Abstract
Aspergillus flavus was isolated from soil and exhibited laccase activity under both constitutive and copper induced conditions. Spiking the medium with 1 mM copper sulfate resulted in an increase in the activity which reached 51.84 U/mL, a distinctive protein band was detected at 60 kDa. The extracellular enzyme was purified 81 fold using gel filtration chromatography and resulted in two different laccase fractions L1 and L2, the latter had a higher enzymatic activity which reached 79.57 U/mL and specific activity of 64.17 U/μg protein. The analysis of the spectrum of the L2 fraction showed a shoulder at 330 nm which is characteristic for T2/T3 copper centers; both copper and zinc were detected suggesting that this is an unconventional white laccase. Primers of laccase gene were designed and synthesized to recover specific gene from A. flavus . Sequence analysis indicated putative laccase (Genbank ID: JF683612) at the amino acid level suggesting a close identity to laccases from other genera containing the copper binding site. Decolorization of textile waste water under different conditions showed possible application in bioremediation within a short period of time. The effect of copper on A. flavus was concentration dependent.
Keywords: Aspergillus flavus, laccase, copper induction, textile waste water decolorization
Introduction
Laccases (p-diphenol:dioxygen oxidoreductase; EC 1.10.3.2) belong to the group of blue copper oxidases, they are the most abundant members of the multicopper protein family which include tyrosinase, monooxygenase and dioxygenase. From a phylogenetic perspective, this group of enzymes developed from small prokaryotic azurins to euokaryotic plasma proteins ( Claus 2003 ). Laccases use oxygen as an electron acceptor to remove hydrogen radicals from phenolic hydroxyl groups ( Eggert et al. , 1998 ). The free radicals formed can undergo rearrangements that lead to alkyl-aryl cleavage, oxidation of benzyl alcohols, and cleavage of side chains and aromatic rings, they have an overlapping substrate specificity which could be extended by redox-mediators and therefore, could be used in degrading a wide range of xenoaromatics ( Molianen et al. , 2010 ). Laccases have been widely known for their biotechnological applications, they are used in food industry to modify color and cross linking of biopolymers ( Selinheimo et al. , 2006 ), in pulp and paper industry ( Camarero et al. , 2004 ), in nanobiotechnology for their application in bioelectrochemistry, other applications include soil bioremediation, synthetic chemistry and cosmetics ( Rodriguez Couto and Toca Herrara 2006 ) and bioremediation of polyethylene ( Santo et al. , 2012 ), but the most widespread application is the decolorization of textile dyes ( Telke et al. , 2010 ). Due to the multidisciplinary uses of laccase, there is a dire need to induce both its expression and productivity through up-regulation of the enzyme encoding genes. Contrary to an effective but complex and expensive tools of bioengineering, increasing the enzyme yield by adding inducers is perceived as simple and cost-effective ( Skorobogat’ko et al. , 1996 , Levin et al. , 2010 ). There are many different inducers for laccase production ( Lorenzo et al. , 2002 ), but the most common of which is copper, the effect of copper is considered the most efficient of the putative inducers tested ( Palmieri et al. , 2000 ). Generally, blue copper oxidases are characterized by having four cupric (Cu 2+ ) ions coordinated in such a manner that each of the known magnetic species (type 1, type 2 and type 3) is associated with a single polypeptide chain ( Telke et al. , 2010 ). The Cu 2+ -binding domains are highly conserved in blue copper oxidases. Laccases are widely distributed in higher plants and fungi, they are also present in insects, bacteria ( Brijwani et al. , 2010 ) and cyanobacteria ( Palanisami and Lakshmanan 2011 ), but their abundance is mainly in white rot fungi ( Lorenzo et al. , 2002 , Vanhulle et al. , 2007 , Moilanen et al. , 2010 ), nevertheless, brown rot fungi are capable of producing laccases too ( D’Souza et al. , 1996 ), Aspergillus niger and genetically modified Aspergillus are among the mentioned brown rot fungi which produce laccases with wide industrial and biotechnological applications ( Rodriguez Couto and Toca Herrara 2006 ), Aspergillus ochraceus are capable of producing laccase and degrading textile dyes ( Telke et al. , 2010 ). Over expression of fungal laccases is done in Aspergillus niger and Aspergillus oryzae where its successfully cloned and heterologously expressed ( Rodriguez Couto and Toca Herrara 2007 ).
In the present work we characterize and investigate laccase-related enzyme in the brown rot fungus Aspergillus flavus and test its response to copper and its industrial application in terms of textile waste water treatment.
Materials and Methods
Isolation and identification of the microorganism
Aspergillus AF2 was isolated from agricultural soil at AGERI, Giza, Egypt. The fungus was grown and maintained on malt extract agar medium with the following components per litre: 20 g malt extract, 20 g glucose, 1 g peptone, 20 g agar, incubated at 30 °C for 5 days, 4 mm fungal discs were cut from the actively growing outer circumference of the fungal colony and were used to inoculate media in the following experiments. Identification of the fungus was performed microscopically by examining the hyphae on water agar according to Barnett and Hunter (1999) .
Induction of laccase under different copper sulfate concentrations
Two 4 mm A. flavus discs were used to inoculate 250 mL Erlenmeyer flasks containing 100 mL of basal GYP medium ( Galhaup et al. , 2002 ) which contained the following components per litre: 20 g glucose, 5 g yeast extract, 5 g peptone from casein and 1 g MgSO 4 .7H 2 O. The culture pH was adjusted to 4.5 prior to sterilization. Laccase activity was assayed, as described later, after 6 days of incubation at 30 °C under submerged conditions (150 rpm). Three different copper concentrations of filter sterilized (0.45 μm) CuSO 4 .5H 2 O were added to the medium after 24 h of fungal growth to avoid growth inhibition by copper. The concentrations used were: 1, 5 and 10 mM, were each added under aseptic conditions to triplicate flasks (n = 3) and left to incubate as described earlier. Control flasks contained no copper. The plotted results are the mean values for the obtained data.
Mycelial growth, enzyme assay and protein determination
The mycelial growth was monitored by weighing the filtered and washed mycelia on pre-weighed filter paper and drying them to a constant weight at 70 °C, and calculating the difference. Laccase activity was measured using ABTS [2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)] as the substrate ( Srinivasan et al. , 1995 ). The quantity of ABTS converted to product was measured spectrophotometrically at 420 nm (absorption coefficient value ɛ = 36000 M −1 cm −1 ) using a Schimadzu UV 2100 spectrophotometer. One unit of activity in international units (U) is defined as the amount of enzyme required to produce 1 μmol product/min at 30 °C and expressed as U/mL, while specific activity was expressed as U/μg protein. Protein concentration (μg/mL) was estimated using Lowry method (1951) using bovine serum albumin (BSA) as the standard.
Polyacrylamide gel electrophoresis (PAGE)
For purification of extracellular laccase, each culture medium was amended with the optimal copper concentration cultivated as previously described, mycelia were separated by filtration on miracloth, the culture filtrate was frozen, thawed and centrifuged to remove precipitated polysaccharides. The filtrate was concentrated using 30 kDa cutoff membrane filters to concentrate each culture, each were dialyzed twice against 50 mM phosphate buffer pH 4.8. An amount of 100 μg protein was loaded in each lane, samples were then electrophoresed at room temperature using Tris-glycine buffer (pH 8.3) at 50 V through the stacking polyacrylamide gel (6%) and at 100 V through the resolving polyacrylamide gel (10%) ( Laemmli 1970 ). After electrophoresis, the polyacrylamide gels were stained with Coomassie Brilliant blue R-250; the image was captured by a CCD camera.
Spectral properties and metal content
The resulting filtrate from cultures containing 1 mM copper were the ones used for spectral and metal content determination. The protein concentrate was further chromatographed on Sephadex G-100 fast flow column (1.5 x 30 cm). The column was equilibrated at a flow rate of 1 mL/min with phosphate buffer. The resulting fractions were scanned for protein at 280 nm and laccase activity at 420 nm using ABTS substrate as described earlier. The fractions with enzymatic activity were pooled, concentrated by sucrose, dialyzed again against water. The fraction with the highest activity was used for spectroscopic characterization of the Cu (II) centers. Spectrophotometric measurements were carried out between 280 and 700 nm using a Schimadzu UV 2100 spectrophotometer. The concentrations for Cu 2+ , Zn 2+ , Fe 2+ and Mn 2+ were detected using Unicam 939 Atomic Absorption Spectrometer (England). Prior to determination, the purified fraction was dialyzed against 10 mM sodium acetate buffer (pH 6) for 16 h.
pH and temperature optima and enzyme kinetic data
The pH optima of the purified laccase fraction were determined in phosphate buffer in a pH range from 2–9. The temperature optimum was determined by incubating the enzyme in the same buffer at different temperatures ranging from 25–60 °C and activity was assayed using ABTS as previously described. Determination of K m and V max were carried out under optimal conditions for the conversion of ABTS at 50 °C and pH 3. The substrate concentrations ranged from 2 to 10 μM ABTS. Plots were performed as Lineweaver-Burke plot.
PCR amplification and sequencing of genomic laccase
Mycelia of optimal laccase production which was 6 days Aspergillus flavus were filtered on miracloth, liquid nitrogen. The frozen mycelia were ground in sterilized mortar and DNA extraction took place using Genomic DNA extraction kit (Fermentas, EU). DNA was amplified using PCR (Perkin Elmers) with the following primers: (FW) 5′-CAC TGG CAC GGN TTC TTC CA, (RV) 5′-GTG ACT ATG ATA CCA GAA NGT ( D’Souza et al. , 1996 ). Amplification was performed under the following PCR conditions: an initial cycle of denaturation 5 min at 94 °C, annealing 2 min at 54 °C, and extension 20 min at 72 °C, followed by 35 cycles of denaturation 1 min at 94 °C, annealing 2 min at 54 °C and extension 5 min at 72 °C and then final incubation 10 min at 72 °C. Samples were separated on 1% agarose gel, amplified laccase band was recovered. Sequence analysis of the recovered putative laccase was done using ABI Prism BigDye TM Terminator Cycle Sequencing Ready Reaction kit according to manufacturer’s instructions (PE Applied Biosystems) and an ABI Prism TM 377XL DNA sequencer (Perkin Elmer). The DNA sequence was submitted to the National Center for Biotechnology Information (NCBI) database and deposited in the Gene Bankit nucleotide sequence database under accession number: JF683612. The DNA fragment sequence was reverse transcribed, open reading frame amnio acid sequence was deduced using European Molecular biology Laboratory EMBL ( www.embl.de/services/ ). Comparative analysis for different laccase amino acid sequences were obtained from NCBI database for alignment and generation of phylogenetic tree.
Phylogenetic analysis
A phylogenetic tree was generated using PhyML and then visualized using the TreeDyn online program ( www.phylogeny.fr ). The molecular evolutionary genetics analysis (MEGA 4.0 software) was used to detect the amino acid frequencies in percentage ( Tamura et al. , 2007 ).
A. flavus laccase application in textile waste water treatment
A. flavus under laccase induced conditions was used for decolorization of textile waste water under different copper sulfate concentrations (0, 1, 5 and 10 mM). Flasks containing the waste water were inoculated with 48 h spore suspension (10 6 ) and were left to incubate in a rotatory shaker (150 rpm) under the previously mentioned conditions. The waste water was obtained at the end of a dyeing process where the dyes belonged to the azo group, the absorption peak was at 540 nm. The textile waste water was obtained from a textile factory located near Cairo, Egypt. The decolorization was performed using a UV-Vis spectrophotometer (Schimadzu UV 2100 spectrophotometer) and was calculated from the equation [( A 0 - A / A 0 )] x 100, where A 0 is the initial dye absorption on the day of inoculation; A is the final dye absorption after incubation. All experiment were performed in triplicates (n = 3).
Degradation velocity was calculated from the equation
where A 0 is the initial concentration and A t is the degradation at time t (48 h or 2880 min).
Statistical treatment of data
Each experiment was performed in triplicate. Analysis of the variance (ANOVA) was performed using MS Excell statistiXL version 1.8.
Results
The effect of copper on Aspergillus flavus laccase
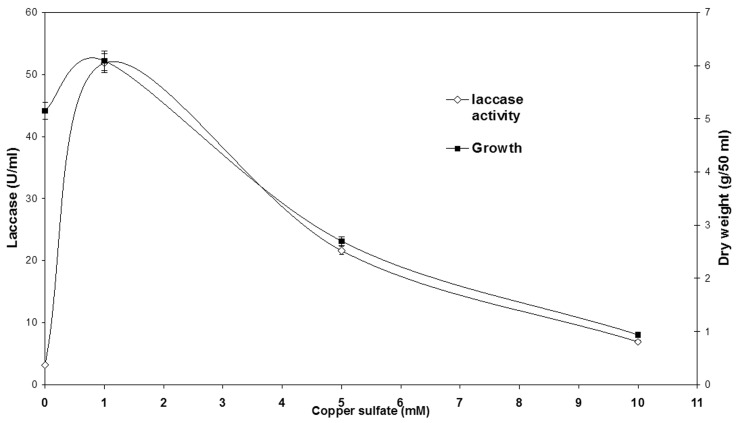
The addition of copper to the cultivation medium showed an initial increase from 5.1 U/mL to 51.84 U/ mL when 1 mM copper sulfate was added, this was followed by a gradual decrease which reached 21.6 U/mL at 5 mM copper sulfate and a further decrease which reached 6.91 U/mL when 10 mM copper sulfate was added to the cultivation medium. The growth pattern was initially high at 0 and 1 mM copper sulfate (5.1 and 6.09 g/50 mL respectively) but there was a drop in mycelial growth when 5 mM copper was added that reached 2.7 g/50 mL while a further drop which reached 0.94 g/50 mL when 10 mM copper sulfate was added ( Figure 1 ).
Figure 1.
Growth and laccase activity in Aspergillus flavus after 6 days of incubation at 30 °C under submerged conditions (150 rpm). Different concentrations of CuSO 4 .5H 2 O were added to the medium after 24 h of fungal growth.
Protein electrophoresis and enzyme characterization
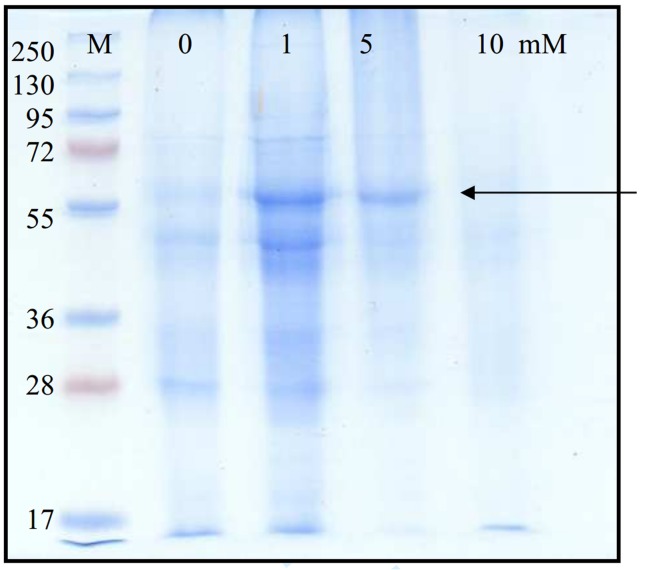
Figure 2 shows a distinctive band was detected at 60 kDa in SDS-PAGE electrophoresis of laccase produced under copper inducing conditions, this band was vaguely present in control cultures containing no copper and in 5 mM, cultures amended with copper, on the other hand, it disappeared from cultures containing 10 mM.
Figure 2.
SDS-PAGE (10%) for control, and media supplemented with 1, 5 and 10 mM copper sulfate, added after 24 h of cultivation for Aspergillus flavus . A prestained molecular marker was used in the range from 250 to 17 kDa (M).
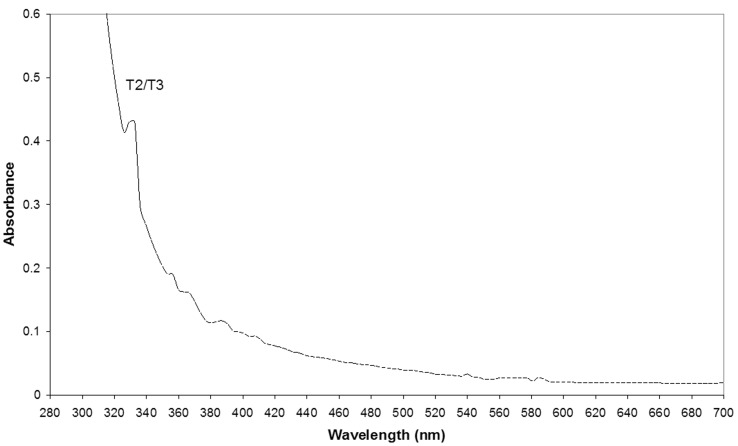
Purification data of the extracellular laccase is given in Table 1 . The enzyme was purified 81-fold, purification resulted in two laccase fractions, L2 being more abundant with an activity of 64.17 U/μg protein. The nature of the catalytic centre of L2 fraction was investigated spectrophotemetrically by UV-Visible spectrum. Figure 3 shows a small shoulder at 330 nm suggesting the presence of type II/III binuclear Cu (II) and the lack of a strong peak of absorption at 610 nm which is typical for type I Cu (II) confirms the absence of copper type I in the purified fraction. The enzyme lacked the usual blue color characteristic of typical laccases. The metal content of L2 fraction did not contain Iron or Manganese but Copper (7.49 μg/mL) and Zinc (0.28 μg/mL) were present.
Table 1.
Partial purification of extracellular laccase from Aspergillus flavus grown in basal GYP medium amended with 1 mM copper sulfate.
| Fraction | Volume (mL) | Activity (U/mL) | Protein (μg/mL) | Specific activity (U/μg) | Purification fold |
|---|---|---|---|---|---|
| Culture filtrate | 1200 | 320.85 | 401 | 0.8 | 1 |
| Ultrafiltration | 100 | 157.025 | 18 | 8.72 | 11 |
| Gel filtration, Sephadex G-100 | |||||
| Fractions:L1 | 15 | 25.92 | 6 | 4.32 | 5.5 |
| L2 | 10 | 79.57 | 1.24 | 64.17 | 81 |
Figure 3.
UV- visible spectrum of the purified L2 fraction.
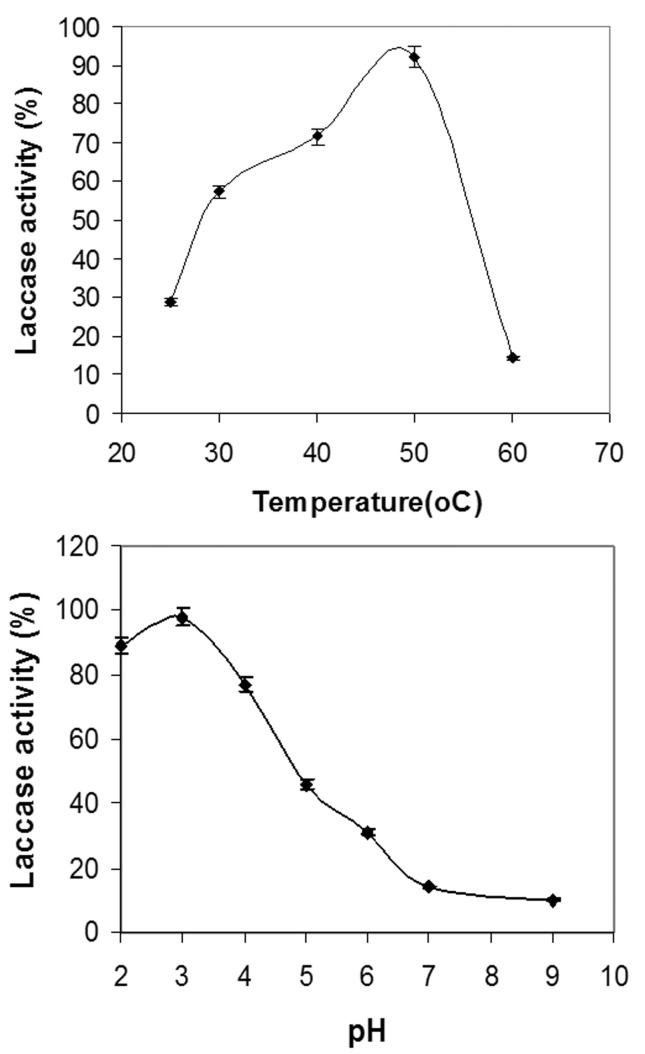
The enzyme showed a temperature optimum at 50 °C after which the activity dropped. The enzyme showed ≤ 50% activity at 30–50 °C. The pH optima was in the highly acidic region at pH 3, the activity dropped but was still showed some activity in the alkaline region ( Figure 4 ). The enzyme showed a calculated K m value of 2.48 μM while V max was calculated to be 4.78 x 10 −2 mM.s −1 .
Figure 4.
Temperature and pH optima for laccase calculated as percentage activity. K m was calculated to be 2.48 μM while V max was calculated to be 4.78 x 10 −2 mM.s −1 .
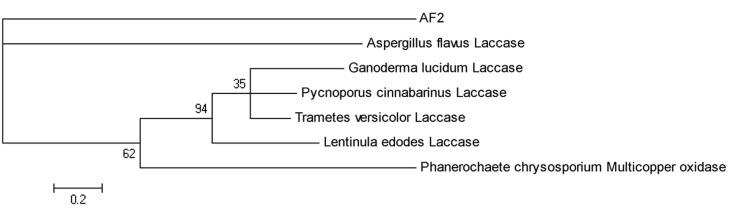
The relatedness of A. flavus laccase with those of other genera was compared ( Figure 5 ). The figure shows that the closest resemblance for the strain under study is Aspergillus flavus , the relatedness to other genera was 62% representing other white rot fungi.
Figure 5.
Phylogenetic tree obtained by neighbor-joining analysis of Laccase gene sequence and its relationships to other fungal laccases. AF2, Aspergillus flavus under investigation (JF683612), Aspergillus flavus laccase (XP_002382290), Ganoderma lucidum laccase (AAR82934), Pycnoporus cinnabarinus laccase (AAC39469), Trametes versicolor laccase (BAA23284), Lentinula edodes laccase (ACR24356), Phanerocheate chrysosporium multicopper oxidase (AAS21659).
The effect of copper on textile waste water treatment by A. flavus laccase
Table 2 shows that the increase in copper concentration was concomitant with the increase in decolorization. The results show 71% decolorization when no copper was added, but an increase that reached 78 and 82% when 1 and 5 mM copper was added to the culture medium, the highest decolorization was 92% when 10 mM copper was added, this is contrary to the results shown in Figure 1 which showed a decrease in laccase activity after the addition of 5 and 10 mM copper to the cultivation medium. The degradation velocity in the presence of copper was as follows: 0.0273, 0.0285 and 0.0319 A. min −1 for 1, 5 and 10 mM copper, respectively as compared to 0.0173 in cultures devoid of copper.
Table 2.
The decolorization of real textile waste water effluent under different copper sulfate concentrations.
| Copper sulfate (mM) | Decolorization (%) | Degradation velocity ( A . min −1 ) |
|---|---|---|
| 0 | 51 ± 1 | 0.0173 |
| 1 | 78.6 ± 0.577 | 0.0273 |
| 5 | 82.1 ± 0.288 | 0.0285 |
| 10 | 92 ± 1.1 | 0.0319 |
Discussion
Laccases have been discovered and studied since the nineteenth century, they occur in bacteria, various plant parts and insects, but the majority is found in higher fungi, these laccases are produced by fungi which cause white rot, plant pathogens or soil saprophytes ( Fernandez-Fernandez et al. (2012) . The isolate used in this study was obtained from agriculture soil and was tested for its laccase production; it was identified as Aspergillus flavus . Usually, extracellular laccases are formed in fungi during secondary metabolism and are constitutively produced in small amounts; enhancing laccase-producing ability of the microorganisms is considered important for industrial applications ( Levin et al. , 2010 ); therefore, copper was used in this study for this purpose as it is considered one of the common putative laccase inducers ( Palmieri et al. , 2000 ). The addition of copper sulfate on the day of inoculation had a negative impact on the decolorization and fungal growth, while after 24 h; both fungal growth and decolorization were observed (data not shown). This suggests that copper sulfate might act as an inhibitor or inducer depending on the time of addition to the cultivation media and the microorganism used. Gallhaup et al. (2002) added copper after 64–96 h cultivation for Trametes pubescens , while copper was added on the day of inoculation for Pleurotus ostreatus ( Palmieri et al. , 2000 ) and for Corliolus hirsutus ( Skorobogat’ko 1996 ). It is plausible to say that the addition of copper at this stage had an inducing effect, but that there is also constitutive laccase since there was low laccase activity in control cultures devoid of copper. The results show that crude laccase activity in the presence of copper reached 51 U/mL. Brijwani et al. (2010) stated that typical fungal strains show activity ranging from 4–100 U/mL. The concentrations used to induce laccase are variable, Zhuo et al. (2011) tested the effect of copper at 1 to 5 mM, Fonesca et al. (2010) used 0.5 and 1 mM, Schuckel et al. (2011) used 1 mM, Gallhaup et al. (2002) used 2 mM while Litvintsva and Henson (2002) used 0.4 mM and only 150 μM was required to induce laccase in the white rot fungus Pleurotus ostreatus ( Palmieri et al. , 2000 ).
Although most laccases isolated from fungi show similar structural properties, yet the physiology of their production is markedly different in even closely related organisms ( Grazillo et al. , 1998 ). Trametes trogii showed a single polypeptide chain with a molecular mass of 70 kDa ( Grazillo et al. , 1998 ), Coriolus hisrustus showed a 55 kDa laccase ( Skorobogat’ko et al. , 1996 ), on the other hand Aspergillus ochraceus produced a 65 kDa laccase ( Telke et al. , 2010 ). The most significant result that describes A. flavus laccase is the spectral and metal content of the purified fraction. The majority of laccases are blue containing four copper atoms per enzyme molecule. The typical UV-Visible spectrum for the four differently coordinated copper ions give a maximum absorption at 610 nm which is typical to type 1 plus an absorption maximum at 330 nm which is usually formed by the coupled binuclear site type 3 ( Salony et al. , 2006 and Telke et al. , 2010 ). However, a number of untypical yellow and white laccases were also reported ( Palmieri et al. , 1997 , Pozdnyakova et al. , 2006 , Giardina et al. , 2009 , Shuckel et al. , 2011 ). The presence of a shoulder at 320–340 nm is characteristic to the yellow laccases, even though they contain four copper atoms and lack a shoulder at 610 nm. It was suggested that the formation of yellow laccases result from binding of the lignin degradation aromatic products which in turn results in the reduction of type 1 copper and loss of the characteristic blue copper of laccase ( Giardina et al. , 2009 ). While white laccase is characteristic by the presence of a single copper atom in addition to other metal ions such as zinc, iron or manganese ( Palmieri et al. , 1997 ). The enzyme isolated from A. flavus lacked the typrical blue color, did not show a shoulder at 610 nm and Zn was present in addition to the copper, therefore, it is suggested that it is a white laccase. Type I copper was also reported at 604 nm for Chaetomium thermophilium (Chefetz et al. , 1998). Other characteristics of the obtained laccase is that the optimal temperature (50 °C) and pH (3) are similar to other laccase ( Schuckel et al. , 2011 ), the low K m value 2.84 μM for ABTS as the substrate is quite low, but this not uncommon ( Baldrian et al. , 2006 ), the low K m reflects a high catalytic efficiency. A white laccase was produced from Marasmius sp . with K m value 3.9 μM for ABTS as the substrate. Generally, the majority of research done on fungi are almost exclusive to white rot fungi, which are not available in Egypt, therefore, Laccase gene-specific sequences are detected not only in white rot fungi, but are also detected in brown rot fungi ( D’Souza et al. , 1996 ). Therefore, we consider A. flavus laccase under study is closely related to A. flavus and 62% related to white rot laccase.
The increase in decolorization and degradation velocity was correlated to the increase in copper concentration added to the medium. The addition of copper sulfate at low concentrations seems to induce laccase enzyme which is usually the primary mechanism responsible for decolorization of a number of dyes ( Hou et al. , 2003 , Murugesan et al. , 2009 , Telke et al. , 2010 , Schukel et al. , 2011 ), but the increase in decolorization at high copper concentrations seems irrelevant to laccase production suggesting that there is another mode of action involved in this process. Copper is known to be a pro-oxidant, its addition to the fungal cultivation media and its interaction with the fungal cells results in the formation of hydrogen peroxide as an oxidative stress response (Palansiami and Lakshmanan 2010). Copper ions were reported to interact with dioxygen in the medium in a Fenton-like reaction ( Urbanski and Berjesewicz 2000 ), its presence may have contributed to increasing the attack to the dye molecules present in the effluent. Copper is an important component of laccase active site could enhance laccase activity under certain conditions, it is also an important metal that acts as a catalytic oxidant, Copper-dioxygen complexes have been suggested to play important roles in a number of catalytic oxidation reactions, it is the major component protein involved in dioxygen metabolism and a main contributor to several physiological functions such as dioxygen carriers, oxidases, oxygenases and superoxide dismutases, it is therefore considered plausible to consider copper dioxygen adducts as the main key reaction intermediates in enzymatic reactions ( Akyilmaz et al. , 2010 ).
Conclusion
Aspergillus flavus laccase is not only constitutive but also inducible under low copper concentrations; spectral and metal characteristics of the obtained laccase suggest that it belongs to the unconventional white laccases. A. flavus laccase possesses characteristics very close to those of other laccase containing fungi. The enhanced decolorization and high degradation velocity suggest that there is a laccase-copper synergistic effect that enhances the treatment of textile waste water. Further studies are in progress to examine copper oxidative stress response and the expression of constitutive and induced laccase on the RNA level.
References
- Akyilmaz E, Yorganci E, Asay E. Do copper ions activate tyrosinase enzyme? A biosensor model for the solution. Bioelectrochem. 2010;78:155–160. doi: 10.1016/j.bioelechem.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Baldrian P. Fungal laccases-occurrence and properties. FEMS Microbiol Rev. 2006;30:215–242. doi: 10.1111/j.1574-4976.2005.00010.x. [DOI] [PubMed] [Google Scholar]
- Barnett HL, Hunter BB. Illustrated genera of imperfect fungi. 4th edition. APS Press; St. Paul: 1999. [Google Scholar]
- Brijwani K, Rigdon A, Vadlani VP. Fungal laccases:production, function, and applications in food processing. Enzyme Res. 2010 doi: 10.4061/2010/149748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarero S, Garcia O, Vidal T, et al. Efficient bleaching of non-wood high-quality paper pulp using laccase-mediator system. Enzyme Microb Technol. 2004;35:113–120. [Google Scholar]
- Claus H. Laccases and their occurrence in prokaryotes. Arch Microbiol. 2003;179:145–150. doi: 10.1007/s00203-002-0510-7. [DOI] [PubMed] [Google Scholar]
- Couto RS, Toca Herrara JL. Industrial and biotechnological applications of laccases: A review. Biotechnol Adv. 2006;24:500–513. doi: 10.1016/j.biotechadv.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Couto SR, Toca-Herrara JL. Laccase production reactor scale by filamentous fungi. Biotechnol Adv. 2007;25:558–569. doi: 10.1016/j.biotechadv.2007.07.002. [DOI] [PubMed] [Google Scholar]
- D’Souza TM, Boominathan K, Reddy CA. Isolation of laccase gene-specific sequences from white rot and brown rot fungi by PCR. Appl Environ Microbiol. 1996;62:3739–3744. doi: 10.1128/aem.62.10.3739-3744.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert C, LaFayette PR, Temp U, et al. Molecular analysis of a laccase gene from the white rot fungus Pycnoporus cinnabarinus . Appl Environ Microbiol. 1998;64:1766–1772. doi: 10.1128/aem.64.5.1766-1772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Fernandez M, Sanroman M, Moldes D. Recent developments and applications of immobilized laccase. Biotechnology Adv. 2012;31:1808–1825. doi: 10.1016/j.biotechadv.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Fonesca MI, Shimizu E, Zapata PD, et al. Copper inducing effect on laccase production of white rot fungi native from Misiones (Argentina) Enzyme Microb Technol. 2010;46:534–539. doi: 10.1016/j.enzmictec.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Galhaup C, Goller S, Pterbauer CK, et al. Characterization of the major laccase isoenzyme from Trametes pubescens and regulation of its synthesis by metal ions . Microbiol. 2002;148:2159–2169. doi: 10.1099/00221287-148-7-2159. [DOI] [PubMed] [Google Scholar]
- Garzillo AM, Colao MC, Caruso C, et al. Laccase from the white-rot fungus Trametes trogii . Appl Microbiol Biotechnol. 1998;49:545–551. doi: 10.1007/s002530051211. [DOI] [PubMed] [Google Scholar]
- Giardina P, Faraco V, Pezzella C, et al. Laccases: a never-ending story. Cell Mol Life Sci. 2009;67:369–385. doi: 10.1007/s00018-009-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou H, Zhou J, Wang J, et al. Enhancement of laccase production of Pleurotus ostreatus and its use for the decolorization of anthraquinone dye . Process Biochem. 2003;39:1415–1419. [Google Scholar]
- Kapdan IK, Kargi F, McMullan G, et al. Effect of environmental conditions on biological decolorization of textile dyestuff by C. versicolor . Enzyme Microb Technol. 2008;26:381–387. doi: 10.1016/s0141-0229(99)00168-4. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin L, Melignani E, Ramos AM. Effect of nitrogen sources and vitamins on ligninolytic enzyme production by some white-rot fungi. Dye decolorization by selected culture filtrates. Bioresource Technol. 2010;101:4554–4563. doi: 10.1016/j.biortech.2010.01.102. [DOI] [PubMed] [Google Scholar]
- Litvintseva AP, Henson JM. Cloning, characterization, and transcription of three laccase genes from Gaeumannomyces graminis var. tritici , the take-all fungus . Appl Environ Microbiol. 2002;68:1305–1311. doi: 10.1128/AEM.68.3.1305-1311.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo M, Moldes D, Rodriguez Couto S, et al. Improving laccase production by employing different lignocellulosic wastes in submerged cultures of Trametes versicolor . Bioresource Technol. 2002;82:109–113. doi: 10.1016/s0960-8524(01)00176-6. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:256–275. [PubMed] [Google Scholar]
- Moilanen U, Osma JF, Winquist E, et al. Decolorization of simulated textile dye baths by crude laccases from Trametes hirsuta and Cerrena unicolor . Eng Life Sci. 2010;10:242–247. [Google Scholar]
- Murugesan K, Kim YM, Jeon JR, et al. Effect of metal ions on reactive dye decolorization by laccase from Ganoderma lucidum . J Haz Mat. 2009;168:523–529. doi: 10.1016/j.jhazmat.2009.02.075. [DOI] [PubMed] [Google Scholar]
- Palanisami S, Lakshmanan U. Role of copper in poly R-478 decolorization by the marine cyanobacterium Phormidium valderianum BDU140441 . World J Microbiol Biotechnol. 2011;27:669–677. [Google Scholar]
- Palmieri G, Giardina P, Bianco C, et al. A novel white laccase from Pleurotus ostreatus . J Biol Chem. 1997;271:31301–31307. doi: 10.1074/jbc.272.50.31301. [DOI] [PubMed] [Google Scholar]
- Palmieri G, Giardina P, Bianco C, et al. Copper induction of laccase isoenzymes in the ligninolytic fungus Pleurotus ostreatus . Appl Environ Microbiol. 2000;66:920–924. doi: 10.1128/aem.66.3.920-924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozdnyakova NN, Rodakiewicz-Nowak J, Turkovskaya OV, et al. Oxidative degradation of polyaromatic hydrocarbons and their derivatives catalyzed directly by the yellow laccase from Pleurotus ostreatus D1 . J Mol Catal B: Enzym. 2006;41:8–15. [Google Scholar]
- Salony S, Mishra V, Bisaria S. Production and characterization of laccase from Cyathus bulleri and its use in decolorization of recalcitrant textile dyes . Appl Microbiol Biotechnol. 2006;71:646–653. doi: 10.1007/s00253-005-0206-4. [DOI] [PubMed] [Google Scholar]
- Santo M, Weitsman R, Sivan A. The role of the copper binding enzyme laccase in the biodegradation of polyethylene by the actinomycete Rhodococcus rubber . Int Biodeter Biodegrad. 2012;208:1–7. [Google Scholar]
- Schukel J, Matura A, Van Pee KH. One copper laccase related enzyme from Marasmius sp.: purification, characterization and bleaching of textile dyes . Enzyme Microbial Technol. 2011;48:278–284. doi: 10.1016/j.enzmictec.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Selenheimo E, Kurus K, Buchert J, et al. Effects of laccase, xylanase and their combination on the rheological properties of wheat doughs. J Cereal Sci. 2006;43:152–159. [Google Scholar]
- Sokorobogat’ko OV, Stepanova EV, Gavrilova VP, et al. Effects of inducers on the synthesis of extracellular laccase by Coriolus hirsutus , a basidial fungus . Appl Biochem Microbiol. 1996;32:524–528. [Google Scholar]
- Srinivasan C, D’souza T, Boomathan K, et al. Demonstration of laccase in the white rot basidiomycete Phanerochaete chysosporium BKM-F-1767 . Appl Environ Microbiol. 1995;61:4274–4277. doi: 10.1128/aem.61.12.4274-4277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, et al. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software. Version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Telke AA, Kadam AA, Jagtap SS, et al. Biochemical characterization and potential for textile dye degradation of blue laccase from Aspergillus ochraceus NCIM-1146 . Biotechnol Bioprocess Eng. 2010;15:696–703. [Google Scholar]
- Urbanski NK, Berjesewicz A. Generation of • OH initiated by interaction of Fe +2 and Cu +2 with dioxygen; comparison with the Fenton chemistry . Acta Biochimica Polonica. 2000;47:951–962. [PubMed] [Google Scholar]
- Vanhulle S, Enaud E, Trovaslet M, et al. Overlap of laccase/cellobiose dehydrogenase activities during the decolorization of anthraquinone dyes with close chemical structures by Pycnoporus strains . Enzyme Microbiol Technol. 2007;40:1723–1731. [Google Scholar]
- Zhuo R, Ma L, Fan F, et al. Decolorization of different dyes by newly isolated white-rot fungi strain Ganoderma sp. En3 and cloning and functional analysis of its laccase gene . J Hazard Mat. 2011;192:855–873. doi: 10.1016/j.jhazmat.2011.05.106. [DOI] [PubMed] [Google Scholar]