least 1 area must manifest before age 3 years. In this large population-based cohort study, we evaluated the association between maternal use of valproate during pregnancy and the risk of autism spectrum disorder and childhood autism in the offspring, taking parental history of epilepsy and psychiatric conditions into account.
METHODS
We conducted a population-based cohort study on the risk of autism in children exposed prenatally to valproate. The risk estimates were adjusted for known risk factors for autism. We also estimated the effects of valproate dose, trimester of exposure, valproate polytherapy, congenital malformations, and maternal history of epilepsy.
Ethical Approval
All data were analyzed at Statistics Denmark using encrypted identification numbers with no contact with the individuals. The study was approved by the Danish Data Protection Agency and a Danish Regional Ethical Committee. The requirement for informed consent was waived because of the use of deidentified data.
Study Population
We identified all children born alive in Denmark between January 1, 1996, and December 31, 2006, using data from the Danish Civil Registration System.14 The unique personal identification number includes information on sex and age and was used to ensure complete linkage of individual information in all national registries used in this study.
Medication Exposure
The Danish Prescription Register holds information on all prescriptions filled since January 1, 1996. Information about medical treatment given only in hospitals is not part of the register. It is not possible to purchase antiepileptic drugs in Denmark without a prescription. We defined the exposure window from 30 days before the estimated day of conception to the day of birth and included children with an estimated time of conception after February 1, 1996, who were born up until December 31, 2006. Exposure for antiepileptic medication was defined as any filled prescription with Anatomical Therapeutic Classification codes N03A (antiepileptic drugs) and N05BA09 (clobazam).
Children were defined as having been exposed to monotherapy if their mothers had filled prescriptions for only 1 type of antiepileptic drug and as exposed to polytherapy if their mothers had filled prescriptions for more than 1 type of antiepileptic drug. For both monotherapy and polytherapy, the mothers may have filled prescriptions for other types of medicine. We also estimated the risk of autism spectrum disorder and childhood autism among the offspring of users of frequently used antiepileptic drugs other than valproate (carbamazepine, clonazepam, lamotrigine, and oxcarbazepine).
The estimated mean daily dose of antiepileptic drugs was calculated from the total amount of antiepileptic drug filled during the time from 30 days before pregnancy to birth divided by the number of days in the same period. The defined daily dose (DDD)36 is the assumed average maintenance dose per day for a drug used for its main indication in adults. Based on the DDD, the estimated daily antiepileptic drug dose was dichotomized into high (>50% of DDD) and low (≤50% of DDD).
Diagnostic and Covariate Information
The Danish Psychiatric Central Register was used to identify children diagnosed for the first time with autism spectrum disorder (International Statistical Classification of Diseases, Tenth Revision [ICD-10] codes F84.0, F84.1, F84.5, F84.8, and F84.9]) and with childhood autism (ICD-10 code F84.0). The same register was used to identify parents diagnosed with psychiatric disorders before birth of the child (International Statistical Classification of Diseases, Eighth Revision [ICD-8] codes 290-315 and ICD-10 codes F0.00-F99.9). Gestational age, birth weight, and parity information were obtained from the Danish Birth Register. We used the National Hospital Register to identify children diagnosed with congenital malformations (ICD-10 codes Q0 to Q99 [except for Q53, undescended testicle, and Q65, congenital deformities of hip, because of low validity of these diagnoses])15 and also to identify parents diagnosed with epilepsy before birth of the child (ICD-8 code 345 and ICD-10 codes G40 and G41).
Analytical Strategy
Children were followed up from birth to death, emigration, diagnosis of autism spectrum disorder or childhood autism, or December 31, 2010, whichever came first. Risk and prevalence estimates were reported with 95% CIs. P < .05 was used to define significance, using 2-sided testing.
The primary outcome was the hazard ratio (HR) of autism spectrum disorder and childhood autism after valproate exposure in pregnancy. We used Cox regression to estimate the HR for autism spectrum disorder, with age of the child as the underlying time scale and with separate baseline diagnostic rates (strata) for each birth year group to adjust for the increasing prevalence of autism spectrum disorder. The proportional hazards assumption was evaluated for all variables by comparing estimated log-minus-log survivor curves over the different categories of variables investigated. Control for the lack of independence of children within the same family was obtained using a robust (Huber-White) variance estimator. We adjusted for differences in both length of follow-up and autism spectrum disorder prevalence time trends. Importantly, the HR did not depend on age of the child; thus, age is not expected to bias the HR when the children are followed up into adulthood. Hazard ratios were adjusted for risk factors for autism (parental age at conception, parental psychiatric history, gestational age, birth weight, sex of the child, congenital malformations, and parity).
For the primary outcome we also estimated the cumulative incidence (absolute risk) of autism spectrum disorder and childhood autism in unexposed children using the Kaplan-Meier method and multiplied this estimate with the adjusted HR for the exposed children to estimate the absolute risk in exposed children. The absolute risk for the exposed children is valid under the proportional hazards assumption, because autism spectrum disorder is relatively rare (prevalence <10%).16
The secondary outcome was the risk of autism following valproate exposure after stratifying for maternal epilepsy prior to birth.
Stratified and Sensitivity Analyses
In a sensitivity analysis to assess possible confounding by indication, we compared the association between autism spectrum disorder and childhood autism in offspring of women who used valproate during pregnancy and women who were previous users of valproate but who stopped at least 30 days before the estimated day of conception. This analysis addresses whether the illness leading to valproate use, rather than valproate exposure, was associated with autism in the child. We also estimated the risk of autism after stratifying the risk according to dose, trimester of exposure (first vs later), and use of other antiepileptic drugs and polytherapy and after excluding children with congenital malformations. Because autism is more frequent in males, we calculated the sex ratio of exposed children adjusted for time to diagnosis of autism spectrum disorder and compared this sex ratio with that of unexposed children.
Data analyses were performed using Stata version 12 (StataCorp).
RESULTS
We identified children with an estimated time of conception after February 1, 1996, who were born up to December 31, 2006 (N=668 468). We excluded children with likely errors in gestational age (≤23 weeks or ≥45 weeks) (1489 [0.2%]), those with missing values for gestational age (6275 [0.9%]), those with missing information about the mother (5 [0.001%]), adopted children (3461 [0.5%]), and those who died during the first year of life (1623 [0.2%]). Children who died more than 1 year after birth (40 [0.01%]) and those who emigrated (14 492 [2.2%]) were included in the analyses and censored at time of death or emigration. After exclusion, the study cohort comprised 655 615 children born to 428 407 mothers, including multiple births of 26 418 children (4.0%).
The mean age of the children at end of follow-up was 8.84 years (range, 4-14; median, 8.85). During the study period, 5437 children were diagnosed with autism spectrum disorder, including 2067 with childhood autism. Characteristics of the study participants are shown in Table 1.
Table 1.
Characteristics of Study Participants
| Characteristic | Valproate Exposure, No. (%)
|
|
|---|---|---|
| Exposed (n = 508) | Not Exposed (n = 655 107) | |
| Maternal age at conception, y | ||
| <21 | 30 (5.9) | 26 212 (4.0) |
|
| ||
| 21-25 | 117 (23.0) | 127 230 (19.4) |
|
| ||
| 26-30 | 190 (37.4) | 262 707 (40.1) |
|
| ||
| 31-35 | 129 (25.4) | 180 973 (27.6) |
|
| ||
| ≥36 | 42 (8.3) | 57985 (8.9) |
|
| ||
| Paternal age at conception, y | ||
| ≤25 | 67 (13.6) | 76 700 (12.0) |
|
| ||
| 26-30 | 171 (34.7) | 213 912 (33.4) |
|
| ||
| 31-35 | 149 (30.2) | 211 993 (33.1) |
|
| ||
| ≥36 | 106 (21.5) | 138 337 (21.6) |
|
| ||
| Parental psychiatric historya | ||
| Yes | 43 (8.5) | 22 854 (3.5) |
|
| ||
| No | 465 (91.5) | 632 253 (96.5) |
|
| ||
| Gestational age, wk | ||
| <28 | 2 (0.4) | 1230 (0.2) |
|
| ||
| 28-31 | 9 (1.8) | 4616 (0.7) |
|
| ||
| 32-36 | 37 (7.3) | 36 556 (5.6) |
|
| ||
| ≥37 | 460 (90.6) | 612 705 (93.5) |
|
| ||
| Birth weight, g | ||
| <2500 | 46 (9.1) | 32 225 (4.9) |
|
| ||
| 2500-2999 | 69 (13.7) | 72 735 (11.2) |
|
| ||
| 3000-3499 | 147 (29.2) | 199 583 (30.6) |
|
| ||
| ≥3500 | 242 (48.0) | 346 785 (53.2) |
|
| ||
| Sex | ||
| Boys | 267 (52.6) | 335 843 (51.3) |
|
| ||
| Girls | 241 (47.4) | 319 249 (48.7) |
|
| ||
| Congenital malformationsb | ||
| Yes | 51 (10.0) | 25 568 (3.9) |
|
| ||
| No | 457 (90.0) | 629 539 (96.1) |
|
| ||
| Parity | ||
| 0 | 239 (47.0) | 283 054 (43.2) |
|
| ||
| ≥1 | 269 (53.0) | 372 047 (56.8) |
|
| ||
| Epilepsy (mother) | ||
| Yes | 432 (85.0) | 6152 (0.9) |
|
| ||
| No | 76 (15.0) | 648 955 (99.1) |
One or both parents diagnosed with a psychiatric disorder before birth of the child (International Classification of Diseases, Eighth Revision: codes 290-315; International Classification of Diseases, Tenth Revision [ICD-10]: All F codes).
Child diagnosed with congenital malformations (ICD-10 codes Q0 to Q99, except for Q53 and Q65 [see Methods]).
We identified 2644 children exposed to antiepileptic drugs during pregnancy, including 508 exposed to valproate. The overall use of antiepileptic drugs during pregnancy remained stable during the study period; however, valproate use decreased and lamotrigine use increased (Figure 1) (eTable 1, available at http://www.jama.com). The median number of valproate prescriptions filled during pregnancy was 4 (range, 1-15), and the median time between 2 successive filled valproate prescriptions during pregnancy was 6 weeks (range, 0-38).
Figure 1.
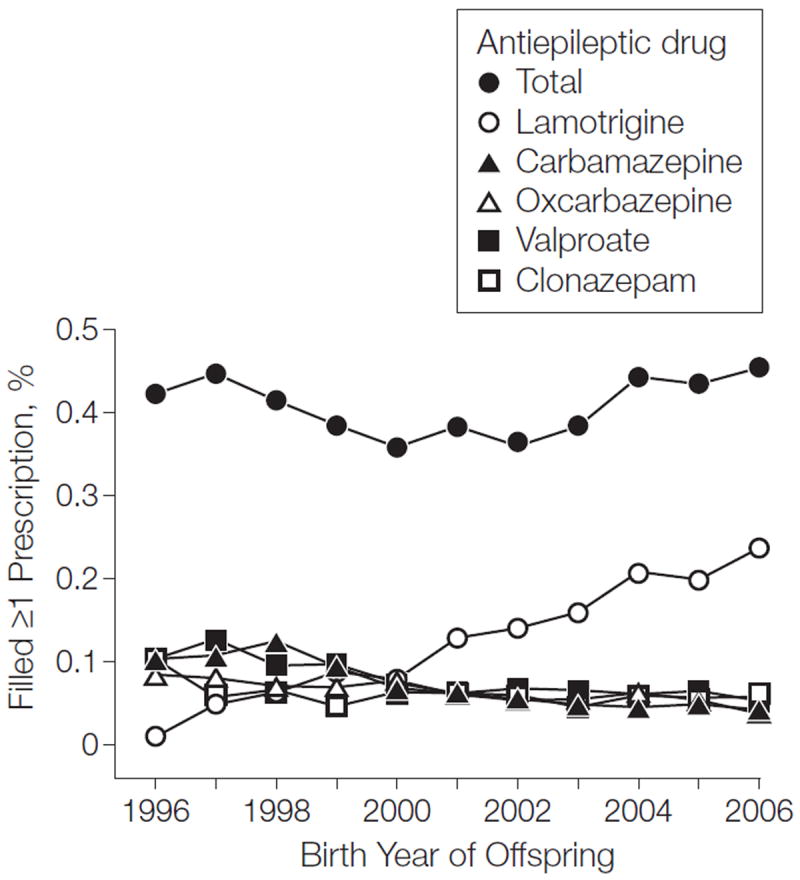
Proportion of Women Who Filled at Least 1 Prescription for Antiepileptic Drugs During Pregnancy, by Birth Year of the Child See eTable 1 for number of live births, congenital malformations, autism spectrum disorder, chilhood autism, and antiepileptic use by the mother during pregnancy.
The absolute risk after 14 years of follow-up was 1.53% (95% CI, 1.47%-1.58%) for autism spectrum disorder and 0.48% (95% CI, 0.46%-0.51%) for childhood autism.
Use of valproate during pregnancy was associated with an absolute risk of 4.42% (95% CI, 2.59%-7.46%) for autism spectrum disorder (adjusted HR, 2.9 [95% CI, 1.7-4.9]) and an absolute risk of 2.50% (95% CI, 1.30%- 4.81%) for childhood autism (adjusted HR, 5.2 [95% CI, 2.7-10.0]) (Table 2). We also found higher risks of autism spectrum disorder (adjusted HR, 2.2 [95% CI, 1.02-4.9]) and childhood autism (adjusted HR, 5.6 [95% CI, 1.7-18.1]) among children of women who used valproate during pregnancy compared with children of women who were previous users of valproate but who stopped at least 30 days before conception (eTable 2).
Table 2.
Association Between Any Maternal Use of Valproate During Pregnancy and Autism Spectrum Disorder and Childhood Autism
| Valproate Exposure | Live Births, No. | Person-Years at Risk | No. With Disorder | Hazard Ratio (95% Cl)
|
Absolute Risk Over 14 y, % (95% CI) | |
|---|---|---|---|---|---|---|
| Crude | Adjusteda | |||||
| Autism Spectrum Disorder | ||||||
| Yes | 508 | 4880 | 14 | 3.0 (1.8-5.1) | 2.9 (1.7-4.9) | 4.42 (2.59-7.46) |
|
| ||||||
| No | 655 107 | 5 793 387 | 5423 | 1 [Reference] | 1 [Reference] | 1.52 (1.47-1.58) |
|
| ||||||
| Childhood Autism | ||||||
| Yes | 508 | 4891 | 9 | 5.4 (2.8-10.4) | 5.2 (2.7-10.0) | 2.50 (1.30-4.81) |
|
| ||||||
| No | 655 107 | 5 803 842 | 2058 | 1 [Reference] | 1 [Reference] | 0.48 (0.45-0.51) |
Adjusted for maternal age at conception, paternal age at conception, parental psychiatric history, gestational age, birth weight, sex, congenital malformations, and parity.
When restricting the analyses to offspring of women with epilepsy, the absolute risk of autism spectrum disorder in 432 children exposed to valproate was 4.15% (95% CI, 2.20%-7.81%) (adjusted HR, 1.7 [95% CI, 0.9-3.2]) and the absolute risk for childhood autism was 2.95% (95% CI, 1.42%-6.11%) (adjusted HR, 2.9 [95% CI, 1.4-6.0]), when compared with the absolute risk of 2.44% (95% CI, 1.88%-3.16%) for autism spectrum disorder and 1.02% (95% CI, 0.70%-1.49%) for childhood autism among 6152 children not exposed to valproate (Table 3).
Table 3.
Association Between Epilepsy in the Mother, Valproate Exposure, and Autism Spectrum Disorder and Childhood Autism
| Epilepsy in Mother | Valproate Exposure | Live Births, No. | No. With Disorder | Hazard Ratio (95% Cl)
|
Absolute Risk Over 14 y, % (95% CI) | |
|---|---|---|---|---|---|---|
| Crude | Adjusteda | |||||
| Autism Spectrum Disorder | ||||||
| Yes | Yes | 432 | 11 | 1.7 (0.9-3.2) | 1.7 (0.9-3.2) | 4.15 (2.20-7.81) |
|
| ||||||
| No | 6152 | 83 | 1 [Reference] | 1 [Reference] | 2.44 (1.88-3.16) | |
|
| ||||||
| No | Yes | 76 | 3 | 4.2 (1.4-12.5) | 4.4 (1.4-13.6) | 6.67 (2.12-20.6) |
|
| ||||||
| No | 648 955 | 5340 | 1 [Reference] | 1 [Reference] | 1.52 (1.46-1.57) | |
|
| ||||||
| Childhood Autism | ||||||
| Yes | Yes | 432 | 8 | 2.8 (1.3-6.1) | 2.9 (1.4-6.0) | 2.95 (1.42-6.11) |
|
| ||||||
| No | 6152 | 37 | 1 [Reference] | 1 [Reference] | 1.02 (0.70-1.49) | |
|
| ||||||
| No | Yes | 76 | 1 | 4.2 (0.6-30.0) | 3.9 (0.5-28.9) | 1.86 (0.24-13.8) |
|
| ||||||
| No | 648 955 | 2021 | 1 [Reference] | 1 [Reference] | 0.48 (0.45-0.51) | |
Adjusted for maternal age at conception, paternal age at conception, parental psychiatric history, gestational age, birth weight, sex, congenital malformations, and parity.
There was also an increased risk among exposed children of mothers without a history of epilepsy when compared with those unexposed to valproate, but the number of exposed cases of mothers without epilepsy was low (Table 3).
In a sensitivity analysis, we changed the valproate exposure window prior to pregnancy. Compared with unexposed women, the adjusted HRs for autism spectrum disorder were 2.3 (95% CI, 1.4-3.9) for the window from 180 days prior to pregnancy, 2.7 (95% CI, 1.6-4.6) for the window from 90 days prior to pregnancy, and 3.0 (95% CI, 1.8-5.2) for the window from 0 days prior to pregnancy; the corresponding adjusted HRs for childhood autism were 4.1 (95% CI, 2.1-8.0), 4.8 (95% CI, 2.5- 9.3), and 5.4 (95% CI, 2.8-10.5), respectively.
The risk of autism spectrum disorder and childhood autism was increased among 441 valproate-exposed children whose mothers filled valproate prescriptions in the first trimester (adjusted HR, 2.9 [95% CI, 1.6-5.1] for autism spectrum disorder and 4.7 [95% CI, 2.2-9.9] for childhood autism). The adjusted HRs among 67 children whose mothers only filled valproate prescriptions later in pregnancy were 3.1 (95% CI, 0.8-12.2) for autism spectrum disorder and 8.3 (95% CI, 2.1-32.4) for childhood autism (eTable 3).
When compared with offspring not exposed to valproate, the adjusted HR of autism spectrum disorder among 388 children exposed to valproate monotherapy was 3.0 (95% CI, 1.7-5.4) and the adjusted HR of childhood autism was 4.9 (95% CI, 2.3-10.3). Among 120 children exposed to valproate polytherapy, the adjusted HR of autism spectrum disorder was 1.9 (95% CI, 0.5- 7.7) and the adjusted HR of childhood autism was 5.2 (95% CI, 1.3-21.3) (eTable 4).
Risks were similar for 183 children of women who used a high valproate dose (>750 mg/d) (adjusted HR, 2.5 [95% CI, 1.03-6.1] for autism spectrum disorder and 4.3 [95% CI, 1.4- 13.4] for childhood autism) compared with those for 325 children of women who used a low valproate dose (≤750 mg/d) (adjusted HR, 3.2 [95% CI, 1.7-6.2] for autism spectrum disorder and 5.4 [95% CI, 2.4-12.1] for childhood autism) (eTable 5).
For other antiepileptic drugs, there was no increased risk of autism spectrum disorder or childhood autism among the offspring of women who used oxcarbazepine as monotherapy during pregnancy (Figure 2 and eTable 4). There were few (<5) exposed cases when stratifying the individual drugs by dose, especially when analyzing the risk of childhood autism (eTables 4 and 5).
Figure 2.
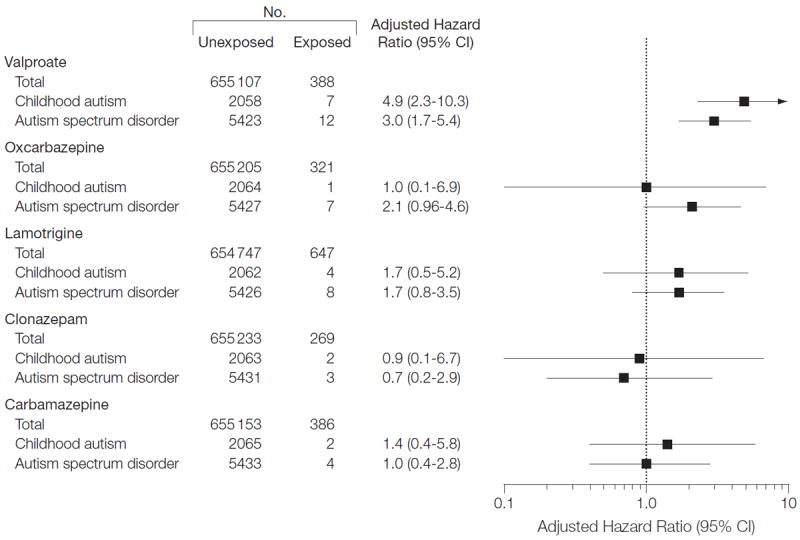
Autism Spectrum Disorder and Childhood Autism in Offspring of Mothers Who Used Antiepileptic Drugs as Monotherapy During Pregnancy Compared With Offspring of Women Who Did Not Use the Individual Antiepileptic Drug
After excluding the 25 619 children with congenital malformations from the analyses (Table 1), the adjusted HRs associated with valproate exposure (n=457), compared with those associated with nonexposure (n=629 539), were 2.8 (95% CI, 1.5-5.1) for autism spectrum disorder and 4.2 (95% CI, 1.9- 9.3) for childhood autism (eTable 6).
The sex ratio (boys to girls; adjusted for time to event) of autism spectrum disorder was 2.4 (95% CI, 0.8-7.7) (10 boys and 4 girls) for children exposed to valproate and 4.4 (95% CI, 4.1-4.7) (4449 boys and 974 girls) for children not exposed to valproate (P=.32 by χ2 test). The sex ratio of childhood autism was 1.2 (95% CI, 0.3- 4.4) (5 boys and 4 girls) for children exposed to valproate and 4.6 (95% CI, 4.1-5.1) (1704 boys and 354 girls) for children not exposed to valproate (P=.04 by χ2 test).
DISCUSSION
In this population-based cohort study, children of women who used valproate during pregnancy had a higher risk of autism spectrum disorder and childhood autism compared with children of women who did not use valproate. Their risks were also higher than those for children of women who were previous users of valproate but who stopped before their pregnancy. Our results are consistent with findings from previous case series, although the risk of autism spectrum disorder following valproate exposure in our study was slightly lower,4-6 probably because our findings were based on a larger and unselected cohort of valproate-exposed women. Because autism spectrum disorders are serious conditions with lifelong implications for affected children and their families, even a moderate increase in risk may have major health importance. Still, the absolute risk of autism spectrum disorder was less than 5%, which is important to take into account when counseling women about the use of valproate in pregnancy.
Valproate is indicated for the treatment of epilepsy, manic episodes associated with bipolar disorder, and prophylaxis of migraine headaches. It was not possible from the prescriptions to identify the disorder for which the women received valproate treatment. However, we assume that women who had a diagnosis of epilepsy received valproate for this indication. An increased risk from valproate use was found in the offspring of mothers both with and without an epilepsy diagnosis, suggesting a biological effect of valproate. In the offspring of women with epilepsy, the risk associated with valproate use was lower compared with the overall risk from valproate. This suggests confounding effects of maternal epilepsy or interaction between genetic susceptibility and valproate. We cannot exclude the possibility that children of women who used valproate during pregnancy may be examined more carefully for autism spectrum disorders, because valproate has been associated with adverse effects.4-6 This potential bias would lead to overestimation of the effect of valproate on the risk of autism in our study.
Risk of Autism According to the Timing of Exposure to Valproate
We found no difference in risk of autism spectrum disorder between offspring of women who redeemed prescriptions for valproate early vs later in pregnancy, but the number of women who redeemed valproate only in the later stages of pregnancy was low. The estimates were based on the trimester when the women redeemed a prescription and not on when the women actually ingested the tablets; therefore, misclassification of timing of the exposure may have occurred. Changing the exposure window prior to pregnancy had only minor influence on the association between valproate exposure and autism spectrum disorder. Misclassification of the timing of conception is also possible, but this misclassification is likely small.17,18
Risk of Autism by Antiepileptic Treatment Regimen
We found increased risks of autism spectrum disorder, both in offspring of women who filled prescriptions for valproate alone and offspring of those who filled prescriptions for valproate as well as other antiepileptic drugs during pregnancy; however, the number of women exposed to valproate polytherapy was low. We assume that women using valproate monotherapy did not change antiepileptic medication during pregnancy, but use of polytherapy may reflect a change.
Previous studies suggest that the teratogenic potential of valproate19 is higher for high daily doses of valproate during pregnancy.20 We found increased risks associated with both a high dose and a low dose. However, we did not have information on the actual dose of valproate or on whether the dose was changed during pregnancy, which may limit the validity of the analyses.
Exposures to carbamazepine, oxcarbazepine, lamotrigine, and clonazepam monotherapy were not associated with increased risks of autism spectrum disorder and childhood autism. Valproate is a fatty acid derivative and chemically different from the other antiepileptic drugs studied, which may explain the different results observed with different antiepileptic drugs.
Women who use valproate in pregnancy may use other types of drugs having a potential effect on the development of the nervous system. However, we did not adjust for use of other prescription medicines in pregnancy, because there is no consensus on what kinds of medicines may be associated with an increased risk of autism in the offspring.
Brain Development, Congenital Malformations, and Autism
The potential harmful effects of valproate on the developing brain give rise to health concerns.1,2 Several animal studies describe autistic-like behavior in offspring of mothers exposed to valproate during pregnancy. In animal studies, some manifestations of autism were reproduced by administering valproate at various stages in pregnancy, eg, increased repetitive or stereotypic activity, higher anxiety, and decreased level of social interaction.9,21 The precisemode of action of valproate is not established, but it may alter neurotransmitter functioning involved in cell migration and differentiation, neuronal apoptosis,22 or synaptic plasticity23 or act via other mechanisms including attenuation of folic acid metabolism, inhibition of histone deacetylases, oxidative stress, and production of arene oxide intermediates.24,25
Like other studies,19,20 ours found an increased risk of congenital malformations in children exposed to valproate, but the risk of autism spectrum disorder and childhood autism associated with valproate exposure persisted after restricting the analysis to children without congenital malformations.
Strengths and Weaknesses of the Data
We assessed a population-based cohort of all children born between 1996 and 2006 for up to 14 years and with less than 3% loss to follow-up, making selection bias an unlikely explanation for our results.4-6
We assumed that most women who filled a prescription for valproate also used themedication at least to some extent. A previous study showed high agreement between maternal report of antiepileptic medication use and filled prescriptions for antiepileptic drugs as registered by the Danish Prescription Register.26
We did not have information on the use of antiepileptic drugs by pregnant women while admitted to hospitals, and we therefore may not have included all valproate-exposed pregnancies. However, valproate is rarely given alone for shorter intervals such as hospital admissions, and failure to include valproate-exposed children in our exposed cohort would tend to underestimate an association with prenatal valproate exposure.
We did not adjust for multiple comparisons but specified a priori that the main outcome was the risk of autism spectrum disorder and childhood autism following prenatal exposure to valproate. Multiple comparisons increase the risk of incidental findings, and the results of the secondary analyses are therefore exploratory and subject to further studies.
The positive predictive value of an epilepsy diagnosis in the Danish Hospital Register was 81%27; therefore, some patients registered with epilepsy in our cohort do not have epilepsy. Also, patients registered with epilepsy in childhood and early adulthood may no longer have epilepsy. However, the associations between valproate exposure and autism spectrum disorders were found among offspring of mothers both with and without epilepsy, indicating an association with valproate exposure. Seizures may influence the well-being of the unborn child, but from the registers it was not possible to identify pregnant women experiencing seizures during pregnancy. We are not aware of studies that have assessed the contribution of seizures in pregnancy to the risk of autism in the offspring of women with epilepsy.
When evaluating the quality of the childhood autism diagnosis in the Danish Psychiatric Central Register, it was found that 94% of the childhood autism diagnoses met the ICD-10 criteria for a correct diagnosis.28 The other autism spectrum disorder diagnoses in the register have not been validated, but the quality is expected to be high. In this study, the prevalence estimate of autism spectrum disorder was 1.53% (95% CI, 1.47%-1.58%) at 14 years of follow-up, similar to the prevalence of 1.1% in US children aged 3 to 17 years based on parent reports29 or of approximately 1% among 8-year-old US children based on data from the Autism and Developmental Disabilities Monitoring Network.30
We adjusted for parental psychiatric history, which is a well-known risk factor for autism in the offspring,31 but the estimates of the risk associated with valproate exposure changed little. We did not have information on use of alcohol in pregnancy, but we adjusted for diagnoses of alcohol abuse from the Danish Psychiatric Central Register. However, not all parents with alcohol abuse or psychiatric disorders were identified from the registers, and residual confounding by unmeasured psychiatric disorders in the mother or father can therefore not be entirely excluded. We did not have information on folic acid use in pregnant women (only high-dose [5 mg] folic acid requires a prescription in Denmark). Studies of folate status during the later stages of pregnancy did not find significant associations with mental and psychomotor development at age 5 years,32 but periconceptional use of folic acid has been associated with a 39% reduced risk of autistic disorder (childhood autism) (adjusted odds ratio for autistic disorder in children of folic acid users, 0.61 [95% CI, 0.41-0.90]),33 and use of folic acid may reduce the risk of autism spectrum disorder in mothers with inefficient folate metabolism.34 However, we expect that pregnant women exposed to valproate would be more likely to take folic acid compared with those not exposed to valproate during pregnancy because of recommendations regarding the use of folic acid in fertile women with epilepsy, which would tend to underestimate the true risk associated with valproate.
CONCLUSIONS
Maternal use of valproate during pregnancy was associated with a significantly increased risk of autism in the offspring, even after adjusting for parental psychiatric disease and epilepsy. For women of childbearing potential who use antiepileptic medications, these findings must be balanced against the treatment benefits for women who require valproate for epilepsy control.
Acknowledgments
Funding/Support: Dr Christensen receives research support from the Danish Epilepsy Association. Dr Pedersen is supported by a Sapere Aude–Postdoctoral grant from the Danish Council for Independent Research. This study was supported by grants from the European Research Council (the European Union Seventh Framework Programme, ERC-2010-StG No. 260242) and the Danish Medical Research Council (09- 072986).
Role of the Sponsors: The funding sources had no role in the design and conduct of the study; the collection, analysis, and interpretation of data; or the preparation, review, or approval of the manuscript.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Author Contributions: Dr Christensen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: All authors.
Acquisition of data: Christensen, Vestergaard.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: Christensen, Grønborg, Sørensen, Parner.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Christensen, Grønborg, Sørensen, Schendel, Parner.
Obtained funding: Christensen, Parner, Vestergaard. Administrative, technical, or material support: Christensen.
Study supervision: Christensen, Parner, Vestergaard.
Online-Only Material: eTables 1-6 and Author Video Interview are available at http://www.jama.com.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Christensen reported receiving honoraria for serving on the scientific advisory boards of UCB Nordic and Eisai AB; receiving lecture honoraria from UCB Nordic and Eisai AB; and receiving travel funding from UCB Nordic. Dr Parner reported receiving grants from Autism Speaks and the National Institutes of Health. No other authors reported disclosures.
References
- 1.Holmes GL, Harden C, Liporace J, Gordon J. Postnatal concerns in children born to women with epilepsy. Epilepsy Behav. 2007;11(3):270–276. doi: 10.1016/j.yebeh.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Meador KJ, Baker G, Cohen MJ, Gaily E, Westerveld M. Cognitive/behavioral teratogenetic effects of antiepileptic drugs. Epilepsy Behav. 2007;11(3):292–302. doi: 10.1016/j.yebeh.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meador KJ, Baker GA, Browning N, et al. NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12(3):244–252. doi: 10.1016/S1474-4422(12)70323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore SJ, Turnpenny P, Quinn A, et al. A clinical study of 57 children with fetal anticonvulsant syndromes. J Med Genet. 2000;37(7):489–497. doi: 10.1136/jmg.37.7.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasalam AD, Hailey H, Williams JH, et al. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev Med Child Neurol. 2005;47(8):551–555. doi: 10.1017/s0012162205001076. [DOI] [PubMed] [Google Scholar]
- 6.Bromley RL, Mawer G, Clayton-Smith J, Baker GA Liverpool and Manchester Neurodevelopment Group. Autism spectrum disorders following in utero exposure to antiepileptic drugs. Neurology. 2008;71(23):1923–1924. doi: 10.1212/01.wnl.0000339399.64213.1a. [DOI] [PubMed] [Google Scholar]
- 7.Bromley RL, Mawer GE, Briggs M, et al. Liverpool and Manchester Neurodevelopment Group. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. doi: 10.1136/jnnp-2012-304270. published online January 31, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markram K, Rinaldi T, La Mendola D, Sandi C, Markram H. Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology. 2008;33(4):901–912. doi: 10.1038/sj.npp.1301453. [DOI] [PubMed] [Google Scholar]
- 9.Wagner GC, Reuhl KR, Cheh M, McRae P, Halladay AK. A new neurobehavioral model of autism in mice: pre- and postnatal exposure to sodium valproate. J Autism Dev Disord. 2006;36(6):779–793. doi: 10.1007/s10803-006-0117-y. [DOI] [PubMed] [Google Scholar]
- 10.Schneider T, Przewłocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology. 2005;30(1):80–89. doi: 10.1038/sj.npp.1300518. [DOI] [PubMed] [Google Scholar]
- 11.Miles JH. Autism spectrum disorders—a genetics review. Genet Med. 2011;13(4):278–294. doi: 10.1097/GIM.0b013e3181ff67ba. [DOI] [PubMed] [Google Scholar]
- 12.Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. 2011;128(2):344–355. doi: 10.1542/peds.2010-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- 14.Pedersen CB, Gøtzsche H, Møller JO, Mortensen PB. The Danish Civil Registration System: a cohort of eight million persons. Dan Med Bull. 2006;53(4):441–449. [PubMed] [Google Scholar]
- 15.Larsen H, Nielsen GL, Bendsen J, Flint C, Olsen J, Sørensen HT. Predictive value and completeness of the registration of congenital abnormalities in three Danish population-based registries. Scand J Public Health. 2003;31(1):12–16. doi: 10.1080/14034940210134194. [DOI] [PubMed] [Google Scholar]
- 16.Symons MJ, Moore DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol. 2002;55(9):893–899. doi: 10.1016/s0895-4356(02)00443-2. [DOI] [PubMed] [Google Scholar]
- 17.Kristensen J, Langhoff-Roos J, Skovgaard LT, Kristensen FB. Validation of the Danish Birth Registration. J Clin Epidemiol. 1996;49(8):893–897. doi: 10.1016/0895-4356(96)00018-2. [DOI] [PubMed] [Google Scholar]
- 18.Olesen AW, Westergaard JG, Thomsen SG, Olsen J. Correlation between self-reported gestational age and ultrasound measurements. Acta Obstet Gynecol Scand. 2004;83(11):1039–1043. doi: 10.1111/j.0001-6349.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 19.Jentink J, Loane MA, Dolk H, et al. EUROCAT Antiepileptic Study Working Group. Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med. 2010;362(23):2185–2193. doi: 10.1056/NEJMoa0907328. [DOI] [PubMed] [Google Scholar]
- 20.Vajda FJ, Hitchcock A, Graham J, et al. Foetal malformations and seizure control: 52 months data of the Australian Pregnancy Registry. Eur J Neurol. 2006;13(6):645–654. doi: 10.1111/j.1468-1331.2006.01359.x. [DOI] [PubMed] [Google Scholar]
- 21.Schneider T, Turczak J, Przewłocki R. Environmental enrichment reverses behavioral alterations in rats prenatally exposed to valproic acid: issues for a therapeutic approach in autism. Neuropsychopharmacology. 2006;31(1):36–46. doi: 10.1038/sj.npp.1300767. [DOI] [PubMed] [Google Scholar]
- 22.Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002;99(23):15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sui L, Chen M. Prenatal exposure to valproic acid enhances synaptic plasticity in the medial prefrontal cortex and fear memories. Brain Res Bull. 2012;87(6):556–563. doi: 10.1016/j.brainresbull.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Ornoy A. Valproic acid in pregnancy: how much are we endangering the embryo and fetus? Reprod Toxicol. 2009;28(1):1–10. doi: 10.1016/j.reprotox.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Walsh CA, Morrow EM, Rubenstein JL. Autism and brain development. Cell. 2008;135(3):396–400. doi: 10.1016/j.cell.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olesen C, Søndergaard C, Thrane N, Nielsen GL, de Jong-van den Berg L, Olsen J EuroMAP Group. Do pregnant women report use of dispensed medications? Epidemiology. 2001;12(5):497–501. doi: 10.1097/00001648-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Christensen J, Vestergaard M, Olsen J, Sidenius P. Validation of epilepsy diagnoses in the Danish National Hospital Register. Epilepsy Res. 2007;75(2-3):162–170. doi: 10.1016/j.eplepsyres.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Lauritsen MB, Jørgensen M, Madsen KM, et al. Validity of childhood autism in the Danish Psychiatric Central Register: findings from a cohort sample born 1990-1999. J Autism Dev Disord. 2010;40(2):139–148. doi: 10.1007/s10803-009-0818-0. [DOI] [PubMed] [Google Scholar]
- 29.Kogan MD, Blumberg SJ, Schieve LA, et al. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 2009;124(5):1395–1403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- 30.Mulvihill B, Wingate M, Kirby RS, et al. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2009;58(10):1–20. 20023608. [PubMed] [Google Scholar]
- 31.Lauritsen MB, Pedersen CB, Mortensen PB. Effects of familial risk factors and place of birth on the risk of autism: a nationwide register-based study. J Child Psychol Psychiatry. 2005;46(9):963–971. doi: 10.1111/j.1469-7610.2004.00391.x. [DOI] [PubMed] [Google Scholar]
- 32.Tamura T, Goldenberg RL, Chapman VR, Johnston KE, Ramey SL, Nelson KG. Folate status of mothers during pregnancy and mental and psychomotor development of their children at five years of age. Pediatrics. 2005;116(3):703–708. doi: 10.1542/peds.2004-2189. [DOI] [PubMed] [Google Scholar]
- 33.Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309(6):570–577. doi: 10.1001/jama.2012.155925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96(1):80–89. doi: 10.3945/ajcn.110.004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders— Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61(3):1–19. [PubMed] [Google Scholar]
- 36.WHO Collaborating Centre for Drug Statistics Methodology (WHOCC) [March 22, 2013];Defined Daily Dose; Definition and General Considerations. WHOCC website. http://www.whocc.no/ddd.


