Abstract
Pleurotus ostreatus is able to bioaccumulate several metals in its cell structures; however, there are no reports on its capacity to bioaccumulate iron. The objective of this study was to evaluate cultivation variables to increase iron bioaccumulation in P. ostreatus mycelium. A full factorial design and a central composite design were utilized to evaluate the effect of the following variables: nitrogen and carbon sources, pH and iron concentration in the solid culture medium to produce iron bioaccumulated in mycelial biomass. The maximum production of P. ostreatus mycelial biomass was obtained with yeast extract at 2.96 g of nitrogen L −1 and glucose at 28.45 g L −1 . The most important variable to bioaccumulation was the iron concentration in the cultivation medium. Iron concentration at 175 mg L −1 or higher in the culture medium strongly inhibits the mycelial growth. The highest iron concentration in the mycelium was 3500 mg kg −1 produced with iron addition of 300 mg L −1 . The highest iron bioaccumulation in the mycelium was obtained in culture medium with 150 mg L −1 of iron. Iron bioaccumulation in P. ostreatus mycelium is a potential alternative to produce non-animal food sources of iron.
Keywords: iron sulfate, functional food, hemoglobin, anemia
Introduction
Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm. (Pleurotaceae) is a basidiomycete with high nutritional ( Muñoz et al. , 2007 ), sensorial and functional value ( Wasser, 2002 ). P. ostreatus can bioaccumulate metals in its structures ( Philpott, 2006 ) using different acquisition strategies like the acidification of the culture medium and the production of chelating molecules ( Haas, 2003 ).
Metal bioaccumulation for Pleurotus genus was described for several ions ( Chiu et al. , 1998 ; Kalac and Svoboda, 2000 ; Muñoz et al. , 2007 ; Assunção et al. , 2012 ; Silva et al. , 2012 ). However, reports on iron bioaccumulation for this species have not been found. Iron is a broadly distributed natural transition metal ( Hass, 2003 ). This compound may occupy multiple valences stably and, therefore, it is essential for different metabolic processes as ATP synthesis in the brain, maintenance of oxygen levels and enzyme co-factors ( Dunn et al. , 2007 ). Although it is abundant in nature, iron deficiency is reported as the most prevalent nutritional problem in the world, affecting more than two billion people and most of them are young children and women of reproductive age living in developing countries ( WHO, 2001 ). Iron bioaccumulation by P. ostreatus could provide the development of functional foods enriched with iron.
P. ostreatus can be cultivated on a diversity of substrates, mainly, agricultural and agro industrial residues ( Gern et al. , 2008 ). However, these residues may have heavy metals and agricultural defensives. Mushrooms can bioaccumulate the contaminants from substrate and it has been a growing concern on mushroom production and consumption. The production of mycelial biomass could be an opportunity to iron enriched fungi. The mycelial production allows a greater control of the cultivation media avoiding bioaccumulation of contaminants ( Mshandete and Mgonja, 2009 ). Furthermore, mushroom production takes several months to complete, and it is often difficult to control the product quality due to the use of different agricultural residues. Therefore the mycelium may be an economic and safe alternative for the production of foods, concentrating specific metals such as iron. Mycelium is widely used as an ingredient in many health foods and therapeutics because of its health benefits ( Lee et al. , 2004 ). Thus, the objective of this study was to evaluate cultivation variables that affect iron bioaccumulation in P. ostreatus mycelium.
Material and Methods
Fungal strain
P. ostreatus from the culture collection of the Paranaense University, cryopreserved at −20 °C in wheat grains with saccharose ( Mantovani et al. , 2012 ), was transferred to agar extract malt medium (39 g L −1 ) and kept at 28 °C in the dark to recover mycelial vigor. Mycelium, from the mycelial growth edge with homogenous branching and without sectioning, was used as inoculum. The strain was also deposited in the Fundação Oswaldo Cruz, Coleção de Micro-organismos de Referência em Vigilância Sanitária (FIOCRUZ/CMRVS), reference number Instituto Nacional de Controle de Qualidade em Saúde (INCQS) 40346 in the Coleção de Culturas de Fungos Filamentosos (CCFF), and registered in the World Data Centre for Microorganisms (WDCM) collection number 720.
Experimental design
The influence of different variables on the mycelial growth and iron bioaccumulation was analyzed by the Full Factorial Design 2 4 (FFD) ( Neto et al. , 2001 ). The variables and inferior (−1) and superior (+1) levels were: pH (4.5 or 6.5), iron concentration (50 or 150 mg L −1 ) as FeSO 4 , carbon source (glucose or carboxymethyl cellulose; 20 g L −1 ) and nitrogen source (hydrolyzed casein or yeast extract; 1 g of nitrogen L −1 ). The total nitrogen content of hydrolyzed casein and yeast extract was determined by Kjeldahl method ( Sáez-Plaza et al. , 2013 ). Two other experiments, optimization of the mycelial biomass production and determination of the optimal range of bioaccumulated iron in the mycelium, were carried out from the results obtained in the FFD.
The optimization of the mycelial biomass production was done by a Central Composite Design 2 2 (CCD) ( Neto et al. , 2001 ). The studied variables and inferior (−α and −1), central (zero) and superior (+1 and +α) levels were: glucose concentrations (3.2, 10.0, 20.0, 30.0 or 37.0 g L −1 ) and the concentrations of yeast extract (0.63, 2.0, 4.0, 6.0 or 7.36 g of nitrogen L −1 ). The best conditions for the mycelial biomass production, determined by CCD, were used to optimize the iron bioaccumulation in the mycelium. In this experiment only iron concentration was used as variable in the cultivation medium (0, 25, 50, 75, 100, 125, 150, 175, 200, 250 or 300 mg L −1 ). All experiments were done in five replications.
Culture media
The basis of culture medium consisted of 1.5 g L −1 KH 2 PO 4 , 1 g L −1 NaCl, 1.023 g L −1 MgSO 4 7H 2 O, 0.01 g L −1 ZnSO 4 7H 2 O and 15.0 g L −1 agar ( Pontecorvo et al. , 1953 ). Culture media with addition of carbon source (glucose or carboxymethyl cellulose) or nitrogen source (hydrolyzed casein or yeast extract) were autoclaved at 121 °C for 20 min. During the cooling, they received different volumes of previously filtered (0.22 μm) aqueous solution of FeSO 4 7H 2 O (1000 mg L −1 ). The pH was adjusted with NaOH or HCl (0.1 M) for both solutions that were previously autoclaved at 121 °C for 20 min. Culture media were homogenized and poured in Petri dishes (90 mm diameter). After total cooling a MAE disk (5 mm diameter) containing mycelium (inoculum) was placed at the center of the Petri dish in direct contact with the culture medium. All phases of the experiment were done under aseptic conditions. The mycelial growth was carried out at 28 °C for 14 days.
Production of mycelial biomass and iron bioaccumulation in the mycelium
The Petri dishes containing grown mycelium were heated in a microwave oven for 10 s. The mycelial biomass was removed from the medium surface and then washed three times in 200 mL of ultrapurified water at 80 °C. The water excess was removed by centrifugation at 1700 g for 15 min at 4 °C. After that the mycelial biomass was dehydrated at 60 °C with air circulation until constant mass. The obtained biomass was mixed with 1:12 (m v −1 ) of HNO 3 (1.06 M) and kept at room temperature for 48 h. The mixture was heated at 100 °C then mixed with 1:6 (m v −1 ) of H 2 O 2 (0.88 M) until total solubilization. The final volume was adjusted to 50 mL with ultrapurified water and the iron concentration was determined by an atomic flame absorption spectrophotometer ( Korn et al. , 2008 ) (GBC, model 932 plus ). The obtained results for both factorial experimental designs were evaluated by Pareto’s analysis ( Neto et al. , 2001 ) using Statistica 6.0 software.
Results
The production of mycelial biomass by P. ostreatus was affected (p ≤ 0.05) only by the carbon and nitrogen sources ( Table 1 ). The negative effect of the carbon source (−7.4080) indicates that the shift from the lower level (glucose) to the upper level (carboxymethyl cellulose) reduced biomass production in 7.4080 g. Similarly the positive effect of the nitrogen source (+3.0794) indicates that the shift from the lower level (hydrolyzed casein) to the upper level (yeast extract) provided higher (p ≤ 0.05) mycelial biomass production of 3.0794 g. Thus, the best carbon and nitrogen sources to produce biomass were glucose and yeast extract. The addition of iron or the pH modification in the culture medium did not affect (p ≤ 0.05) the production of mycelial biomass (within the evaluated levels). Thus, only glucose and yeast extract were used as variables for the next experimental phase of optimization of mycelial biomass production in a CCD due to the positive value of the pH effect. A 6.5 pH value was chosen for biomass production optimization without iron addition.
Table 1.
Effect of the variables in Pontecorvo solid culture medium by full factorial design (2 4 ) on the responses of mycelial biomass production and iron bioaccumulation in the mycelium of Pleurotus ostreatus .
| Variables | Mycelial biomass (g dry basis ) | Iron bioaccumulation in the mycelium (mg kg −1 dry basis ) |
|---|---|---|
| Iron (50 or 150 mg L −1 ) | +0.3228 | +13.5327 * |
| Carbon (glucose or carboxymethyl cellulose) | −7.4080 * | −0.5310 |
| Nitrogen (hydrolyzed casein or yeast extract) | +3.0794 * | +2.9739 |
| pH (4.5 or 6.5) | +0.4656 | −1.0214 |
Significant effect (p ≤ 0.05) on the response.
Iron bioaccumulation in the mycelium was affected (p ≤ 0.05) only by the iron concentration in the culture medium with positive effect (+13.5327) ( Table 1 ). The effects indicates that the highest level (150 mg L −1 of iron in the culture medium) provided higher (p ≤ 0.05) iron accumulation in the mycelial biomass. This makes evident that the iron concentration in the culture medium is essential for the bioaccumulation increase of this metal in the mycelial biomass ( Table 1 ). Thus, the effect of the iron concentration on the production of mycelial biomass and bioaccumulation was analyzed in a broad iron concentration range, from zero to 300 mg L −1 , in the culture medium.
Production of mycelial biomass and iron bioaccumulation in the mycelium
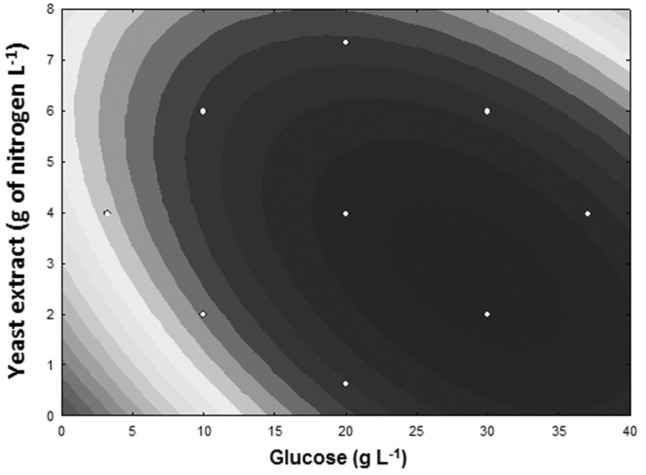
The second-order regression model was developed to predict the optimal point of the biomass production. The optimal range of yeast extract for the production of mycelial biomass was between 1.5 and 4.0 g of nitrogen L −1 , and the optimal point was 2.96 g of nitrogen L −1 . For glucose, the optimal range was from 25 to 35 g L −1 , and the optimal point was 28.45 g L −1 ( Figure 1 ). Knowing that 40% of glucose mass is carbon, the carbon-to-nitrogen ratio in the culture medium for the best range of mycelial production was from 3.5 to 6.6, and the optimal point was 3.8. Thus, the concentrations of the optimal point for the yeast extract and glucose were selected to optimize iron bioaccumulation in the mycelium ( Figure 2 ).
Figure 1.
Effect of yeast extract (g of nitrogen L −1 ) and glucose (g L −1 ) on the production of Pleurotus ostreatus mycelial biomass (g dry basis ). The darkest part of the figure indicates the combination of variables that promotes higher biomass production.
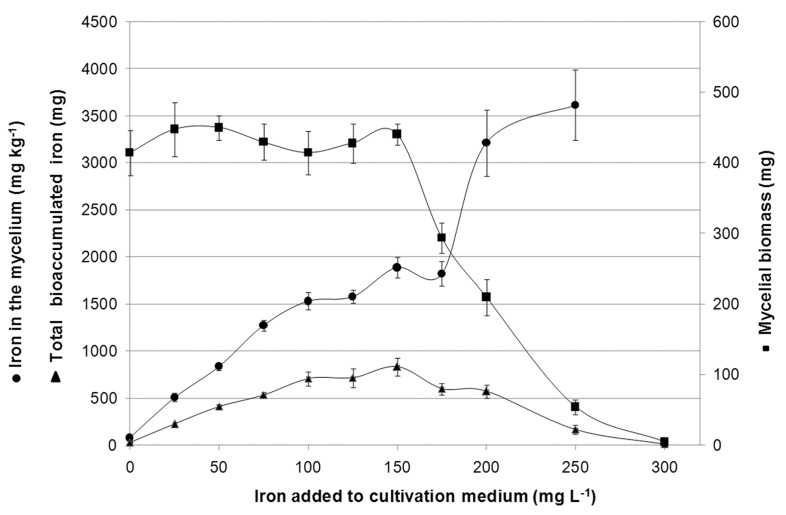
Figure 2.
Mycelial biomass production (mg dry basis ), iron bioaccumulation in the mycelium (mg kg −1 dry basis ) and total bioaccumulated iron (mg) in mycelium of Pleurotus ostreatus grown in culture medium with different concentrations of iron (mg L −1 ).
Culture medium containing 28.45 g L −1 of glucose and 2.96 g of nitrogen L −1 from yeast extract was supplemented with different iron concentrations in order to increase biomass production and iron bioaccumulation. Iron bioaccumulation in the mycelium has increased with the increase of iron concentration in the culture medium ( Figure 2 ). The concentration from zero to 150 mg L −1 of iron in the culture medium did not affect the production of mycelial biomass ( Figure 2 ), which is in agreement with FFD. However a strong inhibition of cellular growth started at 175 mg L −1 , and total inhibition happened at 300 mg L −1 . At 300 mg L −1 , there was no production of mycelial biomass, therefore, it was not possible to evaluate the bioaccumulated concentration of iron in the mycelium. The close relationship between the iron bioaccumulation by the mycelium and the iron concentration in the culture medium are in accordance to the results obtained in FFD ( Table 1 ) where only the iron concentration in the culture medium affected bioaccumulation of this metal in the mycelium.
Iron bioaccumulation in the mycelium occurred from 507 to 3616 mg kg −1 for a culture medium iron concentration ranging from 25 to 250 mg L −1 which represents a transference and accumulation of 20 and 14 times. The bioaccumulation yield (mycelial biomass multiplied by iron concentration) increased up to the concentration of 150 mg L −1 of iron in the culture medium ( Figure 2 ). After this value, the yield was decreasing due to the reduction of the mycelial growth until complete inhibition at 300 mg L −1 .
Discussion
Glucose was the best carbon source for the production of mycelial biomass. Confortin et al. (2008) , working with Pleurotus sajor-caju , and Gbolagade et al. (2006) , with Pleurotus florida , obtained the highest biomass production using glucose rather than sucrose or fructose. The optimal concentration of glucose obtained in this study for the biomass production was 28.45 g L −1 . This value is within the cited range in the literature for the mycelial production by Pleurotus genus, which is 8.18 g L −1 for P. sajor-caju ( Confortin et al. , 2008 ) and 40 g L −1 ( Gern et al. , 2008 ) and 60 g L −1 for P. ostreatus ( Rosado et al. , 2003 ). The best obtained results with glucose rather than carboxymethyl cellulose can be related to the cellular metabolic facility of this molecule whereas carboxymethyl cellulose needs the action of a group of enzymes to be hydrolyzed. Although glucose metabolization is apparently easier, for some microorganisms, metabolic repression can occur by the glucose presence ( Ronne, 1995 ). However, the results found in our study indicate that it did not occur for P. ostreatus in the presence of up to 28.45 g L −1 glucose.
Yeast extract provided higher mycelial biomass production (p ≤ 0.05) with optimal point at 2.96 g of nitrogen L −1 . Gern et al. (2008) reported that the ideal concentration for P. ostreatus varies from 1.86 to 5.00 g nitrogen L −1 . This positive effect of the yeast extract in the basidiomycete production is also reported for Grifola frondosa and Auricularia polytricha ( Lee et al. , 2004 ) and for P. sajor-caju ( Confortin et al. , 2008 ). The appropriateness of this nitrogen source for the production of mycelial biomass in basidiomycetes, especially P. ostreatus , can be associated to its microbial origin with distribution of aminoacids closer to fungal composition. Moreover, hydrolyzed casein, besides being a nitrogen source of animal origin, undergoes denaturation during sterilization with loss of solubility and smaller accessibility to the enzymatic action of the fungus.
The highest production of mycelial biomass in our study was obtained in the culture medium with carbon-to-nitrogen ratio of 3.8. This result is closer to the one reported by Fasidi and Oiorunmaiye (1994) that determined the optimal carbon-to-nitrogen ratio as 4.0 for the production of mycelial biomass of Pleurotus tuber-region in submerged cultivation. This indicates that P. ostreatus mycelium is tolerant to high concentrations of nitrogen and low carbon-to-nitrogen ratio in culture media.
Iron concentration in the culture medium negatively affected the mycelial growth at 175 mg L −1 and caused complete growth inhibition at 300 mg L −1 . Dunn et al. (2007) reported that iron excess causes cell toxicity due to the high reactivity of ions that trigger oxidative stress. The increase of iron concentration in the culture medium also causes the induction of manoprotein synthesis ( Protchenko et al. , 2001 ). These proteins keep at the cell membrane the iron from siderophores, peptic molecules specialized in iron transport ( Haas, 2003 ). Moreover, all these metabolic routes of iron absorption depend on the action of metal reductases to transform Fe +3 to Fe +2 . Therefore, besides the oxidative stress, metabolism is directed towards the synthesis of iron chelating compounds as a defense strategy of the fungus and not to the cell growth.
The increase of iron concentration in the culture medium caused the increase of iron bioaccumulation in P. ostreatus mycelium, and the highest bioaccumulation yield occurred with the addition of 150 mg L −1 of iron. According to Kosman (2003) , fungi depend on iron oxidation to increase bioavailability as well as to reduce the iron cytotoxicity to keep intracellular homeostasis. The versatility and redundancy that basidiomycetes have to bioaccumulate iron are reported ( Haas, 2003 ). Therefore, basidiomycetes use a variety of iron absorption systems such as the ferric iron system that starts by reducing Fe 3+ to Fe 2+ by the iron reductase action. It is likely that the fungus has used this iron absorption system as a defense mechanism to free radicals produced in the oxidative processes mediated by iron. For instance ferric uptake regulation protein acts as a siderophore synthesis repressor and on the expression of iron superoxide dismutase enzyme ( Kosman, 2003 ).
In our experiment, the mycelium bioaccumulated up to 20 times the iron concentration available in the culture medium. Basidiomycete mycelium can bioaccumulate copper and zinc at, 449 and 163 times the medium basal content for Agaricus blazei , respectively ( Rabinovich et al. , 2007 ) and 2 and 11 times for Grifola frondosa ( Figlas et al. , 2010 ). For P. ostreatus basidiocarps, the concentration of mercury was from 65 to 140 times ( Bressa et al. , 1988 ). Several factors affect the bioaccumulation capacity of basidiomycetes like strain, cultivation method, fungus development phase, oxidative state of the mineral, among others ( Baldrian, 2003 ). Nevertheless, P. ostreatus has been the most studied basidiomycete for heavy metal bioaccumulation and nutritional interest due to its growth strength even in highly contaminated substrates.
Mushrooms can bioaccumulate contaminants from natural substrate and it has been a growing concern in mushroom production. For P. ostreatus basidiocarp the concentrations of heavy metals found, in dry basis, are 1.38 mg kg −1 Cd ( Favero et al. , 1990 ) to 3.0 mg kg −1 ( Isildak et al. , 2004 ); 0.02 mg kg −1 Hg ( Bressa et al. , 1988 ); 0.5 to 1.0 mg kg −1 Pb ( García et al. , 2009 ); 1.3 mg kg −1 Cr; 0.4 mg kg −1 Ni and 8.5 mg kg −1 Cu ( Isildak et al. , 2004 ). Therefore, contamination of mushrooms with heavy metals represent a low risk to the public health, in general, but could be a serious problem for those with weakened and immunosuppressed health.
The production of iron enriched mycelial biomass could be an opportunity to use it as a functional food because of its health benefits ( Lee et al. , 2004 ) avoiding the heavy metal accumulation. In our work the bioaccumulated iron concentrations in the mycelium were up to 3500 mg kg −1 . Basidiocarps of P. ostreatus naturaly have iron concentrations, in dry basis, ranging from 48 to 280 mg kg −1 ( Vetter, 1994 ; Tüzen et al. , 1998 ; Demirbas, 2001 ; Isildak et al. , 2004 ; Gençcelep et al. , 2009 ; Patil et al. , 2010 ). The mycelium of P. ostreatus bioaccumulated at least five times more iron than basidiocarp. Thus, iron bioaccumulated mycelium could be an alternative food concentrated with iron of a non-animal source. Future experiments could compare mycelium and basidiocarp bioaccumulation capacity and bioavaibility in vitro and in vivo; include other other functional basidiomycetes; evaluate other biological activities of enriched mycelium and basidiocarp as an alternative source of iron.
Acknowledgments
The authors thank the Paranaense University, the Postgraduate Program in Biotechnology Applied to Agriculture of the Paranaense University, Fundação Araucária, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the financial support and the fellowship.
References
- Assunção LS, Luz JRM, Silva MCS, et al. Enrichment of mushrooms: an interesting strategy for the acquisition of lithium. Food Chem. 2012;134:1123–1127. doi: 10.1016/j.foodchem.2012.03.044. [DOI] [PubMed] [Google Scholar]
- Baldrian P. Interactions of heavy metals with white-rot fungi. Enzyme Microb Technol. 2003;32:78–91. [Google Scholar]
- Bressa G, Cima L, Costa P. Bioaccumulation of Hg in the mushroom Pleurotus ostreatus . Ecotox Environ Safe. 1988;16:85–89. doi: 10.1016/0147-6513(88)90020-6. [DOI] [PubMed] [Google Scholar]
- Chiu SW, Chan YH, Law SC, et al. Cadmium and manganese in contrast to calcium reduce yield and nutritional values of the edible mushroom Pleurotus pulmonarius . Mycologia. 1998;102:449–457. [Google Scholar]
- Confortin FG, Marchetto R, Bettin F, et al. Production of Pleurotus sajor-caju strain PS-2001 biomass in submerged culture . J Ind Microbiol Biotechnol. 2008;35:1149–1155. doi: 10.1007/s10295-008-0394-x. [DOI] [PubMed] [Google Scholar]
- Demirbas A. Heavy metal bioaccumulation by mushrooms from artificially fortified soils. Food Chem. 2001;74:293–301. [Google Scholar]
- Dunn LL, Rahmanto YS, Richardson DR. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 2007;17:93–100. doi: 10.1016/j.tcb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Fasidi IO, Oiorunmaiye KS. Studies on the requirements for vegetative growth of Pleurotus tuber-region (Fr.) Singer, a Nigerian mushroom . Food Chem. 1994;50:397–401. [Google Scholar]
- Favero N, Bressa G, Costa P. Response of Pleurotus ostreatus to cadmium exposure . Ecotox Environ Safe. 1990;20:1–6. doi: 10.1016/0147-6513(90)90039-8. [DOI] [PubMed] [Google Scholar]
- Figlas D, Oddera M, Curvetto N. Bioaccumulation and bioavailability of copper and zinc on mineral-enriched mycelium of Grifola frondosa . J Med Food. 2010;13:469–475. doi: 10.1089/jmf.2008.0284. [DOI] [PubMed] [Google Scholar]
- García MA, Alonso J, Melgar MJ. Lead in edible mushrooms: levels and bioaccumulation factors. J Hazard Mater. 2009;167:777–783. doi: 10.1016/j.jhazmat.2009.01.058. [DOI] [PubMed] [Google Scholar]
- Gbolagade J, Sobowale A, Adejoye D. Optimization of sub-merged culture conditions for biomass production in Pleurotus florida (mont.) Singer, a Nigerian edible fungus . Afr J Biotechnol. 2006;5:1464–1469. [Google Scholar]
- Gençcelep H, Uzun Y, Tunçtürk Y, et al. Determination of mineral contents of wild-grown edible mushrooms. Food Chem. 2009;113:1033–1036. [Google Scholar]
- Gern RMM, Wisbeck E, Rampinelli JR, et al. Alternative medium for production of Pleurotus ostreatus biomass and potential antitumor polysaccharides . Bioresour Technol. 2008;99:76–82. doi: 10.1016/j.biortech.2006.11.059. [DOI] [PubMed] [Google Scholar]
- Haas H. Molecular genetics of fungal siderophore biosynthesis and uptake: the role of siderophores in iron uptake and storage. Appl Microbiol Biotechnol. 2003;62:316–330. doi: 10.1007/s00253-003-1335-2. [DOI] [PubMed] [Google Scholar]
- Isildak Ö, Turkekul I, Elmastas M, et al. Analysis of heavy metals in some wild-grown edible mushrooms from the middle black sea region Turkey. Food Chem. 2004;86:547–552. [Google Scholar]
- Kalac P, Svoboda L. A review of trace element concentrations in edible mushrooms. Food Chem. 2000;69:273–281. [Google Scholar]
- Korn MGA, Morte ESB, Santos DCMB, et al. Sample preparation for the determination of metals in food samples using spectroanalytical methods - A review. Appl Spectrosc Rev. 2008;43:67–92. [Google Scholar]
- Kosman DJ. Molecular mechanisms of iron uptake in fungi. Mol Microbiol. 2003;47:1185–1197. doi: 10.1046/j.1365-2958.2003.03368.x. [DOI] [PubMed] [Google Scholar]
- Lee BC, Bae JT, Pyo HB, et al. Submerged culture conditions for the production of mycelial biomass and exopolysaccharides by the edible basidiomycete Grifola frondosa . Enzyme Microb Technol. 2004;35:369–376. [Google Scholar]
- Mantovani TRD, Tanaka HS, Umeo SH, et al. Cryopreservation at −20 and −70 °C of Pleurotus ostreatus on grains . Indian J Microbiol. 2012;52:484–488. doi: 10.1007/s12088-012-0289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mshandete AM, Mgonja JR. Submerged liquid fermentation of some Tanzanian basidiomycetes for the production of mycelial biomass, exopolysaccharides and mycelium protein using wastes peels media. J Agric Biol. 2009;4:1–13. [Google Scholar]
- Muñoz AHS, Wrobel K, Corona JFG, et al. The protective effect of selenium inorganic forms against cadmium and silver toxicity in mycelia of Pleurotus ostreatus . Mycol Res. 2007;111:626–632. doi: 10.1016/j.mycres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Neto BB, Scarminio IS, Bruns RE. Planejamento e Otimização de Experimentos. 2 edition. Unicamp; Campinas: 2001. [Google Scholar]
- Patil SS, Ahmed SA, Telang SM. The nutritional value of Pleurotus ostreatus (Jacq.: Fr.) Kumm cultivated on different lignocellulosic agro-wastes . Innov Rom Food Biotechnol. 2010;7:66–76. [Google Scholar]
- Philpott CC. Iron uptake in fungi: a system for every source. Biochim Biophys Acta. 2006;1763:636–645. doi: 10.1016/j.bbamcr.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Pontecorvo G, Roper JA, Hemmons LM, et al. The genetics of Aspergillus nidulans . Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- Protchenko O, Ferea T, Rashford J, et al. Three cell wall mannoproteins facilitate the uptake of iron in Saccharomyces cerevisiae . J Biol Chem. 2001;276:49244–49250. doi: 10.1074/jbc.M109220200. [DOI] [PubMed] [Google Scholar]
- Rabinovich M, Figlas D, Delmastro S, et al. Copper- and zinc-enriched mycelium of Agaricus blazei Murrill: bioaccumulation and bioavailability . J Med Food. 2007;10:175–183. doi: 10.1089/jmf.2005.064. [DOI] [PubMed] [Google Scholar]
- Ronne H. Glucose repression in fungi. Trends Genet. 1995;11:12–17. doi: 10.1016/s0168-9525(00)88980-5. [DOI] [PubMed] [Google Scholar]
- Rosado FR, Germano S, Carbonero ER, et al. Biomass and exopolysaccharide production in submerged cultures of Pleurotus ostreatoroseus Sing. and Pleurotus ostreatus “ florida ” (Jack.: Fr.) Kummer . J Basic Microbiol. 2003;43:230–237. doi: 10.1002/jobm.200390026. [DOI] [PubMed] [Google Scholar]
- Sáez-Plaza P, Navas MJ, Wybraniec S, et al. An overview of the Kjeldahl method of nitrogen determination. Part III. Sample preparation, working scale, instrumental finish and quality control. Crit Rev Anal Chem. 2013;43:224–272. [Google Scholar]
- Silva MCS, Naozuka J, Luz JMR, et al. Enrichment of Pleurotus ostreatus mushrooms with selenium in coffee husks . Food Chem. 2012;131:558–563. [Google Scholar]
- Tüzen M, Ozdemiti M, Demirbas A. Study of heavy metals in some cultivated and uncultivated mushrooms of Turkish origin. Food Chem. 1998;63:247–251. [Google Scholar]
- Vetter J. Mineral elements in the important cultivated mushrooms Agaricus bisporus and Pleurotus ostreatus . Food Chem. 1994;50:277–279. [Google Scholar]
- Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002;60:258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- World Health Organization . A guide for programme managers. World Health Organization; Geneva: 2001. [Accessed 6 May 2013]. Iron deficiency anaemia: assessment, prevention and control. Available at: http://whqlibdoc.who.int/hq/2001/WHO_NHD_01.3.pdf. [Google Scholar]