Abstract
In recent years, researchers have turned to transient gene expression (TGE) as an alternative to CHO stable cell line generation for early-stage antibody development. Despite advances in transfection methods and culture optimization, the majority of CHO-based TGE systems produce insufficient antibody titers for extensive use within biotherapeutic development pipelines. Flow electroporation using the MaxCyte STX Scalable Transfection System is a highly efficient, scalable means of CHO-based TGE for gram-level production of antibodies without the need for specialized expression vectors or genetically engineered CHO cell lines. CHO cell flow electroporation is easily scaled from milligram to multigram quantities without protocol reoptimization while maintaining transfection performance and antibody productivity. In this article, data are presented that demonstrate the reproducibility, scalability, and antibody production capabilities of CHO-based TGE using the MaxCyte STX. Data show optimization of posttransfection parameters such as cell density, media composition, and feed strategy that result in secreted antibody titers >1 g/L and production of multiple grams of antibody within 2 weeks of a single CHO-S cell transfection. In addition, data are presented to demonstrate the application of scalable electroporation for the rapid generation of high-yield stable CHO cell lines to bridge the gap between early- and late-stage antibody development activities.
Keywords: transient gene expression, antibody production, flow electroporation, scalable CHO cell transfection, stable cell lines
Introduction
Stable cell lines generated from CHO cells remain the most common system for biomanufacturing of clinical-grade antibodies because of their capacity for high-level production of recombinant proteins with mammalian posttranslational modifications, their proven ability to be cultured at production-scale volumes, and their regulatory track record.1 During early-stage biotherapeutic development, hundreds of candidates may be under evaluation, representing a large investment of time and resources if stable cell lines were created for each candidate. Today’s biopharmaceutical companies must mitigate development risks by reducing such early-stage expenditures while maintaining or lowering late-stage attrition rates. As such, these companies have looked to transient gene expression (TGE) as a means to rapidly screen large numbers of antibodies or antibody-like molecules early in the development process to identify promising candidates for further evaluation.
TGE represents one avenue for early-stage cost savings and risk reduction if the system of choice produces sufficient quantities of antibodies to effectively delay stable cell line generation until later in the development pipeline: the larger the delay in migrating to stable cell line generation, the larger the reduction in risk. Producing sufficient quantities of antibodies relies not only on the overall productivity of the cells but also on the scalability of the system. Consequently, the system of choice must lead to strong antibody production using easily scalable processes. In addition, the TGE system of choice must be universal in nature, enabling the investigation of a large number of antibodies and the identification of highly relevant candidates for further development. Thus, evaluation of TGE systems must include assessment of the universality of the system, the relevance of the identified candidates, the antibody production capacity, and the system scalability.
Initial CHO-based TGE activities within the biopharmaceutical community were limited by poor transfection efficiencies, cell viabilities, and production of insufficient quantities of antibodies. This led to the use of transient expression systems based on HEK cells, which are easily cultured in suspension and generally have higher transfection efficiencies and antibody production capabilities.2 Secreted antibody titers as high as 1 g/L have been demonstrated in HEK293 cells using a combination of vector and HEK cell engineering and optimized posttransfection cell culture conditions.3 Literature suggests, however, that there are differences in posttranslational modifications of proteins produced in HEK cells compared with those produced in CHO cells, thereby increasing the risk of late-stage candidate failure upon migration to CHO-based stable expression because of alterations in manufacturability, affinity, and efficacy.4 In response, there has been strong interest in developing a CHO-based TGE system that can rapidly and efficiently produce gram quantities of antibodies.
There are a number of reported CHO transient transfection protocols using polyethylenimine and lipid-based reagents that vary widely in transfection performance, reproducibility, and scalability. Following optimization of posttransfection cell culture conditions such as temperature, pH, osmolarity, and media additives, these protocols result in antibody titers ranging from 10 to 250 mg/L for typical IgG.5–10 Although these antibody yields represent improvements over historic single-digit milligram-per-liter titers, they remain substantially less than the multigram-per-liter productivity of stable CHO cell lines. Because of this disparity in transient and stable expression productivity, researchers are faced during mid-stage antibody development with balancing production needs versus the expenditure of resources for cell line generation. The decision to migrate to stable cell line–based production could be delayed to later stages of antibody development, and thus the resource expenditure and risk reduced, if further improvements in TGE productivity are made.
One approach for increasing CHO productivity relies on genetic engineering of CHO cell lines and expression vectors through the overexpression of host genes or the introduction of viral elements. For example, researchers have engineered cells to increase transcription rates and transgene expression; reduce protein misfolding, aggregation, and degradation; or promote specific posttranslational modifications.11,12 Others have focused on prolonging CHO cell viability and hence augment antibody production by regulating cellular metabolism and reducing the induction of apoptosis.12–15 Several recent articles report CHO antibody titers between 800 and 1000 mg/L following the creation and optimization of CHO cell lines that overexpress XBP1 and ERO-Lα14 or EBNA-1 and GS,15 respectively. Engineered CHO cell lines such as these are generally not commercially available or are limited by patents and must therefore first be created, characterized, and subsequently optimized prior to use in large-scale antibody production. The universality of these newly engineered CHO cells for the expression of a wide array of antibody candidates is not ensured. A highly productive CHO TGE method that does not require the use or generation of specific CHO cell lines and easily scales from milligram to multigram production would be highly advantageous and enable the use of CHO TGE as a universal tool for early- through late-stage biotherapeutic development.
Flow electroporation using the MaxCyte STX Scalable Transfection System is a universal transient transfection technology for rapid, high-quality cell transfection. MaxCyte electroporation has broad cell compatibility, including CHO cells, and the capacity for large-scale transfection of up to 2 × 1010 cells in a single 30-min electroporation without requiring protocol reoptimization during scale-up. In this article, the application of flow electroporation for high-titer, scalable, CHO-based transient expression of antibodies using unengineered CHO-S cells is described. The ability to rapidly conduct small-scale electroporations to maximize productivity followed by streamlined scale-up to bioproduction is highlighted. CHO transfection efficiency and cell viability levels of greater than 95% using flow electroporation are demonstrated, along with the capacity to reproducibly transfect 1 × 1010 cells for gram-scale protein production. Results indicate that secreted antibody titers routinely exceed 400 mg/L and exceed 1 g/L within 14 d of CHO cell transfection using optimized postelectroporation cell culture conditions that allow for the production of more than 3 g of antibody from less than 3 L of culture. Furthermore, the use of MaxCyte electroporation as a tool for accelerating the generation of high-yield stable CHO cell lines is described, providing for maximum efficiency in the progression to late-stage development and biomanufacturing. Overall, flow electroporation is validated as a single, reproducible, and scalable means of CHO cell antibody production capable of fulfilling the production needs throughout biotherapeutic development pipelines.
Materials and Methods
Cell Culture and Electroporation
CHO-S, suspension-adapted CHO cells (Life Technologies, Carlsbad, CA) were cultured at 37 °C with 5% CO2 in CD-CHO medium (Life Technologies) containing 1% HT supplement and 2 mM GIBCO GlutaMax. Cells were passaged every 2 to 3 d and split 1 d prior to electroporation to ensure they were in log phase growth at the time of transfection.
A green fluorescent protein (GFP) expression plasmid was generated by cloning GFP cDNA into the pCI vector (Promega, Madison, WI). Genes encoding a heavy and light chain of a humanized IgG1 were expressed in tandem via independent copies of the CMV promoter using the pcDNA3 vector (Life Technologies). All plasmid DNA was purified using the Qiagen EndoFree Plasmid Mega Kit (Qiagen, Valencia, CA) and prepared in sterile water.
Prior to electroporation, CHO-S cells were pelleted at 250 × g for 10 min, suspended at 2 × 108 cells/mL in electroporation buffer (MaxCyte, Gaithersburg, MD), and mixed with plasmid DNA (1–2 µg DNA per 1 × 106 cells). Cell-DNA mixtures were transferred to OC-400 (small-scale electroporation) or CL-2 (large-scale electroporation) processing assemblies and loaded onto the MaxCyte STX Scalable Transfection System. Cells were electroporated using the “CHO” protocol provided with the MaxCyte STX and immediately transferred to shake flasks and incubated for 30 to 40 min at 37 °C in a 5% CO2 incubator.
Postelectroporation Cell Culture
Following the 30- to 40-min recovery period, cells were resuspended in culture medium at 4 × 106 cells/mL unless otherwise noted in the Results and Discussion section. All postelectroporation cell culture was carried out in shake flasks. During the optimization process, various culture supplements were added and/or the temperature lowered 24 h after electroporation. CD CHO and CD OptiCHO media (Life Technologies) were tested as well as supplementation of media with commercially available nutrients. The final optimized conditions included the addition of 1 mM sodium butyrate, a temperature shift to 32 °C, and daily feeding using supplemented CD OptiCHO medium.
Cell Viability and GFP Expression Analysis
eGFP expression and cell viability were analyzed 24 h after electroporation by flow cytometry (FACS) using the FACSCalibur and CellQuest software from Becton Dickinson Immunocytometry Systems. Viability was measured by trypan blue exclusion using a hemacytometer. Transfection efficiency was determined as the percentage of viable GFP-positive cells among total cells. Nonelectroporated cells were gated as the control with less than 0.5% of GFP-positive cells. The GFP-transfected cells were also imaged using bright-field and fluorescence microscopy.
Total IgG Measurement
Conditioned media samples were removed without replacement and stored at −80 °C at the indicated times postelectroporation. Total IgG titers were measured by enzyme-linked immunosorbent assay (ELISA). Ninety-six-well plates were coated with 50 µL of diluted (2 µg/mL) goat anti-human IgG, Fcγ fragment capture antibody (Sigma-Aldrich, St. Louis, MO) and incubated overnight at 4 °C. Plates were washed 3 times with phosphate-buffered saline (PBS)/0.1% Tween 20 and blocked overnight with 200 µL of PBS/0.1% Tween 20/bovine serum albumin at 4 °C. Diluted cell supernatant samples were added to the plates and incubated for 1 h at room temperature. Plates were washed 3 times with PBS/0.1% Tween following sample incubation. Peroxidase-conjugated secondary antibody (rabbit anti-human IgG heavy and light chains from Sigma-Aldrich) was diluted 1:20,000 and 50 µL added per well. The plates were incubated at room temperature for 1 h and washed as above. One hundred microliters of TMB substrate (Sigma-Aldrich) was added per well and incubated at room temperature for 5 min, and the reaction was stopped by the addition of 50 µL of 0.25 M sulfuric acid. Plates were read on a standard absorbance microplate reader. Purified human IgG1 (Sigma-Aldrich) was used as a positive control to create a standard curve.
Generation and Screening of Stable Cell Lines
Cells were harvested via centrifugation 24 h postelectroporation and resuspended in CD CHO medium supplemented with G418. Two weeks later, limiting dilution cloning of the stable cell pools was performed in the selection medium in twenty-five 96-well plates seeded at 0.3 cells/well. Two weeks following limiting dilution, 479 clones were screened using a human IgG ELISA to identify the top stable cell line candidates. The top 23 candidates were expanded in shake flasks, and productivity was tested on days 1 to 21 of culture.
Results and Discussion
High-Efficiency, High-Cell Viability Transfection of CHO Cells
High-cell viability and transfection efficiency are key to supporting high-level expression of recombinant proteins. Transient transfection of CHO cells has historically resulted in lower transfection efficiencies than other cell types used for protein expression such as HEK cells. CHO-S cells transfected with a plasmid encoding GFP via electroporation using the MaxCyte STX displayed a 96% transfection efficiency measured via FACS (Suppl. Fig. S1) and greater than 95% cell viability as measured by trypan blue exclusion 24 h after electroporation. Cell viability was greater than 95% for both transfected and untransfected cells, demonstrating that electroporation did not affect cell health.
Reproducible, Scalable, High-Titer Antibody Production Using Unengineered CHO-S Cells
CHO cells have been engineered to overexpress a variety of genes in an effort to increase recombinant protein production for both stable and transient expression systems. This approach, however, requires the creation and characterization of custom cell lines prior to the use as a method for TGE and may require cell line reoptimization for each antibody candidate. CHO-S cells are advantageous as they are commercially available, are grown as suspension cultures, and can be grown in serum-free medium at high cell densities—features that directly affect ease of use and safety during large-scale protein production.
CHO-S cells were electroporated using the MaxCyte STX with1 µg DNA per 1 × 106 cells of an antibody expression plasmid encoding both the heavy and light chains of a humanized antibody. A total of 10 CHO cell electroporations were performed on 4 different days over a 5-mo period to assess transfection reproducibility and overall antibody expression levels. Cells were cultured postelectroporation using unoptimized conditions in shake flasks. The average secreted antibody titer 2 wk after transfection for all 10 electroporation runs was 409 ± 68 mg/L (Suppl. Table S1). These data illustrate not only the high run-to-run and day-to-day reproducibility of electroporation but also the capacity of electroporated CHO cells cultured in unoptimized conditions to produce antibody titers approximately two- to threefold higher than published titers for other transient transfection methods.5–10 To our knowledge, these are the highest reported antibody titers using an unengineered CHO cell line.
The universal nature of MaxCyte electroporation for high-titer, CHO antibody expression was confirmed for a variety of human and murine IgG isotypes, bispecific antibodies, antibody-like molecules, and recombinant proteins such as Fc fusions and Fab fragments (data not shown). In addition, independent studies of the quality of several antibodies and antibody-like molecules via sodium dodecyl sulfate–polyacrylamide gel electrophoresis, isoelectric focusing, and/or chromatography indicate that antibodies produced via MaxCyte electroporation are similar to reference antibodies produced by stable clones and are secreted in monomeric forms that do not show evidence of degradation or aggregation (data not shown).
The amount of antibody required throughout the biotherapeutic development process varies from low-milligram to multigram quantities. Ideally, the transient transfection system of choice will have the scalability and reliably to rapidly produce the full range of required antibody quantities. In the initial study of 10 electroporations, 7 were small scale (8 × 107 cells) and 3 were large scale (1 × 1010 cells) in nature; all performed using identical electroporation parameters. Comparable antibody titers were produced for small- and large-scale electroporation, 417 ± 74 mg/L and 392 ± 63 mg/L, respectively (Suppl. Table 1), highlighting the ability to scale-up electroporation without negatively affecting antibody production. This ability to easily scale-up and scale-down without protocol reoptimization enables rapid alignment of resources with the desired antibody production capacity.
Effects of Postelectroporation Cell Culture Seed Density
Increased laboratory productivity is a combination of maximizing antibody yield while minimizing labor and material resource requirements. Typical transfection methods are performed at cell densities of 1 to 4 × 106 cells/mL at the time of transfection.6–9 In contrast, MaxCyte electroporation is compatible with cell densities as high as 2 × 108 cells/mL. This capability provides the flexibility to seed cultures at higher cell densities posttransfection to reduce material and labor costs for both upstream and downstream processes.
The antibody production capabilities of CHO cells cultured at medium (6 × 106 cells/mL) or high (1 × 107 cells/mL) cell densities postelectroporation were examined in an effort to streamline processing and minimize media usage. Cell cultures seeded at 1 × 107 cells/mL postelectroporation produced antibody titers greater than 2.5-fold higher than cultures seeded at 6 × 106 cells/mL (Fig. 1A). High-density cultures had secreted antibody titers exceeding 800 mg/L by day 15 using unsupplemented CD OptiCHO medium and grown in shake flasks. Although it appears that viable cell densities declined more rapidly in the high-density culture (Fig. 1B), this is likely an artifact of underestimated cell counts due to increased cell aggregation at later time points. The steadily increasing antibody titers in both cultures reveal that there are no adverse effects on productivity resulting from culturing cells at higher densities. Overall, these data demonstrate that optimizing the seed density posttransfection can positively affect antibody yield while reducing the required culture volume.
Figure 1.

Increased antibody titers upon culturing at high cell densities postelectroporation. A total of 1 × 1010 CHO-S cells were electroporated with an antibody expression plasmid (1 µg DNA/1 × 106 cells). Postelectroporation cells were inoculated into 2 shake flasks at high and medium viable cell densities (VCDs: 6 × 106 or 1 × 107 cells/mL, respectively) and cultured with daily feeding using unsupplemented CD OptiCHO medium. Sodium butyrate (1 mM) was added to both cultures and the temperature lowered to 32 °C 24 h postelectroporation. (A) VCD and (B) antibody titers were measured through day 15 postelectroporation.
Optimization of Postelectroporation Cell Culture Conditions
Significant efforts have been made to increase CHO cell antibody yield for both transient and stable expression systems. A range of factors has been identified that influence gene expression, protein folding, posttranslational modification, and ultimately antibody yield. Each candidate antibody often differs in its basal level of expression and can be affected differently by alterations to posttransfection culture conditions. Thus, design-of-experiment studies for a matrix of parameters are frequently conducted for individual candidates to identify custom culture conditions for maximum antibody-specific productivity.9,10 In this study, posttransfection cell culture parameters including temperature shift and a variety of media additives were examined. The combination of a hypothermic temperature shift and the addition of sodium butyrate increased CHO cell productivity by more than sixfold on day 8 following electroporation (Suppl. Fig. S2).
Cell growth, nutrient depletion, and secreted protein titers are interrelated and are influenced by media formulation. To examine these effects, CHO cells were transfected and resuspended in CD CHO or CD OptiCHO media either unsupplemented or supplemented with commercially available nutrients. Sodium butyrate was added to all cultures, and the temperature was lowered to 32 °C 24 h postelectroporation. Although cell viability was not affected by media formulation during the first 12 d of culturing (Fig. 2A), it did significantly affect antibody production (Fig. 2B). CD OptiCHO medium produced antibody titers from three to six times higher than CD CHO medium depending on nutrient supplementation. The addition of nutrient supplements to CD OptiCHO medium generated antibody titers approximately twice those of unsupplemented medium. The combination of culturing cells in CD OptiCHO medium and supplementing with nutrients produced antibody titers greater than 1.2 g/L by day 14 of culture. This represents the first reported antibody titers greater than 1 g/L for an unengineered CHO cell line within 2 wk following any method of transfection.
Figure 2.
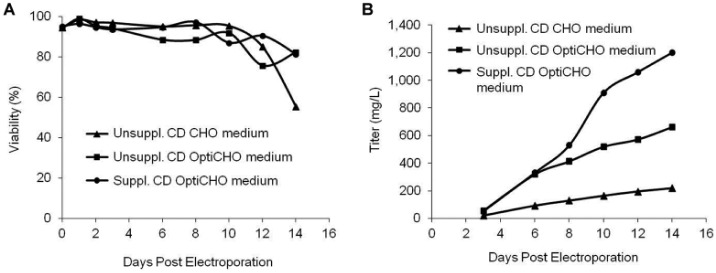
Substantially higher antibody titers using optimized media. A total of 8 × 107 CHO-S cells were electroporated with an antibody expression plasmid (2 µg DNA/1 × 106 cells) using small-scale electroporation. Postelectroporation cells were seeded at 5 to 6 × 106 cells/mL in CD CHO or CD OptiCHO media either supplemented or unsupplemented with commercially available nutrients. Sodium butyrate (1 mM) was added to all cultures and the temperature lowered to 32 °C 24 h postelectroporation. Cells were fed daily. (A) Cell viability was measured via trypan blue exclusion on days 0 to 14 postelectroporation. (B) Antibody titers were measured on days 3 to 14 postelectroporation.
To validate media optimization and demonstrate scalability, side-by-side, small-scale (8 × 107 cells) and large-scale (1 × 1010 cells) CHO electroporations were performed followed by culturing of cells using the optimized media and culture conditions. Electroporated cells were seeded at 6 × 106 cells/mL following transfection corresponding to 20-mL and 2.8-L cell culture volumes, respectively. Both small-scale and large-scale electroporations produced antibody titers greater than 1.0 g/L within 12 d of transfection (Fig. 3A). Twenty-one milligrams of total antibody was produced via small-scale electroporation (Fig. 3B) and 3.5 g of antibody on day 12 following large-scale electroporation (Fig. 3C). This confirms the considerable production capacity of TGE using flow electroporation, particularly at large scale, as a single, 30-min large-scale electroporation of CHO cells produced more than 3 g of antibody from less than 3 L of culture. Importantly, the maintenance of gram-per-liter titers upon scale-up did not require reoptimization of any conditions, clearly demonstrating the ease of method scalability.
Figure 3.
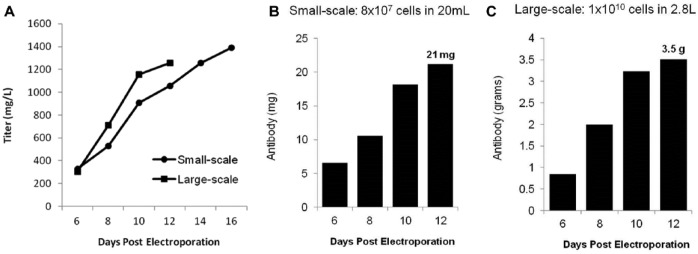
Gram-scale, CHO-based antibody production. CHO-S cells were transfected with an antibody expression plasmid (2 µg DNA/1 × 106 cells) via small-scale (8 × 107 cells) or large-scale (1 × 1010 cells) electroporation. Sodium butyrate (1 mM) was added to cultures and the temperature lowered to 32 °C 24 h postelectroporation. Cultures were fed daily using supplemented CD OptiCHO medium. (A) Secreted antibody titers were measured on days 6, 8, 10, and 12 following large-scale electroporation and on days 6, 8, 10, 12, 14, and 16 following small-scale electroporation. Total IgG production following (B) small-scale and (C) large-scale electroporation was calculated for days 6 to 12.
Overall, the scalability of MaxCyte electroporation enables rapid small-scale studies to identify optimal posttransfection cell culture conditions followed by streamlined scale-up to production-level TGE. The increase in absolute antibody yield achieved through post culture optimization in combination with the reduction in media and media additive usage through higher-density cell culturing represent significant opportunities for increased laboratory productivity and cost reduction.
Stable Cell Line Generation
Stable cell line generation is a lengthy, labor-intensive, multistage process that can take months to complete. Although flow electroporation’s capacity to rapidly produce multiple grams of antibody extends the feasibility of using TGE through later stages in antibody development, companies ultimately migrate to stable cell-based expression for commercial antibody manufacturing. To demonstrate that electroporation using the MaxCyte STX is a viable option for use in stable cell line generation, CHO-S cells were transfected with a plasmid expressing a humanized mAb. The creation of individual clones was performed using limiting dilution at 0.3 cells/well without the aid of advanced tools such as FACS-mediated single-cell deposition, automated clone picking, or cell imaging systems. Primary screening of 479 CHO cell clones was conducted after the initiation of colony formation, and the top 23 performers, representing the top 5%, were further expanded in culture. Twenty-five percent of the top-performing clones had titers ≥1 g/L (Suppl. Table 2). Clone 17 was identified as the top performer and displayed sustained productivity as high as 27 pg/cell/d over a 21-day production period with a final antibody titer of 3.4 g/L (Fig. 4). The high transfection efficiency and cell viability of MaxCyte electroporation enabled the identification of a high-yield stable cell line within 6 wk of the initial cell electroporation without the need to screen thousands of clones.
Figure 4.
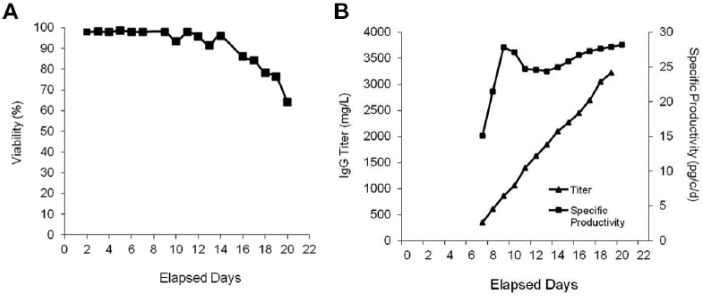
Rapid generation of high-yield stable clones using electroporation. CHO-S cell were electroporated with a humanized mAb DNA plasmid and cultured postelectroporation in G418 selection medium for 2 wk. Limiting dilution cloning was performed in twenty-five 96-well plates at 0.3 cells per well. Following initiation of colony formation, 479 clones were screened for antibody production. The top 23 clones were expanded and productivity assessed (see Suppl. Table 2). The cell viability (A), secreted antibody titers, and specific productivity (B) of the top performer, clone 17, on days 1 to 21 following inoculation of the scaled up culture.
In conclusion, the MaxCyte STX is shown to be a high-performance means of CHO transient transfection that is able to rapidly scale-up and scale-down for the production of milligram to multigram quantities of antibodies via TGE. High transfection efficiencies and cell viabilities enable secreted antibody titers that routinely exceed 400 mg/L within 2 wk of electroporation of unengineered CHO cells and can exceed 1 g/L with optimization of posttransfection cell culture conditions. This unprecedented level of CHO productivity enables the rapid production of multiple grams of antibody, thereby extending the utility of TGE to later stages of therapeutic development and facilitating the efficient identification and progression of quality candidates through preclinical studies.
Separately or in parallel, the same process can be used to generate stable pools with subsequent cloning and selection of high-yield stable clones. The ability to simultaneously conduct early-stage development and initiate creation of quality stable cell lines using a single transfection platform mitigates risk in early-stage development while bridging the gap with manufacturing and decreasing the time to market. Overall, these data demonstrate that flow electroporation has the performance, reproducibility, and scalability needed for candidate selection through commercial implementation during therapeutic antibody development and production.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Functional Genetics for providing plasmids used in this research. We also thank MaxCyte scientists Rama Shivakumar and Angelia Viley for their valuable contributions and experimental work.
Footnotes
Supplementary material for this article is available on the Journal of Biomolecular Screening Web site at http://jbx.sagepub.com/supplemental.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Kim J., Kim Y., Lee G. CHO Cells in Biotechnology for Production of Recombinant Proteins: Current State and Further Potential. Appl. Microbiol. Biotechnol. 2012, 93, 917–930. [DOI] [PubMed] [Google Scholar]
- 2. Geisse S. Reflections on More Than 10 Years of TGE Approaches. Protein Expr. Purif. 2009, 64, 99–107. [DOI] [PubMed] [Google Scholar]
- 3. Backliwal G., Hildinger M., Chenuet S., et al. Rational Vector Design and Multi-pathway Modulation of HEK 293E Cells Yield Recombinant Antibody Titers Exceeding 1 g/L by Transient Transfection Under Serum-Free Conditions. Nucleic Acids Res. 2008, 36, e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Croset A., Delafosse L., Gaudry J. P., et al. Differences in the Glycosylation of Recombinant Proteins Expressed in HEK and CHO cells. J. Biotechnol. 2012, 161, 336–348. [DOI] [PubMed] [Google Scholar]
- 5. Ye J., Kober V., Tellers M., et al. High-level Protein Expression in Scalable CHO Transient Transfection. Biotechnol. Bioeng. 2009, 103, 542–551. [DOI] [PubMed] [Google Scholar]
- 6. Agrawal V., Yu B., Pagila R., et al. A High-Yielding, CHO-K1-Based Transient Transfection System. BioProcess Int. 2013, 11, 28–35. [Google Scholar]
- 7. Liu C., Dalby B., Chen W., et al. Transient Transfection Factors for High-Level Recombinant Protein Production in Suspension Cultured Mammalian Cells. Mol. Biotechnol. 2008, 39, 141–153. [DOI] [PubMed] [Google Scholar]
- 8. Rajendra Y., Kiseljak D., Baldi L., et al. A Simple High-Yielding Process for Transient Gene Expression in CHO Cells. J. Biotechnol. 2011, 153, 22–26. [DOI] [PubMed] [Google Scholar]
- 9. You M., Liu Y., Chen Y., et al. Maximizing Antibody Production in Suspension-Cultured Mammalian Cell by the Customized Transient Gene Expression Method. Biosci. Biotechnol. Biochem. 2013, 77, 1207–1213. [DOI] [PubMed] [Google Scholar]
- 10. Bollin F., Dechavanne V., Chevalet L. Design of Experiment in CHO and HEK Transient Transfection Condition Optimization. Protein Exp. Purif. 2011, 78, 61–68. [DOI] [PubMed] [Google Scholar]
- 11. Jossé L., Smales C., Tuite M. Engineering the Chaperone Network of CHO Cells for Optimal Recombinant Protein Production and Authenticity. Methods Mol. Biol. 2012, 824, 595–608. [DOI] [PubMed] [Google Scholar]
- 12. Lee Y., Wong K., Tan J., et al. Overexpression of Heat Shock Proteins (HSPs) in CHO Cells for Extended Culture Viability and Improved Recombinant Protein Production. J. Biotechnol. 2009, 143, 34–43. [DOI] [PubMed] [Google Scholar]
- 13. Macaraeg N., Reilly D., Wong A. Use of an Anti-Apoptotic CHO Cell Line for Transient Gene Expression. Biotechnol. Prog. 2013, 29, 1050–1058. [DOI] [PubMed] [Google Scholar]
- 14. Cain K., Peters S., Hailu H., et al. A CHO Cell Line Engineered to Express XBP1 and ERO1-Lα Has Increased Levels of Transient Protein Expression. Biotechnol. Prog. 2013, 29, 697–706. [DOI] [PubMed] [Google Scholar]
- 15. Daramola O., Stevenson J., Dean G., et al. A High-Yielding CHO Transient System: Coexpression of Genes Encoding EBNA-1 and GS Enhances Transient Protein Expression. Biotechnol. Prog. 2014, 30, 132–141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


