Abstract
In this study, the characterization and the antimicrobial properties of nano silver (nAg) coating on leather were investigated. For this purpose, turbidity, viscosity and pH of nAg solutions prepared by the sol-gel method were measured. The formation of films from these solutions was characterized according to temperature by Differential Thermal Analysis-Thermogravimetry (DTA-TG) equipment. The surface morphology of treated leathers was observed using Scanning Electron Microscopy (SEM). The antimicrobial performance of nAg coatings on leather materials to the test microorganisms as Escherichia coli , Staphylococcus aureus , Candida albicans and Aspergillius niger was evaluated by the application of qualitative (Agar overlay method) and quantitative (percentage of microbial reduction) tests. According to qualitative test results it was found that 20 μg/cm 2 and higher concentrations of nAg on the leather samples were effective against all microorganisms tested. Moreover, quantitative test results showed that leather samples treated with 20 μg/cm 2 of nAg demonstrated the highest antibacterial activity against E. coli with 99.25% bacterium removal, whereas a 10 μg/cm 2 concentration of nAg on leather was enough to exhibit the excellent percentage reduction against S. aureus of 99.91%. The results are promising for the use of colloidal nano silver solution on lining leather as antimicrobial coating.
Keywords: nano Ag, antimicrobial activity, lining leather, coating
Introduction
Microorganisms such as moulds, yeasts and bacteria are often observed in leather shoes and may cause skin diseases of the feet or undesired odor. A shoe lining leather contacts tightly with the skin of the feet, and the addition of antimicrobial agent may help to form a clean environment inside the shoes. Bunce and Khan (2004) reported that the use of antimicrobial finishes and treatments on leather material can help to avoid or control cross infection and can extend the lifetime of the product, by stopping microbial growth. Antimicrobial treatment can be used in a number of ways including coating to the finished leather. However, Gu et al. (2009) mentioned that because of the disparate antimicrobial spectrum and the problem of toxicity, normal leather fungicides, such as 2-(thiocyanomethylthio) benzothiazole (TCMTB) are not appropriate for use in shoe lining leather.
It is known that if certain functional additives are applied to the surface of leather during the finishing process, the final leather products will have the desired properties on the surface; consequently, adding suitable antimicrobial agents to leather surfaces can provide powerful antimicrobial functions. Therefore, in order to enhance the antimicrobial performance of shoes, a new antimicrobial agent must be found which can be used to inhibit microorganisms in leather.
In recent years, Pal et al. (2007) ; Shahverdi et al. (2007) observed that nanoparticles of silver have been found to exhibit interesting antibacterial activities, and the use of silver nanoparticles as antibacterial agent is relatively new. Because of their high reactivity due to their large surface to volume ratio, nanoparticles play a crucial role in inhibiting bacterial growth in aqueous and solid media. Silver-containing materials can be employed to eliminate microorganisms on medical devices as reported by Babycos (1993) ; Bosetti et al. (2002) and textile fabrics as mentioned by Zhu et al. (2001) ; Pacios et al. (2007) or they can be used for water treatment as claimed by Chou et al. (2005) . Nano silver in solution or supported on appropriate substrates is currently used due to its effective action in adversely affecting cellular metabolism and inhibiting cell growth. Some studies have shown that silver deposits are not toxic to human cells in vivo and they are reported to be biocompatible by Linnert et al. (1990) ; Kusnetsov et al. (2001) ; Vamathevan et al. (2002) ; Naoi et al. (2004) .
The synthesis of silver nanoparticles is well understood and several methods leading to a good control over the size and shape of the particles have been developed by Huang et al. (2004) ; Liu et al. (2005) ; Wang et al. (2005) . The sol-gel method for the preparation of silver solution has some advantages such as good homogeneity, ease of composition control, low processing temperature and good optical properties. In particular, the sol-gel process is efficient in producing thin, transparent, multi-component oxide layers of many compositions on various substrates as reported by Kim et al. (2002) .
In this study, colloidal nAg solutions prepared by the sol-gel method were characterized using a turbidimeter, a pH meter, and a digital rheometer. Characteristics of the films obtained from the nAg solutions were determined by Differential Thermal Analysis-Thermogravimetry (DTA-TG) and Scanning Electron Microscopy (SEM). The antimicrobial effects of nAg coatings on lining leathers against the tested microorganisms, the bacteria Escherichia coli and Staphylococcus aureus and the fungi Candida albicans and Aspergillius niger , were also investigated.
Materials and Methods
Preparation and characterization of nano Ag solution
The nAg solutions were prepared by the sol-gel method according to the following reaction, as described by Lkhagvajav et al. (2011) .
This method is based on the reduction of silver nitrate (AgNO 3 , 99.9% pure, Merck) to metallic silver nanoparticles using glucose (C 6 H 12 O 6 , 99.95% pure, Merck) as the reducing agent. A certain amount of precursor, AgNO 3 , was dissolved in distilled water in a reaction container. An aqueous solution of the reducing agent was prepared in another container, and these two solutions were mixed and stirred at room temperature until a colorless or transparent aqueous solution was formed. The concentration of nAg solutions was varied by diluting the initial stock solution (1000 ppm) with distilled water.
To evaluate solution characteristics, which affect the structure of the thin film on leather, turbidity, pH values, and rheological properties of the prepared sols were determined using a turbidimeter, a pH meter, and a rheometer respectively. Turbidity properties of the solutions were measured in the range of 0–1000 ntu (nephelometric turbidity units) using a VELP TB1 turbidimeter (Ustimate, Italy). After preparation of transparent solutions, their pH values were obtained using a standard pH meter (WTW Inolab, Weilheim, Germany). Additionally, the rheological behavior of the solutions, including the viscosity, was determined with a CVO 100 Digital rheometer (Bohlin Instrument, Worcestershire, UK).
Differential Thermal Analysis-Thermogravimetry (DTA-TG) equipment was used to determine what chemical constituents are liberated on heating, to measure the temperature where changes take place. The surface morphology of treated leathers was observed using Scanning Electron Microscopy (SEM, Philips XL 20 Series accelerating voltage 20.0 kV). Leather samples were coated with thin film of gold using Emitech K550X ion sputtering device at 15 milliamper and 8 × 10 −2 mbar vacuum.
Application of nAg solutions to lining leather
The shoe lining leathers (goat crust) used in this study were produced by a conventional process without any treatment by neither bactericide nor fungicide. Leather samples measuring 2 × 2 cm were cut under sterile conditions and, except the control sample, nAg solutions at different concentrations (0.1 μg/cm 2 , 1 μg/cm 2 , 10 μg/cm 2 , 20 μg/cm 2 ) were applied to the grain side of these samples. After application, the leather specimens were passed through a drying process at 105 °C for 15 min and ironed at 100 °C. In this way, a thin film containing nAg was formed on the leather samples.
Antimicrobial activity
Test microorganisms used in this study were Escherichia coli ATCC 12228, Staphyloccocus aureus ATCC 6538-P, Candida albicans ATCC 10239 and Aspergillus niger (TEM). Bacteria and C. albicans were activated in Muller Hinton Broth (MHB) in a shaking water-bath at 37 °C for 24 h. A. niger was activated in Potato Dextrose Agar at 27 °C for 5 days.
In Agar Overlay Technique, a qualitative test, the control leather sample and the leather samples treated with different concentrations of nAg were placed at the center of Petri dishes. 7 mL of soft MHA containing 0.75% agar inoculated with bacteria and C. albicans (10 5 cfu/mL), to which Triphenyl tetrazolium chloride solution had been added at a final concentration of 50 ppm in order to visualize microbial growth, was poured on to the leather samples on Petri dishes. In the case of A. niger , the leather samples were placed on Petri dishes inoculated with fungal spores (10 5 spore/mL) prepared in physiological saline solution (0.85% NaCl) by the spread plate technique. Plates were incubated inverted in plastic bags at 37 °C for 24 h for bacteria and C. albicans , and at 27 °C for 5 days for A. niger . The plates were visually examined for zones of inhibition around and on the leather samples. The size of the inhibition zone was measured at two cross sectional point and the average was taken as explained in the study of Balogh et al. (2001) . All experiments were performed in triplicate.
In percentage of microbial reduction test, a quantitative analysis, the Gram-negative bacterium E. coli (8.1 × 10 5 cfu/mL) and the Gram-positive bacterium S. aureus (7.3 × 10 5 cfu/mL) were used. The control leather sample and nAg treated 2 × 2 cm leather samples were placed in 250 mL Erlenmeyer flasks with 50 mL physiological saline solution (0.85% NaCl) containing above-mentioned cell numbers of the test strains. The mixtures were cultured at 37 °C in a shaking incubator for 24 h. After incubation, 1 mL of the bacteria containing mixture was serially diluted and 0.1 mL of each dilution was plated on MHA containing 0.5% TTC solution. After 24 h of incubation at 37 °C, viable bacteria were counted based on colony forming units and the mean value of the cells at the lowest dilution was calculated. The reduction rate (%) of the specimen was calculated according to the following equation which was also used by Balogh et al. (2001) ; Kim and Kim (2006) :
where A is the number of bacteria after 24 h in the control (non-treated) sample, and B is the number of bacteria after 24 h in the treated sample.
Results and Ddiscussion
Solution properties
It was understood how well the precursors were dissolved in the solutions by evaluating the turbidity values obtained before the coating process. It was found that powder based precursors are completely dissolved when turbidity value approached 0 ntu, and they are not dissolved and some powder particles are suspended in a sol if it approaches 1000 ntu. The fabrication of a homogeneous, continuous and thin film is directly related to turbidity value. In our experiment, turbidity values of nano silver solutions were found to be in the range 4.29–4.74 ntu. Turbidity values showed that powder based precursors were completely dissolved in the solutions and consequently transparent solutions were obtained. Since very low turbidity values were observed, the solutions had very small silver particles. (Surface topography was conducted on films obtained from colloidal silver solutions by Atomic Force Microscopy in our previous work and the size of particles was observed to be in the range ~20–45 nm ( Lkhagvajav et al. , 2011 ). These values present an important clue for further processing. Therefore, films prepared from solutions containing small particles should be homogeneous, continuous and thin.
Since the pH value of solutions is an important factor influencing the formation of the polymeric three-dimensional structure of the gel during the gelation process, it should be taken into consideration when preparing solutions. While a ramified structure is randomly formed in acidic conditions, separated clusters are formed from the solutions showing basic characters as explained by Brinker and Scherer (1990) . In this study, the pH values of silver based sols had a mildly acidic character with pH values of 4.1–4.6.
The gelation process occurs when aggregation of particles or molecules takes place in a liquid, under the action of Van der Waals forces or via the formation of covalent or noncovalent bonds. The process can be investigated using rheological measurement techniques as claimed by Phonthammachai et al. (2004) . The dependence of the viscosity on the shear rate or test time is a characteristic feature of many sol-gel solutions. It was determined that the viscosity of the Ag solution was approximately equal to 2.55 mPa s. As reported by Huang et al. (2006) , there is a correlation between coating thickness and solution density, liquid-vapor surface tension, and especially viscosity. Due to this fact, the viscosity value is a key factor in controlling film thickness. In our trials, it was expected that thinner films would be produced from silver sols owing to their viscosities.
Thermal analysis
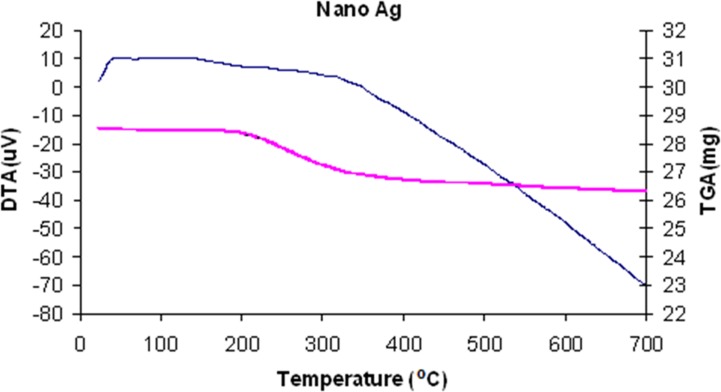
Process optimization is performed in thin film production using DTA-TG results. Seeing that a thin film is formed on leather samples at very low temperatures, DTA-TG results must be evaluated very carefully. Figure 1 illustrates a DTA-TG analysis of Ag solution dried at low temperature for 30 s in air. Similarly, Sen et al. (2005) observed that three or four phenomena take place between room temperature and 700 °C. Similar results were found for Ag powders in our study. As indicated in the DTA curves of the Ag powders, endothermic and exothermic reactions occurred at temperatures between 30 and 700 °C. The first thermal phenomenon occurred between 40 and 150 °C because of solvent removal. The second phenomenon was the combustion of OR groups at temperatures between 185 and 290 °C. The third stage was the formation of ceramic oxides at approximately 470 °C, which was in good agreement with the temperatures mentioned by Sen et al. (2005) ; Celik et al. (2006) . The TG curves for the Ag powders showed weight losses of 7% for temperatures ranging from 30 to 700 °C. In this range of thermal treatment, the weight decrease was due to solvent removal and combustion of carbon-based materials. As can be observed from the TG curves, the largest weight loss occurred during the combustion of carbon-based materials. The slope of the TG curve quickly decreased when the carbon-based materials were burnt out. The variations in the enthalpies and the weight losses occurred because the amounts of the xerogels used in the DTA-TG experiments differed. The amounts affected only the enthalpies and weight losses of the materials. There was no effect on the maximum peak temperatures of combustion and oxidation since these were specific properties of the materials. It is evident from these results that the temperatures of combustion and oxidation depended on the material type. Thus, a processing temperature can be decided as a range of 100 °C and 120 °C for leather application on account of its burning behavior. Within this context, low temperature processing corresponds only to solvent removal.
Figure 1.
DTA-TG curve of powders obtained from Ag solution after drying at low temperature for 30 s in air.
Film characteristics
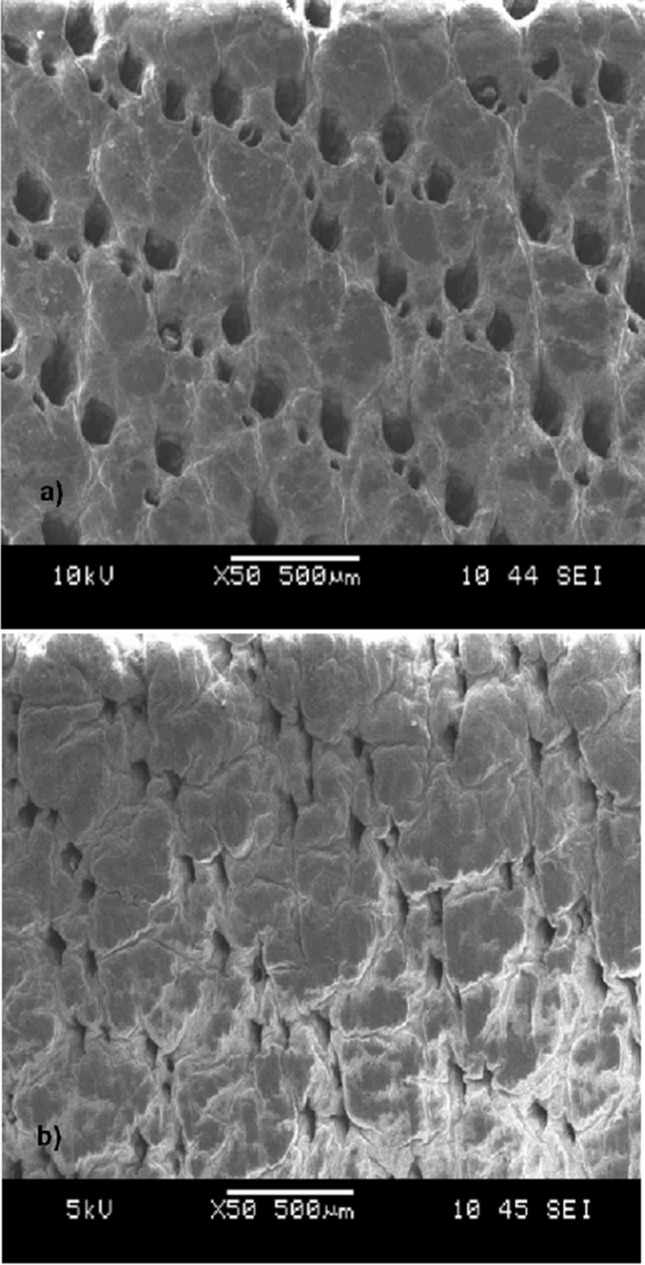
Figure 2a indicates the grain surface of the control leather sample (without any treatment). The SEM micrograph of the grain surface of the leather sample treated with nAg solution (10 μg/cm 2 ) ( Figure 2b ) reveals that a thin, bright, transparent film was obtained on the leather substrate. In fact, the nAg coating was evenly distributed throughout the grain surface and homogenously diffused through the pores. The pore structure of the treated leather sample was tighter than the control sample; the film did not completely fill the leather pores.
Figure 2.
SEM micrographs of control sample (a) and leather sample treated with 10 μg/cm 2 of nAg solution (b).
Thus, it was observed that we have benefited from the advantages of the sol-gel method such as good homogeneity, ease of composition control and low processing temperature and obtained a thin, transparent and evenly distributed films on leather substrate.
Antimicrobial properties of nAg coatings on leather material
The agar overlay test results of the leather samples against test bacteria and fungi are given in Table 1 . According to the results, after appropriate incubation periods, no inhibition zone was seen on the surface of the control sample, moreover they were completely covered by test microorganisms. On the contrary, although the inhibition zones were seen around the leather samples treated with 0.1 μg/cm 2 and 1 μg/cm 2 nAg, there were no microbial growths on the surfaces of the samples. The treatment of the leather samples with 10 μg/cm 2 and 20 μg/cm 2 of nAg was effective and showed good inhibition against both bacteria and fungi, as pronounced and clear inhibition zones were seen around these samples. It is apparent that greater concentrations of nAg used resulted in an increase in antimicrobial activity in the treated leather samples, as indicated by the area of the associated inhibition zones around the samples.
Table 1.
Mean zones of inhibition (mm) of the leather samples.
| Mean zones of inhibition (mm) against microorganisms | ||||
|---|---|---|---|---|
|
|
||||
| Specimens | E. coli | S. aureus | C. albicans | A. niger |
| Control | x a | x a | x a | x a |
| 0.1 μg/cm 2 | o b | o b | o b | o b |
| 1 μg/cm 2 | o b | o b | o b | o b |
| 10 μg/cm 2 | 24 ± 1.0 | 26 ± 0.5 | 22 ± 1.0 | 22 ± 0.6 |
| 20 μg/cm 2 | 26 ± 0.5 | 29 ± 0.7 | 25 ± 1.5 | 25 ± 0.5 |
x - no inhibition zone, microorganism growth on sample.
o - no inhibition zone, but no microorganism growth on sample.
All tested microorganisms were found to be sensitive at the concentrations of nAg of 10–20 μg/cm 2 , because the leather specimens treated with 10–20 μg/cm 2 of nAg displayed strong antimicrobial effects and zones of inhibition were in the range of 22–29 mm. The fact that no growth of any tested bacteria and fungi was observed on the surfaces of the leather samples containing concentrations of 10–20 μg/cm 2 of nAg was evaluated as the result of the good spread of nAg particles on the surface of leather samples. The greater size of inhibition zones around the samples with 20 μg/cm 2 nAg particles can be explained by the homogenous diffusion of nAg into the leather samples.
Table 2 lists the antibacterial properties of leather samples treated with different concentration of the nano sized silver colloids against the bacteria E. coli and S. aureus . At the initial stage, the number of S. aureus cells was 7.3 × 10 5 in all samples; after 24 h, the number of bacterial cells for the control sample and the leather sample treated with 0.1 μg/cm 2 of nAg was 8.1 × 10 7 and 7.2 × 10 7 respectively. The specimens treated by 10 μg/cm 2 and 20 μg/cm 2 of nAg exhibited an excellent percentage reduction against S. aureus of 99.91% and 99.99% respectively. However, in the case of E. coli there was a clear effect of the concentrations of silver nanoparticles. The antibacterial properties increased as the concentration of the nano-sized silver colloids increased. In contrast to S. aureus , specimens treated with 10 μg/cm 2 of nano silver displayed weak antibacterial property against E. coli as 75% reduction. The leather samples treated with 20 μg/cm 2 of nAg showed the highest antibacterial activity against E. coli with 99.25% of bacteria removal ( Table 2 ).
Table 2.
Bacterial reduction on leather samples treated with different concentrations of nAg solution.
| Initial number of bacteria Specimens | Escherichia coli 4 × 10 6 | Staphylococcus aureus 7.3 × 10 5 | ||
|---|---|---|---|---|
|
|
||||
| Reduction rate (%) | Reduction rate (%) | |||
| Control | 4.0 × 10 8 | 8.1 × 10 7 | ||
| 0.1 μg/cm 2 | 3.2 × 10 8 | 20.00% | 7.2 × 10 7 | 11.11% |
| 1 μg/cm 2 | 2.1 × 10 8 | 47.50% | 3.6 × 10 7 | 55.55% |
| 10 μg/cm 2 | 1.0 × 10 8 | 75.00% | 7.2 × 10 4 | 99.91% |
| 20 μg/cm 2 | 1.1 × 10 7 | 99.25% | 6.4 × 10 3 | 99.99% |
The synthesis of silver nanoparticles is well understood and several methods leading to a good control over the size and shape of the particles have been developed ( Huang et al. (2004) ; Liu et al. (2005) ; Wang et al. (2005) . Gaidau et al. (2009) obtained silver nanoparticles (2.69 nm) electrochemically as colloidal silver solutions (29.27 ppm) and treated fur skins by immersion in this solution, which had a content of 490 ppm Ag. The treated skins displayed resistance to S. aureus , E. coli and P. aeruginosa . Gaidau et al. (2011) treated wet-blue leather and metal-free leathers by immersion in nano-Ag based colloidal solutions containing 35 ppm Ag with particles of 4 nm diameter prepared by the electrochemical method. The treated leathers presented inhibitory action against P. aeruginosa (ATCC 9027) and S. aureus (ATCC 6538). Mohajeri-Tehrani et al. (2012) investigated the antimicrobial effects of silver nanoparticles in the form of a colloidal solution of 0.1 mg/mL applied to the surface of leather by the pad-dry cure method, and revealed that the use of such silver-coated leather in shoes for diabetics may have a considerable effect in preventing diabetic foot infections. Smaller numbers of all different types of microorganisms (222 strains of 24 different types of bacteria) responsible for diabetic foot infection grew on nanoparticle-coated leather comparing with natural leather without nanosilver. In this study, nano Ag colloidal solutions synthesized via the sol-gel method were applied to the grain side of the lining leather and the minimum level of nAg (20–45 nm) for obtaining antimicrobial leather was established as 10 μg/cm 2 . Sol-gel is one of the methods used to incorporate silver nanoparticles in the coating. It has several advantages such as high purity, homogeneity, and low processing temperatures ( Ahliah Ismail et al. , 2013 ). The most attractive aspects of this method are that it is an effective preparation method for silver nanoparticles, application on leather is easy, and that it forms a coating which is effective in killing bacteria and fungi.
Maleknia et al. (2010) performed a study to assess the antibacterial properties of nanosized silver colloids on wool fabric. They reported that nanosilver coating on wool fabrics not only had an antibacterial effect against S. aureus and E. coli , but also that its efficiency was still 96% after 20 washings. In our work, leather treated with 20 μg/cm 2 of nAg demonstrated the highest antibacterial activity against E. coli with 99.25% bacterium removal, while a 10 μg/cm 2 nAg concentration applied to leather was enough to exhibit an excellent percentage reduction of S. aureus of 99.91%.
The spectrum of antimicrobial activity of silver is much wider than that of many antibiotics and sulfonamides, and its bactericidal effect is created by minimum doses of the substance. In this connection, Bryzgunov and co-authors revealed that silver demonstrates a more powerful antimicrobial effect than penicillin, biomycin and other antibiotics, and it has a pernicious effect on antibiotic-resistant strains of bacteria ( Beklemyshev et al. , 2009 ). The quantitative test results indicating that the silver nanoparticles are responsible for the antibacterial activity of the coating on leather and this antibacterial activity is quite strong above 10 μg/cm 2 concentration of nAg against both bacteria tested. Relatively, the antibacterial effect against E. coli is lower than that against S. aureus , probably because of the difference in cell walls between Gram-positive and Gram-negative bacteria. The cell wall of E. coli , which consists of lipids, proteins and lipopolysaccharides (LPS), provides effective protection against biocides. However, the cell wall of Gram-positive bacteria, such as S. aureus , does not consist of LPS as observed by Speranza et al. (2004) . Silva and Souca (1973) ; Nikaido and Vaara (1985) ; Raetz (1990) reported that the outer membrane of E. coli cells is predominantly constructed from tightly packed LPS molecules, which provide an effective permeability barrier and render to the surface a density of negative charges. Recently, Amro and co-workers (2000) have shown that metal depletion may cause the formation of irregular-shaped pits in the outer membrane and change membrane permeability, which is caused by progressive release of LPS molecules and membrane proteins.
From the test results, it was established that the effective inhibitory concentration of nAg coating on leather toward all tested microorganisms was 20 μg/cm 2 . At this concentration, not only reductions of 99.25%–99.99% in bacteria but also inhibitions of bacteria and fungi were seen. The antimicrobial performance of nano silver coatings on leather which was performed by a small amount of nAg can be considered as a promising application approach for the leather industry.
Conclusions
In summary, the colloidal nano silver solutions successfully synthesized using the sol-gel method and the turbidity values were in the range of 4.29–4.74 ntu. The pH values of silver-based sols were mildly acidic with a pH value of 4.1 to 4.6. It was determined that the viscosity of the Ag solution was approximately equal to 2.55 mPa.s. The thin and transparent coatings obtained from these solutions were evenly distributed.
In an investigation of the applicability of nAg solution produced by using the sol-gel method as an antimicrobial agent for shoe lining leather, it was found that nAg solutions provide effective antimicrobial properties to leather material. Moreover, their performances were increased by increasing the concentration of nAg. According to the results of the agar overlay method in vitro , the minimum level of nAg for obtaining antimicrobial leather was established as 10 μg/cm 2 . Furthermore, concentrations of nAg which were effective against all tested microorganisms were 20 μg/cm 2 and higher. In addition to this, the leather samples treated with 20 μg/cm 2 of nAg demonstrated the highest antibacterial activity against E. coli with 99.25% bacterium removal, while a 10 μg/cm 2 nAg concentration applied to leather was enough to exhibit the excellent percentage reduction of S. aureus of 99.91%. The results are promising for colloidal nano silver solution use in antimicrobial applications as coatings on lining leathers.
Acknowledgement
We would like to thank TÜBITAK (The Scientific and Technological Research Council of Turkey) for the financial support (Project n. 107M201).
References
- Ahliah Ismail W, Abidin Ali Z, Puteh R. Transparent Nanocrystallite Silver for Antibacterial Coating. J Nanomater. 2013;2013:1–6. [Google Scholar]
- Amro NA, Kotra LP, Wadu-Mesthrige K, Bulychev A, Mobachery S, Liu G. High-resolution Atomic Force Microscopy studies of the Escherichia coli outer membrane: structural basis for permeability . Langmiur. 2000;16:2789–96. [Google Scholar]
- Babycos C. A prospective randomized trial comparing the silver-impregnated cuff with the bedside tunneled subclavian catheter. J Parenter Enteral Nutr. 1993;17:61–63. doi: 10.1177/014860719301700161. [DOI] [PubMed] [Google Scholar]
- Balogh L, Swanson DR, Tomalia DA, Hagnauer GL, McManus AT. Dendrimer-silver complexes and nanocomposites as antimicribial agents. J Nano Letters. 2001;1:18–21. [Google Scholar]
- Beklemyshev VI, Makhonin II, Maugeri UOG. Encyclopedia of Life Support System. Vol. 1. Eolss Publishers Co. Ltd; UK: 2009. Nanomaterials and Coatings with Antimicrobial Properties. [Google Scholar]
- Bosetti M, Masse A, Tobin E, Cannas M. Silver coated materials for external fixation devices: in vitro biocompatibility and genotoxicity. Biomater. 2002;23:887–892. doi: 10.1016/s0142-9612(01)00198-3. [DOI] [PubMed] [Google Scholar]
- Brinker CJ, Scherer GW. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing. Academic Press; San Diego: 1990. 656 [Google Scholar]
- Bunce K, Khan N. The Layman’s Guide to Antimicrobial Fabrics and Testing Methods. Shirley Technologies Ltd; UK: 2004. [Google Scholar]
- Celik E, Yildiz AY, Tanoglu M, Ak Azem NF, Toparli M, Emrullahoglu OF, Ozdemir I. Preparation and characterization of Fe 2 O 3 -TiO 2 thin films on glass substrate for photocatalytic applications . Mater Sci Eng B. 2006;129:193. [Google Scholar]
- Chou WL, Yu DG, Yang MC. The preparation and characterization of silver-loading cellulose acetate hollow fiber membrane for water treatment. Polym Adv Technol. 2005;16:600–608. [Google Scholar]
- Gaidau C, Petica A, Ciobanu C, Martinescu T. Investigation on antimicrobial activity of collagen and keratin based materials doped with silver nanoparticles. Rom Biotechnol Lett. 2009;14:4665–4672. [Google Scholar]
- Gaidau C, Petica A, Dragomir T, Iovu H, Andronescu C. Ag and Ag/TiO 2 nano-dispersed systems for treatment of leathers with strong antifungal properties . Journal of the American Leather Chemists Association. 2011;106:102–109. [Google Scholar]
- Gu H, Zhao C, Wang L, Gong Y, Chen W. A New Combined Antimicrobial Agent: Development and Application in Shoe Lining Leather. XXX Congress IULTCS; Beijing. 2009. [Google Scholar]
- Huang HZ, Yuan Q, Yang XR. Preparation and characterization of metal-chitosan nanocomposites. Coll Surf B Biointerf. 2004;39:31–37. doi: 10.1016/j.colsurfb.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Huang LC, Richman EK, Kirsch BL, Tolbert SH. Direct synthesis of mesoporous TiO 2 modified with phosphotungstic acid under template-free condition . Microporous Mesoporous Mater. 2006;96:301. [Google Scholar]
- Kim DJ, Hahn SH, Oh SH, Kim EJ. Influence of calcination temperature on structural and optical properties of TiO 2 thin films prepared by sol-gel dip coating . Mater Lett. 2002;57:355–360. [Google Scholar]
- Kim S, Kim H. Antibacterial performance of colloidal silver-treated laminate wood flooring. J Intern Biodeter Biodegrad. 2006;57:155–162. [Google Scholar]
- Kusnetsov J, Ivanainen E, Nelomaa N, Zachens O, Martikainen P. Copper and silver ions more effective against legionellae than against mycobacteria in a hospital warm water system. Water Res. 2001;35:4217. doi: 10.1016/s0043-1354(01)00124-5. [DOI] [PubMed] [Google Scholar]
- Linnert T, Mulvaney P, Henglein A, Weller H. Long-lived nonmetallic silver clusters in aqueous solution: preparation and photolysis. J Am Chem Soc. 1990;112:4657. [Google Scholar]
- Liu FK, Ko FH, Huang PW, Wu CH, Chu TC. Studying the size/shape separation and optical properties of silver nanoparticles by capillary electrophoresis. J Chromatogr A. 2005;1062:139–145. doi: 10.1016/j.chroma.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Lkhagvajav N, Yasa I, Çelik E, Koizhaiganova M, Sari Ö. Antimicrobial activity of colloidal silver nanoparticles prepared by sol-gel method. Dig J Nanomater Biostr. 2011;6:149–154. [Google Scholar]
- Maleknia L, Aala A, Yousefi K. Antibacterial properties of nanosized silver colloidal solution on wool fabric. Asian Journal of Chemistry. 2010;22:5925–5929. [Google Scholar]
- Mohajeri-Tehrani MR, Aalaa M, Aalaa AA, Rohipour N, Sanjari M, Khashayar P, et al. Antimicrobial Effects of Silver Nanoparticles Coated Leather on Diabetic Foot Ulcer. Open Access Scientific Reports. 2012;1:538. [Google Scholar]
- Naoi K, Ohko Y, Tatsuma T. TiO 2 films loaded with silver nanoparticles: control of multicolor photochromic behavior . J Am Chem Soc. 2004;196:3664. doi: 10.1021/ja039474z. [DOI] [PubMed] [Google Scholar]
- Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microb Rev. 1985;49:1. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacios R, Marcilla R, Pozo-Gonzalo C, Pomposo JA, Grande H, Aizpurua J, Mecerreyes D. Combined electrochromic and plasmonic optical responses in conducting polymer/metal nanoparticle films. J Nanosci Nanotechnol. 2007;7:2938–2941. doi: 10.1166/jnn.2007.623. [DOI] [PubMed] [Google Scholar]
- Pal S, Kyung Y, Myong Song J. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli . J Appl Environ Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phonthammachai N, Rumruangwong M, Gulari E, Jamieson AM, Jitkarnka S, Wongkasemjit S. Synthesis and rheological properties of mesoporous nanocrystalline CeO 2 via sol-gel process . Coll Surf A: Physicochem Eng Asp. 2004;247:61–68. [Google Scholar]
- Raetz CRH. Biochemistry of endotoxines. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Sen S, Mahanty S, Roy S, Heintz O, Bourgeois S, Chaumont D. Investigation on sol-gel synthesized Ag-doped TiO 2 cermet thin films . Thin Solid Films. 2005;474:245. [Google Scholar]
- Shahverdi AR, Pharm AF, Shahverdi HR, Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli . Nanomed: Nanotechnol Biol Med. 2007;3:168–171. doi: 10.1016/j.nano.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Silva MT, Souca JCF. Ultrastructure of the cell wall and cytoplasmic membrane of gram-negative bacteria with different fixation techniques. J Bacteriol. 1973;113:953–962. doi: 10.1128/jb.113.2.953-962.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speranza G, Gottardi G, Pederzolli C, Lunelli L, Canteri R, Pasquardini L, Carli E, Liu A, Maniglio D, Brugnara M, Anderle M. Role of chemical interaction in bacterial adhesion to polymer surfaces. Biomater. 2004;25:2029–37. doi: 10.1016/j.biomaterials.2003.08.061. [DOI] [PubMed] [Google Scholar]
- Vamathevan V, Amal R, Beydoun D, Low G, McEvoy J. Photocatalytic oxidation of organics in water using pure and silver-modified titanium dioxide particles. J Photochem Photobiol A. 2002;148:233. [Google Scholar]
- Wang HS, Qiao XL, Chen JG, Ding SY. Preparation of silver nanoparticles by chemical reduction method. Coll Surf A. 2005;256:111–115. [Google Scholar]
- Zhu JJ, Liao XH, Zhao XN, Hen HY. Preparation of silver nanorods by electrochemical methods. Mater Lett. 2001;49:91–95. [Google Scholar]