Abstract
The effect of fludioxonil + metalaxyl-M on the mycelial morphology, sporulation and fumonisin B 1 production by Fusarium verticillioides 103 F was evaluated. Scanning electron microscopy analysis showed that the fungicide caused inhibition of hyphal growth and defects on hyphae morphology such as cell wall disruption, withered hyphae, and excessive septation. In addition, extracellular material around the hyphae was rarely observed in the presence of fludioxonil + metalaxyl-M. While promoting the reduction of mycelial growth, the fungicide increased sporulation of F. verticillioides compared to the control, and the highest production occurred on the 14 th day in the treatments and on the 10 th day in the control cultures. Fumonisin B 1 production in the culture media containing the fungicide (treatment) was detected from the 7 th day incubation, whereas in cultures without fungicide (control) it was detected on the 10 th day. The highest fumonisin B 1 production occurred on the 14 th day, both for the control and for the treatment. Fludioxonil + metalaxyl - M can interfere in F. verticillioides mycelial morphology and sporulation and increase fumonisin B 1 levels. These data indicate the importance of understanding the effects of fungicide to minimize the occurrence of toxigenic fungi and fumonisins.
Keywords: toxigenic fungi, mycotoxin, scanning electron microscopy, electron micrographs, extracellular material
Introduction
Fusarium verticillioides (Sacc. Nirenberg) is an economically important pathogen of corn, which causes disease at all the stages of plant development ( Munkvold and Desjardins, 1997 ). The fungus also produces fumonisins, a group of mycotoxins associated with various animal mycotoxicosis such as leukoencephalomalacia in horses ( Marasas et al. , 1988 ), pulmonary edema in swine ( Harrison et al. , 1990 ), renal and liver cancer in rats ( Voss et al. , 2002 ), weight loss and reduced development in poultry ( Ledoux et al. , 1992 ; Weibking et al. , 1993 ). Epidemiological studies have suggested the occurrence of esophageal and liver cancer in humans who consumed contaminated maize in South Africa ( Gelderblom et al. , 1988 ) and China ( Sun et al. , 2007 ) and neural tube defects in embryos from the Texas-Mexico border ( Missmer et al. , 2006 ).
Although 28 fumonisin analogues have been characterized, fumonisins B 1 (FB 1 ) and B 2 (FB 2 ) are detected as natural contaminants at significant levels in maize and maize-based products, and FB 1 is found at highest concentrations ( Rheeder et al. , 2002 ).
Several efforts have been made in the development and use of fungicides for Fusarium sp. control ( Magan et al. , 2002 ) in cereals, but there are few reports on F. verticillioides . The most effective fungicides for F. verticillioides control in vitro were captan + thiabendazole, followed by fludioxonil + metalaxyl - M ( Moraes et al. , 2003 ), which provided an increase of 56% in corn kernel yield ( Goulart and Fialho, 2001 ). Munkvold and O’Mara (2002) reported that fludioxonil was more effective in promoting rapid maize root growth compared with the fungicides captan and difeconazole. Many studies, however, have shown that fungicide application can increase mycotoxin levels. Tridemorph in concentrations from 30 to 50 μg/mL inhibited F. sporotrichioides growth by more than 50%, but increased T-2 toxin production five-fold ( Moss and Frank, 1985 ). Prochloraz and tebuconazole at concentrations of 2 and 8 μg of active ingredient/mL caused an increase in Tri5 gene expression in F. culmorum , which encodes the enzyme that catalyzes the first reaction in trichothecenes biosynthesis ( Doohan et al. , 1999 ). The natural antifungal Trans-2-hexenal was effective for F. verticillioides control in maize, but did not reduce fumonisin production ( Menneti et al. , 2010 ).
Fludioxonil + metalaxyl-M is one of the most used fungicides for the corn crop in Brazil, but there are few studies showing its effect on F. verticillioides and FB 1 production. A previous study showed that the recommended fludioxonil + metalaxyl-M dose was not sufficient to inhibit in vitro growth of F. verticillioides strains and there was an increase in mean FB 1 production by three F. verticillioides strains ( Falcão et al. , 2011 ). Therefore the present study aimed to evaluate in more detail the effect of fludioxonil + metalaxyl-M on mycelial morphology, sporulation, biomass production, nitrogen uptake and FB 1 production by F. verticillioides 103 F in a defined liquid culture medium.
Material and Methods
Fusarium verticillioides strain
The F. verticilliodes 103F strain, isolated from feed samples and morphologically identified at the Science University of Tokyo, Japan, belongs to the culture collection of the Department of Food Science and Technology at the State University of Londrina. This strain was selected based on previous studies of toxigenicity performed in corn cultures, which showed that of the 16 strains analyzed, F. verticillioides 103F produced the highest FB 1 levels (3996.36 ± 390.49 μg/g) ( Falcão et al. , 2011 ).
F . verticillioides cultivation and fungicide treatment
The conidial suspension was prepared by washing the 7 day-old colony grown on Potato Dextrose Agar (PDA) plates at 25 °C with sterile distilled water containing 0.1% Tween 80 (v/v). Conidia counts were determined with a haemocytometer and the inoculum concentration was adjusted to 10 6 conidia/mL. An aliquot of conidia suspension (10 6 conidia/ mL) was inoculated in Erlenmeyer flasks containing 50 mL of defined liquid culture medium ( Jiménez et al. , 2003 ). The liquid culture medium composition was: 0.5 g/L malt extract, 1 g/L mycological peptone, 1 g/L KH 2 PO 4 , 0.3 g/L MgSO 4 .7H 2 O, 0.3 g/L KCl, 1 mL CuSO 4 .H 2 O solution (0.005 g/L), 1 mL ZnSO 4 .7 H 2 O solution (0.01 g/L) and 20 g/L fructose. The fludioxonil (2.5% active ingredient) + metalaxyl-M (1.0% active ingredient) fungicide was added into the culture medium after 24 h at the manufacturer’s recommended dose, i.e. , 75 μL fungicide (1.5 μL/mL) in 50 mL liquid culture medium. The control cultures (without fungicide) received 75 μL sterile distilled water 24 h after F. verticillioides inoculation. The cultures were incubated at 28 °C, 180 rpm, for 3, 5, 7, 10, 12, 14, 18 and 21 d. All the cultures were performed in triplicate. After the incubation periods, aliquots were collected aseptically for sporulation analysis and the cultures were subsequently filtered through Whatman No. 1 filter paper (GE Healthcare), separating the cell-free extract to determine FB 1 and nitrogen, and biomass for analysis of mycelial morphology and biomass production.
Scanning Electron Microscopy (SEM)
Samples of F. verticillioides mycelium were fixed with 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.2) at 4 °C for 12 h. The samples were then washed with sodium phosphate buffer (0.1 M, pH 7.2) and treated with 1% osmium tetroxide in sodium phosphate buffer for 1 h, subjected to gradual dehydration in ethanol (70, 80, 90 and 100%), and dried to the critical point (CPD 030 Critical Point BALTEC Dryer, Leica Microsystems, Liechtenstein). After drying, the samples were glued on stubs using carbon tape and coated with gold (Sputter Coater BALTEC SDC 050, Leica Microsystems, Liechtenstein). The mycelia were analyzed using a FEI Quanta 200 scanning electron microscope.
Cell count
Aliquots of 200 μL culture media were collected after 3, 5, 7, 10, 12, 14, 18 and 21 d incubation, and 10 μL were used to count the conidia in a Newbauer chamber by light microscopy. The required dilutions were performed in 0.1% Tween.
Biomass estimation
The biomass was estimated by determining the mycelial dry weight. The mycelia were dried in an oven at 70 °C to a constant weight on Whatman No. 1 filter paper (GE Healthcare). The weight of the mycelia was determined by subtracting the initial weight of the filter paper from the weight of mycelia and filter paper. The fungal biomass was calculated as the mean value of three independent samples.
Fumonisin analysis
FB 1 was determined by high-performance liquid chromatography (HPLC) according to Shephard et al. (1990) with some modification ( Ueno et al. , 1993 ).
One milliliter of the cell-free extract previously mixed with 1 mL methanol-water (3:1, v/v) was applied onto a preconditioned Sep Pak accell plus QMA (quaternary methylammonium) cartridge (Waters Co., USA). After washing the cartridge with methanol-water (3:1, 6 mL) followed by methanol (3 mL), FB 1 was eluted with 10 mL methanol containing 0.5% acetic acid. The eluate was evaporated to dryness under a stream of nitrogen at 45 °C, and the residue was dissolved in methanol-water (3:1, 800 μL). After derivatization with 200 μL o -phthaldialdehyde (OPA) reagent, HPLC injections were made within 1 min. FB 1 was analyzed by a reversed-phase, isocratic HPLC system (Shimadzu LC-10 AD pump and RF-10A XL fluorescence detector (Shimadzu, Japan), using a C18 Nucleosil 100-5 column (4.6 × 250 mm, Macherey-Nagel GmbH & Co., Germany). Excitation and emission wavelengths were 335 nm and 450 nm, respectively. The eluent was CH 3 OH: 0.1 M NaH 2 PO 4 (80:20, v/v) adjusted to pH 3.3 with ortho -phosphoric acid at 1 mL/min flow rate. The detection limit for FB 1 was 27.5 ng/mL.
Nitrogen determination
Nitrogen was determined by the Kjeldahl method according to the official methodology of the American Association of Cereal Chemists (1990) .
Statistical analysis
Differences in mean cell count, residual nitrogen, biomass and FB 1 levels produced in defined liquid culture medium between the control (without fludioxonil + metalaxil-M) and treatment (with fludioxonil + metalaxil-M) were analyzed by one-way ANOVA followed by the Tukey multiple comparison test (p < 0.05). The cell count was transformed to ln (x) to reduce the variability among the data. Statistical analysis was performed by the ‘Statistica’ software version 6.0 (Stat Soft, 4 Inc.).
Results
Effect of fungicide on mycelial morphology
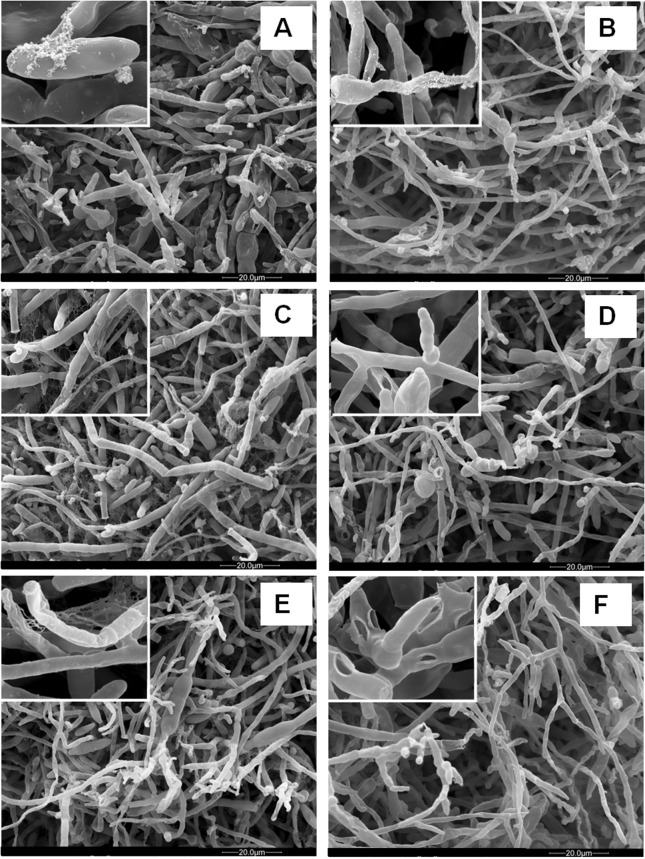
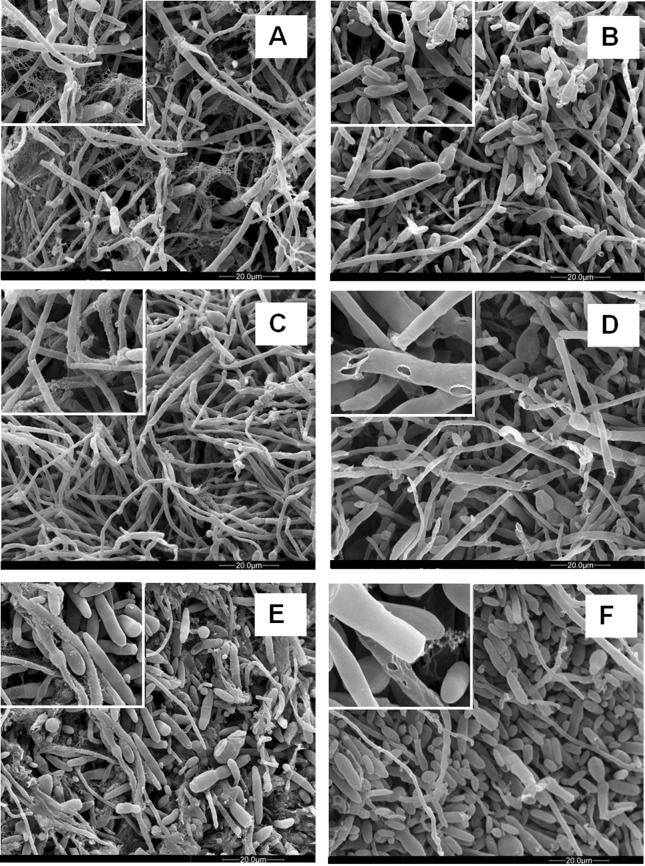
The SEM analysis showed that the fungicide caused inhibition of hyphal growth and defects on hyphae morphology such as cell wall disruption, withered hyphae, and excessive septation ( Figures 1 and 2 - B, D and F ).
Figure 1. Electron micrographs of F. verticillioides 103 F mycelia cultured in defined liquid media in the absence (control) and presence (treatment) of fludioxonil + metalaxyl - M at the dose recommended by the manufacturer (1.5 μL/mL). The details show the fibrillar extracellular material present in control cultures (A = 7 d, C = 10 d, E = 12 d) and disruption of cell walls in the treatments (B = 7 d, D = 10 d, F = 12 d).
Figure 2. Electron micrographs of F. verticillioides 103 F mycelia cultured in defined liquid media in the absence (control) and presence (treatment) of fludioxonil + metalaxyl - M at the dose recommended by the manufacturer (1.5 μL mL -1 ). The details show the fibrillar extracellular material present in control cultures (A = 14 d, C = 18 d, E = 21 d) and disruption of cell walls in the treatments (B = 14 d, D = 18 d, F = 21 d).
The mycelia organization revealed by SEM also showed an extracellular material around the hyphae in the control cultures in all the periods analyzed, which was seen as a flocculent material over the cells or as a fine fibrils attaching hyphae to each other, resembling a biofilm ( Figures 1 and 2 – A, C and E ). Interestingly, in the presence of fludioxonil + metalaxyl - M, that material was rarely observed suggesting that the fungicide affected its formation ( Figures 1 and 2 - B, D and F ).
Effect of fungicide on cell count (sporulation)
In F. verticillioides, while promoting reduction in mycelial growth, fludioxonil + metalaxyl - M increased sporulation in the treatments (10 8 conidia/mL) compared to the control (10 7 conidia/mL) in all the incubation periods (p < 0.05), except for the 5 th d ( Table 1 ).
Table 1. Fusarium verticillioides 103 F conidia count in defined liquid media in the absence (control) and presence (treatment) of fludioxonil + metalaxyl - M at the recommended dose (1.5 μL/mL) in different incubation periods.
| Incubation period (days) | Control | Treatment | ||
|---|---|---|---|---|
|
|
|
|||
| Cell count (spores/mL) x | Ln cell count x | Cell count (spores/mL) x | Ln cell count x | |
| 3 | 4.9 × 10 7 | 17.7 b | 1.1 × 10 8 | 18.5 a |
| 5 | 7.4 × 10 7 | 18.1 a | 1.2 × 10 8 | 18.6 a |
| 7 | 9.8 × 10 7 | 18.4 b | 1.9 × 10 8 | 19.0 a |
| 10 | 7.3 × 10 7 | 18.1 b | 1.9 × 10 8 | 19.0 a |
| 12 | 6.5 × 10 7 | 18.0 b | 1.9 × 10 8 | 19.1 a |
| 14 | 6.3 × 10 7 | 18.0 b | 2.2 × 10 8 | 19.2 a |
| 18 | 4.1 × 10 7 | 17.5 b | 2.2 × 10 8 | 19.2 a |
| 21 | 5.2 × 10 7 | 17.7 b | 2.0 × 10 8 | 19.1 a |
Mean of three repetitions. Means followed by different letters (in the same line) indicate significant difference by the Tukey test (p < 0.05).
Effect of fungicide on biomass production
Table 2 shows the biomass produced by F. verticillioides 103 F in different incubation periods. The maximum biomass production occurred on the 10 th d in the control cultures (0.4 g) and only on the 18 th d in the treatments (0.35 g). There was no significant difference concerning biomass production between the control and the treatment by the Tukey test (p < 0.05) except for the 5 th d, but a decreasing and a delaying trend in biomass production was observed in the cultures to which fludioxonil + metalaxyl - M was added.
Table 2. Biomass production by F. verticillioides 103 F in defined liquid culture media in the absence (control) and presence (treatment) of fludioxonil + metalaxyl - M at the recommended dose (1.5 μL/mL) in different incubation periods.
| Incubation period (days) | Biomass (g) x | |
|---|---|---|
|
|
||
| Control | Treatment | |
| 3 | 0.200 a | 0.160 a |
| 5 | 0.355 a | 0.150 b |
| 7 | 0.370 a | 0.300 a |
| 10 | 0.400 a | 0.295 a |
| 12 | 0.360 a | 0.310 a |
| 14 | 0.365 a | 0.310 a |
| 18 | 0.360 a | 0.350 a |
| 21 | 0.320 a | 0.275 a |
Mean of three repetitions. Means followed by different letters (in the same line) indicate significant difference by the Tukey test (p < 0.05).
Effect of fungicide on fumonisin production
FB 1 production in defined liquid culture medium containing fludioxonil + metalaxyl - M (treatment) was detected from the 7 th d incubation, whereas in the cultures without fungicide (control), it was only detected from the 10 th d ( Table 3 ). The highest FB 1 production occurred on the 14 th d, both for the control (0.72 μg/mL) and for the treatment (2.58 μg/mL). FB 1 production decreased from the 18 th d both in the control cultures and the treatments, possibly due to the decline phase of growth. FB 1 levels were higher (p < 0.05) in the presence of fludioxonil + metalaxyl - M from the 14 th d of incubation ( Table 3 ).
Table 3. Fumonisin B 1 production by Fusarium verticillioides 103 F cultured in defined liquid media in the absence (control) and presence (treatment) of fludioxonil + metalaxyl-M fungicide at the recommended dose (1.5 μL/mL) in different incubation periods.
| Incubation period (days) | Fumonisin B 1 (μg/mL) x | |
|---|---|---|
|
|
||
| Control | Treatment | |
| 3 | ND | ND |
| 5 | ND | ND |
| 7 | ND | 0.56 |
| 10 | 0.03 a | 0.57 a |
| 12 | 0.23 a | 0.45 a |
| 14 | 0.72 b | 2.58 a |
| 18 | 0.35 b | 1.40 a |
| 21 | 0.11 b | 1.19 a |
ND = Not detected.
Mean of three repetitions. Means followed by different letters (in the same line) indicate significant difference by the Tukey test (p < 0.05).
Nitrogen concentration
Taking into account that the nitrogen concentration is an important factor for FB 1 production, analyses were performed to determine residual nitrogen in the absence and presence of fludioxonil + metalaxyl - M in the culture medium. The residual nitrogen concentration in cultures obtained during 21 d cultivation is shown in Table 4 . Even though there was no significant difference (p < 0.05) between the control and the treatment in any of the incubation periods, nitrogen concentration decreased over time.
Table 4. Residual nitrogen in defined liquid media cultured with F. verticillioides 103F in the absence (control) and presence (treatment) of fludioxonil + metalaxyl - M at the recommended dose (1.5 μL/mL) in different incubation periods.
| Incubation period (days) | Nitrogen (%) x | |
|---|---|---|
|
|
||
| Control | Treatment | |
| 3 | 0.015 a | 0.011 a |
| 5 | 0.009 a | 0.008 a |
| 7 | 0.008 a | 0.009 a |
| 10 | 0.009 a | 0.008 a |
| 12 | 0.009 a | 0.009 a |
| 14 | 0.009 a | 0.010 a |
| 18 | 0.008 a | 0.008 a |
| 21 | 0.008 a | 0.010 a |
Mean of three repetitions. Means followed by different letters (in the same line) indicate significant difference by the Tukey test (p < 0.05).
The initial nitrogen from the control medium was 0.022%, and 0.026% in the culture media with fludioxonil + metalaxyl - M added (treatment). On the 3 rd d incubation, the nitrogen concentration decreased to 0.015% and 0.011% respectively. From the 5 th d, the nitrogen concentration decreased to 0.008% in cultures with fludioxonil + metalaxyl-M and 0.009% in control cultures and these values were maintained until 21 st d of incubation.
Discussion
The effect of fludioxonil + metalaxyl - M on mycelial morphology ( Figures 1 and 2 ) are in accordance to those reported by Kang et al. (2001) and Ochiai et al. (2002) . Kang et al. (2001) evaluated the effect of tebuconazole on F. culmorum mycelial ultrastructure and demonstrated inhibited and irregular mycelia growth, besides morphological changes, and excessive hyphal septation. Ochiai et al. (2002) demonstrated that fludioxonil (25 μg/mL) caused severe defects in Candida albicans hyphal formation. In fact, in C. albicans , tunicamycin, even at low concentrations, blocks the N-linked glycosylation and the formation of protein - carbohydrate linkage ( Kuo and Lampen, 1974 ). This linkage is important for the formation of mannoproteins, major cell wall components, and for the formation, development and maintenance of the biofilm matrix, indicating that this was the probable mechanism by which tunicamycin inhibited the biofilm formation by 90% and also decreased cellular growth ( Pierce et al. , 2008 ; Thomas et al. , 2006 ). Furthermore, the antifungal farnesol (300 μM) and miconazole also inhibited biofilm formation by C. albicans ( Ramage et al. , 2002 ; Vandenbosch et al. , 2010 ). Since extracellular materials are important for nutrient uptake, promoting orderly hyphae growth and resistance to antifungal agents ( Blankenship and Mitchell, 2006 ), the influence of fludioxonil + metalaxyl-M ( Figures 1 and 2 - A, C and E ) on their formation may also be related to the decreasing trend in biomass production ( Table 2 ) and consequently in fungal growth.
Some studies have shown a relationship between the onset of sporulation and mycotoxin production. Chemical compounds that inhibit sporulation in Aspergillus parasiticus and A. nidulans also promoted the inhibition of aflatoxin and sterigmatocystin production, respectively ( Reiss, 1982 ; Guzman-de-Peña and Ruiz-Herrera, 1997 ; Guzman-de-Peña et al. , 1998 ). Even though those studies showed a reduction in sporulation and mycotoxin levels after treatment with chemical compounds, the results obtained with F. verticillioides 103F indicated an increase in both sporulation and FB 1 production ( Tables 1 and 3 ). This was probably due to a genetic link between sporulation and mycotoxin production in F. verticillioides , because the mutation in the FCC1 gene resulted in reduction in sporulation and FB 1 biosynthesis ( Shim and Woloshuk, 2001 ). In addition, Costa et al. (2010) showed that sporulation was increased by A. flavus in the presence of neem oil ( Azadirachta indica ), but germination and growth was decreased.
Data on the effect of fungicide on fumonisin production ( Table 3 ) are in accordance to those reported by Falcão et al. (2011) who showed an increased mean FB 1 production by F. verticillioides 103 F (3.5-fold) after 14 d incubation in culture medium with fludioxonil + metalaxyl-M. Moreover, Moss and Frank (1985) showed that the addition of 0.6 to 0.8 μg/mL tridemorph inhibited T-2 toxin and diacetoxyscirpenol (DAS) production, but when added at 30 to 50 μg/mL it stimulated T-2 toxin production by F. sporotrichioides (five-fold), despite having reduced growth by 50%. According to Hasan (1993) , 100 μg/mL vinclozolin decreased the mycelial growth of F. graminearum and production of DAS and zearalenone. Therefore, these studies suggest that the effectiveness of the chemical control agent depends on the fungicide dosage and the mycotoxin in question. Increased FB 1 production in the presence of fludioxonil + metalaxyl-M may be related to the fungicide action mechanism. Fludioxonil is a broad-spectrum fungicide that acts on histidine kinases named Mitogen Activated Protein (MAP). The MAP kinases (MAPKs) are involved in the transduction of many extracellular signals and are important for maintenance, growth regulation, cell differentiation, invasive hyphae growth, conidial germination and virulence ( Xu, 2000 ). Since fludioxonil acts on MAPKs, fludioxonil + metalaxyl - M could alter cell morphology and cause cell lysis in the samples treated with fungicide, releasing intracellular fumonisins. The effects of fludioxonil on Neurospora crassa ( Zhang et al. , 2002 ) showed that the fungicide acts on a MAPK related to osmoregulation, overstimulating the expression of this enzyme and causing hyperosmotic stress, with consequent accumulation of intracellular glycerol, cell swelling and disruption. In C. albicans , the addition of fludioxonil to the culture medium also affected osmoregulation, leading to accumulation of intracellular glycerol and inhibiting hyphae formation ( Ochiai et al. , 2002 ).
Residual nitrogen concentration ( Table 4 ) was not statistically different between the control and the treatment in all the periods analyzed (p < 0.05). However, according to Shim and Woloshuk (1999) , the limiting nitrogen (1.25 or 2.5 mM ammonium phosphate) for F. verticillioides in defined culture medium triggers FB 1 production after 18 h cultivation, while the addition of 20 mM ammonium phosphate inhibits its production. Therefore, the presence of fludioxonil + metalaxyl - M in the culture medium and the limited nitrogen source may exert a synergistic effect in anticipating FB 1 production in the treatments ( Table 3 ).
In summary, the recommended dose of fludioxonil + metalaxyl-M caused inhibition of hyphal growth and extracellular material formation but enhanced sporulation and FB 1 production by F. verticillioides 103F in defined liquid culture medium. The results ratify the importance of understanding the effect of fungicide to minimize the occurrence of toxigenic fungi and fumonisins.
Acknowledgments
The authors thank the CNPq (the Brazilian Government Organization for grant aid and fellowship to Brazilian researchers), CNPq/Ministry of Agriculture, the Araucária Foundation, PPSUS/Brazilian Ministry of Health, Paraná Fund/SETI and CAPES (Co-ordination for Formation of High Level Professionals) - Nanobiotechnology Network Program (04/CII-2008) for the financial support. The CNPq research productivity fellowship is greatly appreciated by E.Y.S. Ono, M.A. Ono and E.Y. Hirooka, as well the CAPES/MSc scholarship by Miguel, T.A.
References
- American Association of Cereal Chemists . Approved Methods of the American Association of Cereal Chemists. St. Paul, Minnesota: 1990. [Google Scholar]
- Blankenship JR, Mitchell AP. How to build a biofilm: a fungal perspective. Curr Opin Microbiol. 2006;9:588–594. doi: 10.1016/j.mib.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Costa CL, Geraldo MRF, Arrotéia CC, et al. In vitro activity of neem oil [ Azadirachta indica A. Juss ( Meliaceae )] on Aspergillus flavus growth, sporulation, viability of spores, morphology and aflatoxin B 1 and B 2 production . Adv Biosci Biotechnol. 2010;1:292–299. [Google Scholar]
- Doohan FM, Weston G, Rezanoor HN, et al. Development and use of a reverse transcription - PCR assay to study expression of tri5 by Fusarium species in vitro and in plant . Appl Environ Microbiol. 1999;65:3850–3854. doi: 10.1128/aem.65.9.3850-3854.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcão VCA, Ono MA, Miguel TA, et al. Fusarium verticillioides : evaluation of fumonisin production and effect of fungicides on in vitro inhibition of mycelial growth . Mycopathologia. 2011;171:77–84. doi: 10.1007/s11046-010-9339-9. [DOI] [PubMed] [Google Scholar]
- Gelderblom WCA, Jaskiewicz K, Marasas WFO, et al. Fumonisins: novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme . Appl Environ Microbiol. 1988;54:1806–1811. doi: 10.1128/aem.54.7.1806-1811.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulart AC, Fialho WFB. Tratamento de sementes de milho com fungicidas para o controle de patógenos. Summa Phytopathol. 2001;27:414–420. [Google Scholar]
- Guzman-de-Peña D, Ruiz-Herrera J. Relationship between aflatoxin biosynthesis and sporulation in Aspergillus parasiticus . Fungal Genet Biol. 1997;21:198–205. doi: 10.1006/fgbi.1996.0945. [DOI] [PubMed] [Google Scholar]
- Guzman-de-Peña D, Aguirre J, Ruiz-Herrera J. Correlation between the regulation of sterigmatocystin biosynthesis, assexual and sexual sporulation in Emericella nidulans . Antonie Leeuwenhoek. 1998;73:199–205. doi: 10.1023/a:1000820221945. [DOI] [PubMed] [Google Scholar]
- Harrison L, Colvin BM, Green JT, et al. Pulmonary edema and hydrothorax in swine produced by fumonisin B 1 , a toxic metabolite of Fusarium moniliforme . J Vet Diagn Invest. 1990;2:217–221. doi: 10.1177/104063879000200312. [DOI] [PubMed] [Google Scholar]
- Hasan HAH. Fungicide inhibition of aflatoxins, diacetoxyscirpenol and zearalenone production. Folia Microbiol. 1993;38:295–298. doi: 10.1007/BF02898597. [DOI] [PubMed] [Google Scholar]
- Jiménez M, Mateo JJ, Hinojo MJ, et al. Sugar and amino acids as factors affecting the synthesis of fumonisins in liquid cultures by isolates of the Gibberella fujikuroi complex . Int J Food Microbiol. 2003;89:185–193. doi: 10.1016/s0168-1605(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Kang Z, Huang L, Krieg U, et al. Effects of tebuconazole on morphology, structure, cell wall components and trichothecene production of Fusarium culmorum in vitro . Pest Manag Sci. 2001;57:491–500. doi: 10.1002/ps.310. [DOI] [PubMed] [Google Scholar]
- Kuo SC, Lampen JO. Tunicamycin - An inhibitor of yeast glycoprotein synthesis. Biochem Biophys Res Commun. 1974;58:287–295. doi: 10.1016/0006-291x(74)90925-5. [DOI] [PubMed] [Google Scholar]
- Ledoux DR, Browm TP, Weibking TS, et al. Fumonisin toxicity in broiler chicks. J Vet Diag Invest. 1992;4:330–333. doi: 10.1177/104063879200400317. [DOI] [PubMed] [Google Scholar]
- Magan N, Hope R, Colleate A, et al. Relationship between growth and mycotoxin production by Fusarium species, biocides and environment . Eur J Plant Pathol. 2002;108:685–690. [Google Scholar]
- Marasas WFO, Kellerman TS, Gelderblom WCA, et al. Leukoencephalomalacia in a horse induced by fumonisin B 1 isolated from Fusarium moniliforme . Onderstepoort J Vet Res. 1988;55:197–203. [PubMed] [Google Scholar]
- Menneti AM, Gregori R, Neri F. Activity of natural compounds on Fusarium verticillioides and fumonisin production in stored maize kernels . Int J Food Microbiol. 2010;136:304–309. doi: 10.1016/j.ijfoodmicro.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Suarez L, Felkner M, et al. Exposure to fumonisins and the occurrence of neural tube defects along the Texas - Mexico border. Environ Health Persp. 2006;114:237–241. doi: 10.1289/ehp.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes MHD, Menten JOM, Gravena JC, et al. Controle químico de Fusarium moniliforme em sementes de milho: metodologia de avaliação e efeitos sobre a qualidade fisiológica . Fitopatol Bras. 2003;28:626–632. [Google Scholar]
- Moss MO, Frank M. The influence of the fungicide tridemorph on T-2 toxin production by Fusarium sporotrichioides . Trans Br Mycol Soc. 1985;54:585–590. [Google Scholar]
- Munkvold GP, Desjardins AE. Fumonisins in maize: can we reduce their occurrence? Plant Dis. 1997;81:556–565. doi: 10.1094/PDIS.1997.81.6.556. [DOI] [PubMed] [Google Scholar]
- Munkvold GP, O’Mara JK. Laboratory and growth chamber evaluation of fungicidal seed treatments for maize seedling blight caused by Fusarium species . Plant Dis. 2002;86:143–150. doi: 10.1094/PDIS.2002.86.2.143. [DOI] [PubMed] [Google Scholar]
- Ochiai N, Fujimura M, Oshima M, et al. Effects of iprodione and fludioxonil on glycerol synthesis and hyphal development in Candida albicans . Biosci Biotechnol Biochem. 2002;66:2209–2215. doi: 10.1271/bbb.66.2209. [DOI] [PubMed] [Google Scholar]
- Pierce CG, Thomas DP, López-Ribot JL. Effect of tunicamycin on Candida albicans biofilm formation and maintenance . J Antimicrob Chemother. 2008;63:473–479. doi: 10.1093/jac/dkn515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G, Saville SP, Wickes BL, et al. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule . Appl Environ Microbiol. 2002;68:5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss J. Development of Aspergillus parasiticus and formation of aflatoxin B 1 under the influence of conidiogenesis affecting compounds . Arch Microbiol. 1982;133:236–238. doi: 10.1007/BF00415008. [DOI] [PubMed] [Google Scholar]
- Rheeder JP, Marasas WFO, Vismer HF. Production of fumonisin analogs by Fusarium species . Appl Environ Microbiol. 2002;68:2101–2105. doi: 10.1128/AEM.68.5.2101-2105.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard GS, Sydenham EW, Thiel PG, et al. Quantitative determination of fumonisins B 1 and B 2 by high-performance liquid chromatography with fluorescence detection . J Liq Chromatogr. 1990;13:2077–2087. [Google Scholar]
- Shim W-B, Woloshuk CP. Nitrogen repression of fumonisin B 1 biosynthesis in G ibberella fujikuroi . FEMS Microbiol Lett. 1999;177:109–116. doi: 10.1111/j.1574-6968.1999.tb13720.x. [DOI] [PubMed] [Google Scholar]
- Shim W-B, Woloshuk CP. Regulation of fumonisin B 1 biosynthesis and conidiation in Fusarium verticillioides by a cyclin-like (C-type) gene, FCC1 . Appl Environ Microbiol. 2001;67:1607–1612. doi: 10.1128/AEM.67.4.1607-1612.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Wang S, Hu X, et al. Fumonisin B 1 contamination of home-grown corn in high-risk areas for esophageal and liver cancer in China . Food Addit Contam. 2007;24:181–185. doi: 10.1080/02652030601013471. [DOI] [PubMed] [Google Scholar]
- Thomas DP, Bachmann SP, Lopez-Ribot JL. Proteomics for the analysis of the Candida albicans biofilm lifestyle . Proteomics. 2006;6:5795–5796. doi: 10.1002/pmic.200600332. [DOI] [PubMed] [Google Scholar]
- Ueno Y, Aoyama S, Sugiura Y, et al. A limited survey of fumonisins in corn and corn-based products in Asian countries. Mycotoxin Res. 1993;9:27–34. doi: 10.1007/BF03192229. [DOI] [PubMed] [Google Scholar]
- Vandenbosch D, Braeckmans K, Nelis HJ, et al. Fungicidal activity of miconazole against Candida spp. biofilms . J Antimicrob Chemother. 2010;65:694–700. doi: 10.1093/jac/dkq019. [DOI] [PubMed] [Google Scholar]
- Voss KA, Howard PC, Riley RT, et al. Carcinogenicity and mechanism of action of fumonisin B 1 : a mycotoxin produced by Fusarium moniliforme (= F. verticillioides ) . Cancer Detect Prev. 2002;26:1–9. doi: 10.1016/s0361-090x(02)00011-9. [DOI] [PubMed] [Google Scholar]
- Weibking T, Ledoux DR, Bermudez AJ, et al. Effects of feeding Fusarium moniliforme culture material, containing known levels of fumonisin B 1 , on the young broiler chick . Poult Sci. 1993;72:456–466. doi: 10.3382/ps.0720456. [DOI] [PubMed] [Google Scholar]
- Xu J-R. MAP kinases in fungal pathogens. Fungal Genet Biol. 2000;31:137–152. doi: 10.1006/fgbi.2000.1237. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lamm R, Pillonel C, et al. Osmorregulation and fungicide resistance: the Neurospora crassa os - 2 gene encodes a HOG1 mitogen - activated protein kinase homologue . Appl Environ Microbiol. 2002;68:532–538. doi: 10.1128/AEM.68.2.532-538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]