Abstract
The practice of refrigerating raw milk at the farm has provided a selective advantage for psychrotrophic bacteria that produce heat-stable proteases and lipases causing severe quality problems to the dairy industry. In this work, a protease (AprX) and a lipase (LipM) produced by Pseudomonas fluorescens 041, a highly proteolytic and lipolytic strain isolated from raw milk obtained from a Brazilian farm, have been purified and characterized. Both enzymes were purified as recombinant proteins from Escherichia coli . The AprX metalloprotease exhibited activity in a broad temperature range, including refrigeration, with a maximum activity at 37 °C. It was active in a pH range of 4.0 to 9.0. This protease had maximum activity with the substrates casein and gelatin in the presence of Ca +2 . The LipM lipase had a maximum activity at 25 °C and a broad pH optimum ranging from 7.0 to 10. It exhibited the highest activity, in the presence of Ca +2 , on substrates with long-chain fatty acid residues. These results confirm the spoilage potential of strain 041 in milk due to, at least in part, these two enzymes. The work highlights the importance of studies of this kind with strains isolated in Brazil, which has a recent history on the implementation of the cold chain at the dairy farm.
Keywords: raw milk, food deterioration, Pseudomonas fluorescens, extracellular protease, extracellular lipase
Introduction
In Brazil, the practice of refrigerating raw milk at the dairy farm started in the 90s, was officially instituted by the government in 2002 and it is still being implemented in some areas of the country [ Brasil, 2002 ; Brasil, 2011 ]. The refrigeration of raw milk in the farm and dairy industries has improved the quality and shelf life of milk and dairy products. However, it does not prevent the growth of psychrotrophic microorganisms that produce heat-stable enzymes such as proteases and lipases ( Cousin 1982 ; Sorhaug and Stepaniak 1997 ; Decherni et al. , 2005 ; De Jonghe et al. , 2010; Corrêa et al. , 2011 ; Baglinière et al. , 2013 ; Quigley et al. , 2013 ).
Many of these enzymes are produced by Pseudomonas fluorescens , a frequent psychrotrophic spoilage bacterium found in milk ( Wiedmann et al. , 2000 ; Dogan and Boor, 2003 ; Pinto et al ,. 2006 ; Dufour et al .; 2008 ; Marchand et al. , 2009 ; Baglinière et al. , 2013 ). As hydrolytic enzymes from this bacterium are generally not inactivated by pasteurization or even by Ultra-High Temperature (UHT) treatment ( Griffiths et al. , 1981 ; Chen et al. , 2003 ; De Jonghe et al. , 2010; Baglinière et al. , 2013 ), they can cause severe problems in the dairy industry such as milk protein hydrolysis, development of off-flavors, shelf-life reduction, decrease of yield during cheese production, milk heat-stability loss, and gelation of UHT milk ( Fairbairn and Law, 1986 ; Datta and Deeth, 2001 ; Chen et al. , 2003 ; Dufour et al. , 2008 ; Baglinière et al. , 2013 ).
A commom type of protease produced by P. fluorescens is metalloprotease. This class of enzyme contains one zinc atom and up to eight calcium atoms, conferring thermostability to the protein ( Sorhaug and Stepaniak, 1997 ). These authors listed some important characteristics of the metalloproteases secreted by strains of P. fluorescens including temperature optimum between 30 and 45 °C, a significant residual activity at 4 °C, and a pH optimum in the neutral range. These authors pointed out that a heat treatment of milk sufficient to fully inactivate these enzymes would also create unacceptable changes in the product and it is therefore unpractical for the dairy industry.
Microorganisms that produce lipolytic enzymes, such as P. fluorescens , are important in the dairy industry because they can produce rancid flavors and odors in milk and dairy products that make these foods unacceptable to consumers ( Cousin 1982 ). Bacterial lipases generally have molecular masses between 30 to 50 kDa, and the pH optimum is slightly alkaline (in the range of 7 to 9) ( Chen et al. , 2003 ; Chakraborty and Paulraj, 2009 ; Boran and Ugur 2010 ; Anbu, 2014 ). Lipase production by P. fluorescens is influenced by the type and concentration of carbon and nitrogen sources, iron, pH, dissolved oxygen concentration, and growth temperature ( Cousin 1982 ; Burger et al. , 2000 ; Woods et al. , 2001 ; Rajmohan et al. , 2001).
The present work aimed at the molecular characterization of a protease and a lipase produced by P. fluorescens 041, a highly milk deteriorating strain isolated from refrigerated raw milk obtained from a Brazilian farm. Both enzymes were overexpressed in Escherichia coli , purified to homogeneity by affinity chromatography and biochemically characterized in order to evaluate their role in the spoilage of milk components.
Material and Methods
Bacterial strains and plasmids
The bacterial strains and plasmids used in this study are listed in Table 1 . P. fluorescens 041 and 07A strains were isolated from refrigerated raw milk as highly proteolytic and lipolytic psychrotrophic bacteria ( Martins et al. , 2005 ; Pinto et al. , 2006 ; Pinto et al. , 2010 ).
Table 1. Bacterial strains and plasmids.
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli XL1-Blue | Cloning and subcloning host sup E44, hsd R17, end A1, rec A1, g yr A96, thi 1, rel A1, lac -F[ pro AB+, lac Iq, lac Z-M15, Tn10 ( tet F)] | Bullock et al., 1987 |
| P. fluorescens 07A | Wild type | Martins et al. , 2005 |
| P. fluorescens 041 | Wild type | Martins et al. , 2005 |
| Plasmids | ||
| pCR2.1-TOPO | Cloning vector, lacZ α fragment containing MCS, f1 origin, ColE1, Km r Ap r | Invitrogen |
| pQE30-Xa | Vector for the insertion of a Factor Xa Protease recognition site C-terminal of the 6xHis tag, T5 promoter, lac operator, ribosome binding site, ATG start codon, His tag sequence, multiple cloning sites, stop codons in all three reading frames, Col E1 origin of replication, Ap r | Qiagen |
| pQE30-Xa-aprX041 | 1.43 kb fragment containing aprX from P. fluorescens 041 in pQE30-Xa, Ap r | This study |
| pQE30-Xa-lipM041 | 1.42 kb fragment containing lipM from P. fluorescens 041 in pQE30-Xa, Ap r | This study |
Growth conditions
P. fluorescens was cultured in TYEP (tryptone 1%, yeast extract 0.25%, KH 2 PO 4 0.1%, K 2 HPO 4 0.1%, and CaCl 2 0.25%) broth at 25 °C with aeration or in 12% (w/v) reconstituted skim milk powder. E. coli XL1-Blue was cultured in Luria-Bertani (LB) broth or on LB agar plates at 37 °C, as required.
DNA manipulations, PCR reactions and sequencing
DNA manipulations
Cloning, restriction enzyme analysis, and transformation of E. coli were performed using established procedures ( Sambrook et al. , 1989 ). PCR was performed with TaKaRa Ex Taq polymerase (TaKaRa Shuzo, Shiga, Japan). Plasmid DNA was isolated using the QIAprep Spin Miniprep kit, and chromosomal DNA was purified with the DNeasy tissue kit. DNA fragments were purified from agarose gels by using the QIAquick gel extraction kit (all kits from Qiagen, Hilden, Germany).
Amplification and sequencing of the protease and lipase genes by PCR
The reaction consisted of 2.0 mM MgCl 2 , 5.0 μL of 10X buffer Ex Taq , 2.5 mM deoxynucleotide triphosphates (dNTPs), 0.5 μM of each primer, 1 U of Ex Taq DNA polymerase, and 40 ng of DNA in a final volume of 50 μL. Primers based on the sequences of the aprX (GenBank accession numbers DQ146945 , AY298902 , AF216700 , AY973251 ) and lip gene (GenBank accession numbers AF216702 , AY694785 , M86350 , S77830 , D11455 , AB063391 , AY304500 , AY673674 , M74125 , AY700013 ) of other P. fluorescens strains were designed ( Table 2 ), and synthesized by Microsynth (Zürich, Switzerland). The reactions were carried out in a T3 thermocycler (Biometra ® , Biolabo Scientific Instruments, Zürich, Switzerland).
Table 2. Primers used to amplify the aprX and lipM gene by PCR.
| Primer | Sequence (5′-3′) | Aplication |
|---|---|---|
| Apr-F | TTATGTCAAAAGTAAAAGAC | Amplification of aprX gene |
| Apr-R | TCAGGCTACGATGTCACTG | Amplification of aprX gene |
| APRX-F | ATT GGATCC AAAGCTATTGTATCTGCCGCG | Amplification of aprX gene and preparation for cloning in pQE-30Xa |
| APRX-R | ATT GAGCTC TCAGGCTACGATGTCACTGGC | Amplification of aprX gene and preparation for cloning in pQE-30Xa |
| Lip-F | ATGGGTRTSTTYGACTATAAAAACC | Amplification of lipM gene |
| Lip-R | TTAACCGATCACAATCCCCTCC | Amplification of lipM gene |
| LIPM-F | ATT GGATCC AACCTCGGTACCGAGGACTC | Amplification of lipM gene and preparation for cloning in pQE-30Xa |
| LIPM-R | ATT GAGCTC TTAACCGATCACAATCCCCTCCC | Amplification of lipM gene and preparation for cloning in pQE-30Xa |
The introduced restriction sites BamHI and Sac I are underlined.
The M13 Forward and Reverse Primers were used to sequence the aprX and lipM genes of P. fluorescens 041 cloned into pCR2.1-TOPO according to description of Invitrogen.
Cloning, heterologous expression and purification of P. fluorescens 041 protease and lipase
Once the complete sequences of the aprX and lipM genes were obtained, primers were designed ( Table 2 ) to amplify the open reading frames (ORF) by PCR using the bacterial genomic DNA as a template and TaKaRa Ex Taq as DNA-polymerase. The primers generated Bam HI and Sac I sites at the 5′ and 3′ ends of the amplified fragments, respectively.
The amplified DNA fragments of 1,434 bp and 1,422 bp, containing the aprX and lipM structural genes, respectively, were digested with Bam HI and Sac I and ligated into vector pQE-30Xa (Qiagen) previously cut with the same restriction enzymes. Plasmids harbouring the aprX or lipM ORFs inserted downstream of the T5 promoter were named pQE-30Xa-aprX041 or pQE-30Xa-lipM041. The plasmids were subsequently transformed into the expression strain E. coli XL1-Blue.
For overproduction of AprX and LipM, E. coli XL1-Blue cells carrying pQE-30Xa-aprX041 or pQE-30Xa-lipM041 were grown in dYT medium (tryptone 1.6%, yeast extract 1.0%, NaCl 0.5%, and glucose 0.2%) containing ampicillin (100 μg mL −1 ) at 37 °C under shaking at 300 rpm. At an optical density of 0.5 at 600 nm, isopropyl-β-D-thiogalactopyranoside (IPTG) was added to the culture to a final concentration of 1 mM, in order to induce the expression of aprX and lipM . After 5 h incubation at 37 °C, the cells were collected by centrifugation at 10,000 g for 30 min, resuspended in 50 mM Tris-HCl (pH 8.0) and centrifuged at 10,000 g for 30 min, followed by two washing steps with 50 mM Tris-HCl pH 8.0, NaCl 150 mM. The resulting cell pellets were finally resuspended in lysis buffer (8 M urea, 0.1 M NaH 2 PO 4 , 0.01 M Tris-HCl, pH 8.0) and the recombinant histidine-tagged enzymes were purified under denaturing conditions using the Ni-NTA Spin Columns (Qiagen) according to the suppliers’ instructions. After purification, the enzymes were subjected to overnight dialysis with Tris-HCl 20 mM, pH 8.0, CaCl 2 5 mM at 4 °C to allow renaturation.
Protein quantification, SDS-PAGE and zymograms
Protein concentration of the purified AprX and LipM enzyme solutions was determined by using the method of Bradford (1976) . Proteins were analysed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis [ Laemmli, 1970 ]. After electrophoresis the gels were stained with Coomassie brilliant blue.
Exoprotease activities of P. fluorescens culture supernatants, resolved proteases after precipitation with ammonium sulfate, and recombinant expressed AprX protease were visualized in SDS-PAGE-gels supplemented with 0.2% (w/v) azocasein ( Christensen et al. , 2003 ). After electrophoresis, proteins were renaturated by washing twice in 50 mM Tris-HCl, pH 7.5, 25% (v/v) isopropanol for 15 min at room temperature and once in 50 mM Tris-HCl, pH 7.5. After overnight renaturation at 4 °C, the zymogram was incubated for 4 h in 5 mM CaCl 2 and 50 mM Tris-HCl, pH 8.0 at 40 °C. Prior to detection, the gel was washed in 1 M NaOH for 5 min. Protease activity was detected as colourless zones in an orange background.
For the analysis of the lipase pattern after SDS-PAGE, proteins were renaturated as above described. The gels were overlaid with the fluorescent substrate methylumbelliferyl-butyrate (0.01 M in dimethylformamide) in order to detect lipolytic activity using UV-light (360 nm) to visualize blue fluorescent bands.
Identification of proteins by mass spectrometry
P. fluorescens 041 was grown in TYEP medium at 25 °C for 48 h. The cells were removed from the medium by centrifugation at 10,000 g for 30 min, the supernatant was sterilized by filtration, and the proteins were precipitated with ammonium sulfate. Samples were centrifuged 20 min at 10,000 g and the supernatant was discarded. Pellets were washed twice with an 85% ammonium sulfate solution (w/v), and again centrifuged. The pellets were dissolved in 50 Mm Tris-HCl, pH 8.0 and dialysed overnight at 4 °C against Tris-HCl 50 mM, pH 8.0, CaCl 2 5 mM. Aliquots of 15 μL of the dialysed samples were separated on SDS-PAGE (12%) gels. Coomassie-stained protein bands were excised, digested with trypsin and analysed by mass spectrometry ( Riedel et al. , 2006 ).
Enzyme assays
Proteolytic activity was investigated on azocasein, according to Christensen et al. (2003) , by incubating 250 μL of 2% azocasein (w/v) with 150 μL sterile filtered culture supernatant or with 75 μL of the purified AprX. Lipolytic activity on p -nitrophenyl palmitate was investigated by incubating 1 mL of substrate with 100 μL supernatant from overnight cultures or with 50 μL of the purified lipase LipM.
Characterization of purified enzymes
The proteolytic and lipolytic activities of purified AprX and LipM were determined as described above at various incubation temperatures (4, 25, 30, 37, 40, 45, 50, and 60 °C) and at various pH values using the following buffer systems: sodium succinate (pH 4.0, 5.0, 6.0), Tris-HCl (pH 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, 9.0), and glycine-NaOH (pH 9.0,10.0,11.0,12.0,13.0).
In order to determine heat stability of purified AprX and LipM, they were incubated for 5, 10, 15, 20, 30 and 60 min at 50, 60, 70, 80, 90, and 100 °C. They were also incubated at 65 °C for 30 min and 72 °C for 20 s to simulate the milk pasteurization treatments.
To investigate the effect of metal ions on purified AprX and LipM, the reaction mixture was supplemented with 1 mM of each of the following compounds: MnS0 4 , CoCl 2 , ZnSO 4 , FeSO 4 , MgSO 4 , or FeCl 3 . The effect of protease inhibitors on the proteolytic activity of purified AprX was determined by supplementing the reaction mixture with 1 mM PMSF, 1 mM EDTA, 1 mM Pefabloc SC, 2% (w/v) SDS, 4 M urea, 0.1% (w/v) DTT, and 0.1% (v/v) β-mercaptoethanol and subsequent measuring the residual activities on azocasein substrate.
The substrate specificity of purified AprX was determined on casein, elastin, collagen, bovine serum albumin, and gelatin. The reaction mixture consisted of 0.4% (w/v) of each protein in 400 μL of 50 mM Tris-HCl, pH 6.5 and 150 μL of enzyme solution. After incubation at 37 °C for 1 h, the mixture was withdrawn and the increase in the amount of free amino groups was determined by the ninhydrin method according to Setyorini et al. (2006) .
Activities of purified LipM on different p -nitrophenyl fatty acid esters ( p -nitrophenyl acetate, p -nitrophenyl butyrate, p -nitrophenyl palmitate, and p -nitrophenyl phosphorylcholine) were also measured according to the assay for lipolytic activity as described above.
Results
Milk-deteriorating hydrolytic activities of P. fluorescens
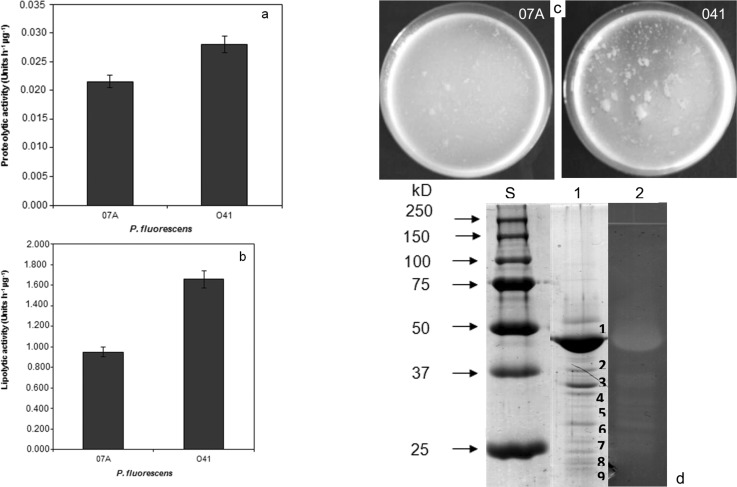
P. fluorescens 041 showed higher proteolytic ( Figure 1A ) and lipolytic ( Figure 1B ) activities than the strain 07A. Moreover, strain 041 exhibited a higher capacity to hydrolyze milk than P. fluorescens 07A when both strains were inoculated into 12% (w/v) reconstituted skim milk ( Figure 1C ). Therefore, P. fluorescens 041 was selected for further analysis of its hydrolytic extracellular enzymes.
Figure 1. Production of extracellular hydrolytic enzymes by P. fluorescens . A: Proteolytic activity in the supernatant of TYEP medium; B: Lipolytic activity in the supernatant of TYEP medium; C: Samples of reconstituted skin milk powder (12%) inoculated with P. fluorescens 07A and 041 after 18 h of incubation at 25 °C. Data represent the average of duplicate experiments; D: Coomassie-stained SDS-PAGE and azocasein zymogram on 12% PAA-gels visualizing protease production by P. fluorescens grown in TYEP medium supplemented with 0.25% CaCl 2 . Lanes S: molar mass standard (Biorad); lane 1: SDS-PAGE of ammonium sulfate precipitated proteins of P. fluorescens 041 supernatant; lane 2: azocasein zymogram of ammonium sulfate precipitated proteins of P. fluorescens 041 after proteins precipitation with ammonium sulfate.
SDS-PAGE analysis of ammonium sulfate precipitated protein from supernatants of TYEP cultures of P. fluorescens 041 demonstrated the presence of multiple protein bands ( Figure 1D ). Proteolytic activity of the dominant 50 kDa band was demonstrated by a zymogram incorporating azocasein ( Figure 2 ). Mass spectrometry analysis of the major proteolytic protein band identified this protein as a metalloprotease, which is commonly referred to as AprX. Analysis of the bands with lower molecular weight that showed proteolytic activity ( Figure 1D ) revealed that these bands were actually degradation products of AprX.
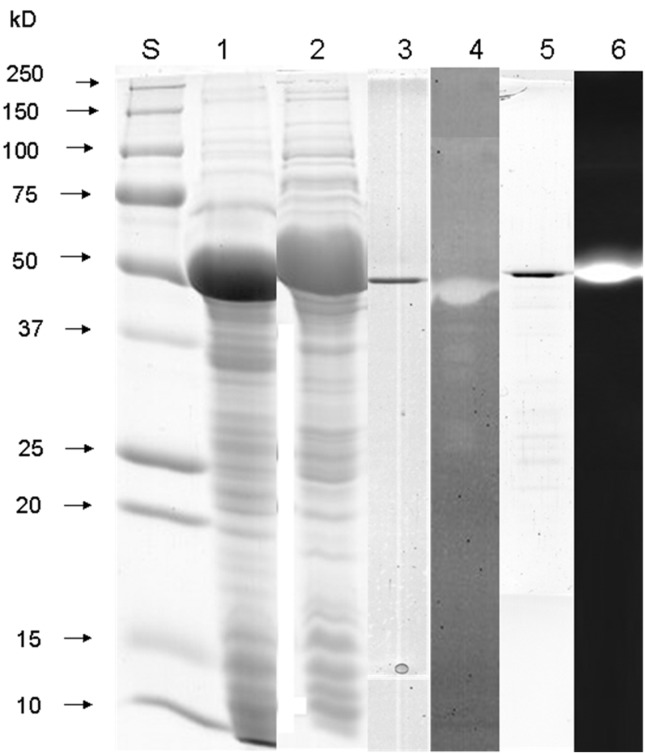
Figure 2. Coomassie-stained SDS-PAGE and zymogram gel on 12% PAA-gels visualizing recombinant AprX and LipM. Lane S: molecular mass standard (Biorad); lane 1: SDS-PAGE of crude extract of E. coli XL1-Blue carrying pQE30-Xa-aprX-041; lane 2: SDS-PAGE of crude extract of E. coli XL1-Blue carrying pQE30-Xa-lipM-041; lane 3: SDS-PAGE of purified AprX; lane 4: azocasein zymogram of purified AprX; lane 5: SDS-PAGE of purified LipM; lane 6: MU-butyrate zymogram of purified LipM.
Cloning and sequencing of protease and lipase genes
Primers based on sequences of homologous proteases and lipases from other P. fluorescens strains were synthesized and used to PCR amplify a segment encoding these enzymes from P. fluorescens 041 genome. Electrophoresis of the PCR products revealed a single product of about 1,500 bp for both genes. These PCR products were sequenced to reveal their identity as aprX and lipM genes. The aprX and the lipM genes of P. fluorescens 041 comprised open reading frames of 1,434 bp and 1,425 bp and coded for proteins with 477 and 474 amino acids, respectively. Based on the amino acid sequences, the molecular mass of both enzymes was predicted to be 49.365 kDa and 49.811 kDa, which was confirmed by SDS-PAGE analysis of the purified enzymes ( Figure 2 , line 3 and 5). The isoeletric point for AprX was 4.46 and 4.36 for LipM, as determined by using Protean (DNA Star Lasergene 7). They were active on zymograms after renaturation in buffer containing 1 mM of CaCl 2 ( Figure 2 , line 4 and 6).
The aprX gene of P. fluorescens 041 showed 97% identity with the extracellular alkaline metalloprotease ( aprX ) gene of P. fluorescens strain A506 and with the protease ( aprX ) gene of P. fluorescens strain F. The lipM gene of P. fluorescens 041 showed 93% identity with polyurethanase lipase A ( pulA ) gene and 86% with the lipase ( lipA ) gene of P. fluorescens strain A506.
Biochemical characterization of AprX and LipM
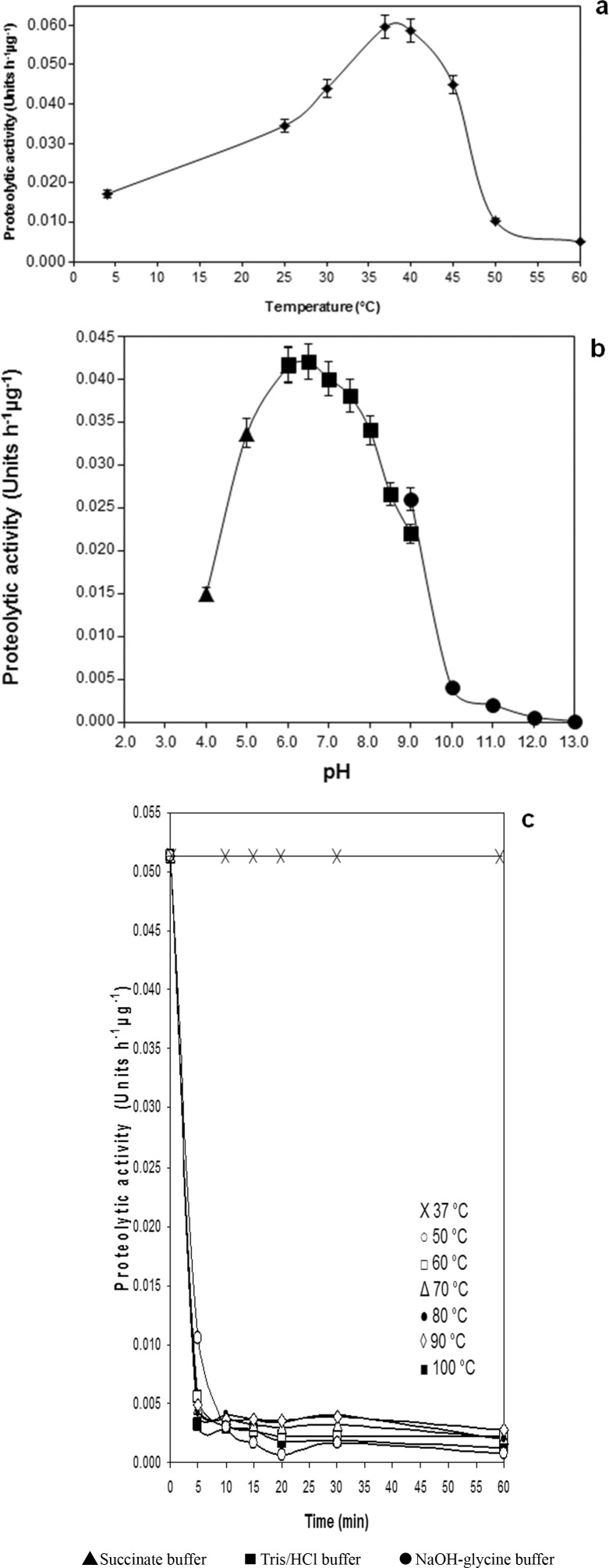
The temperature optimum of activity of the purified protease of P. fluorescens 041 was 37 °C ( Figure 3A ). Moreover it showed activity under conditions of refrigeration from 4 °C to 7 °C, and low activity in temperatures higher than 45 °C ( Figure 3A ).
Figure 3. Biochemical characteristics of AprX. A: Temperature optimum of purified AprX on azocasein; B: pH-optimum of purified AprX on azocasein. C: Effect of heat treatment on proteolytic activity of purified AprX. Data represent the average of duplicate experiments.
The pH optimum of AprX is between 6.0 and 6.5 ( Figure 3B ). The protease still exhibits 36% residual activity at pH 4.0 and 62% at pH 9.0.
The protease activity was strongly decreased by pre-incubating the enzyme at different temperatures ( Figure 3C ); the residual activity of the protease after 60 min at 50, 60, 70, 80, 90 and 100 °C was between 2 and 4%. Inactivation of the metalloprotease at temperature and time conditions used during the pasteurization process were also evaluated: AprX showed 70% residual activity when it was treated at 75 °C for 20 s (HTST treatment: high temperature and short time) and 4% residual activity when it was incubated at 65 °C for 30 min (LTLT: low temperature and long time).
Proteolytic activity of AprX was strongly dependent on the presence of Ca 2+ . However, other metal ions reduced the proteolytic activity ( Table 3 ). The activity of AprX was decreased when 1 mM EDTA, an inhibitor that specifically acts on metalloproteases, was added to the reaction mixture, confirming the type of enzyme. In addition, AprX was strongly inhibited by denaturing and reducing agents such as SDS, dithiothreitol (DTT), β-mercaptoethanol, and urea ( Table 4 ).
Table 3. Effect of metal ions on the activities of alkaline metalloprotease and lipase.
| Metal ion | Relative activity (%) | |
|---|---|---|
|
|
||
| Alkaline metalloprotease a | Lipase b | |
| None | 100 ± 2 | 100 ± 2 |
| Mn 2+ | 73 ± 1 | 61 ± 1 |
| Co 2+ | 48 ± 3 | 59 ± 8 |
| Zn 2+ | 86 ± 3 | 49 ± 0 |
| Fe 2+ | 90 ± 5 | 48 ± 3 |
| Fe 3+ | 102 ± 1 | 65 ± 2 |
| Mg 2+ | 100 ± 1 | 50 ± 3 |
A reaction mixture containing 250 μL of 2% (w/v) azocasein in 50 mM Tris/HCl (pH 8.0), 75 μL of AprX, and 1 mM of each metal ion was incubated at 37 °C for 12 h. The remaining activity was then measured, as described in the text. Results show the mean value (n = 3) plus or minus the standard deviation.
A reaction mixture containing 1 mL of substrate (one volume of 0.3% (w/v) p -nitrophenyl palmitate in isopropanol and nine volumes 0.2% (w/v) sodium desoxycholate and 0.1% (w/v) gummi arabicum in 50 mM sodium phosphate buffer, pH 8.0), 50 μL of LipM, and 1 mM of each metal ions was incubated at 25 °C for 20 min. The remaining activity was then measured, as described in the text. Results show the mean value (n = 3) plus or minus the standard deviation.
Table 4. Effect of inhibitors, denaturing and reducing agents on the activity of alkaline metalloprotease.
| Compound | Relative activity (%) c |
|---|---|
| Inhibitor a | |
| None | 100 ± 1 |
| PMSF | 95 ± 2 |
| EDTA | 51 ± 3 |
| Pefabloc SC | 89 ± 1 |
| Denaturing and reducing agent b | |
| None | 100 ± 2 |
| SDS | 6 ± 1 |
| Urea | 38 ± 5 |
| DTT | 24 ± 3 |
| β-mercaptoethanol | 44 ± 2 |
A reaction mixture containing 250 μL of 2% (w/v) azocasein in 50 mM Tris/HCl (pH 8.0), 75 μL of AprX, and 1 mM of each inhibitor was incubated at 37 °C for 12 h. The remaining activity was then measured, as described in the text.
A reaction mixture containing 250 μL of 2% (w/v) azocasein in 50 mM Tris/HCl (pH 8.0), 75 μL of AprX, and 2% (w/v) SDS, 4 M urea, 0.1% (w/v) DTT, or 0.1% (v/v) β-mercaptoethanol in 50 mM Tris/HCl (pH 8.0) was incubated at 37 °C for 12 h. The remaining activity was then measured, as described in the text.
Results show the mean value (n = 3) plus or minus the standard deviation.
The alkaline metalloprotease was further tested for its capability to hydrolyze different substrates such as casein, bovine serum albumin, collagen, elastin, and gelatin. The highest activities were found on gelatin (100%) and casein (87.6%), followed by collagen (57%), elastin (41.2%), and bovine serum albumin (39.8%).
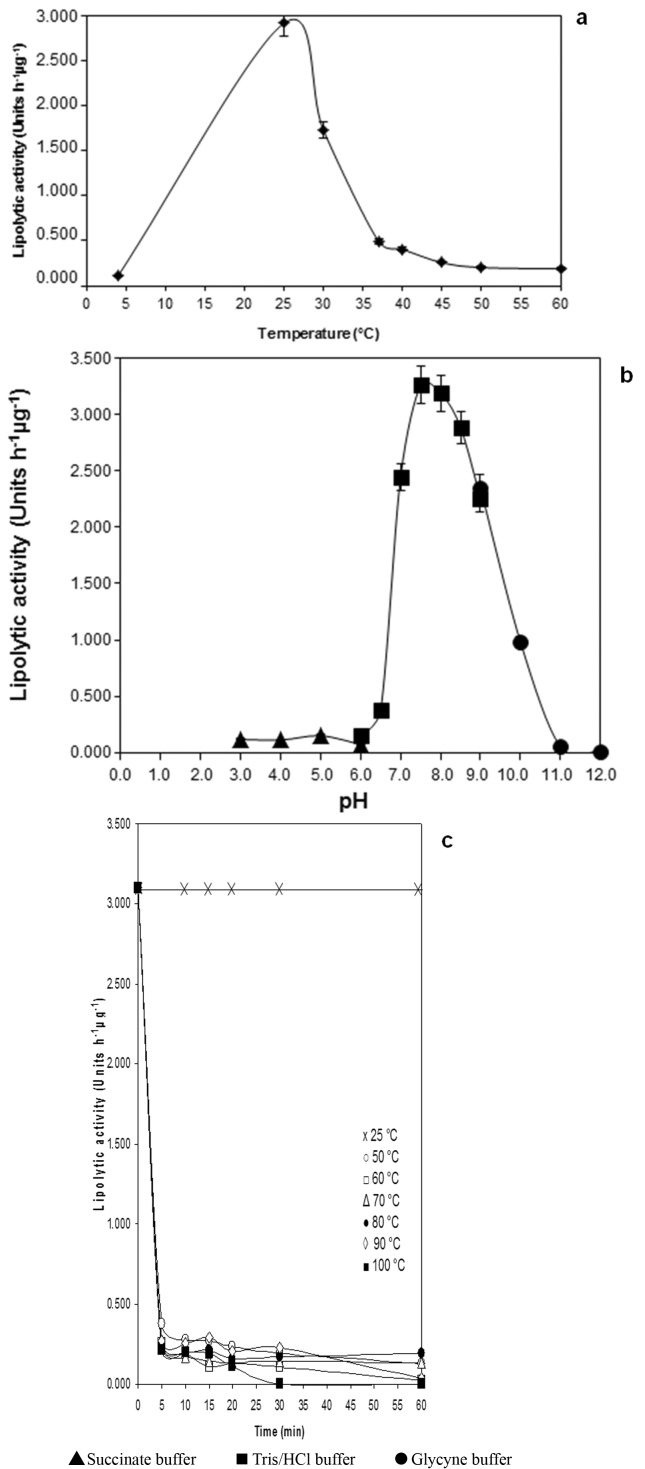
The temperature optimum of the purified lipase was 25 °C ( Figure 4A ). LipM showed a residual activity of 3.7% at 4 °C and exhibited low activities at temperatures higher than 37 °C ( Figure 4A ). Besides, LipM showed the highest lipase activity at pH 7.5 ( Figure 4B ). At pH values lower than 6.0 and higher than 11.0 only residual lipase activities could be detected ( Figure 4B ).
Figure 4. Biochemical characteristics of LipM. A: Temperature optimum of purified LipM; B: pH-optimum of purified LipM. C: Effect of heat treatment for 60 min on lipolytic activity of purified LipM. Data represent the average of duplicate experiments.
The lipase activity after 60 min of pre-incubation at 50, 60, 70, 80, 90 and 100 °C was nearly undetected ( Figure 4C ). The treatment of 65 °C for 30 min (LTLT), and 75 °C for 20 s (HTST), reduced the lipolytic activity to 13.2% and 25.4%, respectively.
Lipase activity was dependent on Ca +2 ions in the renaturation buffer. The same was observed for the protease activity. The presence of ions other than Ca +2 reduced the lipolytic activity ( Table 3 ). Among the tested substrates, LipM exhibited the highest activity for p -nitrophenyl palmitate (100%), followed by p -nitrophenyl butyrate (73%), p -nitrophenyl acetate (20%), and p -nitrophenyl phosphorylcholine (11%). The highest activity on substrates with long-chain fatty acid residues such as p -nitrophenyl palmitate indicates that the enzyme has esterolytic and lipolytic activities.
Discussion
Numerous Pseudomonas spp. have been shown to produce and secrete hydrolytic enzymes ( McCarthy et al. , 2004 ; Burger et al. , 2000 ; Woods et al. , 2001 ; Pinto et al. , 2006 ; Pinto et al. , 2010 ; Liao and McCallus 1998 ; Koka and Weimer, 2000 ; Maunsell et al. , 2006 ; Mu et al. , 2009 ; Jankiewicz et al. , 2010 ; De Jonghe et al. , 2011 ). Interestingly, in this study it was verified that aprX encodes for the major, if not the only extracellular protease produced by P. fluorescens 041. Mass spectrometry analysis of low molecular weight bands that showed proteolytic activity on the azocasein zymogram were identified as degradation products of the metalloprotease AprX. These results are in agreement with the findings of Liao and McCallus (1998) who observed that P. fluorescens CY091 produces a unique extracellular 50 kDa protease, AprX. Our results are also in agreement with several other studies ( Koka and Weimer, 2000 ; Mu et al. , 2009 ; Jankiewicz et al. , 2010 ). In contrast, Rajmohan et al. (2002) reported that another P. fluorescens isolated from milk produces five distinct proteases when they used the ultrafiltration technique to purify these enzymes. Nicodeme et al. (2005) observed the presence of more than one protease band for some strains of Pseudomonas, while others produced just one protease, as revealed by a zymogram analyzes . According to Sørhaug and Stepaniak (1997) the number of secreted proteases depends strongly on the P. fluorescens strain. These findings highlight the great diversity of P. fluorescens isolates and reiterate the importance of studies aiming to elucidate the molecular mechanisms of the hydrolytic enzymes produced by these strains.
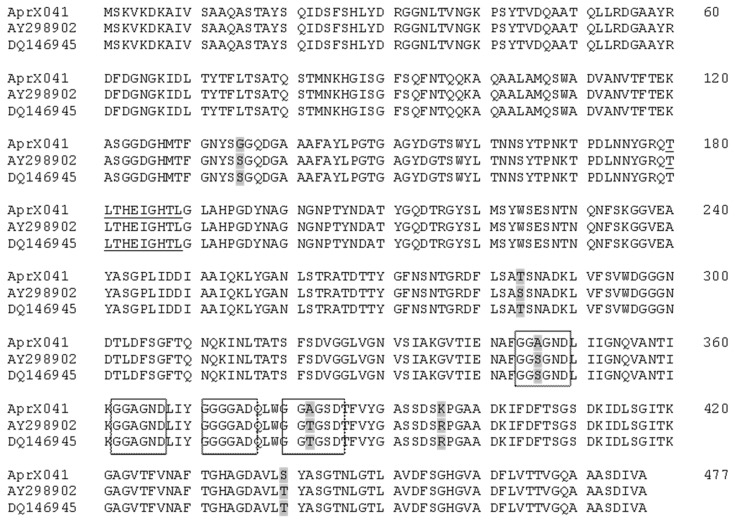
Once the AprX protein produced by strains of P. fluorescens isolated from raw milk showed high similarity with sequences from homologous enzymes in the database ( Figure 5 ), it further confirms the possibility of using the aprX gene as a marker to detect P. fluorescens in milk by using PCR as described by Martins et al. (2005) and Machado et al. (2013) . This approach would reduce the time for detecting these bacteria in raw milk giving flexibility for the dairy manager to choose the best use for a particular milk batch during processing.
Figure 5. Multiple sequence alignment of deduced protease AprX from P. fluorescens 041 (this study), P. fluorescens A506 (Genbank accession number AY298902 ), and P. fluorescens strain F (Genbank accession number DQ146945 ). The differences in amino acid residues are indicated by gray shading, and the catalytic domain of neutral zinc metalloprotease is underlined. Boxed residues are thought to participate in Calcium binding.
Unlike many proteolytic and lipolytic enzymes described in the literature ( Makhzoum et al. , 1996 ; Liao and McCallus, 1998 ; Rajmohan et al. , 2002 ; Chen et al. , 2003 ; Kojima and Shimizu, 2003 ; Nornberg et al. , 2009 ; Baglinière et al. , 2013 ; Anbu 2014 ), the protease and lipase evaluated in this study were relatively more sensitive to heat treatment. This could be attributed due to differences in the enzymes structures or to differences in experimental procedures, as the above mentioned studies have used purified enzymes from culture supernatants and we have purified those from overexpressing E. coli strains. However, some authors ( Teo et al. , 2003 ; Jing et al. , 2010 ) verified that His-tag did not affect the metalloprotease activities of some strains, indicating that the recombinant metalloprotease was in an active form. Affinity tags have become essential tools for the production of recombinant proteins in a wide variety of settings ( Waugh, 2011 ).
As the heat treatment and refrigeration processes adopted by the dairy industry during milk processing and storage do not fully inhibit enzymatic activity nor the growth of psychrotrophic bacteria, it is important to produce milk under stringent good manufacturing practices to limit contamination and bacterial spoilage.
Although LipM exhibits the conserved serine lipase catalytic domain, it presented somewhat lower similarity, as compared to AprX alignment, to the sequences described in the data base ( Figure 6 ). LipM was also less heat stable than some lipases described by other authors ( Knaut, 1978 ; Cousin, 1982 ; Makhzoum et al. , 1996 ; Boran and Ugur, 2013; Anbu, 2014 ), although low heat stability has also been observed ( Chakraborty and Paulraj, 2009 ; Dahiya et al. , 2010 ). According to Cousin (1982) , complete inactivation of lipases was only obtained by autoclaving milk at 121 °C for 15 min. Knaut (1978) observed that lipases from P. fluorescens species were stable even above 100 °C. A heat-treatment of 98 °C for 14 to 25 min was necessary to inactivate lipases from some Pseudomonas species, including P. fluorescens and P. fragi ( Cousin, 1982 ).
Figure 6. Multiple sequence alignment of deduced lipase LipM from P. fluorescens 041 (this study), Lip (Genbank accession number DQ305493 ), Lip68 (Genbank accession number AY694785 ), and LipA (Genbank accession number AF216702 ) from P. fluorescens . The differences in amino acid residues are indicated by gray shading and the catalytic domain of serine lipase is underlined.
Overall, the biochemical properties of the purified protease and lipase from this work were similar to those found for proteases and lipases of other P. fluorescens strains isolated from raw milk ( Makhzoum et al. , 1996 ; Kim et al. , 1997 ; Schokker and van Boekel, 1997 ; Liao and McCallus, 1998 ; Rajmohan et al. , 2002 ; Chen et al. , 2003 ; Kojima and Shimizu, 2003 ; Dufour et al. , 2008 ; Correa et al. , 2011 ; Baglinière et al. , 2013 ). It is worth to mention that AprX still exhibited 36% residual activity at pH 4.0, so if present in milk, this enzyme would not only affect the quality of pasteurized milk products but also of fermented products such as yogurt and cheese.
Surprisingly, no lipolytic activity could be detected when the renaturated SDS-PAGE was overlaid with the lipase substrate methylumbeliferyl-butyrate (results not shown). Probably this occurred due to the degradation of the enzyme by proteases or because Ca +2 was not added into the renaturation buffer, and the lipase may need this ion for correct folding.
The purification of AprX and LipM was important for the characterization of these spoilage enzymes and it would be interesting to use them to develop tools for improving their detection in milk. Nowadays, there is a great need for developing fast and reliable methods to detect spoilage enzymes directly from samples in order to determine the quality of milk that arrives at the dairy industry platform ( Datta and Deeth, 2001 ). Most approaches currently available are time consuming, do not have good sensitivity or have detection limits that are too high. Besides characterizing these spoilage enzymes, it is important to estimate the extent of degradation of milk components, and thus further improve enzymatic methods to access milk quality.
In this work a protease and a lipase produced by P. fluorescens 041, a highly milk spoilage strain, isolated from cooled raw milk were purified and characterized. The study showed that both enzymes presented similar biochemical properties to other enzymes from P. fluorescens strains isolated from raw milk. The differences that were observed could be accounted for the experimental procedures, especially the use of overexpressed recombinant proteins. The study confirms the spoilage potential of strain 041 in milk due to, at least in part, these two enzymes. The work highlights the importance of studies of this kind with P. fluorescens strains, the major spoilage bacteria contaminating milk produced in Brazil ( Martins et al. , 2005 ) which has a recent history on the implementation of the cold chain at the dairy farm.
Acknowledgments
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil) for a doctoral scholarship provided to M.L. Martins and a post-doctoral scholarship to U.M. Pinto. We are thankful to Leo Eberl from the University of Zurich - Switzerland for his assistance with this work.
References
- Anbu P. Characterization of an extracellular lipase by Pseudomonas koreensis BK-l07 isolated from soil . Prep Biochem Biotechnol. 2014;44:266–280. doi: 10.1080/10826068.2013.812564. [DOI] [PubMed] [Google Scholar]
- Baglinière, et al. Proteolysis of ultra high temperature-treated casein micelles by AprX enzyme from Pseudomonas fluorescens F induces their destabilization . Int Dairy J. 2013;31:55–61. [Google Scholar]
- Boran R, Ugur A. Partial purification and characterization of the organic solvent-tolerant lipase produced by Pseudomonas fluorescens RB02-3 isolated from milk . Prep Biochem Biotechnol. 2010;40:229–241. doi: 10.1080/10826068.2010.488929. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–274. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRASIL . Diário Oficial da República Federativa do Brasil. Brasília, 20 set. 2002, Seção I. 2002. Ministério da Agricultura, Pecuária e Abastecimento. Departamento de Inspeção de Produtos de origem Animal. Instrução Normativa n° 51, de 18 de setembro de 2002. Aprova os Regulamentos Técnicos de Produção, Identidade e Qualidade do Leite tipo A, do Leite tipo B, do Leite tipo C, do Leite Pasteurizado e do Leite Cru Refrigerado e o Regulamento Técnico da Coleta de Leite Cru Refrigerado e seu Transporte a Granel.13 [Google Scholar]
- BRASIL . Diário Oficial da República Federativa do Brasil. Brasília, 30 dez. 2011. Seção I. 2011. Ministério da Agricultura, Pecuária e Abastecimento. Departamento de Inspeção de Produtos de origem Animal. Instrução Normativa n°62, de 29 de dezembro de 2011. Aprova o Regulamento Técnico de Produção, Identidade e Qualidade do Leite tipo A, o Regulamento Técnico de Identidade e Qualidade de Leite Cru Refrigerado, Leite Pasteurizado e o Regulamento Técnico da Coleta de Leite Cru Refrigerado e seu Transporte a Granel. [Google Scholar]
- Bullock WO, Fernandez JM, Short JM. XL-1 Blue: a high efficiency plasmid transforming recA Escherichia coli strain with β-galactosidase selection . Bio Techniques. 1987;5:376–377. [Google Scholar]
- Burger M, et al. Temperature regulation of protease in Pseudomonas fluorescens LD107d2 by an ECF sigma factor and a transmembrane activator . Microbiology. 2000;146:3149–3155. doi: 10.1099/00221287-146-12-3149. [DOI] [PubMed] [Google Scholar]
- Chakraborty K, Paulraj R. Purification and Biochemical Characterization of an Extracellular Lipase from Pseudomonas fluorescens MTCC 2421 . J Agri Food Chem. 2009;57:3859–3866. doi: 10.1021/jf803797m. [DOI] [PubMed] [Google Scholar]
- Chen L, Daniel RM, Coolbear T. Detection and impact of protease and lipase activities in milk and milk powders. Int Dairy J. 2003;13:255–275. [Google Scholar]
- Christensen, et al. Quorum-sensing-directed protein expression in Serratia proteamaculans B5a . Microbiology. 2003;149:471–483. doi: 10.1099/mic.0.25575-0. [DOI] [PubMed] [Google Scholar]
- Correa APF, Daroit DJ, Velho RV, Brandelli A. Hydrolytic potential of a psychrotrophic Pseudomonas isolated from refrigerated raw milk . Braz J Microbiol. 2011;42:1479–1484. doi: 10.1590/S1517-838220110004000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin MA. Presence and activity of psychrotrophic microorganisms in milk and dairy products. J Food Protect. 1982;45:172–207. doi: 10.4315/0362-028X-45.2.172. [DOI] [PubMed] [Google Scholar]
- Datta N, Deeth HC. Age gelation of UHT milk. Trans Inst Chem Eng. 2001;79:197–210. [Google Scholar]
- Dahiya P, Arora P, Chaudhury A, Chand S, Dilbaghi D. Characterization of an extracellular alkaline lipase from Pseudomonas mendocina M-37 . J Basic Microbiol. 2010;50:420–426. doi: 10.1002/jobm.200900377. [DOI] [PubMed] [Google Scholar]
- De Jonghe V, et al. Influence of storage conditions on the growth of Pseudomonas species in refrigerated raw milk . Appl Environ Microbiol. 2011;77:460–70. doi: 10.1128/AEM.00521-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decherni S, Benjelloun H, Lebeault JM. Effect of modified atmospheres on the growth and extracellular enzyme activities of psychrotrophs in raw milk. Eng Life Sci. 2005;5:350–356. [Google Scholar]
- Dogan B, Boor KJ. Genetic diversity and spoilage potentials among Pseudomonas spp. isolated from fluid milk products and dairy processing plants . Appl Environ Microbiol. 2003;69:130–138. doi: 10.1128/AEM.69.1.130-138.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour, et al. Molecular typing of industrial strains of Pseudomonas spp. isolated from milk and genetical and biochemical characterization of an extracellular protease produced by one of them . Int J Food Microbiol. 2008;125:188–196. doi: 10.1016/j.ijfoodmicro.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Fairbairn DJ, Law BA. Proteinases of psychrotrophic bacteria: their production, properties, effects and control. J Dairy Res. 1986;53:139–77. doi: 10.1017/s0022029900024742. [DOI] [PubMed] [Google Scholar]
- Griffiths MW, Philips JD, Muir DD. Thermostability of proteases and lipases from a number of species of psychotrophic bacteria of dairy origin. J Appl Bacteriol. 1981;50:289–303. doi: 10.1111/j.1365-2672.1981.tb00894.x. [DOI] [PubMed] [Google Scholar]
- Jankiewicz U, Szawlowska U, Sobanska Biochemical characterization of an alkaline metallopeptidase secreted by a Pseudomonas fluorescens isolated from soil . J Basic Microbiol. 2010;50:125–34. doi: 10.1002/jobm.200900054. [DOI] [PubMed] [Google Scholar]
- Jing Y, Toubarro D, Hao Y, Simões N. Cloning, characterisation and heterologous expression of an astacin metalloprotease, Sc-AST, from the entomoparasitic nematode Steinernema carpocapsae . Mol Biochem Parasit. 2010;174:101–108. doi: 10.1016/j.molbiopara.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Jonghe, et al. Influence of storage conditions on the growth of Pseudomonas species in refrigerated raw milk . App Env Microbiol. 2010;77:460–470. doi: 10.1128/AEM.00521-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, et al. Purification and characterization of an extracellular metalloprotease from Pseudomonas fluorescens . J Biochem. 1997;121:82–88. doi: 10.1093/oxfordjournals.jbchem.a021575. [DOI] [PubMed] [Google Scholar]
- Knaut T. Heat resistance of Pseudomonas lipases in milk. XX International Dairy Congress 1978 [Google Scholar]
- Kojima Y, Shimizu S. Purification and characterization of the lipase from Pseudomonas fluorescens HU380 . J Biosci Bioeng. 2003;96:219–26. [PubMed] [Google Scholar]
- Koka R, Weimer BC. Isolation and characterization of a protease from Pseudomonas fluorescens RO98 . J Appl Microbiol. 2000;89:280–8. doi: 10.1046/j.1365-2672.2000.01108.x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liao CH, McCallus DE. Biochemical and genetic characterization of an extracellular protease from Pseudomonas fluorescens CY091 . Appl Environ Microbiol. 1998;64:914–921. doi: 10.1128/aem.64.3.914-921.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado SG, Bazzolli DMS, Vanetti MCD. Development of a PCR method for detecting proteolytic psychrotrophic bacteria in raw milk. Int Dairy J. 2013;29:8–14. [Google Scholar]
- Makhzoum A, Owusu-Apenten RK, Knapp JS. Purification and properties of lipase from Pseudomonas fluorescens strain 2D . Int Dairy J. 1996;6:459–472. [Google Scholar]
- Marchand, et al. Heterogeneity of heat-resistant proteases from milk Pseudomonas species . Int J Food Microbiol. 2009;133:68–77. doi: 10.1016/j.ijfoodmicro.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Martins ML, et al. Detection of the apr gene in proteolytic psychrotrophic bacteria isolated from refrigerated raw milk. Int J Food Microbiol. 2005;102:203–211. doi: 10.1016/j.ijfoodmicro.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Maunsell B, Adams C, O’Gara F. Complex regulation of AprA metalloprotease in Pseudomonas fluorescens M114: evidence for the involvement of iron, the ECF sigma factor, PbrA and pseudobactin M114 siderophore . Microbiology. 2006;152:29–42. doi: 10.1099/mic.0.28379-0. [DOI] [PubMed] [Google Scholar]
- McCarthy CN, Woods RG, Beacham IR. Regulation of the aprX-lipA operon of Pseudomonas fluorescens B52: differential regulation of the proximal and distal genes, encoding protease and lipase, by ompR-envZ . FEMS Microbiol Lett. 2004;241:243–248. doi: 10.1016/j.femsle.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Mu Z, Du M, Bai Y. Purification and properties of a heat-stable enzyme of Pseudomonas fluorescens Rm12 from raw milk . Eur Food Res Technol. 2009;228:725–734. [Google Scholar]
- Nicodeme, et al. Extracellular protease activity of different Pseudomonas strains: dependence of proteolytic activity on culture conditions . J Appl Microbiol. 2005;99:641–8. doi: 10.1111/j.1365-2672.2005.02634.x. [DOI] [PubMed] [Google Scholar]
- Nornber MFBL, Friedrich RSC, Weiss RDN, Tondo EC, Brandelli A. Proteolytic activity among psychrotrophic bacteria isolated from refrigerated raw milk. Int J Dairy Technol. 2009;63:41–46. [Google Scholar]
- Pinto CLO, Martins ML, Vanetti MCD. Qualidade microbiológica de leite refrigerado e isolamento de bactérias psicrotróficas proteolíticas. Cienc Tecnol Aliment. 2006;26:1–7. [Google Scholar]
- Pinto UM, Costa ED, Mantovani HC, Vanetti MCD. The proteolytic activity of Pseudomonas fluorescens 07A isolated from milk is not regulated by quorum sensing signals . Braz J Microbiol. 2010;41:91–96. doi: 10.1590/S1517-838220100001000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley, et al. The complex microbiota of raw milk. FEMS Microbiol Rev. 2013;37:664–698. doi: 10.1111/1574-6976.12030. [DOI] [PubMed] [Google Scholar]
- Rajmohan S, Dodd CE, Waites WM. Enzymes from isolates of Pseudomonas fluorescens involved in food spoilage . J Appl Microbiol. 2002;93:205–13. doi: 10.1046/j.1365-2672.2002.01674.x. [DOI] [PubMed] [Google Scholar]
- Riedel K, Carranza P, Gehrig P, Potthast F, Eberl L. Towards the proteome of Burkholderia cenocepacia H111: setting up a 2-DE reference map . Proteomics. 2006;6:207–216. doi: 10.1002/pmic.200500097. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor Laboratory; Cold Spring Harbor: 1989. [Google Scholar]
- Schokker EP, van Boekel MAJS. Production, purification and partial characterization of the extracellular proteinase from Pseudomonas fluorescens 22F . Int Dairy J. 1997;7:265–271. [Google Scholar]
- Setyorini E, Takenaka S, Murakami S, Aoki K. Purification and characterization of two novel halotolerant extracellular proteases from Bacillus subtilis strain FP-133 . Biosci Biotechnol Biochem. 2006;70:433–40. doi: 10.1271/bbb.70.433. [DOI] [PubMed] [Google Scholar]
- Sorhaug T, Stepaniak L. Psychrotrophs and their enzymes in milk and dairy products: quality aspects. Trends Food Sci Tech. 1997;8:35–37. [Google Scholar]
- Teo JWP, Zhang LH, Poh CL. Cloning and characterization of a metalloprotease from Vibrio harveyi strain AP6 . Gene. 2003;303:147–56. doi: 10.1016/s0378-1119(02)01151-4. [DOI] [PubMed] [Google Scholar]
- Waugh DS. An overview of enzymatic reagents for the removal of affinity tags. Protein Expres Purif. 2011;80:283–93. doi: 10.1016/j.pep.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmann M, Weilmeier D, Dineen SS, Ralyea R, Boor KJ. Molecular and phenotypic characterization of Pseudomonas spp. isolated from milk . Appl Environ Microbiol. 2000;66:2085–95. doi: 10.1128/aem.66.5.2085-2095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RG, Burger M, Beven CA, Beacham IR. The aprX-lipA operon of Pseudomonas fluorescens B52: a molecular analysis of metalloprotease and lipase production . Microbiology. 2001;147:345–54. doi: 10.1099/00221287-147-2-345. [DOI] [PubMed] [Google Scholar]