Abstract
Americans like to believe that their healthcare system is the best in the world, but the information they receive about the healthcare systems of other countries is limited. This is unfortunate because experiences abroad—both good and bad—can provide important lessons for the United States.
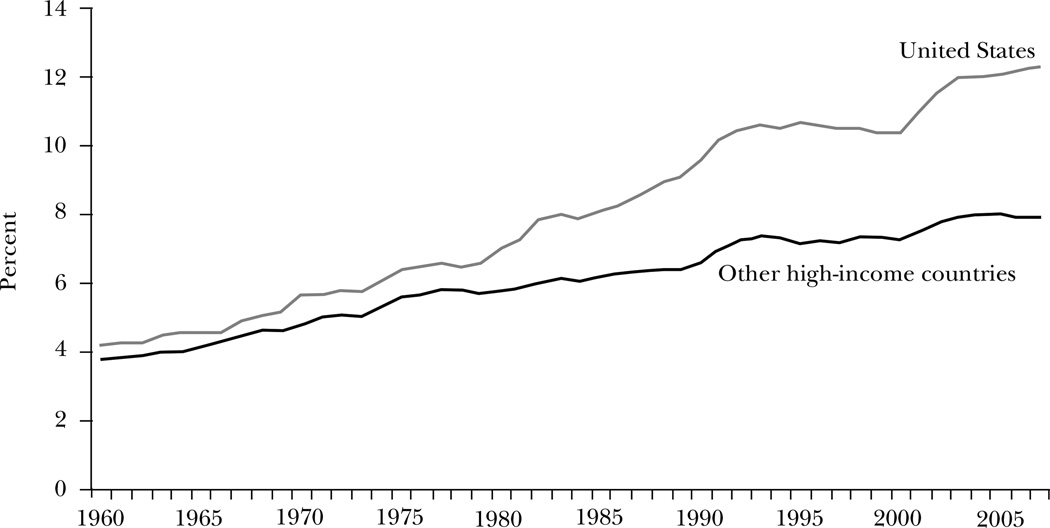
Other high-income countries are notable in their lower levels and less-rapid growth rates of medical spending, as shown in Figure 1. In 1960, U.S. spending on acute medical care as a share of GDP—hospitals, physicians, and pharmaceuticals, but not long-term care—was only 10 percent above that of other high-income countries. By 1980, the gap had doubled to 21 percent. The gap has since more than doubled again. By 2007, U.S. spending on acute medical care was 25 percent higher than the next-highest country (Switzerland) and 55 percent above the average of other high-income countries.1 When coupled with the well-known finding that life expectancy and quality of life are relatively similar across high-income countries, the enormously higher healthcare spending in the United States raises the possibility of substantial waste.
Figure 1. Acute Medical Spending as a Share of GDP, 1960–2007.
Source: Data are from OECD (2010).
Note: “Other high-income countries” refers to the unweighted average of spending as a share of GDP in 14 OECD countries: Australia, Austria, Canada, Finland, France, Germany, Italy, Japan, the Netherlands, Norway, Spain, Sweden, Switzerland, and the United Kingdom. Because of differences in the treatment of the residential component of long-term care services, we present data for acute care services only—generally hospitals, physicians, and prescription drugs. Some countries changed the methodology for estimating medical spending in various years. These breaks in the data were adjusted for increasing or decreasing spending in each year prior to the break, assuming that spending in the year of the break grew by the average of the year before and the year after the break.
Data from within the United States also suggest that a considerable share of U.S. healthcare spending is not purchasing much or any additional health. For example, Fisher, Wennberg, Stukel, Gottlieb, Lucas, and Pinder (2003a, b) show that Medicare spending varies greatly across regions of the country, but this extra spending has no association with better health outcomes. Estimates of excessive spending based on this geographic variation range from 20 percent (Skinner, Fisher, and Wennberg, 2005) to 30 percent or more (Fisher et al., 2003a,b; McKinsey Global Institute, 2008). Since even these lower-spending areas in the United States may spend more than is necessary to care for people, the savings from a better-functioning medical system could be even larger.
This paper draws on international evidence on medical spending to examine what the United States can learn about making its healthcare system more efficient. To start, we should consider whether we are primarily interested in the level of spending or in its growth rate. Over the long term, the growth rate of medical costs is driven predominantly by changes in the technological capacity of medicine (Newhouse, 1992; Cutler and McClellan, 2001). At a point in time, however, other factors vary more—including price and administrative cost differences. We focus primarily on understanding contemporaneous differences in the level of spending, generally from the 2000s, for two reasons: First, the level of spending is so different—more than $3,000 per person annually—that understanding why the level is so different is important in itself. Second, the savings from bringing the level of spending down would be enormous. If 30 percent of medical spending is not necessary, then the potential waste is more than $700 billion annually.
Medical spending differs across countries either because the price of services differs (for example, a coronary bypass surgery operation may cost more in the United States than in other countries) or because people receive more services in some countries than in others (for example, more bypass surgery operations). Within the price category, there are two further issues: whether factors earn different returns across countries and whether more clinical or administrative personnel are required to deliver the same care in different countries.
We first present the results of a decomposition of healthcare spending along these lines in the United States and in Canada. We then delve into each component in more detail—administrative costs, factor prices, and the provision of care received—bringing in a broader range of international evidence when possible. Finally, we touch upon the organization of primary and chronic disease care and discuss possible gains in that area.
Healthcare Spending Differences between the United States and Canada
A number of authors have examined medical spending differences across countries. Anderson and colleagues (2003) noted that Americans received no more physician visits or hospital days than people in other countries and concluded that “It’s the Prices, Stupid.” However, physicians’ visits and hospital days do not pick up the intensity of interactions with the medical system. In addition, the price per visit may reflect administrative inefficiencies as much as differential factor incomes. Thus, a more-detailed comparison of international spending differences is useful.
Pozen and Cutler (2010) conducted an analysis decomposing the factors leading to differential spending between Canada and the United States. Canada has a single-payer system, while the United States has a mixture of public and private insurance. Because these forms of insurance are so different, they looked only at provider costs for hospital and physician care (setting aside, for example, insurer overhead and long-term care). For these provider costs, spending was $1,589 per capita higher—that is, 120 percent higher—in the United States than in Canada in 2002. Table 1 summarizes hospital and physician spending differences between the United States and Canada.
Table 1.
Summary of Hospital and Physician Spending Differences between the United States and Canada in 2002
| Dollars per capita | Percent of total difference | |
|---|---|---|
| Total difference | $1,589 | — |
| Provider incomes | $490 | 31% |
| Additional procedures for hospitalized patients | $224 | 14% |
| Administration | $616 | 39% |
| Total accounted for | $1,330 | 84% |
Source: Data are from Pozen and Cutler (2010).
Note: The data are for hospital and physician care only.
The largest quantitative difference in healthcare spending between the United States and Canada is in administrative costs. Adjusting for population size, there are 44 percent more administrative staff in the U.S. healthcare system than in the Canadian system. In addition, physicians devote more of their time to administration in the United States than in Canada (13 percent versus 8 percent, respectively) (Remler, Gray, and Newhouse, 2000; CMA, 2003). Finally, the level of nonstaff spending—malpractice insurance, office space, and utilities—is higher in the United States. All told, differences in administrative expenses compose 39 percent of the total spending difference. This figure probably underestimates the extent of administrative costs, because a substantial share of nursing time is also spent on administrative tasks. For example, typical hospital-based nurses spend one–third of their day on documentation (Hendrich, Chow, Skierczynski, and Lu, 2008). This documentation, however, is counted as clinical care, not administration.
Another part of the spending difference between the United States and Canada is the higher salaries for healthcare providers in the United States. Generalist physicians earn one–third more in the United States than in Canada, and specialists earn 50 percent more. Nursing and other staff salaries are higher as well. Taken together, the difference in worker earnings translates into 31 percent of the total spending difference.
Lastly, Americans also receive more-intensive care than do Canadians. While the population-adjusted hospital admission rates are about the same in the two countries, additional procedures are provided to those with the same diagnosis in the United States. For example, people with a heart attack in the United States are twice as likely to receive bypass surgery or angioplasty than are similar people in Canada (Ko et al., 2007). At the inpatient level, these differences amount to about 14 percent of total spending. There are likely differences in outpatient care as well, like greater imaging and more frequent specialist consultations in the United States, but these are harder to capture.
As shown in the last row of Table 1, administrative expenses, prices, and intensity together add up to 84 percent of the total spending difference between the United States and Canada. The residual likely reflects some undercounting of additional technology provided in non-inpatient settings and possibly mismeasurement of factor returns.
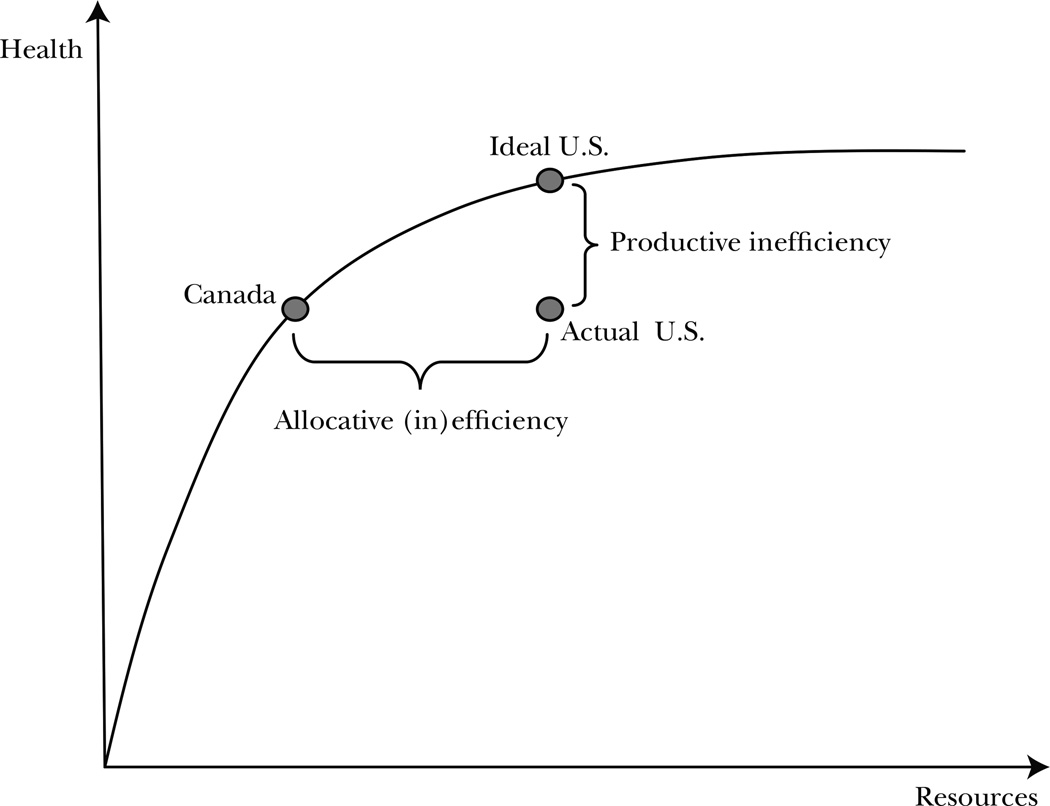
Greater need for administrative personnel is a form of productive inefficiency. In a standard production possibility framework, a country with higher administrative needs would be inside the production possibility frontier. Figure 2 shows this as the difference between the actual U.S. levels of inputs and outputs and the ideal U.S. levels. Higher factor returns may reflect productive inefficiency as well if the excess payment is a pure rent and if there is a social cost to making the transfer (for example, if distortionary taxes need to be raised to finance the additional transfers). Greater care received may be allocatively efficient or inefficient, depending on whether the additional care provided in the United States is valued highly. In Figure 2, the key issue is the dollar value of the health improvement that accompanies the additional care.
Figure 2. Productive and Allocative Efficiency.
Garber and Skinner (2008) present a diagram similar to Figure 2 and argue that the United States is on a production possibility frontier that is interior to that of other countries. In essence, the United States may simply be unable to produce the same health outcomes per dollar input as other countries. They suggest that allocatively, the United States is efficiently using extra resources in some cases and inefficiently using them in others; the average product of such a system is considerably less than an integrated system with the same overall costs. Our results argue more strongly that both productive and allocative efficiency in the United States could be improved were the health system to change in the appropriate way.
In the remaining sections of the paper, we explore administrative costs, factor returns, and differences in the provision of care across countries, and we consider what these differences imply for U.S. healthcare policy.
Healthcare Administrative Expenses
Perhaps the most troubling difference between the U.S. and Canadian healthcare systems is the differential amount spent on administration (see also Woolhandler, Campbell, and Himmelstein, 2003). For every office-based physician in the United States, there are 2.2 administrative workers. That exceeds the number of nurses, clinical assistants, and technical staff put together. One large physician group in the United States estimates that it spends 12 percent of revenue collected just collecting revenue (Blanchfield, Heffernan, Osgood, Sheehan, and Meyer, 2010). Canada, by contrast, has only half as many administrative workers per office-based physician.
The situation is no better in hospitals. In the United States, there are 1.5 administrative personnel per hospital bed, compared to 1.1 in Canada. Duke University Hospital, for example, has 900 hospital beds and 1,300 billing clerks. On top of this are the administrative workers in health insurance. Health insurance administration is 12 percent of premiums in the United States and less than half that in Canada (Davis, Schoen, Guterman, Shih, Schoenbaum, and Weinbaum, 2007).
International comparisons of medical care occupations are difficult, but they suggest that the United States has more administrative personnel than other countries do. Data from the Luxembourg Income Study indicate that the United States has 25 percent more healthcare administrators than the United Kingdom, 165 percent more than the Netherlands, and 215 percent more than Germany. The number of clerks of all forms (including data entry clerks) is much higher in the United States as well.
To put the number of administrative staff in perspective, the number of clinical staff is roughly the same in the United States as elsewhere. The United States has 10 percent more physicians per capita than Canada, but 25 percent fewer physicians than the typical high-income country. Similarly, the United States has 17 percent more nurses per capita than Canada and 8 percent more nurses per capita than the typical rich country.
What are all these administrative personnel doing? There are several functions of the administrative staff (Medical Group Management Association, 2005). One part is credentialing—receiving permission to practice medicine in a particular hospital or for a particular health plan. The average physician submits 18 credentialing applications annually—each insurer, hospital, ambulatory surgery facility, and the like, requires a different one—consuming 70 minutes of staff time and 11 minutes of physician time per application. Verifying eligibility for services is also costly. Insurance information must be verified for 20 to 30 patients daily, including three or four patients for whom verification must be sought orally. Because people change insurance plans frequently and the cost-sharing they are charged varies with plan and with past utilization (for example, how much of the deductible have they spent?), the determination of what to charge a patient is especially difficult. Because of lags in claim reporting, providers often have to collect additional money from patients well after care has been delivered.
Finally, significant time is spent on billing and payment collection (LeCuyer and Singhal, 2007). On average, about three claims are denied per physician per week and need to be rebilled. Often, claims are denied because additional documentation is required, which often cannot be supplied electronically due to outdated computer systems, or because coverage status is uncertain. Three-quarters of denied bills are ultimately paid, but the administrative cost of securing the payment is very high. Provider groups in the United States employ 770 full-time equivalent workers per $1 billion collected, compared to an average in other U.S. industries of about 100. By all indications, the administrative burden is rising over time as insurance policies have become more complex, while the technology of administration has not kept pace.
The administrative burden differs by payer. Medicare imposes very low administrative burdens; there is no utilization review or pre-authorization requirement for Medicare-covered services. Billing is also straightforward. In exchange, however, the fees Medicare pays are lower than private fees. Private insurers impose higher administrative costs, but also pay more generously.
The persistence of high administrative expenses in health care requires some explanation; in many industries, administrative costs have declined substantially over time. Broadly speaking, there are two explanations for the failure of the healthcare industry to simplify administrative costs. First, from a public good perspective, insurers have little incentive to coordinate their credentialing and billing requirements because the costs of imposing different rules are spread across insurers as a whole, not partitioned to any single insurer. This type of coordination failure is not limited to health care. Agreeing on technology standards in many industries has been difficult.
Coordination failures of this type have several possible solutions. Government can mandate a solution, as it sometimes does for technology standards. Or one participating organization may be large enough to impose standardization on the industry, as Wal-Mart has essentially done in retail trade (Johnson, 2006). The equivalent to Wal-Mart in health care is the federal government. Government spending is nearly half of medical spending in the United States and is an even larger part of acute care. The federal government has mandated some common forms and basic processes, such as electronic prescribing capability, but its involvement has been limited. Finally, private actors can come together to agree on standards. This approach is common in many high-tech industries, where standards organizations ensure comparability of different platforms (for example, the Institute of Electrical and Electronics Engineers). Such organizations do exist in health care, but they have not been enormously successful to date.
A second potential reason for the persistence of high administrative costs in health care is that complexity might be valuable to insurance payers if it lowers what they ultimately pay for health care. For example, denying claims saves an insurance company money if a service is never reimbursed or if the present value of payments for services that are eventually reimbursed is reduced. Delay may also discourage physicians from providing some services entirely. Moreover, higher administrative costs may be necessary to prevent fraud, which may be more prevalent in a fee-for-service reimbursement system without overall constraints (as in the case of Medicare). The theory of social insurance justifies imposing at least some hassles to screen out less-valuable from more-valuable care (Nichols and Zeckhauser, 1982); physicians may be willing to pay the administrative costs of care for services that are really worth it to their patients. The question is whether there are better ways to enforce these limits. It would be valuable to compare the equilibrium with overall supply limits, as in Canada, to the equilibrium where screening is used to limit service provision.
The literature in the United States provides little guidance on whether the public good model or the complexity model is a more accurate description of the U.S. experience with persistently high administrative expenses in health care. Nor does international experience provide much guidance. Countries with a single payer have lower insurer administrative costs than countries with multiple payers (Davis, Schoen, Guterman, Shih, Schoenbaum, and Weingaum, 2007). However, even countries with private insurers have very strict regulation of insurance policies and of the operation of insurers themselves. These rules generally prohibit having many different policies and access rules.
Because the federal government is involved in so much of health care, it would be natural for the federal government to take the lead in addressing administrative issues. For example, the government could require physicians’ offices, hospitals, and insurers that participate in Medicare, Medicaid, or the soon-to-be-created insurance exchanges to use common credentialing forms, to expand the range of electronic interchange they accept, and to standardize billing, enrollment, and renewal information. There is precedent for the U.S. government in taking the lead on health information technology issues. Responding to slow diffusion of electronic medical record systems, the American Recovery and Reinvestment Act of 2009 allocated $30 billion to support investment in electronic medical records. To ensure that the new systems benefited patients, the Department of Health and Human Services was charged with developing guidelines for receiving federal support. The department has done this, and functional electronic medical records are likely to become far more widespread.
The recent health reform legislation partially addressed the issue of administrative simplification. Section 1104 of the Patient Protection and Affordable Care Act (a.k.a. “the health reform legislation”) signed by President Obama in March 2010 requires the adoption of common operating rules for eligibility verification and claims status checks, and it mandates electronic flow of information and money. This requirement will address some of the administrative bottlenecks, but not all. For example, approval procedures are likely to continue to be quite variable, and issues such as credentialing and preauthorization will still require personal interaction. In either the public good model or the complexity model, the government will need to do more. Again, the solution is for the government to lay out a set of milestones and a path for meeting them, as it has sought to do with electronic medical records. Additional money can be provided up front, or providers and insurers could be penalized for not meeting the goals. With the greater emphasis now being put on reducing spending in health care, this type of action is likely more palatable than in the past.
Healthcare Factor Prices
Physicians in the United States are among the best paid in the world (Bodenheimer, 2005). The average U.S. specialist physician earns $230,000 annually—78 percent above the average in other countries, as shown in Table 2. Primary care physicians earn less (they earn $161,000 on average), but the same percentage more than their peers in other countries.2 A spending differential remains true relative to average incomes in the economy. Specialist U.S. physicians earn 5.8 times what the average worker does, compared to the non-U.S. average of 4.3 times. The ratio of primary care physician earnings to per capita GDP is higher in the United States as well. If we reduced all physician incomes in the United States to match the international ratio of physicians’ incomes to per capita GDP, U.S. healthcare spending would be lower by roughly 2 percent.
Table 2.
Comparison of Physician Earnings across Countries
| Country | Specialists | General practitioners: Ratio to high earners |
||
|---|---|---|---|---|
| Average earnings (1,000s) |
Ratio of earnings to: | |||
| GDP per capita | High earners | |||
| United States | $230 | 5.8 | 1.37 | 0.92 |
| Australia | $173 | 5.3 | 2.54 | 0.98 |
| Canada | $161 | 5.0 | 2.11 | 1.41 |
| France | $131 | 4.4 | 1.47 | 0.92 |
| Germany | $155 | 5.4 | 1.45 | 1.06 |
| Italy | $84 | 3.0 | 1.31 | — |
| Netherlands | $286 | 8.7 | 2.56 | 1.06 |
| New Zealand | $87 | 3.5 | 1.47 | 0.86 |
| Norway | $79 | 1.9 | 0.78 | 0.68 |
| Portugal | $79 | 4.3 | 1.11 | 0.69 |
| Sweden | $71 | 2.3 | 0.98 | 0.86 |
| Switzerland | $130 | 3.7 | 0.87 | 0.77 |
| United Kingdom | $114 | 3.7 | 0.80 | 1.02 |
| Non-U.S. average | $129 | 4.3 | 1.45 | 0.94 |
| Ratio: U.S./Non-U.S. average | 1.78 | 1.35 | 0.94 | 0.98 |
Sources: Data on physician earnings are from the OECD (2010). Average incomes for high earners are based on data in Alvardo, Atkinson, Piketty, and Saez (2011).
Notes: Data on physician earnings are adjusted to 2004 as described in U.S. Congressional Research Service (2007). High earners are people in the 95th to 99th percentile of the earnings distribution. Primary care and specialist incomes are reported combined for Norway and Portugal. They are distributed to general practice and specialty based on the general practitioner-specialist differential in Sweden (for Norway) and the differential in France (for Portugal).
However, these seemingly high salaries for U.S. physicians appear less high in the context of the broader income distribution. To illustrate this point, we combine data on physician earnings from the OECD (2010) with data on high-income earners from Alvaredo, Atkinson, Piketty, and Saez (2011; see also Atkinson and Piketty 2007). For a number of countries, Alvaredo, Atkinson, Piketty, and Saez estimate the share of total income accruing to people at various points in the income distribution, including between the 95th and 99th percentile. We use these data, along with total income in the country and the number of tax filing units, to estimate the average income of tax filing units between the 95th and 99th percentile, which we define as “high income.” The ratio of physician earnings to the incomes of other high earners is a rough measure of the relative returns to becoming a doctor.3
Table 2 shows the resulting calculation for countries for which we have both income data and physician earnings data. Outside of the United States, the average specialist earns 45 percent more than the income of the average high-income family. Relative to this, specialist U.S. physicians earn only 37 percent more. For generalists, the United States is virtually identical to other countries; as shown in Table 2, general practice physicians in the United States and those in other high-income countries both earn about 90 percent of what high-income families earn in their respective countries.
In addition, U.S. physicians generally have more medical school debt than physicians in other countries. Medical school education is generally privately financed in the United States but publicly financed elsewhere. While the overall debt of U.S. physicians is not large relative to their income, the debt must be paid back at a relatively young age and thus may be particularly salient for the decisions young adults make about going to medical school and choice of specialty.
Currently, the United States imports a significant share of its physicians. Almost one-quarter of U.S. physicians were trained abroad (Simoens and Hurst, 2004). The leading supplier is India, though a significant number are U.S. residents who go abroad for training. The shortfall of U.S. doctors seems to be driven not by a lack of supply but by a lack of medical school openings (AAMC, 2010a). The United States annually has 2.3 applicants per medical school slot, and these applicants are generally of very high quality. At least 20 percent of applicants to medical school who do not get in have MCAT (Medical College Admission Test) scores above the mean of admitted students (AAMC, 2010b). Evidence suggests that U.S. physicians trained abroad have comparable outcomes to those trained in the United States, although the outcomes are better for non-U.S. citizen (upon entering medical school) physicians trained abroad than for U.S. citizen physicians trained abroad (Norcini, Boulet, Dauphinee, Opalek, Krantz, and Anderson, 2010).
The one major country that appears to be paying its physicians too little is the United Kingdom. As Table 2 shows, the relative earnings of U.K. specialist physicians are below those of most other countries.4 The effects are apparent in several ways. First, many doctors trained in the United Kingdom move to work in other countries. Four percent of Canadian doctors and 9 percent of Australian doctors were trained in the United Kingdom (Mullan, 2005). In addition, the United Kingdom imports a substantial number of doctors trained elsewhere. Twenty-eight percent of British physicians were trained outside the United Kingdom, with India being the single largest provider.5 In recent years, salaries for British physicians have increased by over a quarter in a bid to strengthen the health system (Day, 2007).
Nurses are also paid more in the United States than in other countries, but the picture is again similar; the higher salary reflects the greater opportunity cost of being a nurse in the United States rather than reflecting pure rents. The average U.S. nurse earns 70 percent more than nurses in other countries. Compared to per capita GDP, however, the difference is only 10 percent, and it would be smaller still comparing nurses to other workers in the upper half of the income distribution (Congressional Research Service, 2007).
Pharmaceutical prices are a price category that has received considerable attention. Branded drugs sell for much higher prices in the United States than in most other countries (Japan being the primary exception), though generic drugs are cheaper in the United States. The discount off U.S. prices in other countries for branded medications is 25 to 40 percent (Danzon and Furukawa, 2003). The proximate reason for this discount is that other countries are monopsony purchasers, and because pharmaceutical prices are well above marginal cost, other countries can obtain a significant discount off U.S. list prices. However, because pharmaceuticals are only about 10 percent of U.S. healthcare spending, the overall amount that could be saved by moving to U.S. government monopsony purchasing of drugs is relatively small—perhaps 20 to 30 percent of pharmaceutical spending, or 2 to 3 percent of total medical costs.
These cost savings also would have to be weighed against the possibility of reduced incentives for investment and innovation in the pharmaceutical industry. The dollar amount of excess pharmaceutical payments in the United States is approximately the total amount of pharmaceutical company research and development (R&D). Furthermore, pharmaceutical company profits are correlated with R&D, even when both are detrended (Scherer, 2001). Thus, the long-term effects of price reductions on R&D spending might be large. However, excess payments are also approximately equal to sales and marketing expenses. In a system with better incentives for physicians to prescribe the right drugs and for patients to take them, such advertising might be needed less frequently. In any case, the potential cost savings for the United States from constraining overall factor prices seem relatively small.
The Provision of Medical Services
On some measures of healthcare service utilization, the United States is average or below average compared to other countries. As noted, Americans receive fewer physician visits and have fewer days of hospital care than people in other countries (Anderson, Reinhardt, Hussey, and Petrosyan, 2003). However, the intensity of care at the physician’s office or at the hospital is much greater in the United States than in other countries (OECD, 2003).
By almost every metric, the U.S. medical sector is one of the most technologically intensive in the world. The United States has the third-highest number of CT scanners per capita (behind Japan and Australia), the second-highest number of MRI scanners per capita (behind Japan), and the second-highest number of PET scanners (behind Japan). The United States is also the second-highest in stent insertions per capita (behind Germany) and the third-highest in bypass surgery per capita (behind Belgium and Germany) (OECD, 2010).
Supply Side
Understanding why the United States practices medicine so intensively is not difficult; it is a direct function of the supply side of the market. Most countries, unlike the United States, ration technological availability by dictating a certain level of technology (for example, a certain number of open heart surgery facilities) across a region.6 The difference between the United States and Canada is again informative: the province of Ontario has 11 open-heart surgery facilities (Cardiac Care Network of Ontario, 2010), while the state of Pennsylvania, with roughly the same population as Ontario, has more than five times the number of heart surgery facilities (Medicare, 2011). California is three times larger in population but has 10 times the number of heart surgery facilities. Given this difference in the number of facilities, it is simply impossible for physicians in Ontario to perform as many open heart surgery operations as those in Pennsylvania or California.
Some of this lower capacity shows up as waiting lists. Canadians wait longer for some services than Americans do, especially for seeing a specialist or getting an elective surgery (Davis, Schoen, Schoenbaum, Doty, Holmgren, Kriss, and Shea, 2007). Waiting lists are particularly common in countries where technology constraints are tighter. The queue in the United Kingdom used to be quite large, while it was smaller in continental European countries that spent more.
Reimbursement policy affecting healthcare technology complements the lack of direct controls in explaining the intensity of U.S. medical care. Traditional U.S. medical care payment has been on a piece-rate basis, termed “fee-for-service.” In the Medicare program, physicians are paid for each service they provide. The exact amount is determined by an administratively set fee schedule, but price remains above marginal cost. Prior to the early 1980s, hospitals were paid on a piece-rate basis as well. In 1983, hospital reimbursement under Medicare moved to a partial bundle payment system, in which hospitals are paid a single amount per admission, depending on the diagnosis of the patient (sicker patients are reimbursed more) and whether the patient received an operation. The latter adjustment is particularly important (McClellan, 1997), because paying more when operations occur allows hospitals to continue making a profit as the intensity of care rises.
Provided that price is above marginal cost, fee-for-service reimbursement encourages overprovision of care at the margin. A good deal of medical care is provided in discretionary situations: Is a scan really needed or not? Is a stent really necessary to prop open an artery, or will the patient improve with diet, exercise, and medications? Fee-for-service payment is more likely to lead to additional care in these discretionary situations.
The alternative to fee-for-service payment is to bundle services into broader pricing units and to pay one price for the bundle as a whole. Where a physician in a fee-for-service payment system will be paid for each patient visit, office test, and any other service provided, the same physician in a bundled payment system might receive one payment for the treatment of a condition as a whole—for example, a fixed amount to manage the hip fracture of a patient. At the extreme, physicians could be paid a single amount for all the care needed by the patient in a year. The latter is termed a “capitated” payment, or a “global budget” for hospitals. Capitated payments are closely related to salary reimbursement, though in the former, physician income can fall when more services are used, while salary earnings are independent of service utilization.
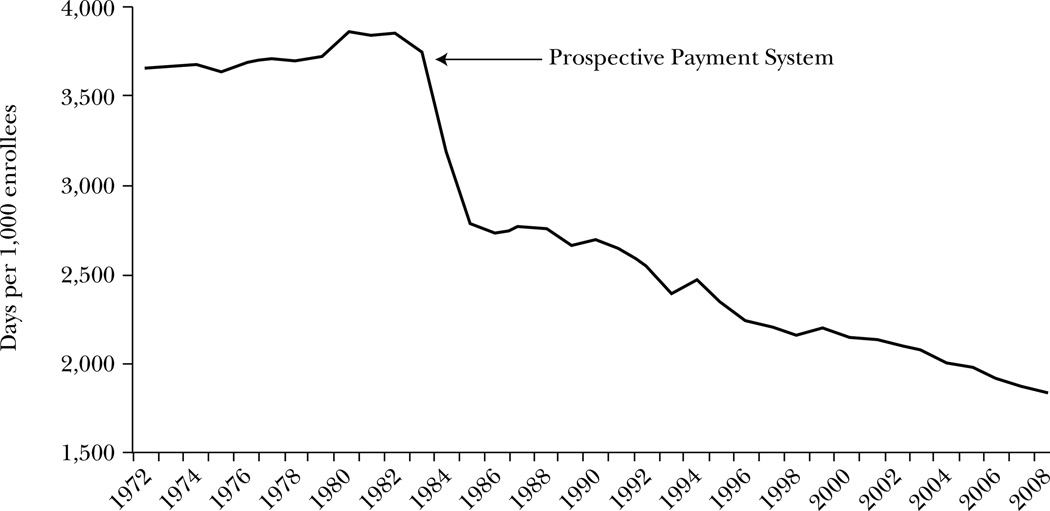
The U.S. experience illustrates the potential effects of changing payment incentives. When the Medicare system transformed hospital payments from piece-rate pricing to bundled payments on an admission basis in the early 1980s, additional days of care and additional inpatient tests went from well-reimbursed to not being reimbursed at all. Within three years, the number of inpatient days for Medicare recipients fell by one-quarter, as shown in Figure 3. Similarly, when managed care provided incentives to use less care, fewer or less-intensive services were provided in areas with high managed care penetration compared to low-penetration areas in the early 1990s (Baker, 1999).
Figure 3. Inpatient Days for Aged Medicare Beneficiaries.
Source: Data are from Centers for Medicare and Medicaid Services (2009).
Note: The figure shows the number of inpatient days per 1,000 aged Medicare beneficiaries. Data exclude enrollees in managed care plans.
Many other countries use more of a bundled payment system than does the United States (Cutler, 2002; OECD, 2003). In France (in the public sector), Italy, Sweden, and the United Kingdom, physicians are paid on a salary or capitated basis. Hospitals receive a global budget in Canada, Denmark, Sweden, and the United Kingdom. When countries do have fee-for-service payment, they often do so in the context of an overall technology limit (as in Canada). Other than the United States, the only high-income country with significant piece-rate pricing and few barriers to acquiring technology is Japan. Indeed, the Japanese government sets prices very low for basic office visits, but allows higher mark-ups on imaging and pharmaceuticals. Hence, it is not surprising that the Japanese healthcare system leads the world in imaging (as noted above) and has high medication usage as well.
The major question about supply-side changes is not whether changes in care provision are possible, but whether they are desirable. When patients receive less-intensive care, do their health outcomes suffer? If so, is the reduction in health outcomes worth the cost savings? Several studies have considered these questions, with mixed results. In many healthcare settings, marginal services appear to have very low health value. For example, several studies have looked at the health effect of the move by Medicare to a more-bundled payment for hospital stays. The general conclusion is that the shift was accomplished without significant adverse effects for Medicare beneficiaries (Rogers, Draper, Kahn, Keeler, Rubenstein, Kosecoff, and Brook, 1990; Cutler, 1995). The evidence on the effect of the spread of managed care similarly suggests no changes in outcomes (Glied, 2000).
International evidence is more mixed. On one side, the greater use of intensive therapies after a heart attack in the United States compared to Canada is not associated with improved mortality, though morbidity is more difficult to determine (Ko et al., 2007). Similarly, a recent study concluded that there was no systematic difference in outcomes in favor of the United States over Canada; if anything, Canadians had better outcomes in most circumstances (Guyatt et al., 2007).
In other settings, however, the differences in health outcomes resulting from differences in treatment intensity may be important. The United States is more aggressive in screening for and treating cancer than are other countries. Mammography rates in Europe are 40 to 80 percent below those in the United States, and rates of screening for colon cancer are 50 to 65 percent lower (Howard, Richardson, and Thorpe, 2009). The difference in screening is especially large among older patients. Treatment with expensive chemotherapy agents is also higher in the United States. This is particularly true for the newest therapies, which can be extremely expensive and are not approved for use in all countries. Consistent with these differences, cancer mortality has declined more rapidly in the United States than in other countries (Preston and Ho, 2009). Compared to 15 other high-income countries, the United States went from a higher rate of prostate cancer death in the early 1990s to 20 percent lower mortality in 2003. Over the same time period, mortality from breast cancer fell by 13 percent more in the United States than in other rich countries.
Significant technology regulation seems unlikely in the United States, but payment reform is definitely possible. Indeed, one of the major goals of the Affordable Care Act is to encourage a transition from the existing fee-for-service payment system to a more-bundled payment structure, ideally beginning with Medicare and then spreading to the private sector (Cutler, 2010). For example, the law requires Medicare to bundle post-acute care services into acute care payments for a number of conditions and creates an Accountable Care Organization program for provider groups that wish to share in the profits from increased delivery efficiency. It also establishes a Center for Medicare and Medicaid Innovation that will experiment with new payment models and expand successes throughout the Medicare program. Finally, it creates an Independent Payment Advisory Board to recommend structural changes to the Medicare program.
Private insurers are not directly included in these efforts, although they have a history of following Medicare reimbursement policy. The intention is for Medicare changes to lead a transformation of the payment system that will encompass private payers as well. Following the effects of these policy changes on the U.S. healthcare system should be a research priority.
Demand Side
This supply-side emphasis is in contrast to a long tradition among health economists of focusing on the demand side of the market. Differences in demand do not appear to drive much of the international variation in use of medical services. While the U.S. population is sicker than the Canadian population in some ways (for example, obesity rates are higher in the United States), the difference in care provision seems to occur even conditional on health status. For example, the greater use of intensive medical therapy after a heart attack is true even controlling for the characteristics of the heart attack patient (Ko et al., 2007). Similarly, people in the United States use mental health services more than people in Canada, but the additional use is entirely accounted for by the population with less-severe illness (Kessler, Frank, Edlund, Katz, Lin, and Leaf, 1997). People with severe needs are treated similarly in the two countries. Nor are out-of-pocket prices lower in the United States; out-of-pocket payments in the United States are actually higher than in most countries.
However, it is also the case that changes in the cost-sharing facing consumers affect the care they demand. The Rand Health Insurance Experiment and a variety of studies since then make clear that medical spending is responsive to the out-of-pocket price (Newhouse et al., 1993). This pattern holds particularly true in settings where supply is not constrained, so that the demand side of the market is the chief limitation on what is done. Thus, many argue for cost-sharing changes to complement changes in reimbursement policy.
As with changes in provider payment policy, the issue with cost-sharing is whether the right services are reduced when cost-sharing is increased. For example, raising copayments for branded prescription drugs induces more people to take generic drugs. But it also leads people to stop taking medications entirely, even when less-expensive generics are available (Huskamp, Deverka, Epstein, Epstein, McGuigan, and Frank, 2003). Generalizing from this experience, higher across-the-board cost-sharing will almost certainly have drawbacks as well as benefits.
Even more-targeted cost-sharing is likely to be far more valuable. For example, insurers might increase cost-sharing for those services that are wasteful and can be avoided, such as nonemergency visits to emergency departments, admission to high-cost hospitals where lower-cost hospitals offer the same services, and discretionary imaging procedures. Such cost-sharing is often termed “value-based insurance design” (Pauly and Blavin, 2008). The 2010 Affordable Care Act has some allowance for value-based insurance, but it does not push this change for the Medicare population. Private insurers, in contrast, have been more active in this area. Because insurers can adjust policies readily, movement to value-based copayment might occur relatively rapidly in the private market.
Malpractice and Defensive Medicine
Many lay observers attribute the overprovision of healthcare to the medical liability system. The concern is that doctors afraid of being sued will practice defensive medicine—ordering additional tests or procedures to avoid being sued. However, the evidence is not particularly favorable to the view of excessive spending due to defensive medicine. Mello, Chandra, Gawande, and Studdert (2010) estimate the costs of this defensive medicine at $46 billion, and the costs of the malpractice system as a whole (including attorneys’ fees and patient compensation) at $56 billion, or 2.4 percent of total healthcare spending. Even significant malpractice reform would not reduce medical spending by a large amount.
Summary
Overall, the international and U.S. experience shows that significant cost savings are possible from changing the way providers are reimbursed and the way cost-sharing is structured. The major issue in both strategies is whether we are able to identify effective care from ineffective care. Doing so would allow us to target care received such that we reward the former and discourage the latter.
The Organization of Care
Most of the literature on international medical care utilization has focused on the number or frequency of specific services, such as whether people are more likely to receive surgery for a particular condition in the United States or in another country. This focus, though, misses an essential element of medical systems: the extent to which they are organized around providing appropriate care.
Consider a person with diabetes. Diabetic patients should monitor their blood sugar regularly, keep their cholesterol levels low, and get regular screenings for blindness, kidney disease, and lower extremity complications. The necessary interaction with the medical system is regular, but not continuous, and it includes a variety of providers.
Medical systems oriented around fee-for-service reimbursement are ill-equipped to care for such patients. Physicians paid on a fee-for-service basis are happy to see patients in their office (assuming price is above marginal cost) and to recommend needed therapies, but they have no pecuniary incentive to make sure that follow-up referrals are actually scheduled (they are not paid for outreach) nor that advice is acted upon (again, no reimbursement). Good chronic care management is very difficult to achieve in a medical care system based on reimbursing only face-to-face interactions.
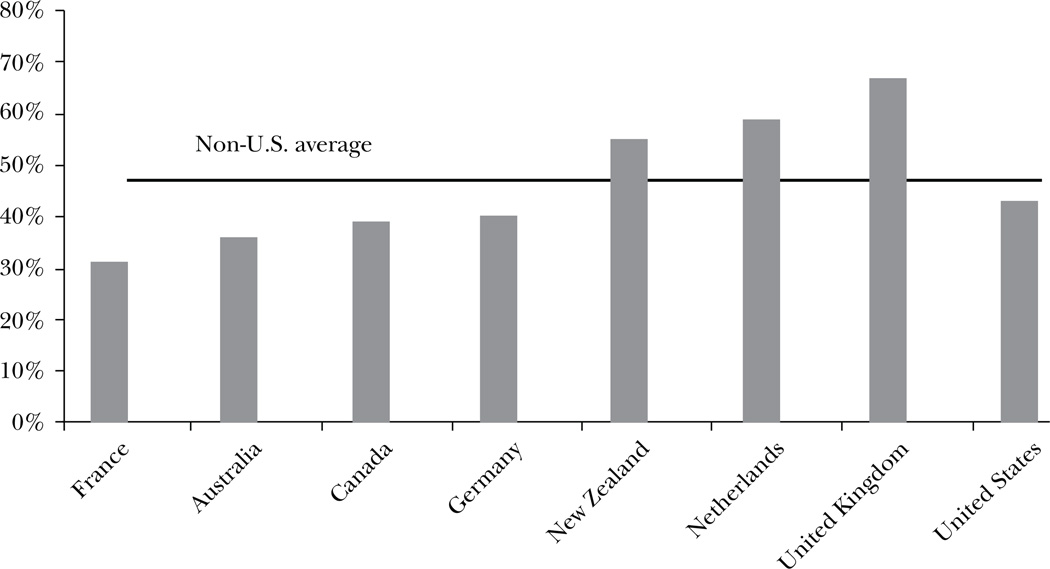
Figure 4 uses international evidence to show the implication of this mispricing on diabetes care. In the United States, only 43 percent of diabetic patients report receiving recommended screening at regular intervals. In France, Australia, Canada and Germany, the level of screening is below the level of the United States. However two-thirds of diabetics in the United Kingdom and nearly 60 percent of diabetics in the Netherlands report having received all recommended screenings. Using an index that averages across standardized rates of kidney failure, stroke, heart attacks and lower-extremity amputations in the diabetic population, the Netherlands the United Kingdom rank first and third, respectively, for outcomes for diabetics out of the eight countries for which we have data. The United States ranks seventh.7
Figure 4. Share of Diabetics Receiving Recommended Care.
Source: Data are from Schoen, Osborn, How, Doty, and Peugh (2009).
Note: The figure shows the share of people with diabetes who had their HbA1c checked in the past six months, had their feet examined for sores or irritations in the past year, had an eye exam for diabetes in the past year, and had their cholesterol checked in the past year.
Several features of the British and Dutch healthcare systems likely contribute to their better performance on these metrics. First, providers in these countries regularly use information technology. Physicians cannot help patients manage their care if they do not know what care their patients have and have not received. Eighty-nine percent of British physicians and 54 percent of Dutch physicians have extensive access to electronic medical records and decision support systems. In contrast, only 26 percent of U.S. physicians have such access (Schoen, Osborn, Doty, Squires, Peugh, and Applebaum, 2009). Indeed, well over half of primary care physicians use sophisticated electronic medical record systems in Austria, Italy, and New Zealand, in addition to England and the Netherlands.
Second, both the United Kingdom and the Netherlands have a team-based approach to care provision. In the Netherlands, physicians have established after-hours cooperatives to provide care on nights and weekends. The United Kingdom has national call centers for the same purpose. Third, physicians are rewarded for care coordination in both countries. Performance on various quality measures has been an important part of physician compensation in the United Kingdom since the early 2000s, and the same is true—to a more limited extent—in the Netherlands. Neither care coordination nor good outcomes are rewarded in the fee-for-service U.S. healthcare system.
In addition, nurses are allowed to play a key role in organizing care in the United Kingdom and the Netherlands. While neither the United Kingdom nor the Netherlands have more nurses than the United States, both countries are notable for allowing nurses greater autonomy in helping care for patients. For instance, teams of general practitioners and nurses provide most of the primary care services in the United Kingdom. The United Kingdom also employs specialist nurses called “community matrons” who act as mostly autonomous case managers of patients with complex needs. The Netherlands also has specialist nurses who take care of patients with specific chronic conditions like diabetes. In fact, in one Dutch program, nurses take care of patients with unstable diabetes while general practitioners are responsible for those with stable disease. Moreover, both countries have nurse-led clinics (Nolte, Knai, and McKee, 2008).
The U.S. healthcare system has seen a push to expand the role of nurses, especially as the number of medical students and residents entering primary care has declined. Several institutions have had success in reconceptualizing the role of nurses. For example, when in 1996, Congress greatly increased the number of veterans eligible to receive services through the Department of Veterans Affairs, more nurse practitioners were hired to meet this demand, and they were conceived of as primary care providers. The results are illustrative: process quality-of-care (receiving appropriate care) was found to be better for the VA relative to Medicare fee-for-service, while spending growth has been much lower (Institute of Medicine, 2011). The Geisinger Health System in Pennsylvania and Kaiser Pemanente have also had success expanding the role of nurses. Geisinger employs nurses as coordinators of care and was one of the first institutions to create care clinics staffed by nurse practitioners, while nurses for Kaiser in San Diego have full authority over the discharge process and nurses in Kaiser’s Riverside Medical Center, as part of healthcare teams, take on the additional roles of healthcare coach and educator to improve the management of chronic care (Institute of Medicine, 2011).
However, state regulations pose a significant barrier to expanding and re-conceiving the role of nurses. In these regulations, spheres of practice authority are legislatively “carved out” of the domain of medicine at the state level, such that scopes of practice for nurses are both relatively circumscribed and vary widely across the country. Practice barriers include on-site physician oversight requirements, chart review requirements, and maximum nurse/physician ratios for physicians who collaborate with more than a single nurse. In addition, nurses are often restricted from prescribing medications, admitting patients to hospitals, and ordering and evaluating tests. Federal efforts may be needed to standardize and expand the role of nurses (Institute of Medicine, 2011).
In part because of this greater flexibility of personnel, better management of care in other countries may offset some of the downside of lower service provision, thus allowing outcomes to be the same or superior at a lower overall spending level. Of course, there is no reason that a country could not have extensive use of acute care along with well-managed chronic care; that is, the United States could lead the world in health outcomes if it succeeded in adding better care management to its greater use of treatments.
Conclusions
Our brief tour of international medical care highlights four primary directions for reform of the U.S. healthcare system. First, the U.S. healthcare system is in great need of administrative simplification. There are few other areas of the U.S. economy where waste is so apparent and the possibility of savings is so tangible. Second, the U.S. healthcare system needs information technology investments. It is virtually impossible to improve care without knowing when it is appropriate, where it falls short, and what care each patient has already received. Third, payment reform is essential to encourage providers to carefully consider which care is of high and low value and to provide incentives for better chronic disease management. Finally, value-based cost-sharing is important to provide people with incentives to use valuable care and discourage less-valuable care.
Together, these changes would almost certainly lower medical care costs, even as they improve the quality of care that people receive. The effect of this change could be sizable. Productivity growth in the United States has averaged 2.5 percent annually since the mid-1990s. In medical care, over the same period, reported productivity growth is negative: official data indicate that we are spending more to get less. While this finding is certainly overstated (for reasons argued in Berndt, Cutler, Frank, Griliches, Newhouse, and Triplett, 2000), few argue that medical care is a high-productivity industry. If medical care could achieve the productivity growth of even the average industry, the excess of private premium growth over GDP growth would be cut in half. If the one-third or more of spending that is wasteful could be eliminated more rapidly, we could see a sustained period of cost reduction. This possibility makes economic research on creating value in health care all the more important.
Acknowledgments
We are grateful to the National Institute on Aging for research support and to the editors, Amitabh Chandra, and Jon Skinner for helpful comments.
Footnotes
To examine spending as a share of GDP is to implicitly assume an income elasticity of 1. Macro income elasticities are generally a bit above 1, but the true income elasticity is difficult to determine (Getzen, 2000). Figure 1 is not adjusted for demographics, but these change only slowly—and European countries are aging more rapidly than the United States.
The definition of primary care and specialty is subject to some uncertainty. The OECD counts the following as specialty care: medical specialists; surgical specialists; pediatricians; psychiatrists; obstetricians/gynecologists; and other nongeneralists. The inclusion of pediatrics as a specialty lowers specialist income in the United States.
The calculation is rough for several reasons. Most importantly, the physician earnings are for an individual, while the income data are for a tax filing unit, typically a family. Still, if these differences are common across countries, we can still examine the relative returns to being a physician in the United States.
Interestingly, primary care physicians in the U.K. earn more than their specialist colleagues, likely due to greater political influence.
In addition to its effect on British medicine, the international migration of physicians has a large effect on global resource availability. All told, 11 percent of doctors trained in the Indian subcontinent practiced outside that area, as did 14 percent of doctors trained in sub-Saharan Africa (with the United States and the United Kingdom being the largest employer in each case). Outside of the five English-speaking countries—Australia, Canada, New Zealand, the United Kingdom, and the United States—the only countries with any significant international medical graduates are Norway and Switzerland, which each employ a moderate share of German-trained physicians.
Some U.S. states regulate acquisition of expensive technologies through Certificate of Need regulation, but this is not frequent and generally not very stringent (Sloan, 1988).
We drew data of the incidence of heart attacks, stroke, lower-extremity amputation, and end-stage renal disease in the diabetic population from the EUCID (European Core Indicators in Diabetes) and the United States (from the Centers for Disease Control and Prevention).
Contributor Information
David M. Cutler, Email: dcutler@fas.harvard.edu.
Dan P. Ly, Email: dan_ly@hks11.harvard.edu.
References
- AAMC. U.S. Medical School Applicants and Students 1982–1983 to 2010–2011. [Data charts accessed October 13, 2010];2010a at https://www.aamc.org/data/facts/. Direct link: https://www.aamc.org/download/132334/data/table24-mcatgpagridall2007-09.pdf.pdf. [Google Scholar]
- AAMC. MCAT and GPA Grid for Applicants and Acceptees for Applicants and Acceptees to U.S. Medical Schools, 2007–2009. 2010b Accessed at: http://www.aamc.org/data/facts/. [Google Scholar]
- Alvaredo Facundo, Atkinson Tony, Piketty Thomas, Saez Emmanuel. Paris School of Economics; 2011. Top Income Database. http://g-mond.parisschoolofeconomics.eu/topincomes/. [Google Scholar]
- Anderson Gerald F, Reinhardt Uwe E, Hussey Peter S, Petrosyan Varduhi. It’s the Prices, Stupid: Why the United States is So Different from Other Countries. Health Affairs. 2003;22(3):89–105. doi: 10.1377/hlthaff.22.3.89. [DOI] [PubMed] [Google Scholar]
- Atkinson Anthony, Piketty Thomas. Top Incomes over the Twentieth Century: A Contrast between European and English-Speaking Countries. Oxford: Oxford University Press; 2007. [Google Scholar]
- Baker Laurence. Association of Managed Care Market Share and Health Expenditures for Fee-for-Service Medicare Patients. JAMA. 1999;281(5):432–437. doi: 10.1001/jama.281.5.432. [DOI] [PubMed] [Google Scholar]
- Berndt Ernst, Cutler David, Frank Richard G, Griliches Zvi, Newhouse Joseph P, Triplett Jack E. Medical Care Prices and Output. In: Culyer Anthony, Newhouse Joseph., editors. Handbook of Health Economics, Volume IA. Elsevier; 2000. pp. 119–180. [Google Scholar]
- Blanchfield Bonnie B, Heffernan James L, Osgood Bradford, Sheehan Rosemary R, Meyer Gregg S. Savings Billions of Dollars—and Physicians’ Time—by Streamlining Billing Practices. Health Affairs. 2010;29(6):1248–1254. doi: 10.1377/hlthaff.2009.0075. [DOI] [PubMed] [Google Scholar]
- Bodenheimer Thomas. High and Rising Health Care Costs, Part 3. Annals of Internal Medicine. 2005;142(12, part 1):996–1002. doi: 10.7326/0003-4819-142-12_part_1-200506210-00009. [DOI] [PubMed] [Google Scholar]
- Canadian Medical Association (CMA) Physician Resource Questionnaire. [Accessed October 23, 2009];2003 Available at: http://www.cmaj.ca/misc/prqindex.shtml. [Google Scholar]
- Centers for Medicare and Medicaid Services. Medicare and Medicaid Statistical Supplement. Washington, DC: Government Printing Office; 2009. [Google Scholar]
- Cardiac Care Network of Ontario. Annual Report, 2009/2010. Toronto: Cardiac Care Network; 2010. [Google Scholar]
- Cutler David M. The Incidence of Adverse Medical Outcomes under Prospective Payment. Econometrica. 1995;63(1):29–50. [Google Scholar]
- Cutler David M. Equality, Efficiency, and Market Fundamentals: The Dynamics of International Medical-Care Reform. Journal of Economic Literature. 2002;40(3):881–906. [Google Scholar]
- Cutler David M. Your Money or Your Life. New York: Oxford University Press; 2004. [Google Scholar]
- Cutler David M. The Simple Economics of Health Reform. The Economists’ Voice. 2010 [Google Scholar]
- Cutler David, McClellan Mark. Is Technological Change in Medicine Worth It? Health Affairs. 2001;20(5):11–29. doi: 10.1377/hlthaff.20.5.11. [DOI] [PubMed] [Google Scholar]
- Danzon Patricia M, Furukawa Michael E. Prices and Availability of Pharmaceuticals: Evidence from Nine Countries. Health Affairs. 2003 Oct 29; doi: 10.1377/hlthaff.w3.521. Web Exclusive, published ahead of print. [DOI] [PubMed] [Google Scholar]
- Davis Karen, Schoen Cathy, Guterman Stuart, Shih Tony, Schoenbaum Stephen C, Weinbaum Ilana. Slowing the Growth of U.S. Health Care Expenditures: What Are the Options? The Commonwealth Fund. 2007 [Google Scholar]
- Davis Karen, Schoen Cathy, Schoenbaum Stephen C, Doty Michelle M, Holmgren Alyssa L, Kriss Jennifer L, Shea Katherine K. Mirror, Mirror on the Wall: An International Update on the Comparative Performance of American Health Care. The Commonwealth Fund. 2007 [Google Scholar]
- Day Michael. So How Much Do Doctors Really Earn? British Medical Journal. 2007;334(7587):236–237. doi: 10.1136/bmj.39112.426481.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher Elliott, Wennberg David E, Stukel Thérèsa A, Gottlieb Daniel J, Lucas FL, Pinder Étoile L. The Implications of Regional Variations in Medicare Spending: Part 1: The Content, Quality, and Accessibility of Care. Annals of Internal Medicine. 2003a;138(4):273–287. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- Fisher Elliott, Wennberg David E, Stukel Thérèsa A, Gottlieb Daniel J, Lucas FL, Pinder Étoile L. The Implications of Regional Variations in Medicare Spending. Part 2: Health Outcomes and Satisfaction with Care. Annals of Internal Medicine. 2003b;138(4):288–298. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- Garber Alan M, Skinner Jonathan. Is American Health Care Uniquely Inefficient? Journal of Economic Perspectives. 2008;22(4):27–50. doi: 10.1257/jep.22.4.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getzen Thomas E. Health Care is an Individual Necessity and a National Luxury Applying Multilevel Decision Models to the Analysis of Health Care Expenditures. Journal of Health Economics. 2000;19(2):259–270. doi: 10.1016/s0167-6296(99)00032-6. [DOI] [PubMed] [Google Scholar]
- Glied Sherry. Managed Care. In: Culyer Anthony J, Newhouse Joseph P., editors. Handbook of Health Economics. Chap. 13. Amsterdam: North-Holland; 2000. [Google Scholar]
- Guyatt Gordon, et al. A Systematic Review of Studies Comparing Health Outcomes in Canada and the United States. Open Medicine. 2007;1(1):27–36. [PMC free article] [PubMed] [Google Scholar]
- Hendrich Ann, Chow Marilyn P, Skierczynski Boguslaw A, Lu Zhenqiang. A 36-Hospital Time and Motion Study: How Do Medical-Surgical Nurses Spend Their Time? The Permanente Journal. 12(3):25–34. doi: 10.7812/tpp/08-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard David H, Richardson Lisa C, Thorpe Kenneth E. Cancer Screening and Age in the United States and Europe. Health Affairs. 2009;28(6):1838–1847. doi: 10.1377/hlthaff.28.6.1838. [DOI] [PubMed] [Google Scholar]
- Huskamp Haiden A, Deverka Patricia A, Epstein Arnold M, Epstein Robert S, McGuigan Kimberly A, Frank Richard G. The Effect of Incentive-Based Formularies on Prescription-Drug Utilization and Spending. New England Journal of Medicine. 2003 Dec 4;:2224–2232. doi: 10.1056/NEJMsa030954. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. The Future of Nursing: Leading Change, Advancing Health. 2011 2011. Available at: http://www.iom.edu/Reports/2010/The-Future-of-Nursing-Leading-Change-Advancing-Health.aspx. [Google Scholar]
- Johnson Fraser P. Supply Chain Management at Wal-Mart. HBS #907D01 Boston, MA: Harvard Business School Publishing; 2006. [Google Scholar]
- Kessler Ronald C, Frank Richard G, Edlund Mark, Katz Steven J, Lin Elizabeth, Leaf Philip. Differences in the Use of Psychiatric Outpatient Services between the United States and Ontario. New England Journal of Medicine. 1997;336(8):551–557. doi: 10.1056/NEJM199702203360806. [DOI] [PubMed] [Google Scholar]
- Ko Dennis T, et al. Regional Differences in Process of Care and Outcomes for Older Acute Myocardial Infarction Patients in the United States and Ontario, Canada. Circulation. 2007;115(2):196–203. doi: 10.1161/CIRCULATIONAHA.106.657601. [DOI] [PubMed] [Google Scholar]
- LeCuyer Nick A, Singhal Shubham. Overhauling the US Health Care Payment System. McKinsey Quarterly. 2007 Jun [Google Scholar]
- Luxembourg Income Study (LIS) Database. 2011 Available at: http://www.lisproject.org/techdoc.htm. [Google Scholar]
- McClellan Mark. Hospital Reimbursement Incentives: An Empirical Analysis. Journal of Economics and Management Strategy. 1997;6(1):91–128. [Google Scholar]
- McKinsey Global Institute. Accounting for the Cost of US Health Care: A New Look at Why Americans Spend More. Washington, DC: McKinsey Global Institute; 2008. [Google Scholar]
- Medical Group Management Association. Administrative Simplification for Medical Group Practices. MGMA Position Paper. 2005 Jun [Google Scholar]
- Medicare. Hospital Structural Measures—Cardiac Surgery Registry. 2011 Available at: http://data.medicare.gov/dataset/Hospital-Structural-Measures-Cardiac-Surgery-Regis/easc-zwde.
- Mello Michelle M, Chandra Amitabh, Gawande Atul A, Studdert David M. National Costs of the Medical Liability System. Health Affairs. 2010;29(9):1–9. doi: 10.1377/hlthaff.2009.0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore James F. The Evolution of Wal-Mart: Savvy Expansion and Leadership. Harvard Business Review. 1993;71(3):82–83. [Google Scholar]
- Mullan Fitzhugh. The Metrics of the Physician Brain Drain. New England Journal of Medicine. 2005;353(17):1810–1818. doi: 10.1056/NEJMsa050004. [DOI] [PubMed] [Google Scholar]
- Newhouse Joseph P. Medical Care Costs: How Much Welfare Loss? Journal of Economic Perspectives. 1992;6(3):13–29. doi: 10.1257/jep.6.3.3. [DOI] [PubMed] [Google Scholar]
- Newhouse Joseph P the Insurance Experiment Group. Free for All? Lessons from the Rand Health Insurance Experiment. Cambridge, MA: Harvard University Press; 1993. [Google Scholar]
- Nichols Albert L, Zeckhauser Richard J. Targeting Transfers through Restrictions on Recipients. American Economic Review. 1982;72(2):372–377. [Google Scholar]
- Nolte Ellen, Knai Cécile, McKee Martin. Managing Chronic Conditions: Experience in Eight Countries. World Health Organization on behalf of the European Observatory on Health Systems and Policies; 2008. [Google Scholar]
- Norcini John J, Boulet John R, Dauphinee W. Dale, Opalek Amy, Krantz Ian D, Anderson Suzanne T. Evaluating the Quality of Care Provided by Graduates of International Medical Schools. Health Affairs. 2010;29(8):1461–1468. doi: 10.1377/hlthaff.2009.0222. [DOI] [PubMed] [Google Scholar]
- OECD. A Disease-based Comparison of Health Systems: What is Best and at What Cost? Paris: Organisation for Economic Co-operation and Development; 2003. [Google Scholar]
- OECD. Organisation for Economic Co-operation and Development; 2010. OECD Health Data 2010: Statistics and Indicators. http://www.oecd.org/document/30/0,3746,en_2649_37407_12968734_1_1_1_37407,00.html. [Google Scholar]
- Pauly Mark V, Blavin Fredric E. Moral Hazard in Insurance, Value-based Cost Sharing, and the Benefits of Blissful Ignorance. Journal of Health Economics. 2008;27(6):1407–1417. doi: 10.1016/j.jhealeco.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Pozen Alexis, Cutler David M. Medical Spending Differences in the United States and Canada: The Role of Prices, Procedures, and Administrative Expenses. Inquiry. 2010;47(2):124–134. doi: 10.5034/inquiryjrnl_47.02.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston Samuel H, Ho Jessica Y. Low Life Expectancy in the United States: Is the Health Care System At Fault? NBER Working Paper 15213. 2009 [Google Scholar]
- Remler Dahlia, Gray Brad M, Newhouse Joseph P. Does Managed Care Mean More Hassle for Physicians? Inquiry. 2000;37(3):304–316. [PubMed] [Google Scholar]
- Rogers William H, Draper David, Kahn Katherine L, Keeler Emmet B, Rubenstein Lisa V, Kosecoff Jacqueline, Brook Robert H. Quality of Care before and after Implementation of the DRG-based Prospective Payment System. A Summary of Effects. JAMA. 1990;264(15):1989–1994. [PubMed] [Google Scholar]
- Scherer EM. The Link between Gross Profitability and Pharmaceutical R&D Spending. Health Affairs. 2001;20(5):216–220. doi: 10.1377/hlthaff.20.5.216. [DOI] [PubMed] [Google Scholar]
- Schoen Cathy, Osborn Robin, Doty Michelle M, Squires David, Peugh Jordon, Applebaum Sandra. A Survey of Primary Care Physicians in Eleven Countries, 2009: Perspectives on Care, Costs, and Experiences. Health Affairs. 2009;28(6):w1171–w1183. doi: 10.1377/hlthaff.28.6.w1171. [DOI] [PubMed] [Google Scholar]
- Schoen Cathy, Osborn Robin, How Sabrina KH, Doty Michelle M, Peugh Jordon. In Chronic Condition: Experiences of Patients with Complex Health Care Needs, in Eight Countries, 2008. Health Affairs. 2009;28(1):w1–w16. doi: 10.1377/hlthaff.28.1.w1. Web Exclusive. [DOI] [PubMed] [Google Scholar]
- Simoens Steven, Hurst Jeremy. Toward High-Performing Health Systems. Chap. 4. Paris: Organisation for Economic Co-operation and Development; 2004. Matching Supply with Demand for the Services of Physicians and Nurses. [Google Scholar]
- Skinner Jonathan S, Fisher Elliott S, Wennberg John. The Efficiency of Medicare. In: Wise David., editor. Analyses in the Economics of Aging. Chicago: University of Chicago Press and NBER; 2005. pp. 129–157. [Google Scholar]
- Sloan Frank A. Containing Health Expenditures: Lessons Learned from Certificate-of-Need Programs. In: Sloan Frank A, Blumstein James F, Perrin James M., editors. Cost, Quality, and Access in Health Care: New Roles for Health Planning in a Competitive Environment. San Francisco: Jossey-Bass Inc.; 1988. pp. 44–70. [Google Scholar]
- U.S. Congressional Research Service. U.S. Health Care Spending: Comparison with Other OECD Countries. 2007 Sep 17; [Google Scholar]
- Woolhandler Steffie, Campbell Terry, Himmelstein David U. Costs of Health Care Administration in the United States and Canada. New England Journal of Medicine. 2003;349(8):768–775. doi: 10.1056/NEJMsa022033. [DOI] [PubMed] [Google Scholar]